Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Prognostic factors | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Peripheral smear description | Peripheral smear images | Positive stains | Flow cytometry description | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Additional references | Practice question #1 | Practice answer #1 | Practice question #2 | Practice answer #2 | Practice question #3 | Practice answer #3Cite this page: Kunnath-Velayudhan S, Geyer JT. MDS with multilineage dysplasia. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/myeloproliferativercmd.html. Accessed September 15th, 2025.
Definition / general
- Myelodysplastic syndrome (MDS) with multilineage dysplasia is characterized by 1 or more cytopenias and dysplastic changes in 2 or more of the myeloid lineages (erythroid, granulocytic and megakaryocytic)
- Myelodysplastic syndrome with ring sideroblasts and multilineage dysplasia (MDS RS MLD) if ring sideroblasts are > 15% (> 5% with SF3B1 mutation)
Essential features
- Dysplastic changes in 2 or more of the myeloid lineages
- Blast counts < 1% in the peripheral blood and < 5% in the bone marrow
- Assess ring sideroblasts (< 15%) and SF3B1 mutation status (if sideroblasts comprise 5 - 15% of erythroid elements) to categorize as MDS MLD or MDS RS MLD
- Monocytes < 1 x 109/L
- Exclusion of other causes of dysplasia
- References: Leukemia 2015;29:66, Leukemia 2007;21:668
Terminology
- Refractory cytopenia with multilineage dysplasia
- Myelodysplastic syndrome with ring sideroblasts and multilineage dysplasia (MDS RS MLD)
ICD coding
Epidemiology
- Median age: 67 - 70 years (M = 70 - 74, F = 75 - 79) (Leuk Res 2012;36:727)
- M > F (Leuk Res 2012;36:727)
- 30% of all cases of MDS (Leuk Res 2013;37:64)
Sites
- Bone marrow
Pathophysiology
- Cell of origin is a hematopoietic stem cell
- Complex interplay between genetic and epigenetic alterations and the bone marrow microenvironment lead to dysregulated hematopoietic differentiation, resulting in impaired differentiation, morphological dysplasia and cytopenia
Etiology
- Age associated risk attributed to hematopoietic stem cell mutations
- Exposure to toxins (e.g., benzene, cigarette smoking, agricultural chemicals or solvents)
- Family history of hematopoietic neoplasms
- Acquired aplastic anemia
- References: N Engl J Med 2014;371:2477, N Engl J Med 2014;371:2488
Clinical features
- Unicytopenia, bicytopenia (common)
- Pancytopenia, milder cytopenia (less common)
Diagnosis
- Peripheral blood analysis
- Bone marrow examination
Laboratory
- Hemoglobin concentration < 10 g/dL (recommended by 2016 WHO)
