Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Clinical features | Radiology description | Radiology images | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Rooper L. SWI / SNF complex deficient sinonasal carcinoma. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/nasalSMARCB1deficient.html. Accessed April 17th, 2024.
Definition / general
- Aggressive carcinoma defined by SMARCB1 gene mutations and associated loss of immunohistochemical expression
- Provisional entity in El-Naggar: WHO Classification of Head and Neck Tumors, 4th Edition, 2017
Essential features
- Sinonasal carcinoma defined by inactivation of SMARCB1 gene
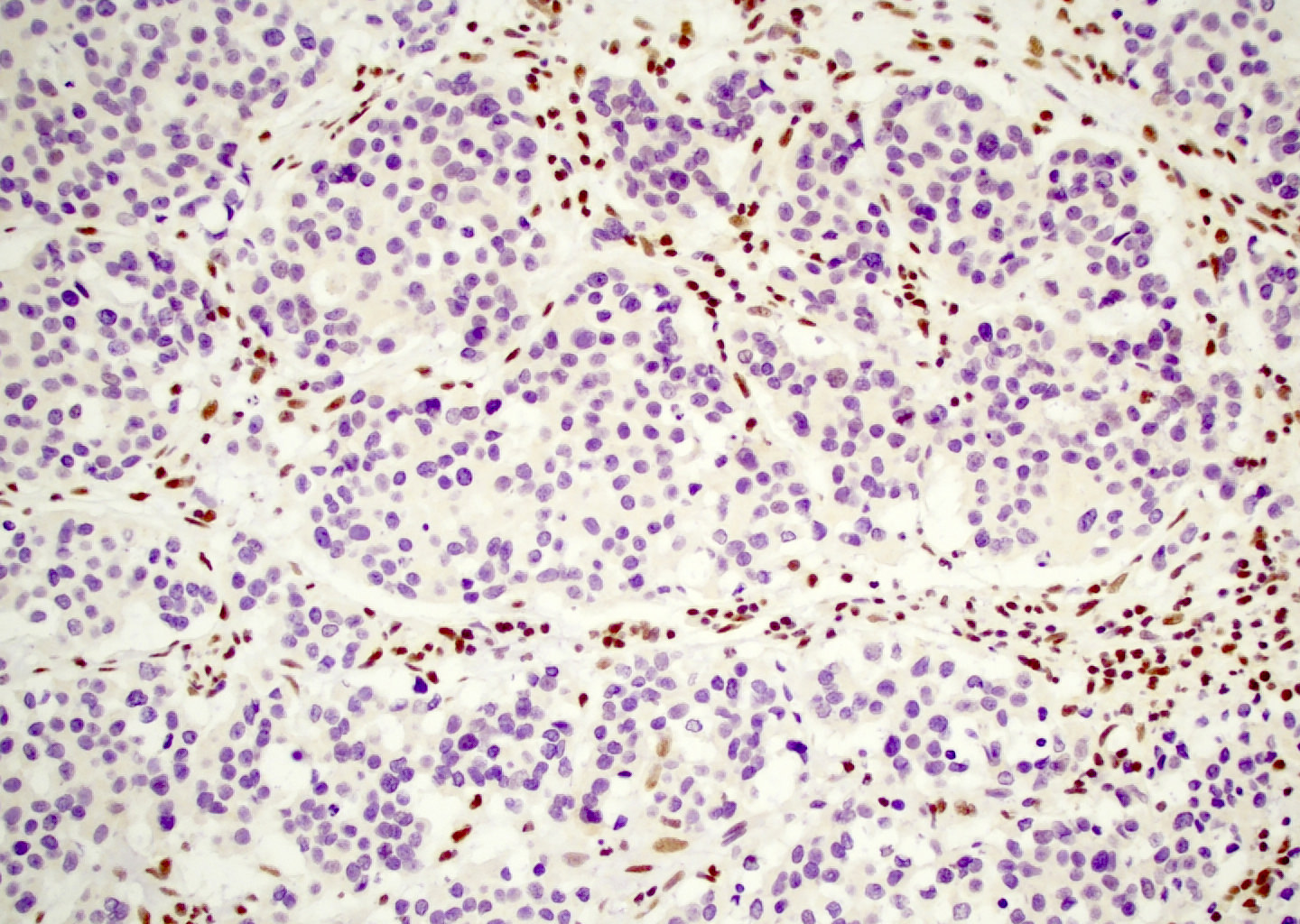
- Loss of SMARCB1 (INI1) immunohistochemical expression is highly sensitive and specific for diagnosis in this site
- Tumors are high grade but demonstrate cytological uniformity
