Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Pathophysiology and etiology | Clinical features | Diagnosis | Laboratory | Prognostic factors | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Peripheral smear description | Peripheral smear images | Positive stains | Electron microscopy description | Electron microscopy images | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Walia R, Yeung CCS. MDS with mutated SF3B1. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/myeloproliferativeRARS.html. Accessed April 25th, 2024.
Definition / general
- Myelodysplastic syndrome (MDS) characterized by morphological dysplasia with presence of ring sideroblasts (RS); may or may not have an SF3B1 mutation
Essential features
- Characterized by cytopenias, morphological dysplasia and ring sideroblasts, often with concomitant SF3B1 mutation
- In the absence of SF3B1 mutation, ≥ 15% ring sideroblasts are needed, whereas ≥ 5% ring sideroblasts are required for diagnosis in the presence of SF3B1 mutation
- Ring sideroblasts are erythroid precursors with ≥ 5 iron granules encircling a third or more of the nucleus
- Lacks Auer rods or del 5q as an isolated cytogenetic abnormality; myeloid blasts are not increased in the bone marrow or peripheral blood
Terminology
- Formerly called refractory anemia with ring sideroblasts, refractory cytopenia with multilineage dysplasia and ring sideroblasts
- Current nomenclature: MDS RS SLD (single lineage dysplasia) and MDS RS MLD (multilineage dysplasia)
ICD coding