- Platelet count < 100 x 109/L (recommended by 2016 WHO)
- Standard hematologic values for cytopenia (hemoglobin < 13 g/dL [males], < 12 g/dL [females] and platelets < 150 109/L) are also used by some authors
- Absolute neutrophil count < 1.8 x 109/L
- Monocytes < 1 x 109/L
- Blasts < 1% in the peripheral blood
- Reference: Blood 2016;128:2096
Prognostic factors
- Median overall survival 36 months
- Evolution of acute leukemia is ~15% at 2 years and 28% at 5 years
- Revised International Prognostic Scoring System (IPSS-R) scores
- Abnormal findings in flow cytometry analysis (see Flow cytometry description)
- Karyotype
- References: Leuk Res 2013;37:64, J Clin Oncol 2005;23:7594
Case reports
- 17 year old girl with cri du chat syndrome (Int J Hematol 2020;112:728)
- 64 year old woman with longstanding anemia and fatigue (Intern Med 2017;56:1213)
- 70 year old man with a 6 - 8 year history of anemia and thrombocytopenia (Case Rep Hematol 2017;2017:3625946)
- 70 year old woman with pancytopenia (Case Rep Hematol 2012;2012:167653)
Treatment
- Transfusion
- Iron chelation
- Erythropoiesis stimulating factors
- Immunosuppressive treatment
- Lenalidomide for lower risk del(5q) MDS
- Luspatercept for MDS with ring sideroblasts
- Azacytidine
- Decitabine
- Induction chemotherapy
