Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Positive stains | Negative stains | Molecular / cytogenetics description | Molecular / cytogenetics images | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1Cite this page: Sibira R, Khalifa M. Luteinized thecoma associated with sclerosing peritonitis. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/ovaryluteinizedthecomasclerperi.html. Accessed April 23rd, 2024.
Definition / general
- Uncommon syndrome characterized by presence of ovarian (mostly bilateral) stromal tumor(s) resembling theca cells and peritoneal fibrotic lesions
Essential features
- Benign tumor
- Usually bilateral (unilateral cases have been reported)
- Ovarian theca cell proliferation involving the cortex with or without mass formation
- Associated abnormal proliferation of fibroblasts and myofibroblasts in the peritoneum that results in an irreversible fibrosis
- Leads to incarceration and strangulation of the abdominal organs, which is eventually fatal
Terminology
- Luteinized thecomatosis associated with sclerosing (encapsulating) peritonitis
ICD coding
Epidemiology
- Rare
- Age ranges from 10 months to 85 years; median age is 27 (Am J Surg Pathol 2008;32:1273)
Sites
- Ovary, predominantly confined to the ovarian cortex
- Associated peritoneal fibroblastic proliferation
- References: Expert Rev Anticancer Ther 2021;21:23, J Obstet Gynaecol Can 2016;38:41
Pathophysiology
- Still unclear; debatable whether this is a neoplasm or nonneoplastic proliferation
- Suggested that neoplastic theca cells are able to produce substances that, when released into the peritoneal cavity, stimulate fibroblastic proliferation