- ICD-10: D46.1 - refractory anemia with ring sideroblasts
Epidemiology
- 3 - 11% of all MDS cases
- Median age: 60 - 73 years
- M = F
- Myelodysplastic syndrome with ring sideroblasts, multilineage dysplasia (MDS RS MLD) is more common than myelodysplastic syndrome with ring sideroblasts, single lineage dysplasia (MDS RS SLD)
- Reference: Am J Med 2012;125:S2
Pathophysiology and etiology
- SF3B1 gene encodes a component of U2 small nuclear ribonucleoproteins (snRNP) spliceosome
- Mutations in the SF3B1 gene lead to altered splicing of mitochondrial iron transporter gene and other metabolic genes, which in turn leads to the ineffective erythropoiesis and formation of ring sideroblasts
- Ring sideroblasts are erythroid precursors with abnormal iron accumulation within the mitochondria
- These changes lead to morphologic dysplasia, ineffective erythropoiesis and iron overload
- References: Blood 2016;128:462, Blood 2012;120:3173
Clinical features
- Anemia
- Thrombocytopenia and neutropenia in a minority
- Hepatosplenomegaly due to iron overload in some
- Reference: Am J Hematol 2021;96:379
Diagnosis
- Refractory anemia or bicytopenia / pancytopenia on complete blood count; 1 - 2 cytopenias MDS RS SLD, 1 - 3 cytopenias MDS RS MLD
- Bone marrow aspiration and biopsy, < 5% bone marrow blasts, < 1% peripheral blood blasts
- Molecular studies for diagnosis and prognosis
- Cytogenetic studies: any abnormality unless it fulfills criteria for MDS with isolated del(5q)
- References: Am J Hematol 2021;96:379, Blood 2016;127:2391