- Stem cell transplantation
- Reference: Blood 2013;122:2943
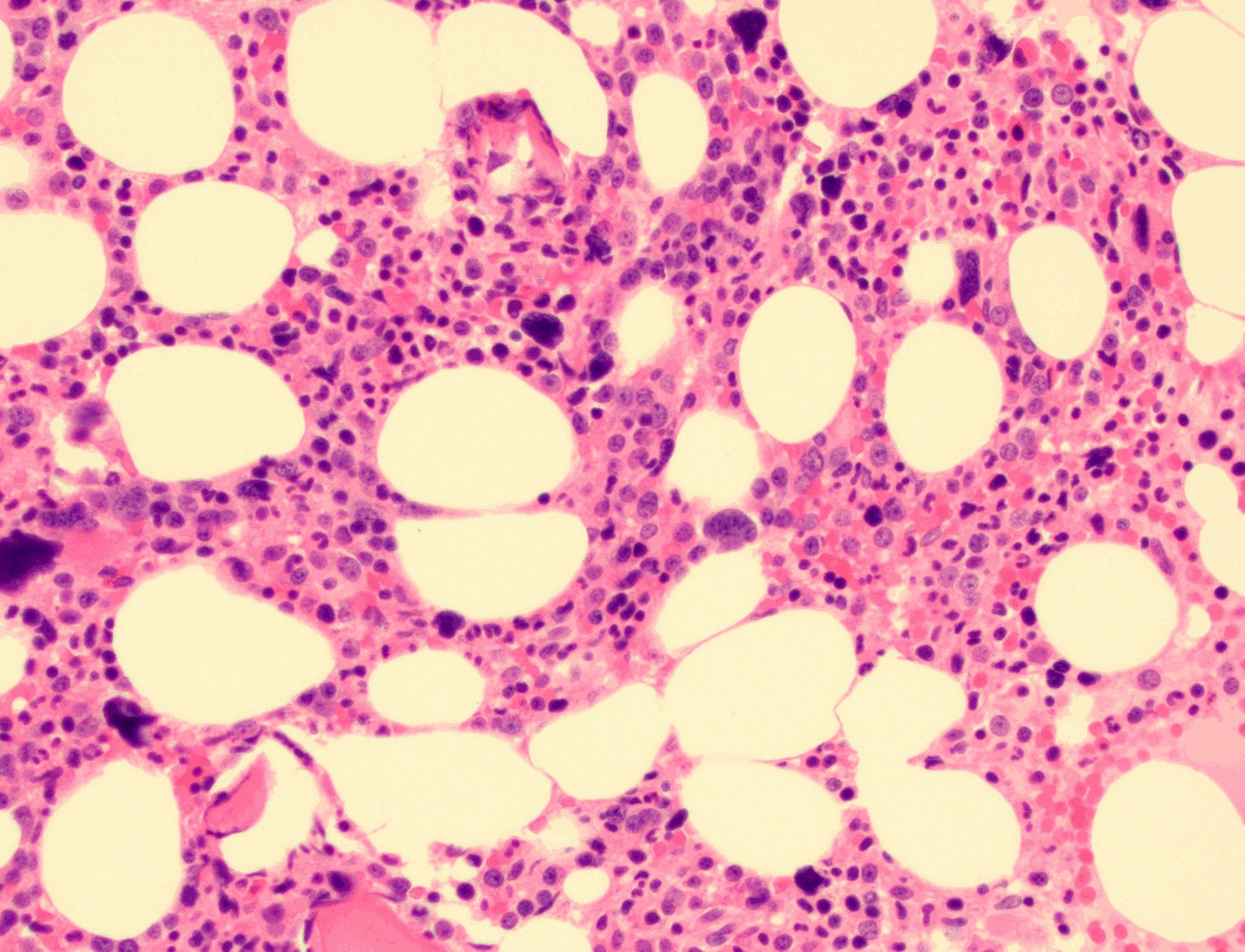
Microscopic (histologic) description
- Bone marrow findings (Leuk Res 2013;37:64)
- General
- Cellularity: majority of cases are normocellular or hypercellular; a subset of MDS-MLD show age adjusted hypocellularity
- Erythroid hyperplasia
- Myeloid hyperplasia with left shift
- Myeloblasts must be < 5%
- Occasional fibrosis (16% of cases)
- Erythroid lineage dysplasia
- Nuclear: nuclear budding, internuclear bridging, karyorrhexis, multinuclearity, megaloblastoid changes
- Cytoplasmic: ring sideroblasts (< 15%, < 5% with SF3B1 mutation)
- Vacuolization
- Myeloid lineage dysplasia
- Small or unusually large size
- Nuclear cytoplasmic asynchrony
- Nuclear hyposegmentation (pseudo Pelger-Huët anomaly)
- Nuclear hypersegmentation
- Decreased granules; agranularity
- Pseudo Chédiak-Higashi granules
- Döhle bodies
- Megakaryocyte dysplasia
- Micromegakaryocytes (most reliable and reproducible)
- Nuclear hypolobation or nonlobation
- Binucleation or multinucleation
- General
Microscopic (histologic) images
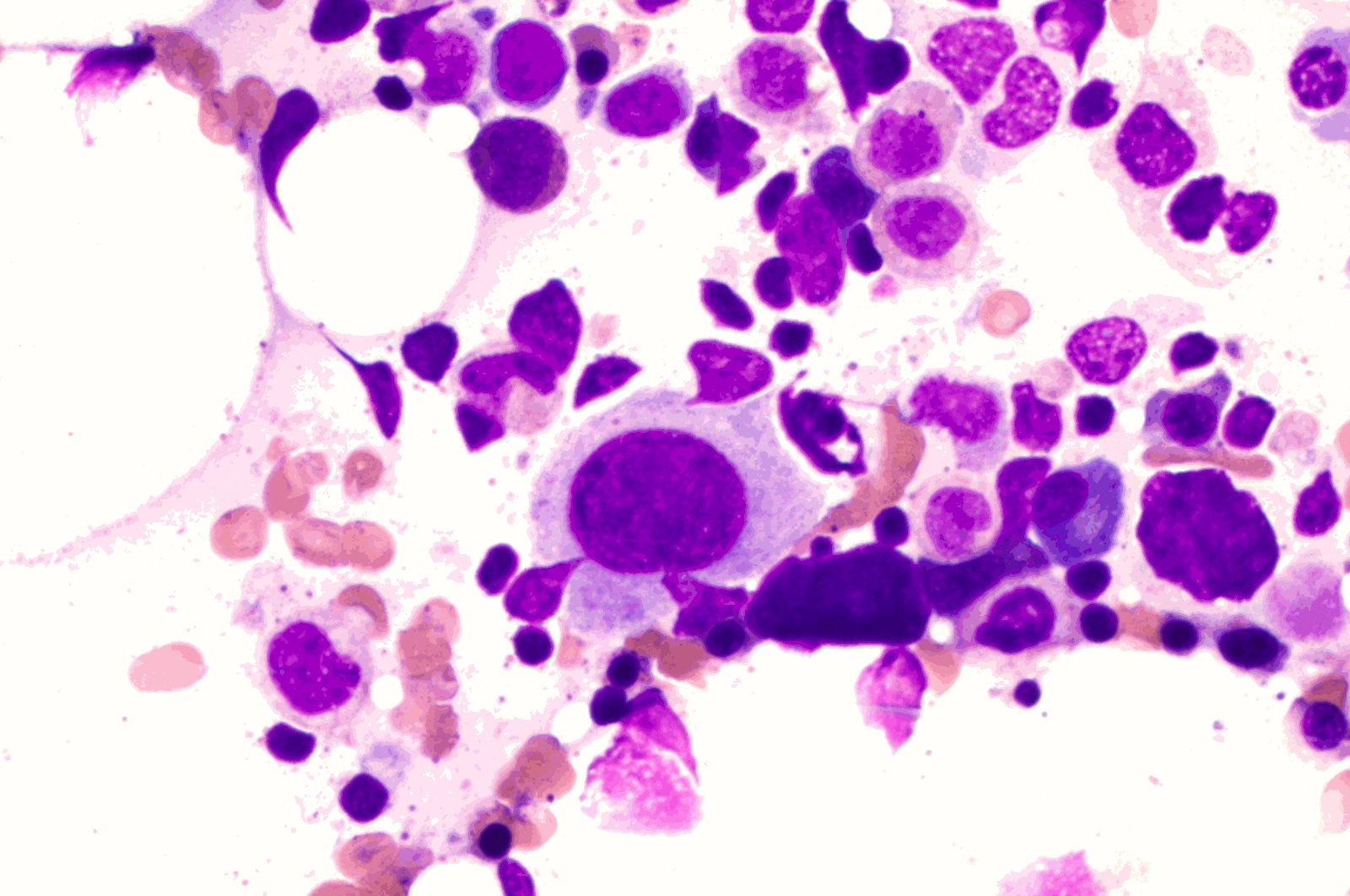
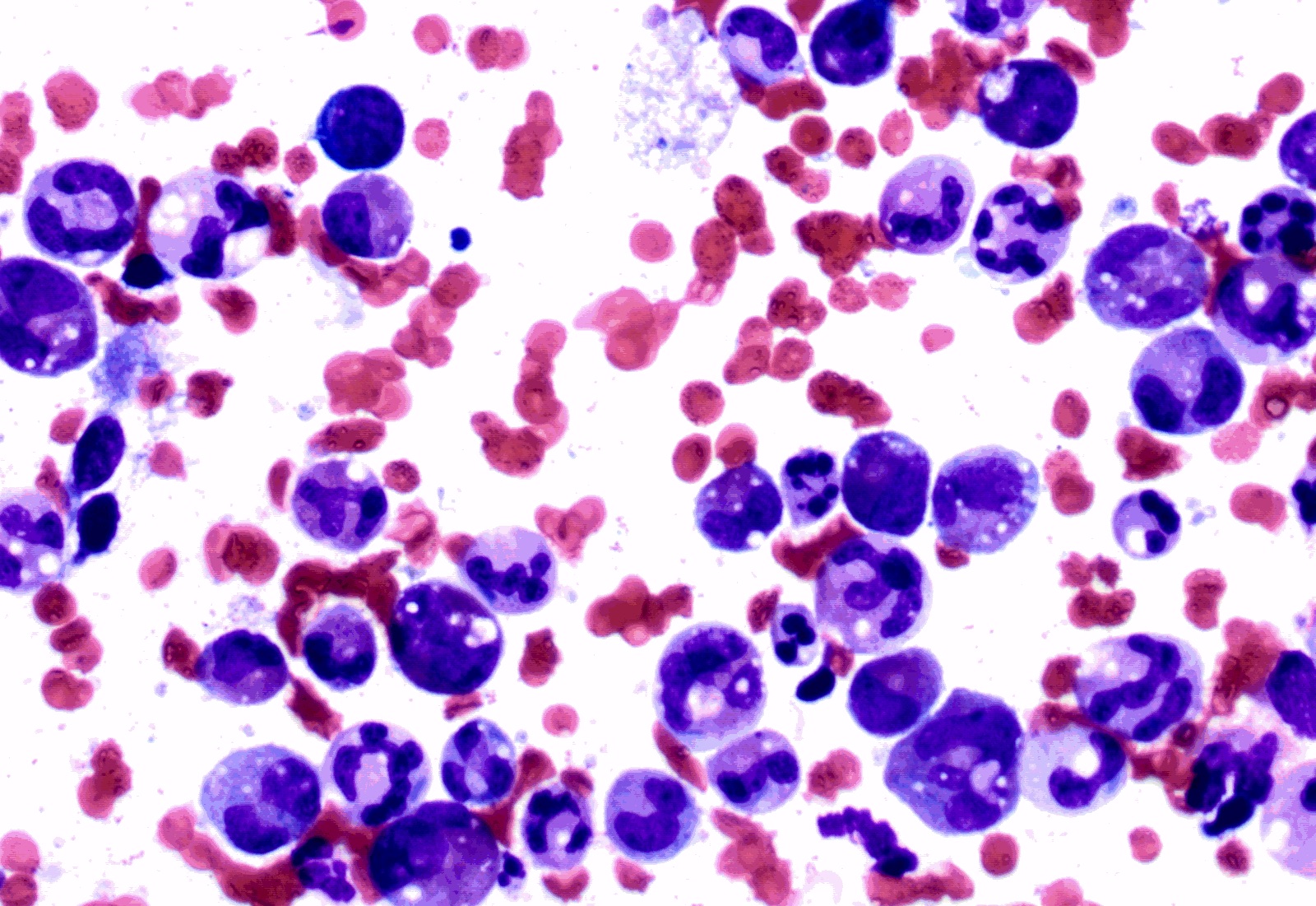
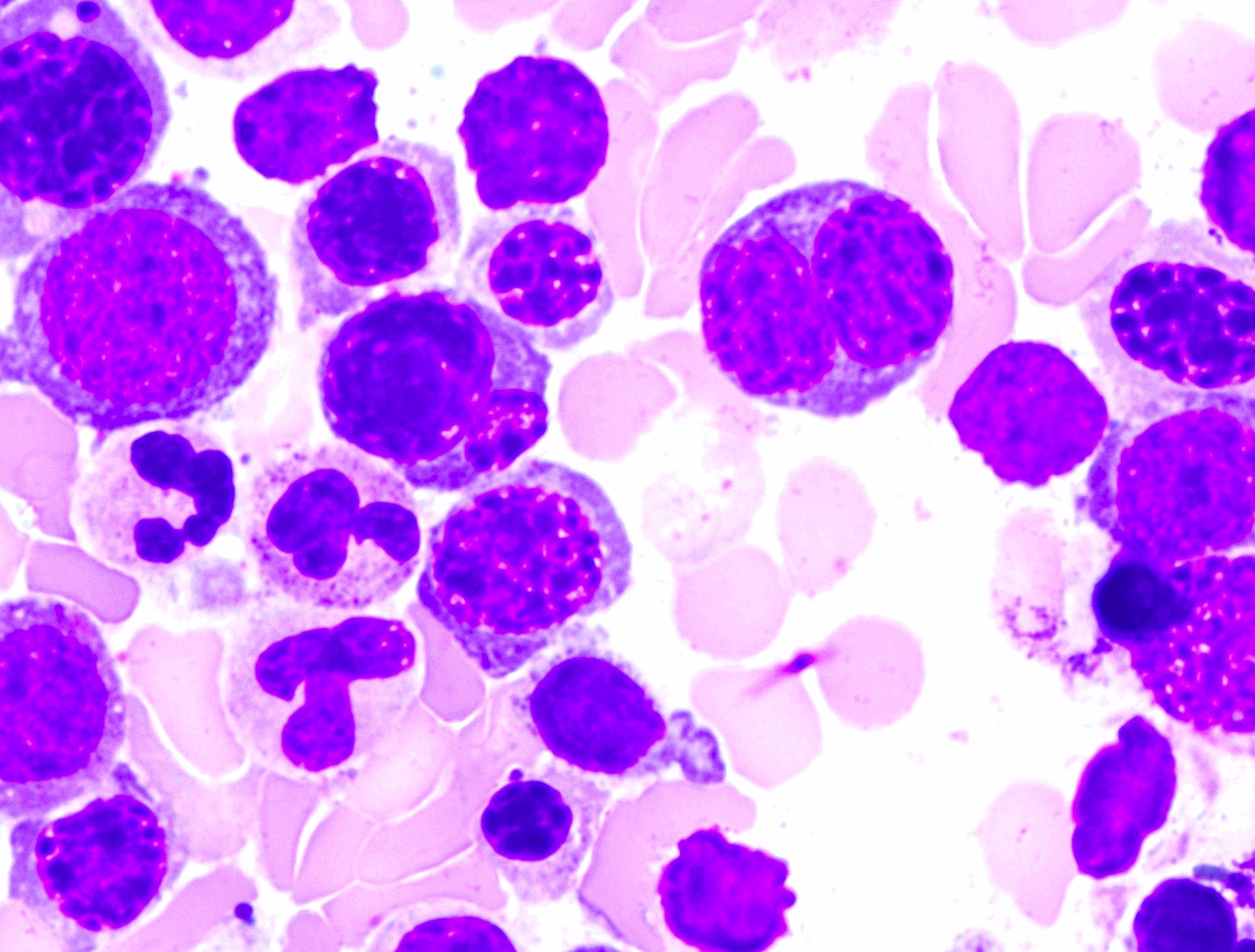
Peripheral smear description