- Has also been suggested that luteinized theca cells secrete steroid hormones (estrogen and progesterone) that would target submesothelial fibroblasts characterized by hormonal sensitivity
- Among the substances produced are cytokines, in particular transforming growth factor beta (TGFβ), which induce a fibrotic reaction within the peritoneum and, rarely, even in distant anatomical sites
- References: Expert Rev Anticancer Ther 2021;21:23
Etiology
- Unknown
Clinical features
- Abdominal pain
- Ascites
- Symptoms of small bowel obstruction (acute / subacute)
- Hormonal symptoms are absent
- Association with anticonvulsant therapy in some cases
- References: Pathology 2018;50:5, Expert Rev Anticancer Ther 2021;21:23
Diagnosis
- Histologic examination
Laboratory
- Occasionally elevated serum CA125 (Expert Rev Anticancer Ther 2021;21:23)
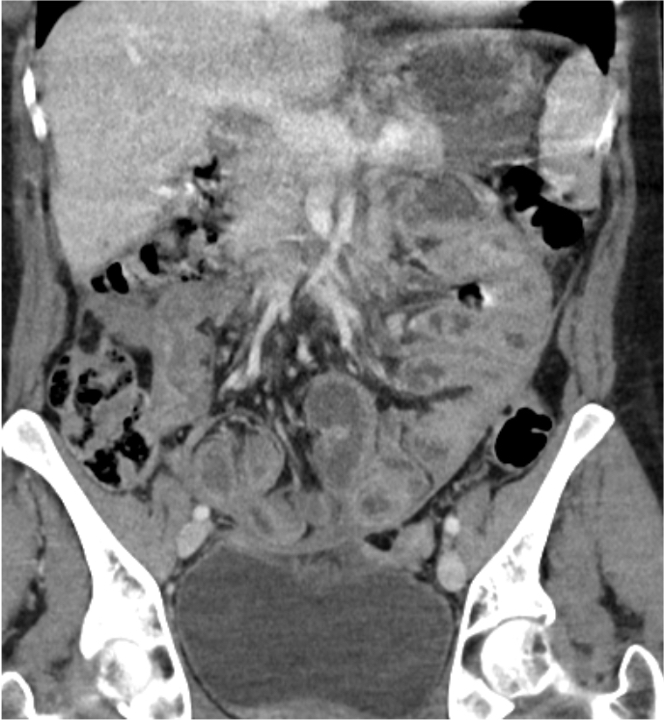
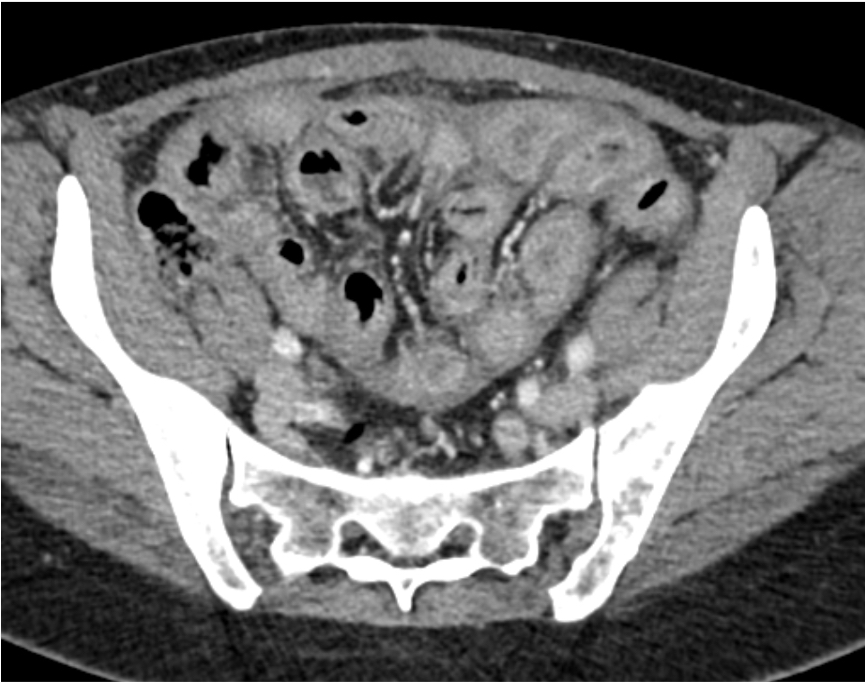
Radiology description
- Magnetic resonance imaging features
- Cystic, solid adnexal region mass
- Cystic component gives a homogeneous iso or hyperintense signal
- Solid component shows moderate enhancement
- Computed tomography (CT) features
- Thickened contrast material enhanced abnormal peritoneal membrane, dilation of bowel loops with clump appearance
- Thickening or calcification of bowel walls, with or without loculated ascites
- References: J Ovarian Res 2013;6:58, Radiographics 2019;39:62
Radiology images
Prognostic factors
- Ovarian luteinized thecoma itself is a benign tumor without risk of recurrence or metastasis
- However, associated peritoneal fibrosis may result in bowel incarceration and strangulation, causing potentially life threatening bowel obstruction
- References: Expert Rev Anticancer Ther 2021;21:23, Pathology 2018;50:5
Case reports