Laboratory
- Normochromic macrocytic or normochromic normocytic anemia (hemoglobin usually is < 10 g/dL), usually no other cytopenias but rarely may have thrombocytosis (correlate with JAK2 mutation status and diagnosis of MDS / myeloproliferative neoplasm with ring sideroblasts and thrombocytosis [MPN RS T]) (Am J Hematol 2021;96:379)
- Concurrent iron deficiency can mask the presence of MDS RS (Blood 2011;117:5793)
- May demonstrate lab values of progressive iron overload - e.g. elevated serum ferritin and transferrin saturation (Hemasphere 2020;4:e357)
Prognostic factors
- 1 - 2% cases evolve into acute myeloid leukemia (AML)
- Age > 70 years, hemoglobin < 8 g/dL in women and < 9 g/dL in men and platelet count < 75 x 109/L are poor prognostic factors
- Absence of SF3B1 mutation, presence of high risk cytogenetics, thrombocytopenia and multilineage dysplasia are poor prognostic factors
- Monosomal karyotype (MK), non-MK other than single or double del(5q) abnormalities, presence of RUNX1 and ASXL1 mutations (Am J Hematol 2019;94:475)
- More recently, SF3B1 mutant MDS proposed as a distinct subtype with relatively good prognosis (Blood 2020;136:157, Am J Clin Pathol 2021 May 12 [Epub ahead of print])
- Reference: Am J Hematol 2021;96:379
Case reports
- 16 year old girl with hypochromic microcytic anemia (Arch Pathol Lab Med 2005;129:e199)
- 59 year old man with beta thalassemia trait and MDS RS (NAJMS 2017;10:32)
- 73 year old woman with MDS with ring sideroblasts (ASH: Reference cases - MDS with ring sideroblasts [Accessed 13 July 2021])