- Red blood cells: anisopoikilocytosis with macrocytosis of red blood cells
- White blood cells
- Nuclear clumping
- Hyposegmentation
- Lack of lobation (pseudo Pelger-Huët anomaly)
- Cytoplasmic hypogranularity or agranularity
- Abnormal granular clumping (similar to Chédiak-Higashi syndrome): infrequent
- No increase in blasts (must be < 1%)
- Reference: Haematologica 2013;98:568
Positive stains
Flow cytometry description
- Flow cytometry findings alone are not diagnostic
- Decreased side scatter of granulocytes
- Erythroid: increased coefficient of variation and decreased intensity of CD71 or CD36
- Aberrant expression of CD56 or CD7 on progenitors, granulocytes or monocytes
- Asynchrony of CD15 and CD16 on granulocytes
- Altered expression of CD13 in relation to CD11b or CD16
- Reference: Leukemia 2014;28:1793
Molecular / cytogenetics description
- Cytogenetics
- Trisomy 8
- Monosomy 7
- del(7q)
- Monosomy 5
- del(5q)
- del(20q)
- Complex karyotypes
- Molecular aberrations
- Cohesion family (STAG2)
- Chromatin modifiers (ASXL1)
- Spliceosome genes (SRSF2)
- Transcription factors (RUNX1)
- Signaling molecules (CBL)
- Tumor suppressors (TP53)
- DNA modifiers (TET2)
- SF3B1 (in setting of MDS RS MLD with at least 5% ring sideroblasts)
- Reference: Leuk Res 2013;37:64
Videos
What is multilineage dysplasia?