- 18 year old woman presented with acute abdomen (Gynecol Oncol Rep 2019;28:44)
- 42 year old presented with progressive respiratory distress, increased abdominal girth and sudden weight gain (J Gynecol Obstet Hum Reprod 2021;50:101734)
- 52 year old woman with erythematous rash, coinciding with the rapid onset of ascites and peripheral edema (J Cutan Pathol 2017;44:898)
Treatment
- Surgical management
- Bilateral salpingo-oophorectomy if localized to ovaries
- Oophorectomy or unilateral salpingo-oophorectomy if unilateral and preserved fertility is desired
- Total hysterectomy with bilateral salpingo-oophorectomy is indicated in postmenopausal patients
- Omentectomy in extensive disease
- Peritoneal biopsy in case of peritoneal nodules only
- Appendectomy or small bowel resection in case of involvement
- Medical management (single or combined therapy)
- Cyclophosphamide, thalidomide, leuprorelin, tamoxifen, goserelin and corticosteroids
- References: Expert Rev Anticancer Ther 2021;21:23, Gynecol Oncol Rep 2019;28:44
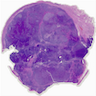
Gross description
- Usually bilateral
- Dimensions range from 2 - 31 cm
- Outer surface is brown, red, purple, pink or gray
- Cut surface is solid, yellow, pink or gray, very often characterized by cystic hemorrhagic or sometimes soft lobulated, cerebriform or polypoid cut surface
- Peritoneum with brown to white fibrous adhesions
- Omentum with lobulation, nodularities, indurations or thickening
- Thick, white adherent membrane encasing the small bowel (partially or complete)
- Reference: Am J Surg Pathol 2008;32:1273
Frozen section description
- Sheets of bland spindle cell proliferation circumferentially involve the entire cortex, with sparing of the medulla in smaller lesions (Am J Surg Pathol 2008;32:1273)