Treatment
- Recombinant erythropoietin (EPO) for anemia
- Iron chelation therapy for iron overload
- Lusatercept and sotatercept: soluble fusion proteins that increase hemoglobin levels by a different mechanism than EPO (Am J Hematol 2019;94:475)
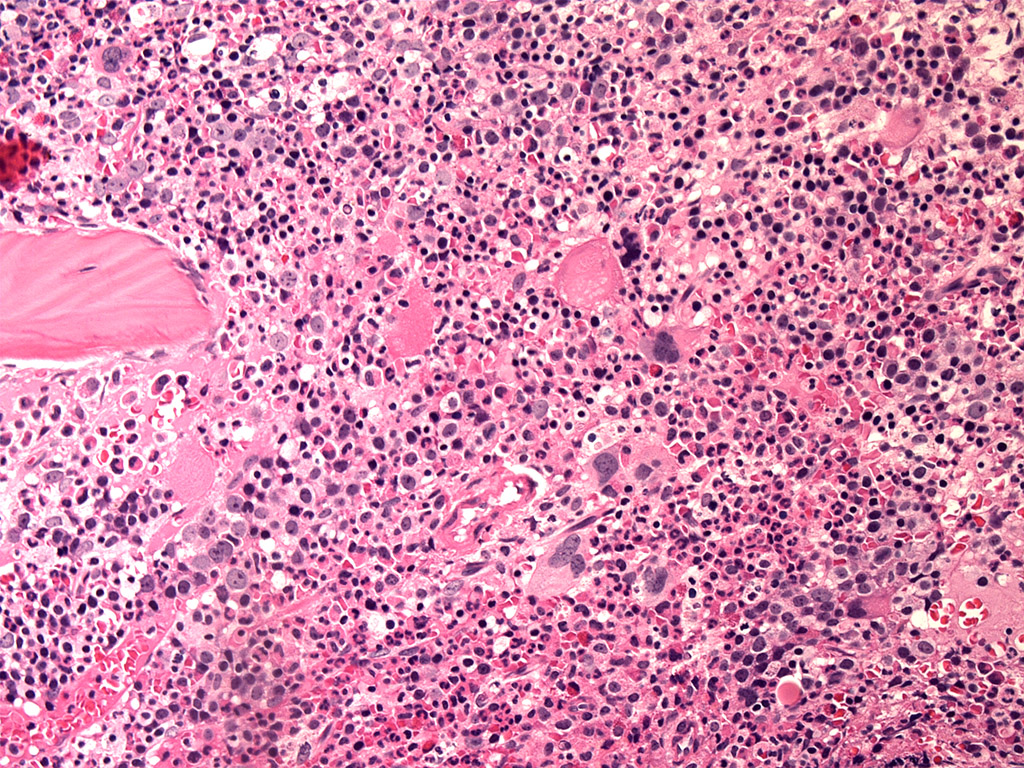
Microscopic (histologic) description
- Microscopic criteria
- Characterized by cytopenias (1 - 2 MDS RS SLD, 1 - 3 MDS RS MLD)
- Ring sideroblasts and morphological dysplasia (1 lineage [erythroid] MDS RS SLD, 2 - 3 lineages MDS RS MLD), often with concomitant SF3B1 mutations
- In the absence of SF3B1 mutations, ≥ 15% ring sideroblasts are needed, whereas > 5% ring sideroblasts are required for diagnosis in the presence of SF3B1 mutations (Blood 2012;119:5674)
- Ring sideroblasts are erythroid precursors with ≥ 5 iron granules encircling a third or more of the nucleus (Haematologica 2008;93:1712)
- Lacks Auer rods, < 5% bone marrow blasts, < 1% peripheral blood blasts
- Does not meet criteria for MDS with isolated del (5q); other cytogenetic abnormalities allowed
- Bone marrow histology
- Bone marrow general:
- Hypercellular or normocellular marrow with erythroid hyperplasia
- Bone marrow erythroid:
- Erythroid hyperplasia (may be mild to moderate dysplasia, including irregular nuclear contours, nuclear budding, megaloblastic change), markedly increased iron stores
- Bone marrow myeloid:
- Myeloblasts < 5% (or call MDS with excess blasts)
- Bone marrow megakaryocytes:
- Normal numbers and morphology (in SLD)