- Tumors have variable proportions of basaloid and rhabdoid / plasmacytoid cells
- Highly aggressive clinical behavior
Terminology
- SMARCB1 stands for SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily B, member 1
- The protein product of the SMARCB1 gene is also sometimes known as INI1, which stands for integrase interactor 1
ICD coding
Epidemiology
- Described in patients from 16 to 89 years old (Virchows Arch 2015;467:649, Pathol Res Pract 2017;213:133)
- M:F = 3:2 (Am J Surg Pathol 2017;41:458)
- No clear clinical risk factors documented to date
Sites
- Exclusively occurs in sinonasal tract
- Most commonly arises in nasal cavity or ethmoid sinus but frequently involves multiple sites (Am J Surg Pathol 2017;41:458)
Clinical features
- Most present with symptoms of mass effect such as nasal obstruction, headache, epistaxis, proptosis or visual deficits (Am J Surg Pathol 2017;41:458)
- More than 80% present with stage T4 disease with bone, skull base or periorbital invasion
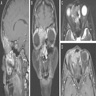
Radiology description
- Avid contrast enhancement with intermediate to low T2 signal and fluorodeoxyglucose (FDG) avidity
- Frequent calcification with occasional hair on end periosteal reaction (Am J Surg Pathol 2014;38:1274)
Case reports
- 44 year old woman with tumor showing yolk sac differentiation (Int J Surg Pathol 2018;26:245)
- 53 year old man with highly necrotic tumor (Ann Otol Rhinol Laryngol 2019;128:676)
- 53 year old man with endobronchial metastases (Acta Cytol 2019 May 27 [Epub ahead of print])
- 61 year old woman with orbital compartment syndrome (Surv Ophthalmol 2019 Apr 10 [Epub ahead of print])
- 77 year old man with retropharyngeal lymph node metastasis (Diagn Cytopathol 2016;44:700)