- Luteinized cells are arranged in small nests or singly with small to moderate amounts of clear or eosinophilic cytoplasm (Am J Surg Pathol 2008;32:1273)
- Mitotic activity is typically prominent (Am J Surg Pathol 2008;32:1273)
- Edema with extravasated erythrocytes, prominent small vessels and microcystic areas
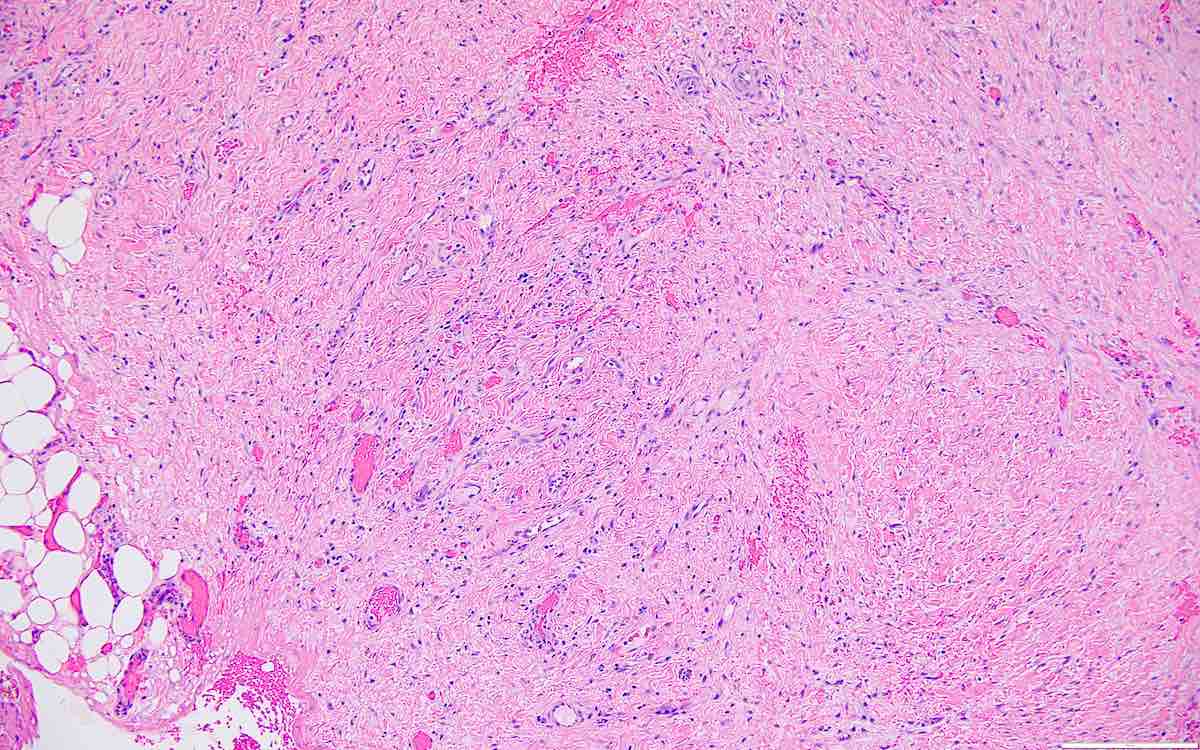
- Peritoneal / omental lesions have proliferation of bland spindled cells with variably fibrillar cytoplasm
- While it is unlikely peritoneal samples are submitted for frozen section examination along with the ovarian tumor, it is very helpful to inquire from the surgeon about the presence of peritoneal lesions or seek this information from the patient's chart as this helps guide diagnosis
Frozen section images
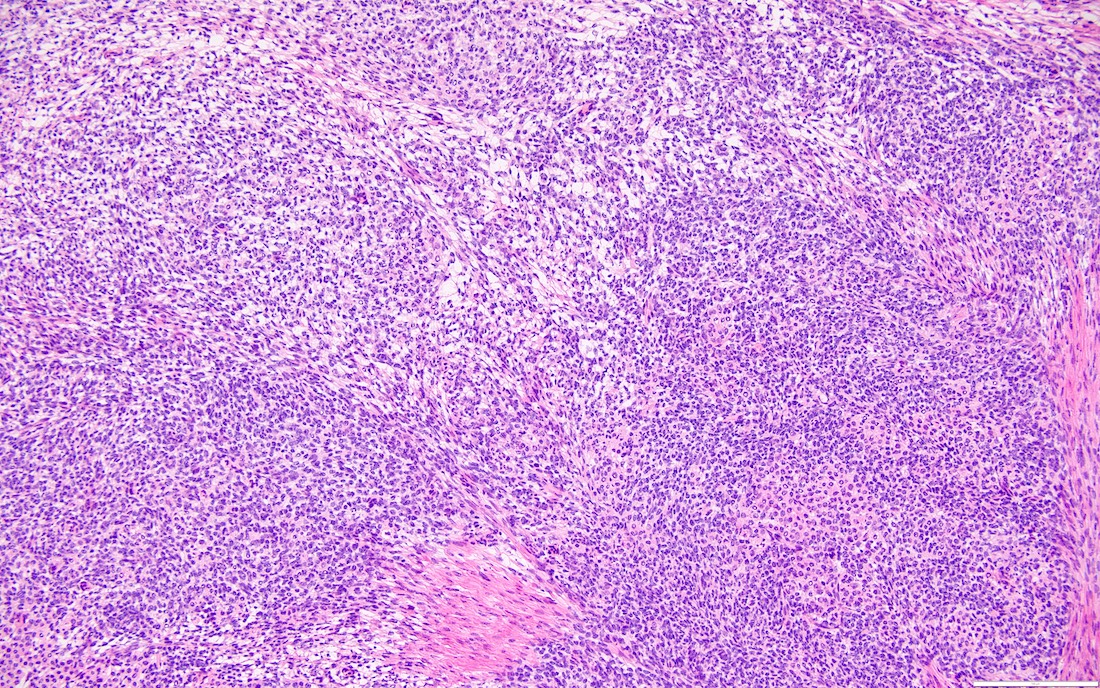
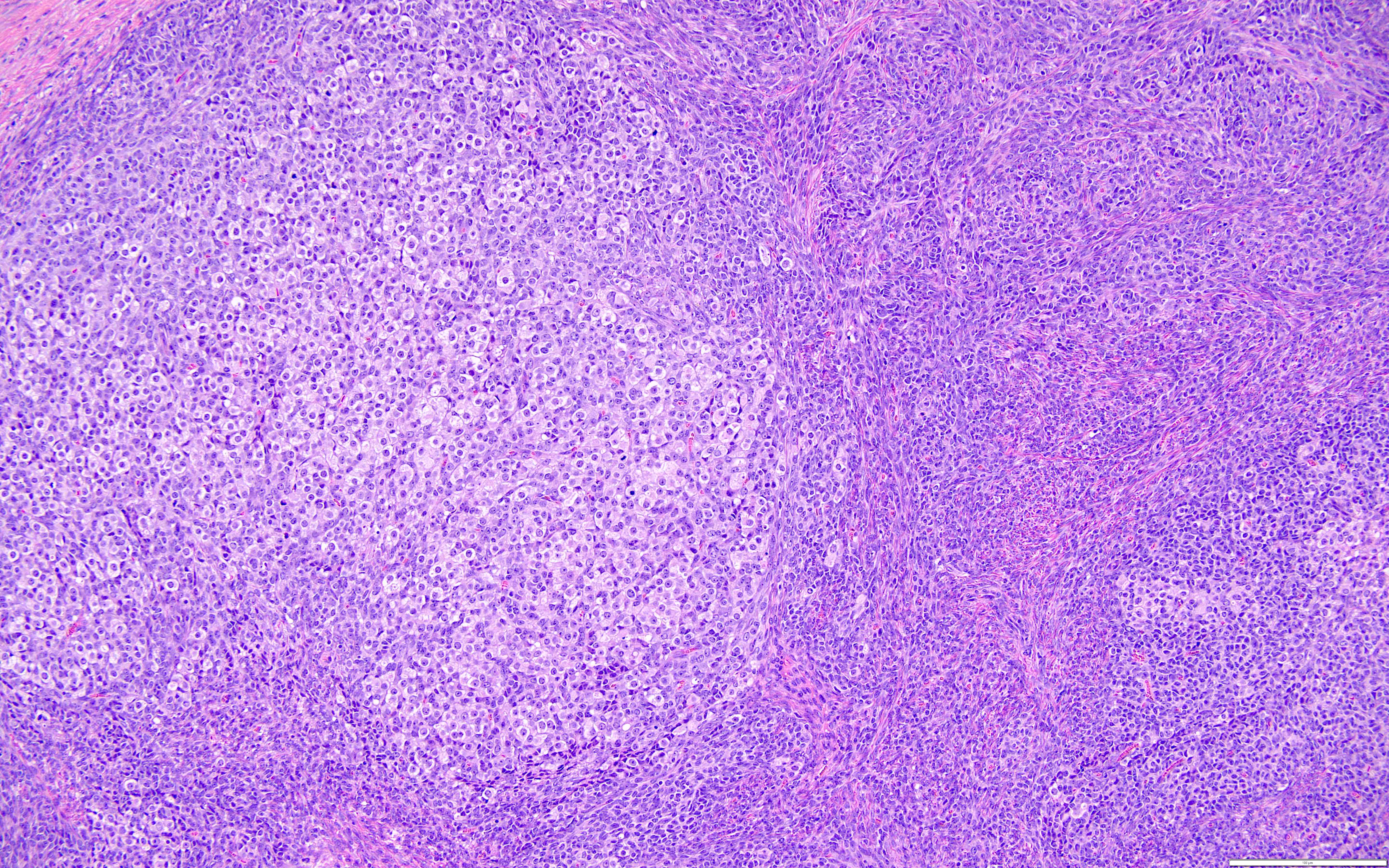
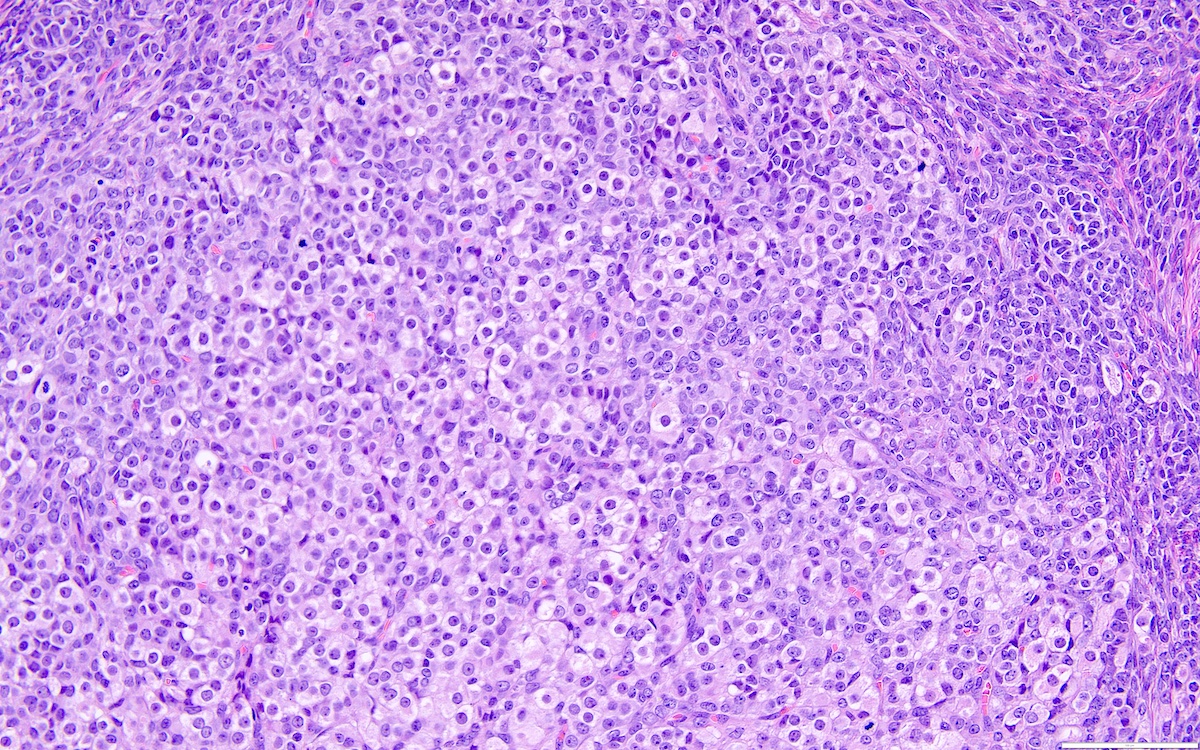
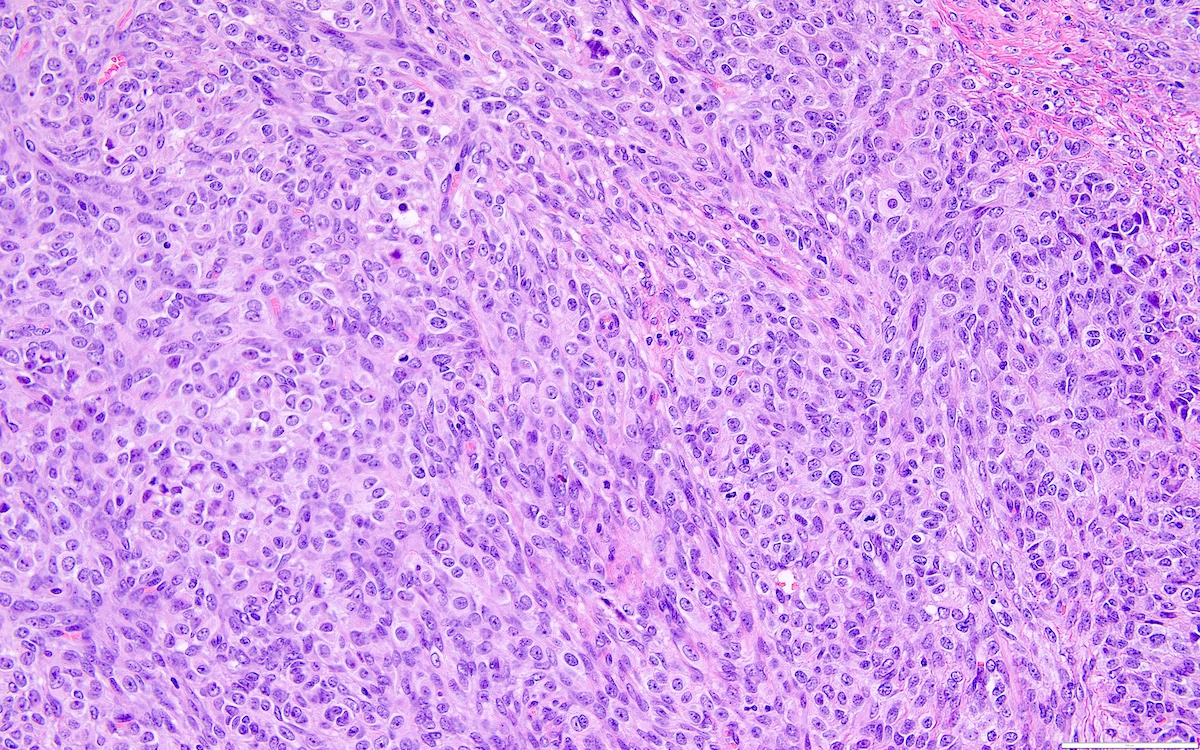
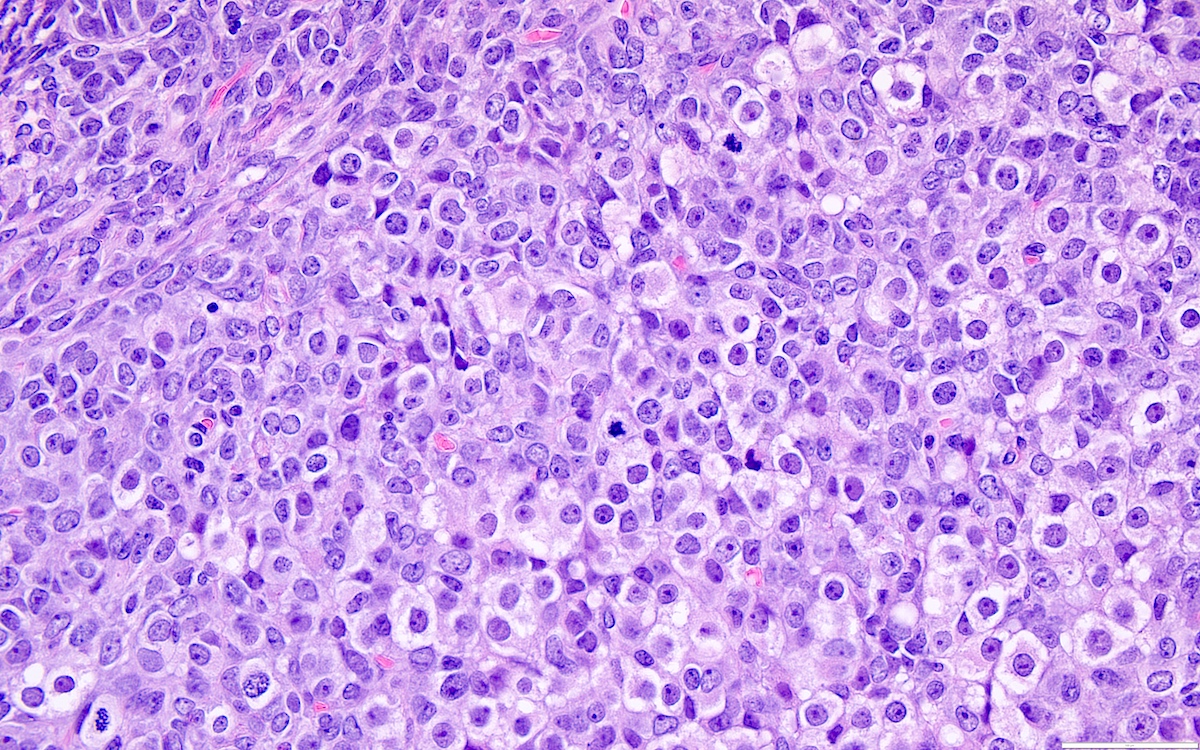
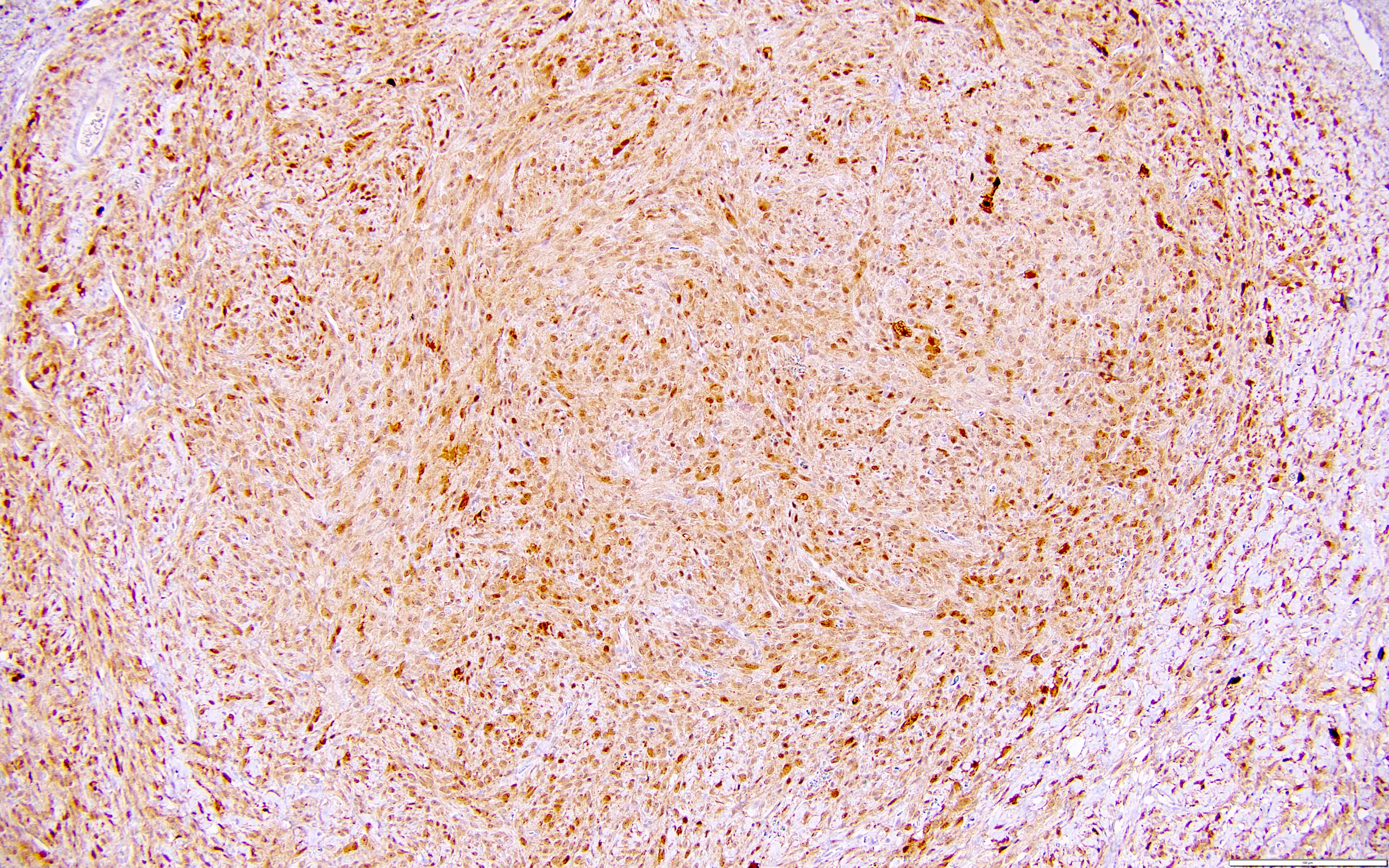
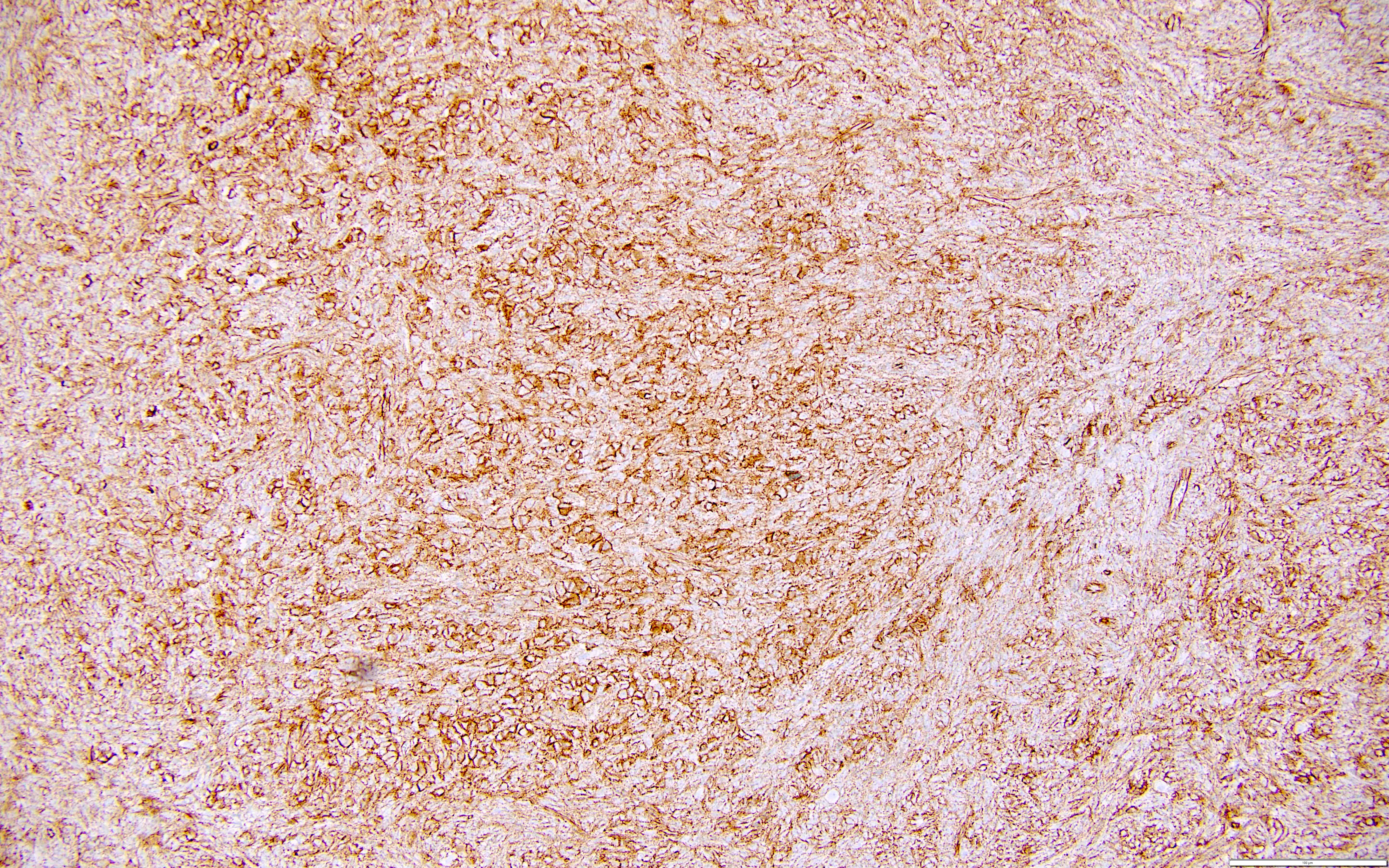
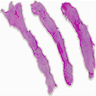
Microscopic (histologic) description
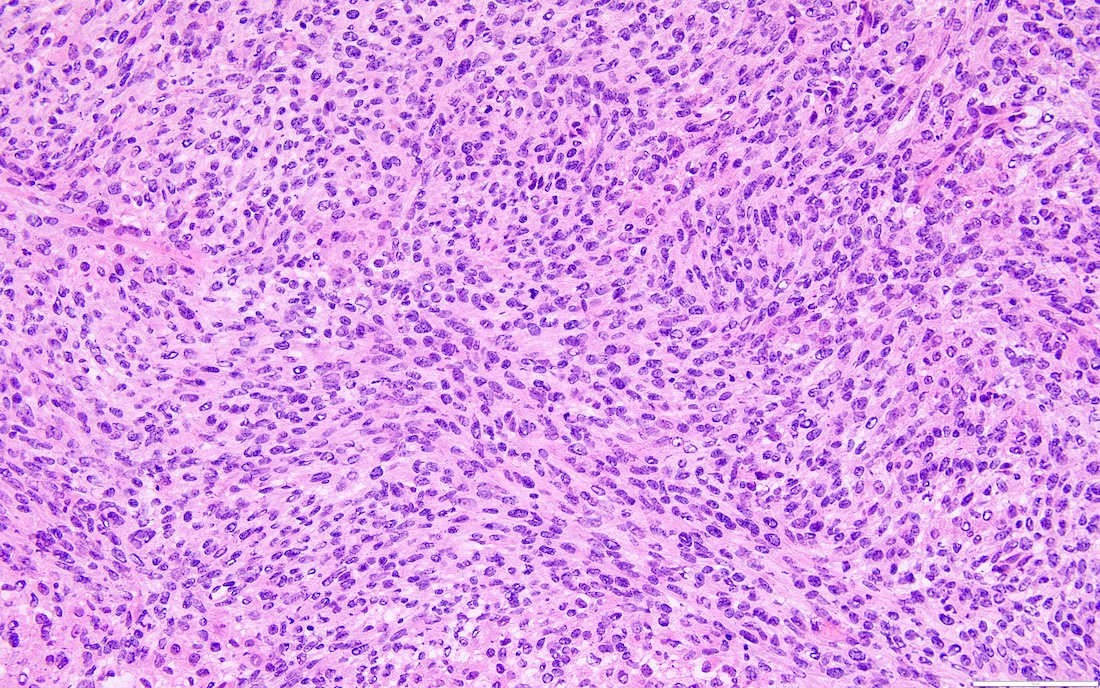
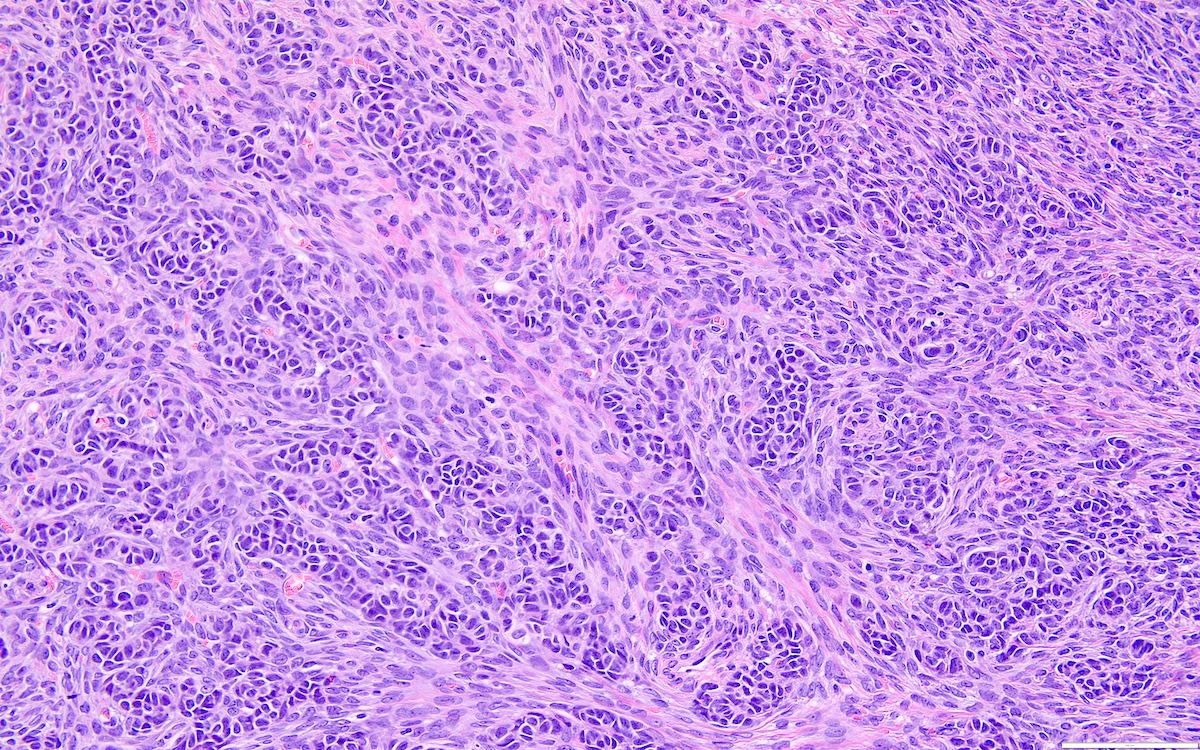
- Highly cellular
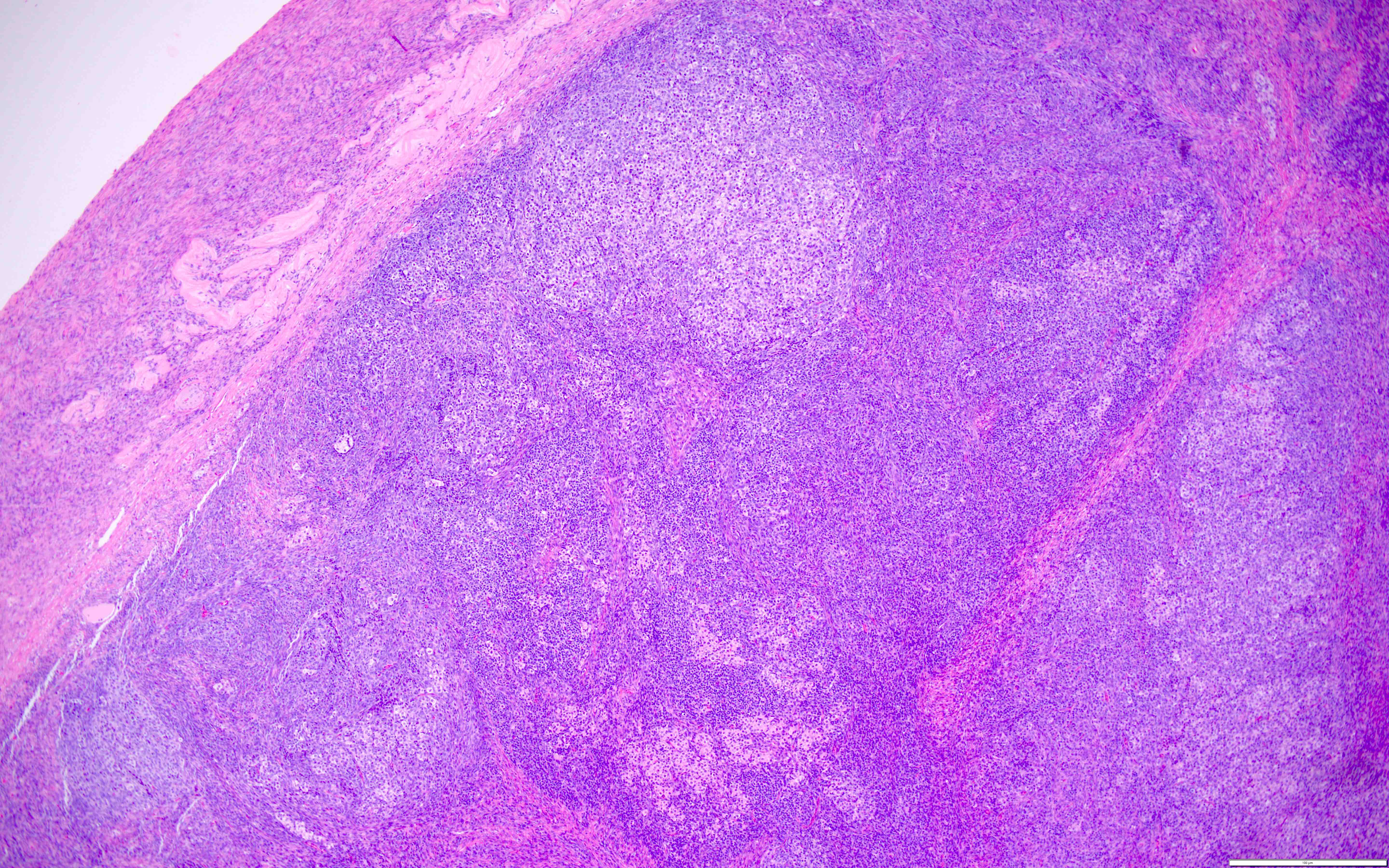
- Sheets of spindle cell proliferation circumferentially involves the entire cortex with sparing of the medulla
- Proliferation arranged haphazardly in small fascicles or short cords and frequently includes pre-existing ovarian structures
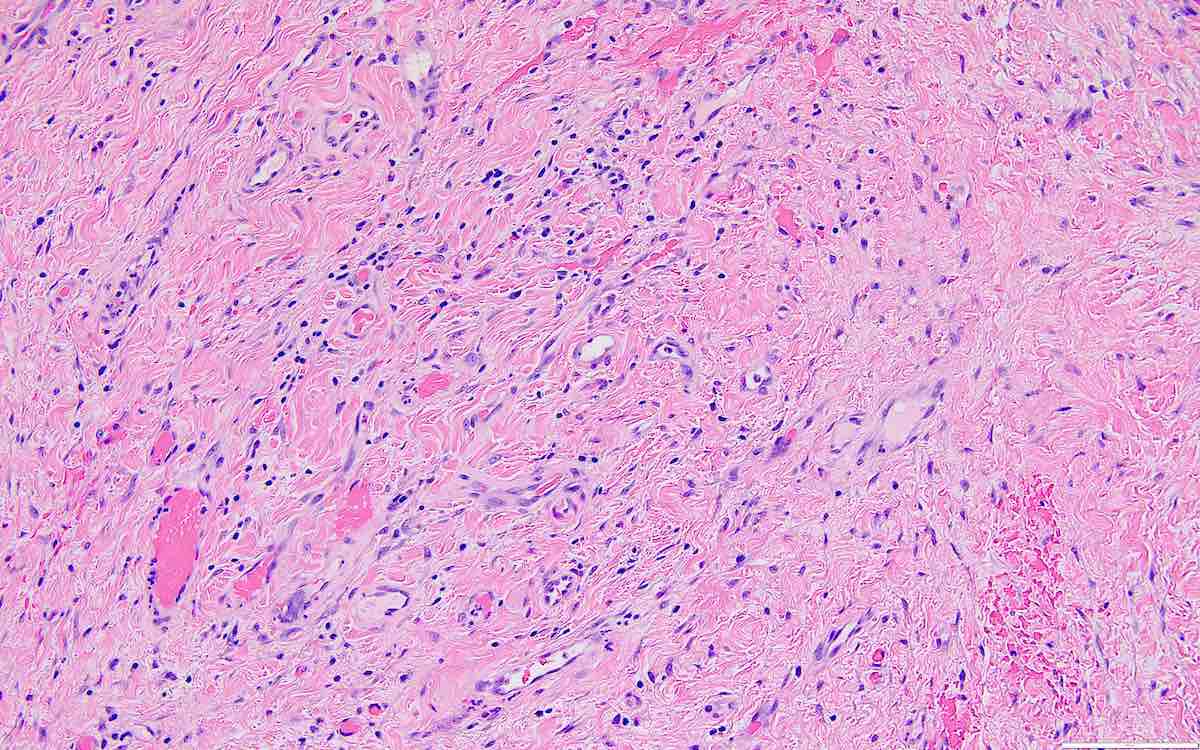
- Less cellular areas show edema with characteristic microcystic pattern