Sample pathology report
- Bone marrow, posterior iliac crest, core biopsy, clot section, aspirate smears and touch imprint:
- Myelodysplastic syndrome with trilineage dysplasia
- Flow cytometry: abnormal myeloid blasts present
- Peripheral blood: anemia, moderate, macrocytic
- Cytogenetics: 46,XY,+8[16]/46,XY[4]
- Next generation sequencing:
- SRSF2 P95R with mutant allele frequency of 31%
- ASXL1 E635%fs*15 with mutant allele frequency of 28%
Differential diagnosis
- Secondary causes of dysplasia:
- Nutritional deficiencies, toxic exposures, drugs and biologic agents, infection, congenital disorders and sideroblastic anemias
- History and physical examination
- Appropriate laboratory investigations
- Myelodysplastic syndrome with single lineage dysplasia:
- Only 1 hematopoietic lineage shows dysplasia
- Myelodysplastic syndrome with excess blasts:
- 5 - 19% myeloblasts in the bone marrow or 2 - 19% blasts in the peripheral blood
- Presence of Auer rods in blasts
- Myelodysplastic syndrome, unclassifiable:
- MDS (MDS with excess blasts excluded) associated with 1% blasts in the peripheral blood on 2 separate occasions
- MDS (MDS with multilineage dysplasia excluded) associated with pancytopenia
- Persistent cytopenia with < 2% blasts in the blood and < 5% in the bone marrow and no significant dysplasia in any myeloid lineage and the presence of cytogenetic abnormalities considered presumptive evidence of MDS
Additional references
Practice question #1
A 60 year old man with anemia underwent bone marrow core biopsy (an H&E image is shown). Which of the following is essential to make the diagnosis of myelodysplastic syndrome?
- Absence of secondary causes of dysplasia
- Presence of dysplasia in the erythroid lineage
- Presence of dysplasia in the megakaryocyte lineage
- Presence of dysplasia in the myeloid lineage
Practice answer #1
Practice question #2
A 90 year old man presents with fatigue and the peripheral blood smear examination shows dysplastic neutrophils. No blasts are present. The evaluation for secondary causes of anemia and dysplastic neutrophils are negative. The bone marrow examination shows dysplasia in erythroid and myeloid lineages and 2% blasts. The iron stain shows 16% ring sideroblasts. This condition is best classified as
- Myelodysplastic syndrome, unclassifiable
- Myelodysplastic syndrome with multilineage dysplasia
- Myelodysplastic syndrome with ring sideroblasts
- Myelodysplastic syndrome with ring sideroblasts and multilineage dysplasia
Practice answer #2
D. Myelodysplastic syndrome with ring sideroblasts and multilineage dysplasia
Comment Here
Reference: MDS with multilineage dysplasia
Comment Here
Reference: MDS with multilineage dysplasia
Practice question #3
Which of the following findings is considered the most reliable and reproducible dysplastic feature in the megakaryocyte lineage?
- Hyperchromatic nuclei
- Micromegakaryocytes
- Multinucleation
- Nuclear hypolobation
Practice answer #3