Treatment
- Best outcomes with trimodality therapy including surgical resection, external beam radiation and systemic chemotherapy (Head Neck Pathol 2017;11:256, Am J Surg Pathol 2017;41:458)
Gross description
- Highly infiltrative tumor that frequently invades multiple sinonasal sites (Head Neck Pathol 2017;11:256)
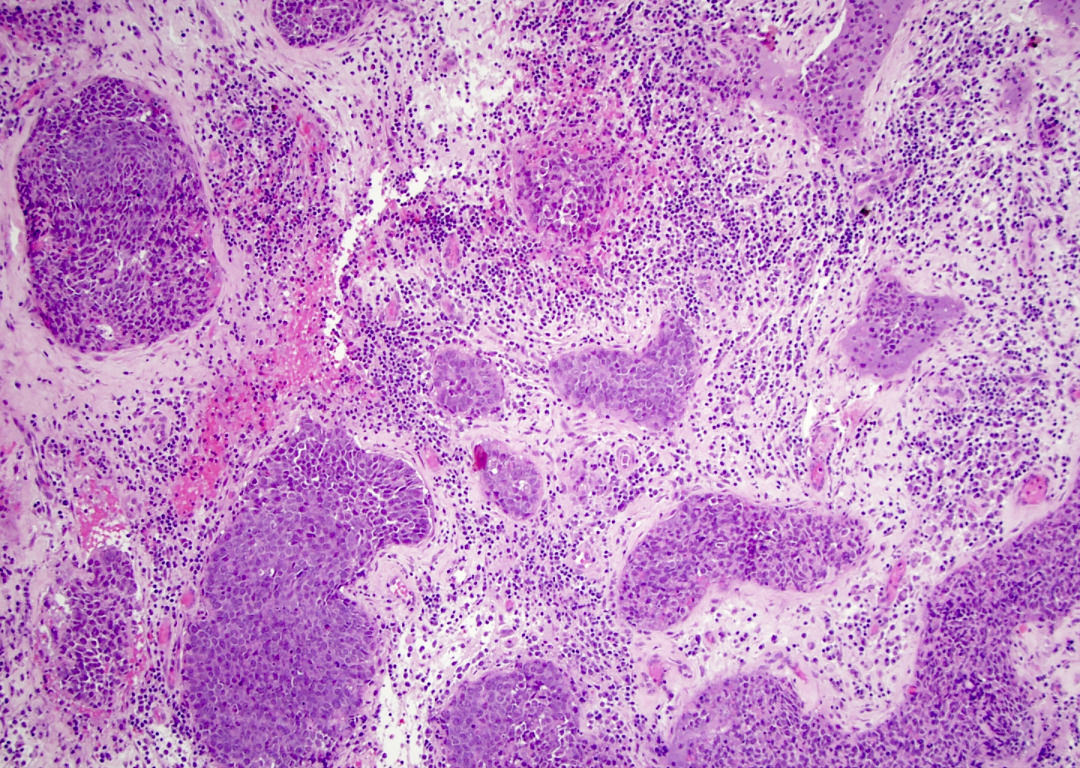
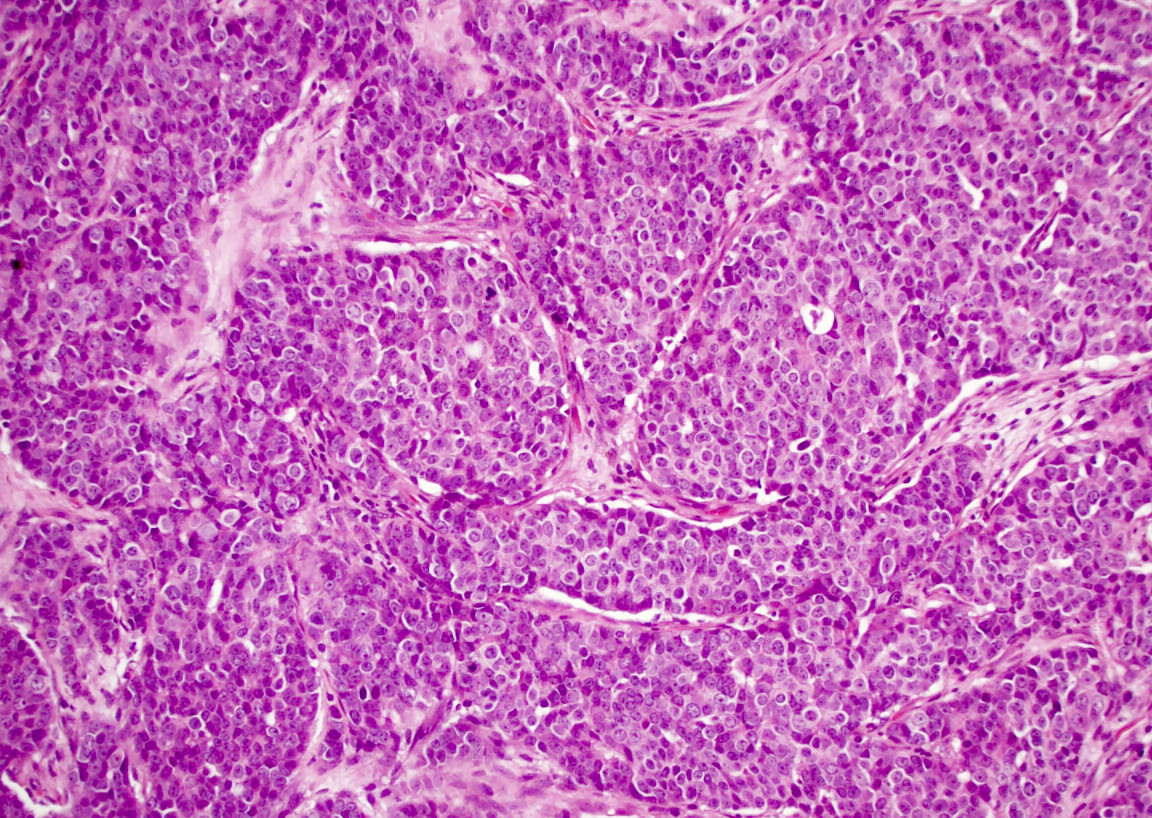
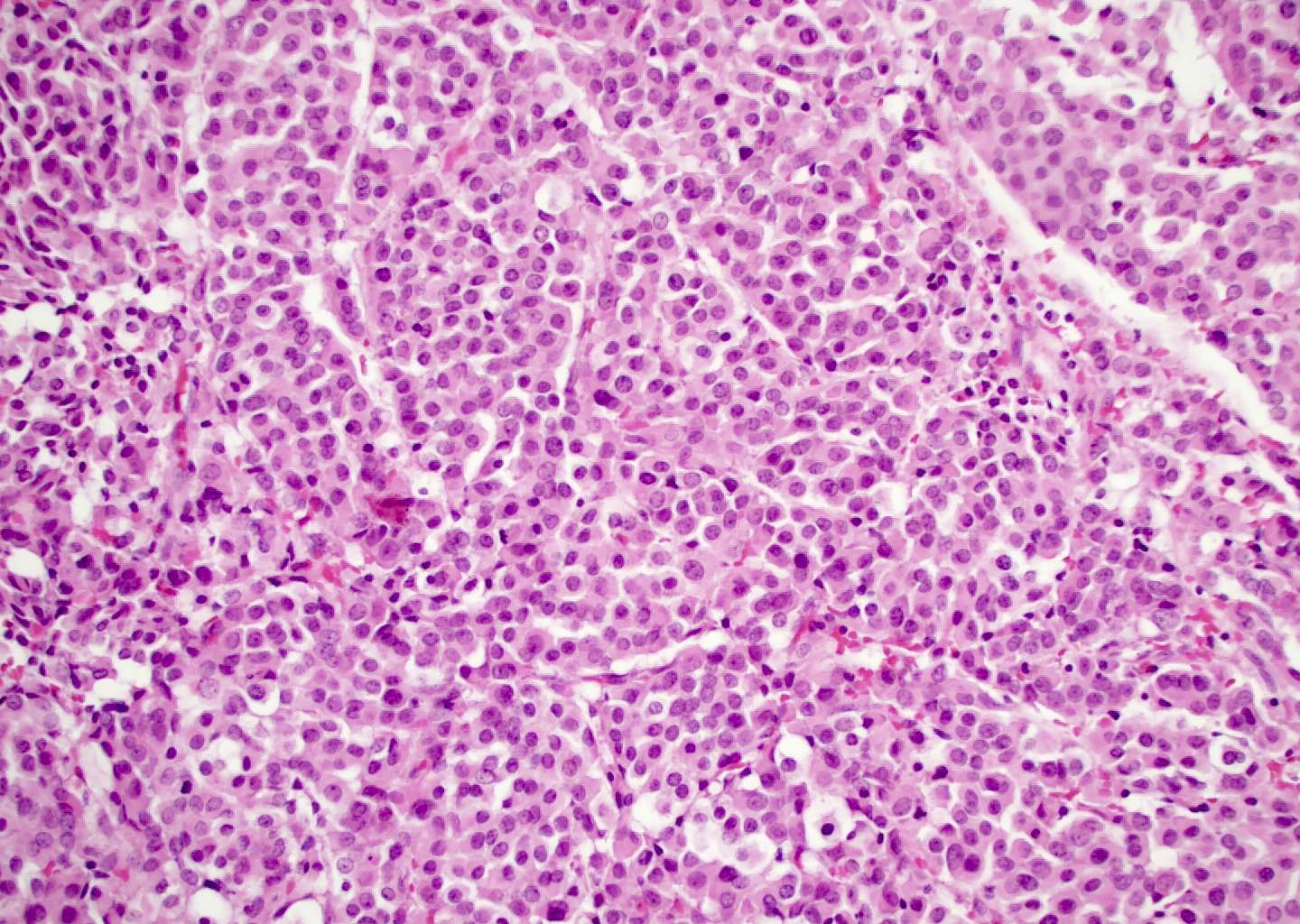
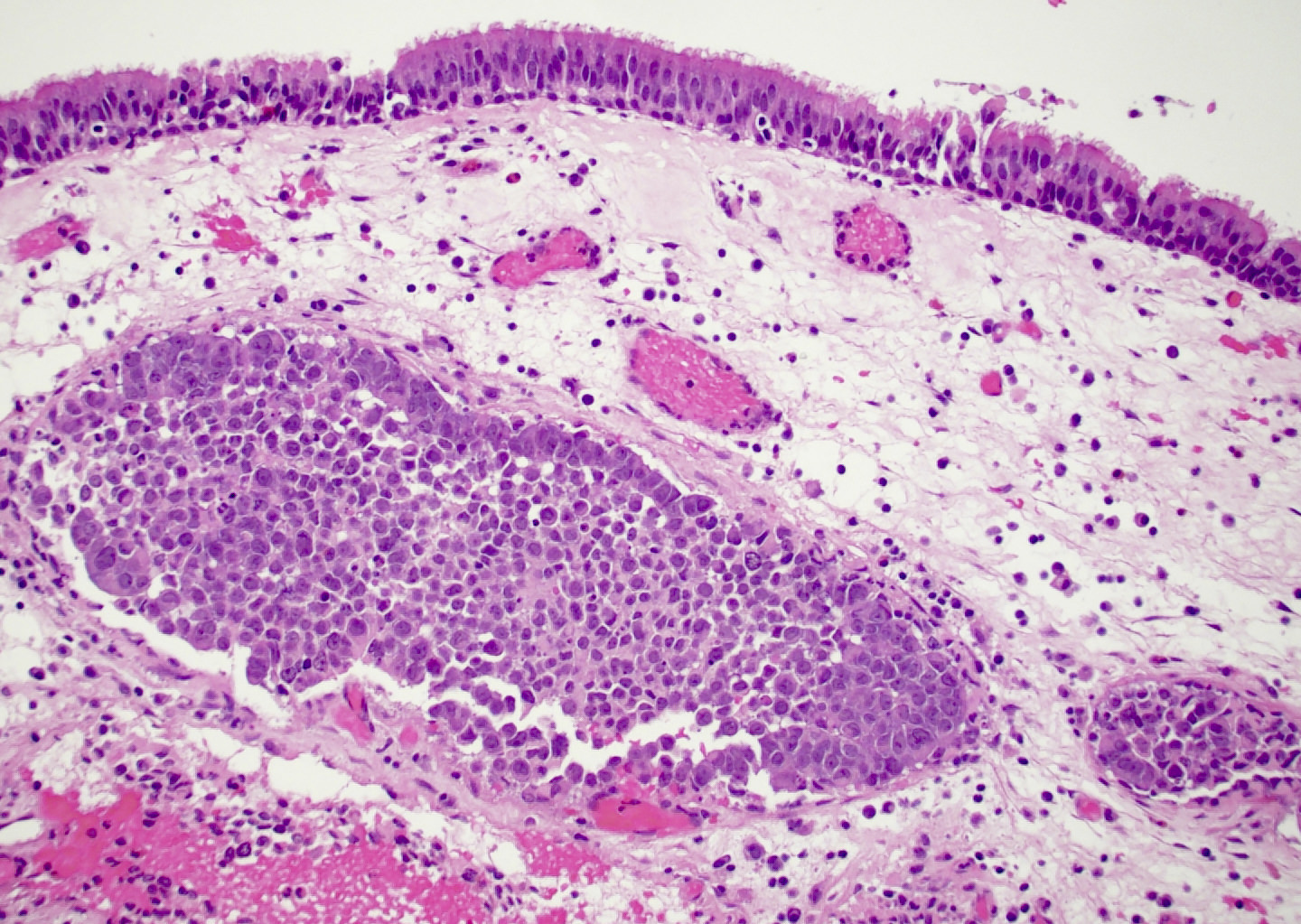
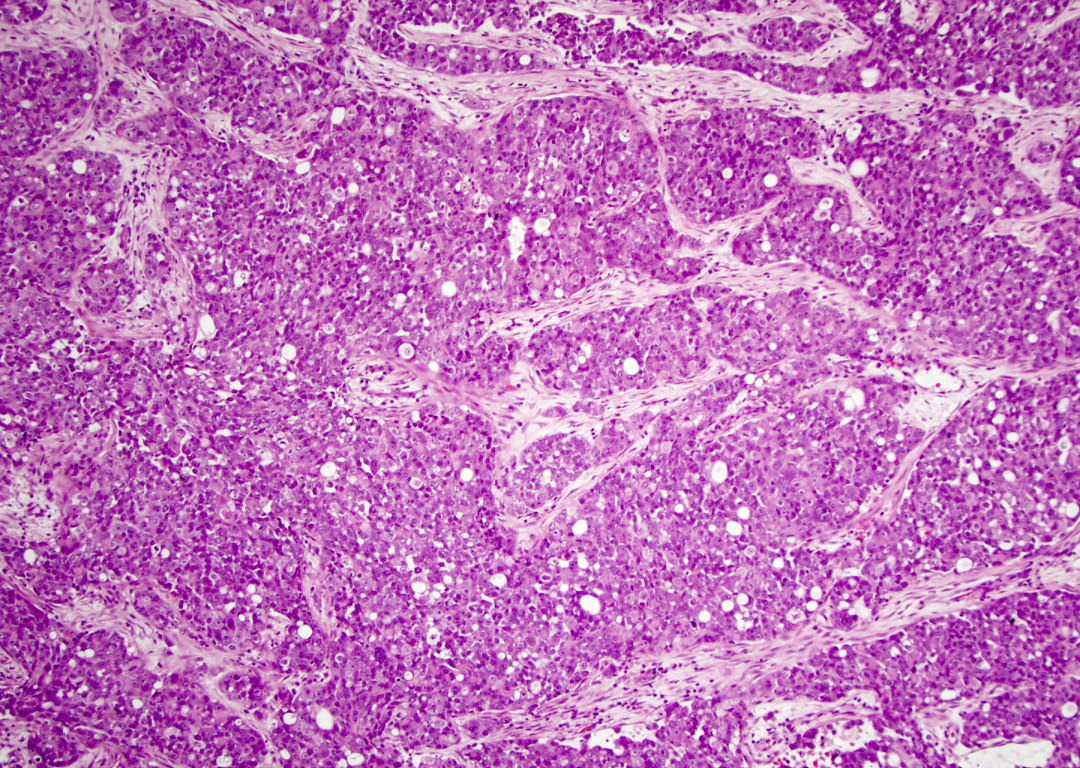
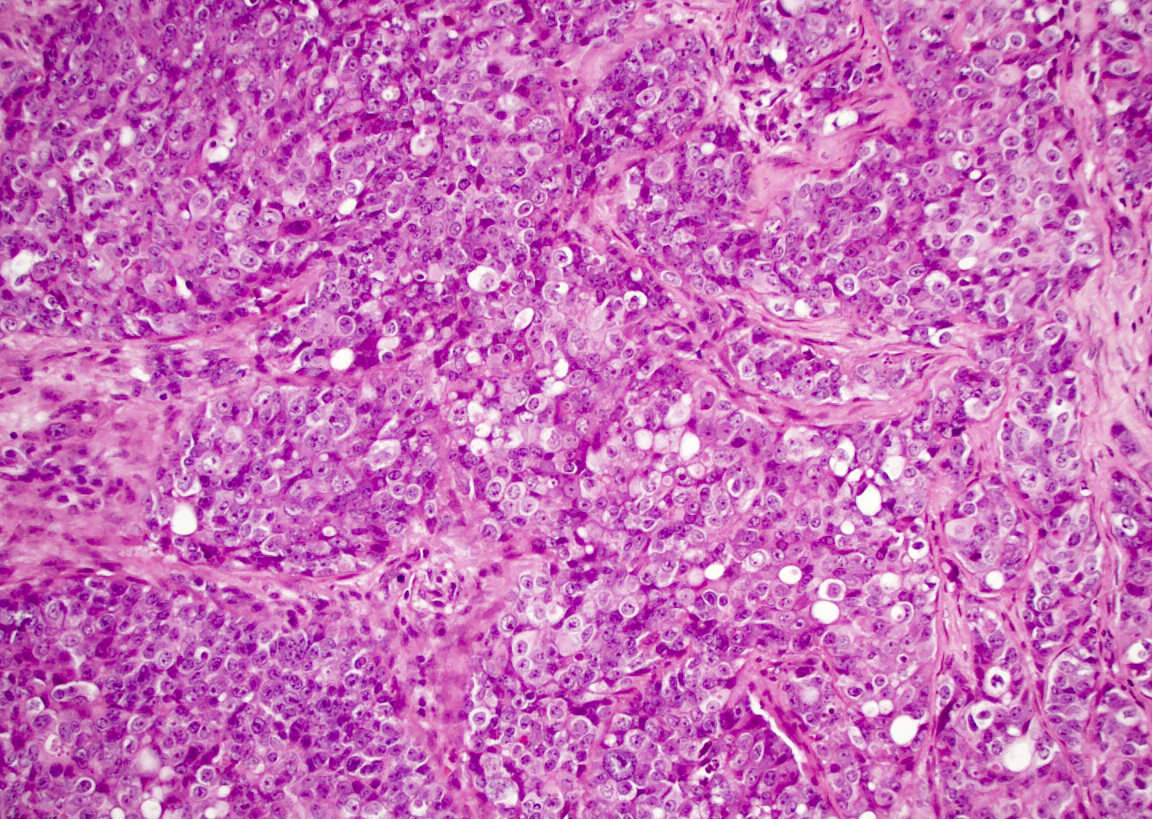
Microscopic (histologic) description
- Although tumors show high grade histologic features including prominent necrosis and mitoses, cytology is relatively uniform
- Nuclei are round to oval with smooth nuclear contours
- Two common morphologic variants: plasmacytoid / rhabdoid (40% of cases) and basaloid (60% of cases) (Am J Surg Pathol 2017;41:458)