- Larger lesions show prominent cellular lobules or vaguely nodular cellular aggregates, often highlighted by hemorrhage, separated by less cellular regions with varying amounts of edema which could give a microcystic appearance
- Edematous areas contain extravasated erythrocytes and prominent small vessels
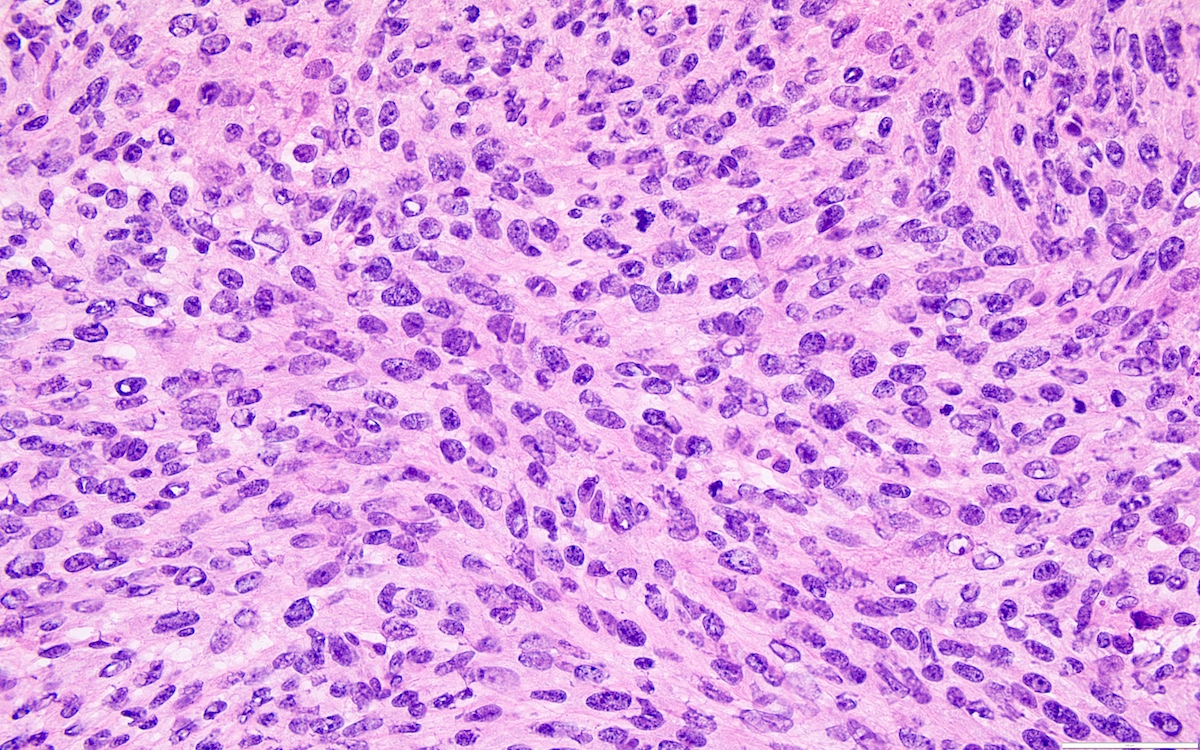
- High power, the cells are rounded, fusiform or stellate and cytoplasm volume ranged from scant to moderate with pale or eosinophilic color
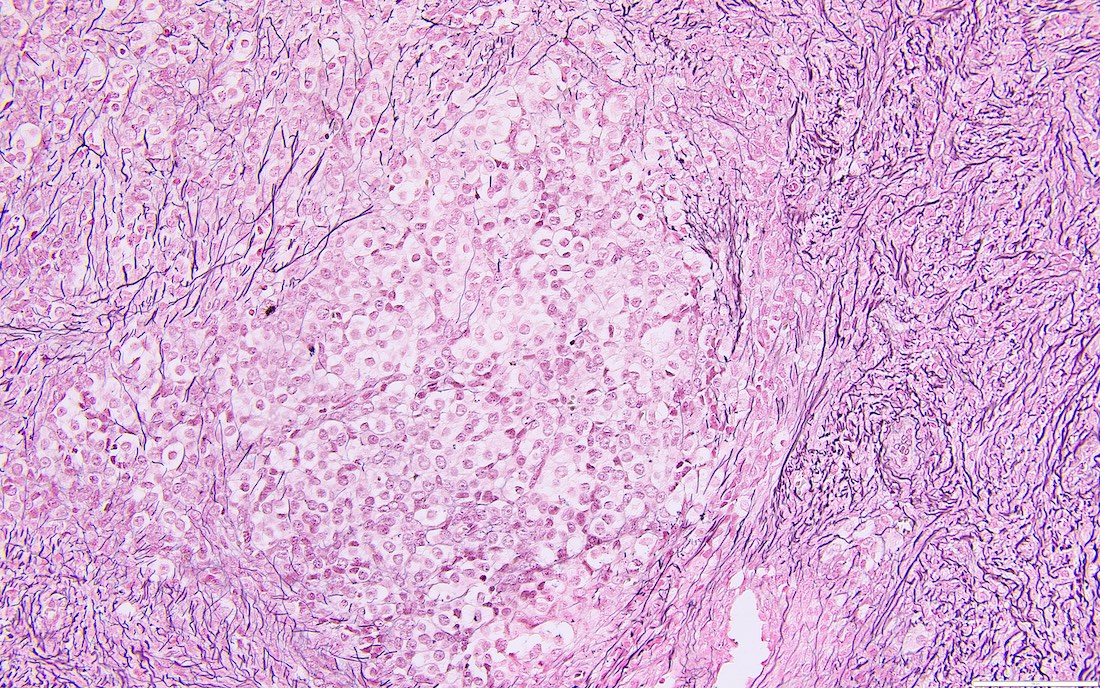
- Ill defined aggregates of luteinized cells with rounded nuclei, eosinophilic to pale cytoplasm, in the background of bland spindle cells
- Brisk mitotic activity may be seen
- Sex cord-like differentiation in rare cases
- Entrapped normal ovarian structures, usually follicles, rarely corpora albicans (Am J Surg Pathol 2008;32:1273)
- Variable amount of hemorrhage, ischemic type necrosis and in rare cases fibrin thrombi in large vessels (Am J Surg Pathol 2008;32:1273)
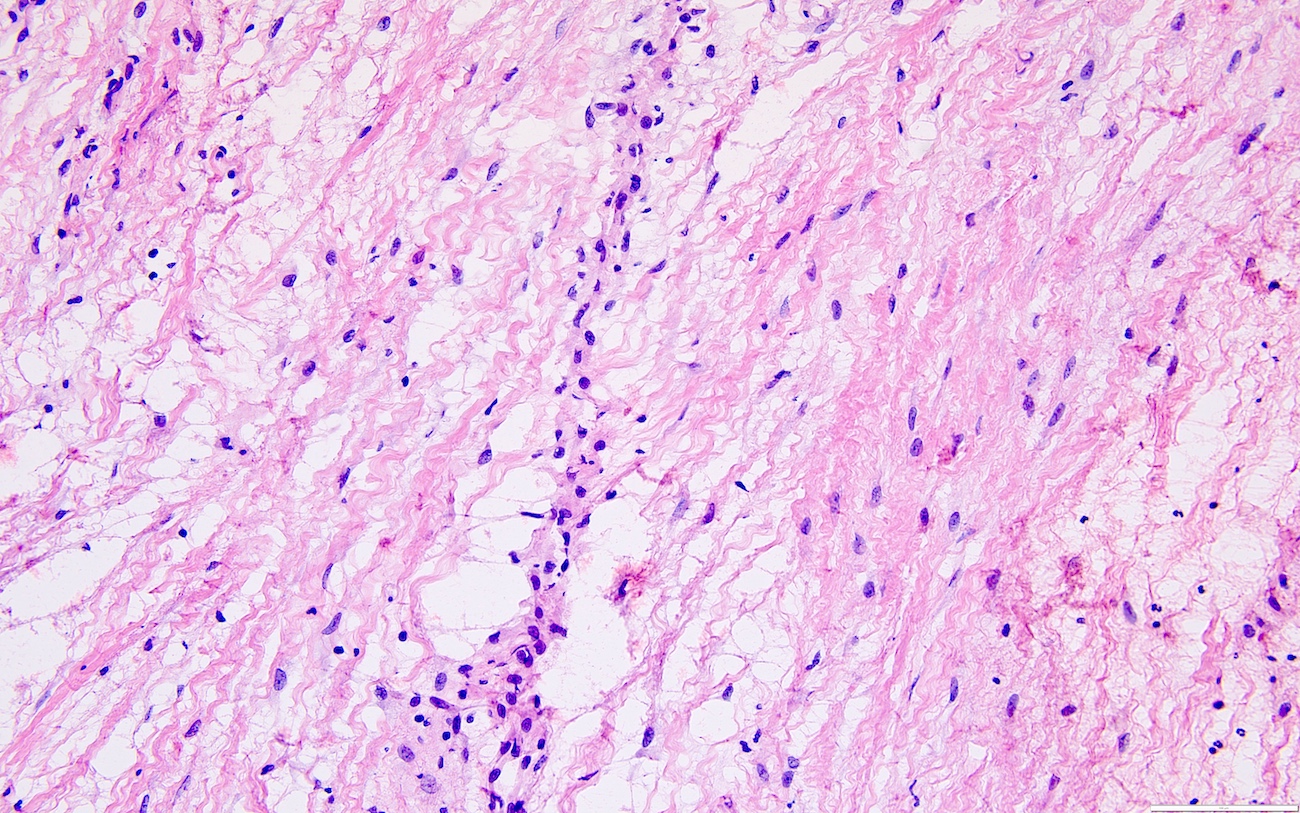
- Peritoneal lesions composed of a proliferation of fibroblastic spindled cells
- Prominent omental lobulation in which the fibrosis expands between the septa of lobules with or without mild chronic inflammatory lymphocytic infiltrate
Microscopic (histologic) images
Virtual slides
Positive stains
- SF1 (positive in spindle cells) (Am J Surg Pathol 2013;37:1458)
- FOXL2 (positive in spindle cells) (Am J Surg Pathol 2013;37:1458)
- Inhibin (positive in luteinized cells)
- Calretinin (positive in luteinized cells)
- CD56 (positive in luteinized cells)
- Acetylglucosaminyltransferase B (MGAT5B) (positive in luteinized cells) (Medicine (Baltimore) 2023;102:e33911)
- Nuclear receptor coactivator 3 (NCOA3) (positive in luteinized cells) (Medicine (Baltimore) 2023;102:e33911)