- In addition to presence of ring sideroblasts and erythroid hyperplasia and dysplasia, > 10% dysplastic cells within the myeloid or megakaryocytic series in multilineage dysplasia subtype
- Bone marrow general:
- Reference: Mediterr J Hematol Infect Dis 2015;7:e2015035, Blood 2016;127:2391
Microscopic (histologic) images
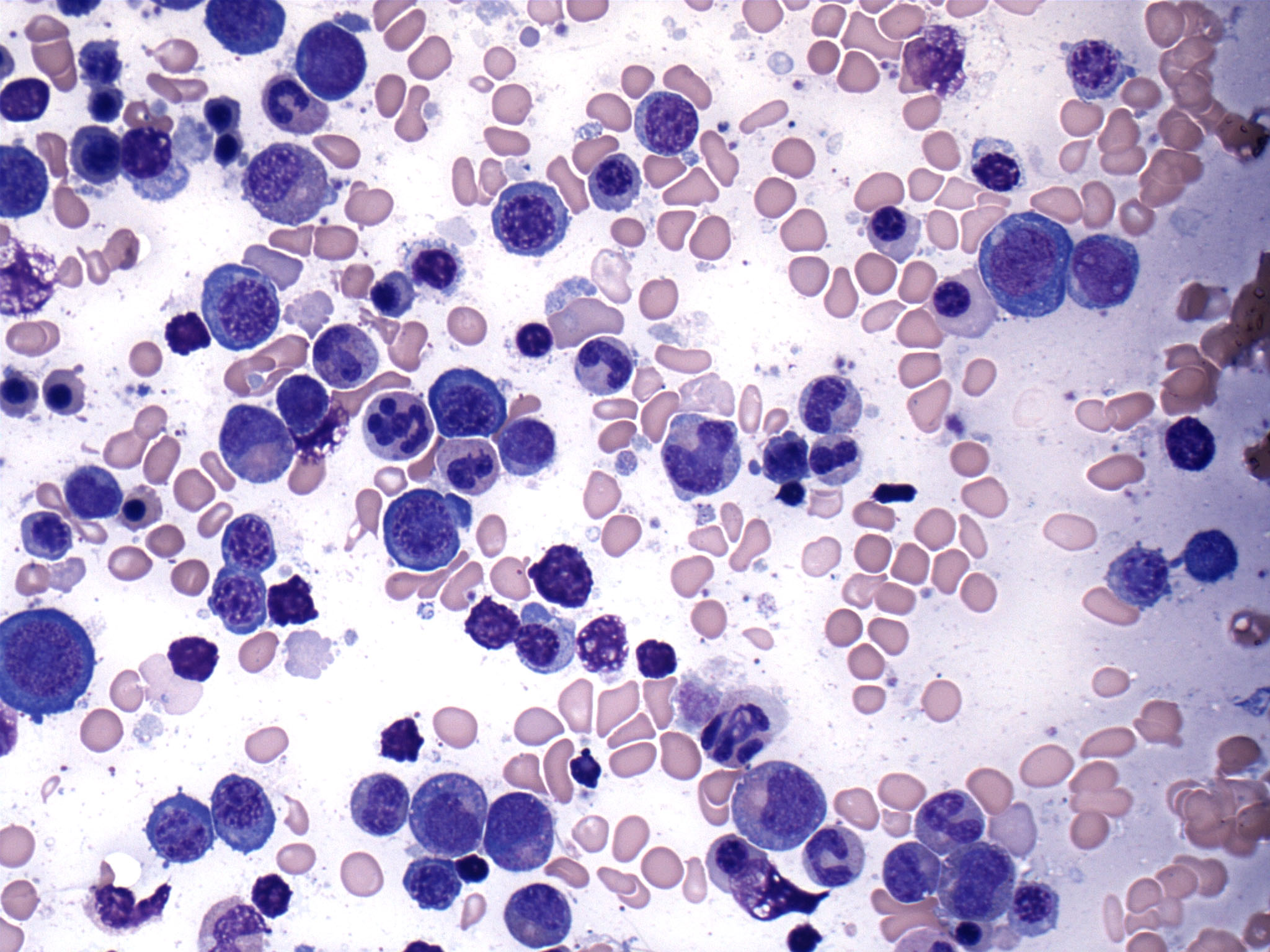
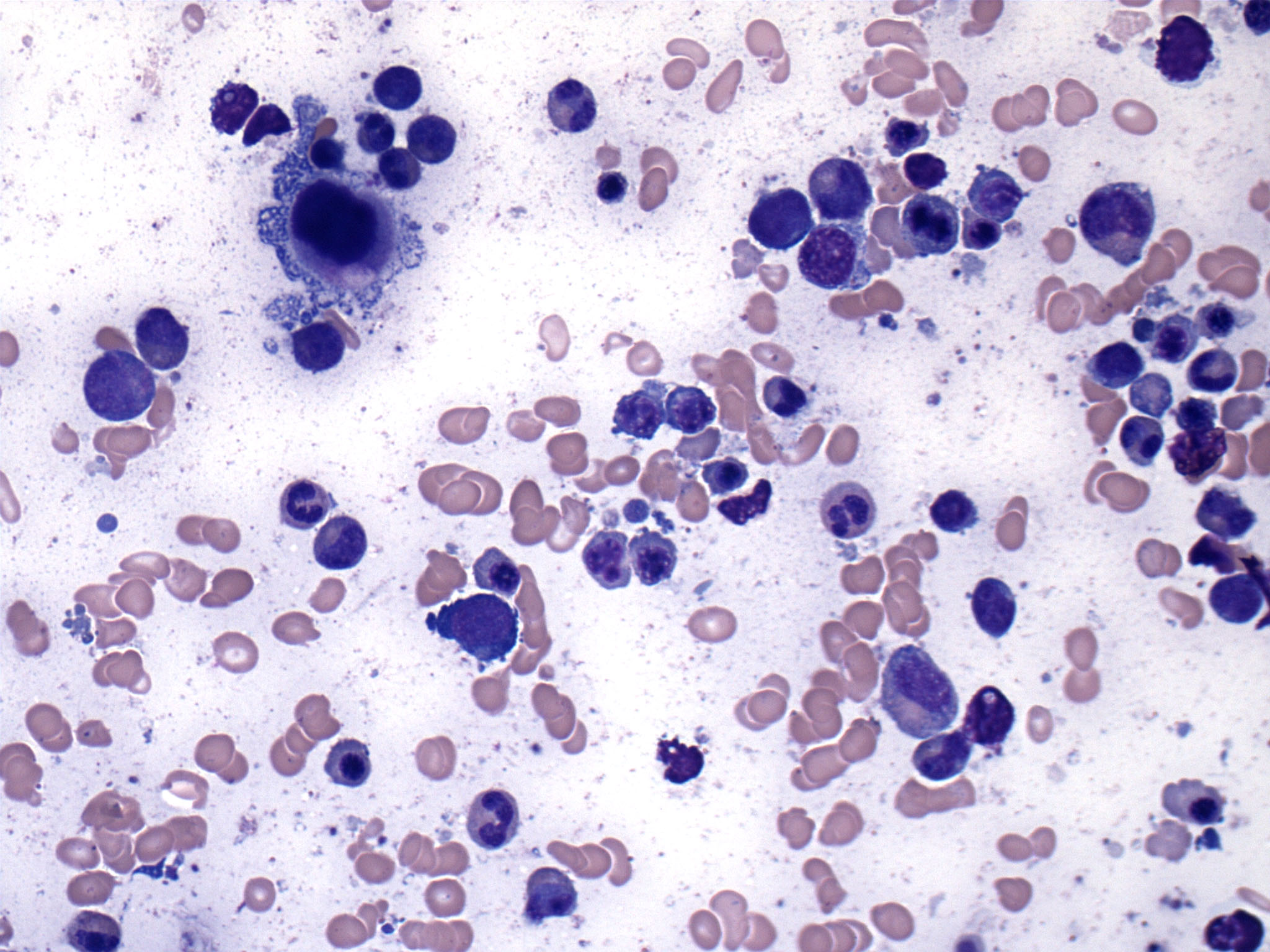
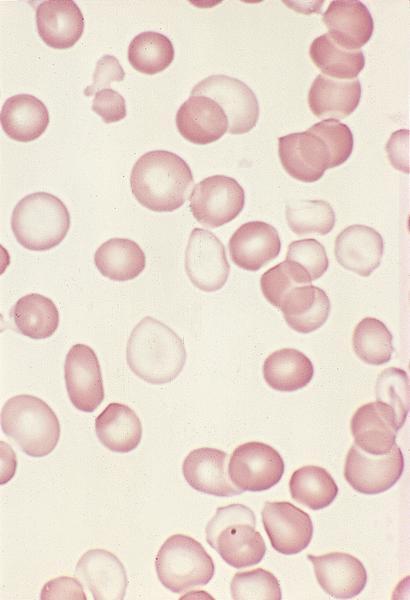
Cytology description
Peripheral smear description
- Peripheral blood:
- Dimorphic red blood cell populations normal and microcytic hypochromic
- Occasional coarse basophilic stippling and Pappenheimer bodies
- < 1% myeloblasts
- Evaluate for cytopenias and granulocytic dysplasia to subclassify
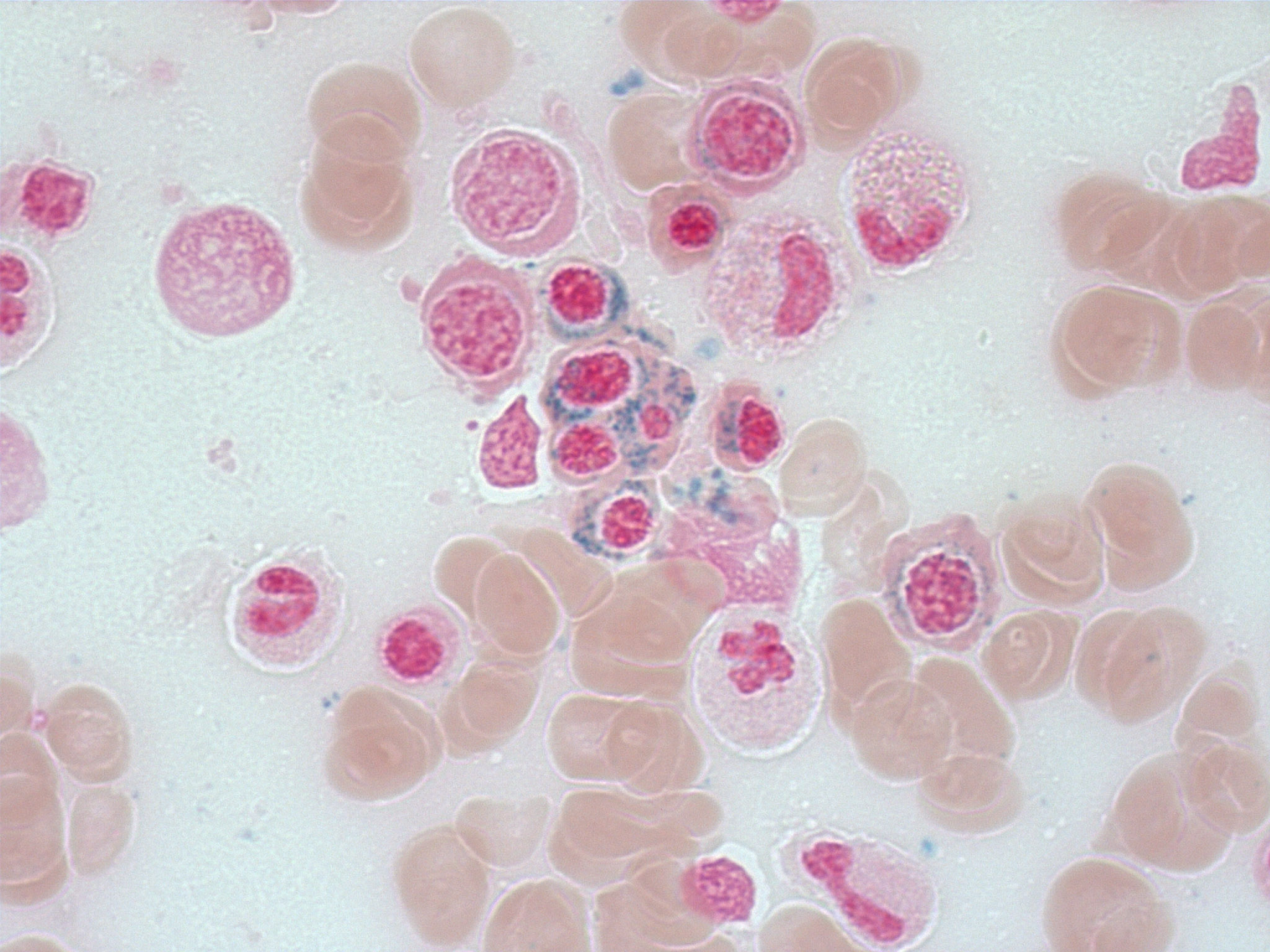
Positive stains
- Iron stain highlights the ring sideroblasts
- Reticulin stain is used to assess the degree of fibrosis in the bone marrow biopsy
- CD34 is used to highlight the blasts in the bone marrow biopsy
- Reference: Hematol Transfus Cell Ther 2019;41:279
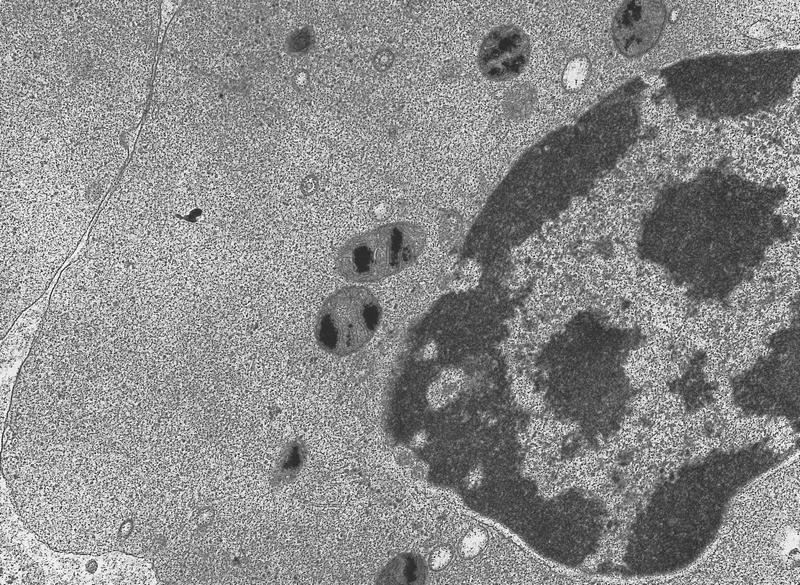
Electron microscopy description
- Transmission electron microscopy analysis of ring sideroblasts reveals features of apoptosis with condensed nuclear chromatin and fragmentation of the erythroid cells because of iron deposition in mitochondria (Oncogene 2006;25:4757)
Molecular / cytogenetics description
- Mutation in the spliceosome gene SF3B1 is found in 90% cases of MDS RS SLD and in 30 - 70% of MDS RS MLD