- Plasmacytoid / rhabdoid variant composed entirely of sheets of plasmacytoid / rhabdoid cells with abundant eosinophilic cytoplasm and eccentric nuclei
- Basaloid variant composed of nests and lobules of basaloid cells with high nuclear cytoplasmic ratio and peripheral palisading and scattered plasmacytoid / rhabdoid cells
- No overt squamous differentiation should be identified (Am J Surg Pathol 2014;38:1282)
- Frequent formation of nonspecific vacuoles and occasional overt glandular differentiation (Hum Pathol 2019;83:59)
- Subset shows papillary / exophytic growth (Virchows Arch 2015;467:649)
- No associated surface dysplasia but pagetoid spread in overlying epithelium can be seen (Am J Surg Pathol 2014;38:1274)
Microscopic (histologic) images
Cytology description
- Hypercellular smears with clusters of cohesive polygonal cells
- Variable proportion of individual rhabdoid / plasmacytoid cells with abundant cytoplasm
- Uniform nuclei with fine chromatin and small nucleoli
- No overt keratinization or other evidence of squamous differentiation (Diagn Cytopathol 2016;44:700)
- Apoptotic bodies common with abundant background necrotic debris (Cancer Cytopathol 2018;126:567)
Positive stains
- Virtually always diffusely positive for pancytokeratin
- Squamous markers p63 / p40 positive in > 50% of cases
- Neuroendocrine markers synaptophysin / chromogranin positive in 30% of cases
- Small subset of cases express diffuse p16 (Am J Surg Pathol 2017;41:458)
Negative stains
Molecular / cytogenetics description
- Defined by inactivating mutations of SMARCB1 gene, a tumor suppressor gene located on chromosome 22q11.2 (Am J Surg Pathol 2014;38:1274, Am J Surg Pathol 2014;38:1282)
- FISH less sensitive for diagnosis than immunohistochemistry, likely due to heterozygous deletions
- Next generation sequencing has not identified other driver mutations within the limited number of cases evaluated (Pathol Res Pract 2017;213:133)
- In situ hybridization for high risk HPV and EBV is consistently negative (Am J Surg Pathol 2017;41:458)
Sample pathology report
- Nasal mass, biopsy:
- SMARCB1 deficient sinonasal carcinoma (see comment)
- Comment: Immunostains show that the tumor cells are positive for AE1/AE3 and p40, focally positive for synaptophysin and INSM1 and negative for NUT1, CD99, p16, S100 and calponin with loss of SMARCB1 (INI1) expression. SMARCB1 deficient sinonasal carcinoma is an aggressive tumor unique to the sinonasal tract that is defined by loss of SMARCB1 (INI1) expression (Am J Surg Pathol 2017;41:458).