- Reticulin stain highlights reticulin fibers separating individual theca cells and around nests in the luteinized cells
Negative stains
- ER (22% positive) (Am J Surg Pathol 2008;32:1273)
- PR (41% positive) (Am J Surg Pathol 2008;32:1273)
- CD117 (33% positive) (Am J Surg Pathol 2008;32:1273)
- Cytokeratin AE1 / AE3
- EMA
- CD34
- Beta catenin (cytoplasmic staining in the luteinized cells)
- TGF beta (Am J Surg Pathol 2008;32:1273)
- WT1 (Medicine (Baltimore) 2023;102:e33911)
- CD99 (Medicine (Baltimore) 2023;102:e33911)
Molecular / cytogenetics description
- MGAT5B::NCOA3 fusion gene recently reported in luteinized thecoma associated with sclerosing peritonitis (LTSP), identified in the luteinized cells (Medicine (Baltimore) 2023;102:e33911)
- Negative for FOXL2 mutation
Sample pathology report
- Right ovary and fallopian tube, right salpingo-oophorectomy:
- Ovarian luteinized thecoma, encased with fibrosis
- Fibrosis involving the surface of the ovary
- Unremarkable right fallopian tube
- Negative for malignancy
- Anterior pelvic peritoneum, excision:
- Peritoneum with fibrosis and mesothelial cell hyperplasia (see comment)
- Negative for malignancy
- Comment: The given picture of peritoneal sclerosis (fibrosis) with hyperplasia of the submesothelial mesenchymal cells can result from a variety of stimuli. Given the finding of luteinized thecoma of the ovary, this favors the diagnosis of luteinized thecoma associated with sclerosing peritonitis (LTSP). LTSP is an uncommon syndrome characterized by the presence of a benign ovarian stromal tumor resembling theca cells and peritoneal fibrotic lesions. In some cases, peritoneal lesions lead to irreversible fibrosis with the incarceration and strangulation of abdominal organs, eventually proving fatal.