- Clonal cytogenetic abnormalities involve a single chromosome in 50% of MDS RS MLD cases, including high risk loss of chromosome 7
- Other MDS related gene mutations are identified in more than half of cases of MDS MLD, including mutations in STAG2, ASXL1, SRSF2, RUNZ1, CBL, TP53 and TET2
Sample pathology report
- Bone marrow, aspiration and biopsy:
- Final diagnosis
- Myelodysplastic syndrome with ring sideroblasts with multilineage dysplasia (see comment)
- Negative for increased blasts
- Positive for SF3B1 mutation
- Peripheral blood with normocytic normochromic anemia and pancytopenia
- Comment: The bone marrow aspirates are cellular and demonstrate trilineage dysplasia with presence of 40% ring sideroblasts and without significant increase in blast count (3% by morphology and flow cytometry). SF3B1 mutations are identified by next generation sequencing. Overall, the morphologic, flow cytometric and molecular features are diagnostic of myelodysplasia with ring sideroblasts with multilineage dysplasia. Cytogenetic studies reveal loss of chromosome 7, which is associated with high risk of leukemic transformation.
- Cytogenetics
- Loss of chromosome 7
- Molecular studies
- SF3B1 mutation
- Flow cytometry
- 3% myeloid blasts (see comment)
- Comment: Bone marrow flow cytometry reveals presence of 3% myeloid blasts expressing CD33, CD13, CD117 and MPO. There is no abnormal B cell clone identified and B cells constitute approximately 4% of all nucleated cells. Plasma cells comprise 2% of all nucleated cells and normally express CD138 and CD38.