Differential diagnosis
- Sinonasal undifferentiated carcinoma:
- Also high grade but shows more prominent cytologic atypia and lacks rhabdoid / plasmacytoid cells
- Negative for p63 / p40 and synaptophysin / chromogranin with frequent IDH2 mutations
- NUT carcinoma:
- Also high grade and frequently positive for p63 / p40
- Shows abrupt keratinization and positivity for NUT1 with retained SMARCB1 (INI1)
- Myoepithelial carcinoma:
- Also demonstrates rhabdoid / plasmacytoid morphology and a subset show loss of SMARCB1 (INI1)
- Positive for myoepithelial markers S100, SMA and calponin
- Adamantinoma-like Ewing sarcoma:
- Also high grade and frequently positive for p63 / p40 and synaptophysin / chromogranin
- Positive for CD99 / NKX2.1 with retained SMARCB1 (INI1)
- High grade neuroendocrine carcinoma:
- Also high grade and positive for synaptophysin / chromogranin
- Speckled chromatin and retained SMARCB1 (INI1)
- HPV related multiphenotypic sinonasal carcinoma:
- Also high grade with basaloid features and positivity for p63 / p40
- Distinct biphasic basal and ductal populations, positive for HPV and retained SMARCB1 (INI1)
Board review style question #1
Board review style answer #1
D. Inactivation of SMARCB1. This photomicrograph depicts a SMARCB1 deficient sinonasal carcinoma, an aggressive sinonasal tumor that is defined by genetic inactivation of the SMARCB1 gene with corresponding immunohistochemical loss of SMARCB1 (INI1) protein expression. The other genetic abnormalities listed here are characteristic of other high grade sinonasal tumors that fall into the differential diagnosis of SMARCB1 deficient sinonasal carcinoma, including IDH2 mutations in sinonasal undifferentiated carcinoma, EWSR1 translocation in adamantinoma-like Ewing sarcoma and NUT1 translocation in NUT carcinoma; these other tumor types all demonstrate retained immunohistochemical expression of SMARCB1.
Comment here
Reference: SMARCB1 deficient sinonasal carcinoma
Comment here
Reference: SMARCB1 deficient sinonasal carcinoma
Board review style question #2
What feature would essentially exclude the diagnosis of SMARCB1 deficient sinonasal carcinoma?
- Basaloid cell nests with peripheral palisading and strong p40 positivity
- Diffuse positivity for p16 immunohistochemistry and high risk HPV RNA in situ hybridization
- Formation of glandular lumina with mucin production
- Retained SMARCB1 signals by FISH
Board review style answer #2
B. Diffuse positivity for p16 immunohistochemistry and high risk HPV RNA in situ hybridization. SMARCB1 deficient sinonasal carcinoma can show a variety of morphologic features and a highly variable immunohistochemical profile. Histologically, SMARCB1 deficient sinonasal carcinoma is composed of various proportions of basaloid and plasmacytoid / rhabdoid cells; a subset of cases can show glandular differentiation. The most sensitive and specific feature for diagnosis is consistent loss of SMARCB1 (INI1) expression by immunohistochemistry; while FISH generally shows loss as well, signals can be retained in rare cases due to heterozygous mutations. Otherwise SMARCB1 deficient sinonasal carcinoma is immunohistochemically heterogeneous with variable positivity for p63 / p40 and synaptophysin / chromogranin; a subset of cases can even demonstrate diffuse p16 positivity. However, in situ hybridization for high risk HPV should be consistently negative.
Comment here
Reference: SMARCB1 deficient sinonasal carcinoma
Comment here
Reference: SMARCB1 deficient sinonasal carcinoma
Back to top