- Other causes of peritoneal sclerosis include chronic ambulatory peritoneal dialysis, the use of peritoneovenous shunt, bacterial or mycobacterial infection, sarcoidosis, carcinoid syndrome, familial Mediterranean fever and exposure to fibrogenic foreign materials. Idiopathic sclerosing peritonitis is diagnosed when all known causes are excluded, with supportive intraoperative and imaging findings.
Differential diagnosis
- Thecoma (Am J Surg Pathol 2014;38:1023):
- Unilateral
- Commonly in perimenopausal and postmenopausal women
- No associated peritoneal lesions
- Associated hormonal manifestations and uterine bleeding
- Bland cytologic features with appreciable pale gray cytoplasm and low mitosis (≤ 4 mitoses/10 HPF)
- Reticulin surrounding individual cells
- Fibroma (Am J Surg Pathol 2006;30:929):
- Mostly unilateral
- Fascicles of bland fibrous spindle cells in a variably collagenous stroma
- No or focal luteinized cells
- Focal weak inhibin and calretinin staining
- Sclerosing stromal tumor (Int J Gynecol Pathol 2016;35:549):
- Pseudolobules contain epithelioid and spindled cells
- Alternating hypo and hypercellular areas
- Hemangiopericytoma-like vessels distributed throughout the tumor
- Microcystic stromal tumor (Histopathology 2019;74:443):
- Small round to oval cystic spaces, in areas coalescing to form larger irregular channels
- Lobulated cellular masses with intervening fibrous or fibro-hyaline stroma
- Mutations in β catenin, with associated nuclear immunoreactivity
- Negative for inhibin and calretinin
- Massive ovarian edema (Diagn Pathol 2016;11:18):
- Diffuse edema; uniformly hypocellular fibromatous stroma
- Preservation of the outer cortex
- Stromal hyperthecosis (Gynecol Endocrinol 2021;37:677):