- 3% myeloid blasts (see comment)
- Peripheral blood
- Differential:
- Complete blood count: 3,500/cumm
- Neutrophils: 60%
- Lymphocytes: 30%
- Eosinophils: 4%
- Monocytes: 4%
- Basophils: 2%
- Blasts: 0%
- Morphology:
- Red blood cells: decreased numbers, normocytic normochromic with mild anisopoikilocytosis and polychromasia
- White blood cells: leukopenia, no blasts, normal morphology
- Platelets: mild thrombocytopenia, few giant platelets seen
- Differential:
- Bone marrow aspirate
- Differential:
- Erythroid: 65%
- Myeloid: 32%
- Lymphocytes: 3%
- Plasma cells: 2%
- Blasts: 3%
- M:E ratio: 1:2
- Morphology:
- Erythroid: erythroid hyperplasia with features of dyserythropoeisis - binucleation, nuclear budding, nucleocytoplasmic asynchrony
- Myeloid: normal maturation with mild dysgranulopoeisis
- Megakaryocytes: normal in numbers with hypolobated and monolobated forms
- Plasma cells: normal in number and morphology
- Lymphocytes: normal in number and morphology
- Iron stain: 40% ring sideroblasts are identified with presence of perinuclear iron granules
- Differential:
- Bone marrow biopsy
- Morphology:
- Hypercellular (70% cellularity) bone marrow with erythroid hyperplasia, dyserythropoiesis, dysgranulopoeisis and dysmegakaryopoeisis
- Reticulin stain shows grade I fibrosis
- Morphology:
Differential diagnosis
- MDS / MPN with ring sideroblasts and thrombocytosis:
- Clonal disorder with diagnostic features of MDS RS SLD
- Platelet count of ≥ 450 × 109/L (persistent)
- Large, atypical megakaryocytes
- Various nonclonal conditions can present with ring sideroblasts in the bone marrow; thorough clinical history and absence of diagnostic criteria for clonal MDS RS (> 15% RS or SF3B1 mutation with > 5% RS) are essential for differential diagnosis
- Genetically inherited congenital sideroblastic anemia (Br J Haematol 2016;174:847):
- Characterized by mutations in 1 of 3 mitochondrial pathways: heme synthesis, iron sulfur cluster biogenesis and protein synthesis
- X linked sideroblastic anemia is the most common form attributed to mutation in aminolevulinic acid (ALA) synthase
- Microcytic hypochromic anemia and iron overload
- Acquired sideroblastic anemia due to chronic alcohol use (Postgrad Med 1992;92:147):
- History of chronic alcoholism
- Elevated serum aspartate aminotransferase (AST):alanine aminotransferase (ALT) ratio
- Macrocytic anemia
- < 15% ring sideroblasts
- Drug induced sideroblastic anemia - chloramphenicol, penicillamine, linezolid, isoniazid (Haematologica 2013;98:e138):
- History of medication use
- May have pure red cell aplasia
- Reversible after discontinuation of drug
- Lead poisoning:
- High serum lead levels, lead lines (Burton lines) on teeth and gingiva
- < 15% ring sideroblasts
- Basophilic stippling in red cells on peripheral smear, microcytic and hemolytic anemia
- Copper deficiency / zinc toxicity (Clin Case Rep 2015;3:325):
- Low serum copper levels or high serum zinc levels
- < 15% ring sideroblasts
- Cytoplasmic vacuoles in erythroid and myeloid cells
- Genetically inherited congenital sideroblastic anemia (Br J Haematol 2016;174:847):
- Reference: Am J Hematol 2021;96:379
Additional references
Board review style question #1
Board review style answer #1
Board review style question #2
How does the finding of an SF3B1 mutation impact the diagnosis of myelodysplastic syndrome (MDS) with ring sideroblasts?
- If no SF3B1 mutation is seen, only 5% ring sideroblasts are needed to make the diagnosis of MDS with ring sideroblasts
- If SF3B1 mutation is seen, only 5% ring sideroblasts are needed to make the diagnosis of MDS with ring sideroblasts
- If SF3B1 mutation is seen, only 10% ring sideroblasts are needed to make the diagnosis of MDS with ring sideroblasts
- There is no impact; 15% ring sideroblasts are required for the diagnosis of MDS with ring sideroblasts
Board review style answer #2
B. If SF3B1 mutation is seen, only 5% ring sideroblasts are needed to make the diagnosis of MDS with ring sideroblasts
Comment Here
Reference: MDS with ring sideroblasts
Comment Here
Reference: MDS with ring sideroblasts