- No ascites or mass; low to absent mitoses
- Commonly in peri and postmenopausal women
- Androgenic manifestations
Additional references
Board review style question #1
A 37 year old woman presents with severe abdominal pain and distention. Laboratory investigations revealed a negative beta hCG and slightly elevated serum CA125. Radiologic images showed thick soft tissue of peritoneal sclerosis encasing the small bowel loops. Exploratory laparotomy reveals a 12.5 cm right ovarian mass and multiple peritoneal nodules, which are resected. Which of the following statements is true regarding the ovarian mass?
- Associated with an unfavorable outcome in the small bowel
- Associated with hormonal manifestations
- Bilateral manifestation indicates metastasis of contralateral ovarian tumor
- Peritoneal nodules are metastasized from the ovarian mass
Board review style answer #1
A. Associated with an unfavorable outcome in the small bowel. Axial abdominopelvic computed tomography (CT) shows peritoneal sclerosis encasing the small bowel loops with a characteristic clumped appearance of small bowel loops within a cocoon. The histopathology picture shows nodules of spindled and luteinized cells. Luteinized thecoma associated with sclerosing peritonitis (LTSP) may result in bowel incarceration and strangulation, causing potentially life threatening bowel obstruction. Answers C and D are incorrect because the ovarian tumor is benign and there is no recurrence or metastasis of the ovarian tumor after excision. Answer B is incorrect because this ovarian tumor usually is not hormonally active.
Comment Here
Reference: Luteinized thecoma associated with sclerosing peritonitis
Comment Here
Reference: Luteinized thecoma associated with sclerosing peritonitis