Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Frozen section description | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Practice question #1 | Practice answer #1 | Practice question #2 | Practice answer #2Cite this page: Jassim T, Allison D. Sclerosing microcystic adenocarcinoma. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/salivaryglandssclerosingmicrocysticadeno.html. Accessed August 29th, 2025.
Definition / general
- Sclerosing microcystic adenocarcinoma (SMA) is an exceedingly rare tumor of presumed minor salivary gland origin that closely resembles cutaneous microcystic adnexal carcinoma (MAC)
- It arises in mucosal sites in the head and neck region
- SMA is made up of deeply infiltrative, variably sized bland ducts, tubules, cords and nests that contain a biphasic population of myoepithelial cells and cuboidal cells within an abundant, densely sclerotic stroma (Head Neck Pathol 2019;13:215, Surg Pathol Clin 2021;14:137)
Essential features
- Sclerosing microcystic adenocarcinoma (SMA) is a unique and rare tumor of presumed salivary origin in the head and neck region that shows significant morphologic overlap with microcystic adnexal carcinoma (MAC) of the skin (Surg Pathol Clin 2021;14:137, Diagnostics (Basel) 2022;12:1288)
- It is composed of a biphasic population of inner cuboidal and outer myoepithelial cells that form highly infiltrative and variably sized ducts, tubules, cords and nests within a dense collagenous stroma; perineural invasion and skeletal muscle involvement is prominent
- Myoepithelial cells are often attenuated but are occasionally clear while the cuboidal component is relatively monomorphic with eosinophilic cytoplasm and round nuclei with even chromatin, occasional nucleoli and no increase in mitosis
- Although follow up data are limited, patients with SMA have had uniformly good outcomes without locoregional recurrence or distant metastases (Surg Pathol Clin 2021;14:137)
Terminology
- Microcystic adnexal (sclerosing sweat duct) carcinoma of intraoral minor salivary gland origin (Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2011;112:284)
- Primary minor salivary gland tumors with microscopic features ascribed to MAC, also referred to as sclerosing sweat duct or syringomatous carcinoma (Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2011;112:284)
- Adenocarcinoma, not otherwise specified (Hum Pathol Rep 2021;26:300577)
ICD coding
Epidemiology
- Exceedingly rare tumor
- Average patient age of 52.6 years (range: 41 - 73 years) (Head Neck Pathol 2016;10:501, Ann Diagn Pathol 2021;54:151806)
- Predilection for women (F:M = 4:1) (Head Neck Pathol 2016;10:501)
- Immunosuppression may play a role in its pathogenesis (Ann Diagn Pathol 2021;54:151806)
Sites
- Most commonly arises from minor salivary glands in the intraoral cavity, including the tongue, lip mucosa, floor of mouth and buccal mucosa, as well as the nasopharynx (Surg Pathol Clin 2021;14:137, Head Neck Pathol 2016;10:501)
- Has also been reported to arise from within the parotid gland (Ann Diagn Pathol 2021;54:151806, Hum Pathol Rep 2021;26:300577)
Pathophysiology
- Pathophysiology is still under investigation
- SMA may show morphologic overlap with cutaneous MAC because normal intraoral minor salivary glands have morphologic and functional overlap with normal eccrine sweat glands
- Recently, whole exome sequencing of a single case revealed a moderate tumor mutational burden, a putative loss of function mutation in CDK11B and no molecular overlap with mutations previously identified in MAC, though more work needs to be done (Anticancer Res 2020;40:6375)
Etiology
- Like MAC, immunosuppression may play a role in the pathogenesis of SMA (several of the cases described have had a history of immunosuppressive therapy for autoimmune disorders, chemotherapy or radiation)
Clinical features
- Typically painless, slow growing mass or submucosal lump (Oral Surg Oral Med Oral Pathol Oral Radiol 2018;125:e94)
- Both SMA and MAC exhibit an infiltrative growth pattern and a highly aggressive local behavior with perineural invasion that can cause numbness (Hum Pathol Rep 2021;26:300577)
- Due to extensive perineural invasion, clear surgical margins may be difficult; patients with positive margins are recommended to undergo adjuvant radiation therapy
Diagnosis
- Tumors may be visualized or palpated on the physical exam while imaging, such as CT and MRI, can be utilized to examine the extent of disease
- Definitive diagnosis will typically be made on an excisional biopsy specimen
Radiology description
- Irregular and amorphous FDG avid lesion with infiltrative pattern that may obscure fat planes (Hum Pathol Rep 2021;26:300577, Ann Diagn Pathol 2021;54:151806, Head Neck Pathol 2019;13:215)
Radiology images
Prognostic factors
- SMA appears to have an indolent clinical course with no documented lymph node involvement or distant metastatic disease, despite the concerning infiltrative histologic appearance (Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2011;112:284)
- Patients with positive surgical margins are recommended to receive adjuvant radiation therapy
- Although follow up data are limited, patients with SMA have had uniformly good outcomes without locoregional recurrence or distant metastases (follow up durations ranging from 21 to 60 months) (Ann Diagn Pathol 2021;54:151806, Surg Pathol Clin 2021;14:137)
Case reports
- 48 year old man with sclerosing microcystic adenocarcinoma of the tongue (Diagnostics (Basel) 2022;12:1288)
- 52 year old woman with an ulcerated palatal nodule of 6 months duration (Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2011;112:284)
- 55 year old woman with left floor of mouth fullness and history of multiple sclerosis on immunotherapy (Head Neck Pathol 2019;13:215)
- 59 year old woman with an induration on her tongue (AJSP: Reviews & Reports 2021;26:329)
- 67 year old man with slowly enlarging right parotid mass of 2 years duration (Hum Pathol Rep 2021;26:300577)
- 73 year old man with a history of nasopharyngeal carcinoma treated with radiotherapy, presented with an ulcerating left tonsillar tumor (Ann Diagn Pathol 2021;54:151806)
- 5 case series of tumors closely resembling MAC occurring in the mucosal surfaces of the head and neck (Head Neck Pathol 2016;10:501)
Treatment
- Wide, local excision with selective neck dissection, though lack of lymph node metastasis in the reported literature may favor against a neck dissection (Hum Pathol Rep 2021;26:300577)
- Adjuvant radiation therapy for patients with positive margins (Head Neck Pathol 2019;13:215)
- Adjuvant radiation therapy because of perineural invasion (AJSP: Reviews & Reports 2021;26:329)
- Due to a lack of data on long term outcomes, prolonged follow up (Oral Surg Oral Med Oral Pathol Oral Radiol 2018;125:e94)
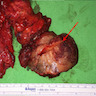
Gross description
- Ill defined, white-tan-gray, firm mass that may show gross extension into adjacent skeletal muscle or adipose tissue (Head Neck Pathol 2019;13:215, Ann Diagn Pathol 2021;54:151806, Hum Pathol Rep 2021;26:300577, AJSP: Reviews & Reports 2021;26:329, Diagnostics (Basel) 2022;12:1288)
Frozen section description
- Assessment of surgical margins, especially during intraoperative evaluation, is extremely challenging if not impossible due to the paucicellular and low grade nature of the tumor (Head Neck Pathol 2019;13:215)
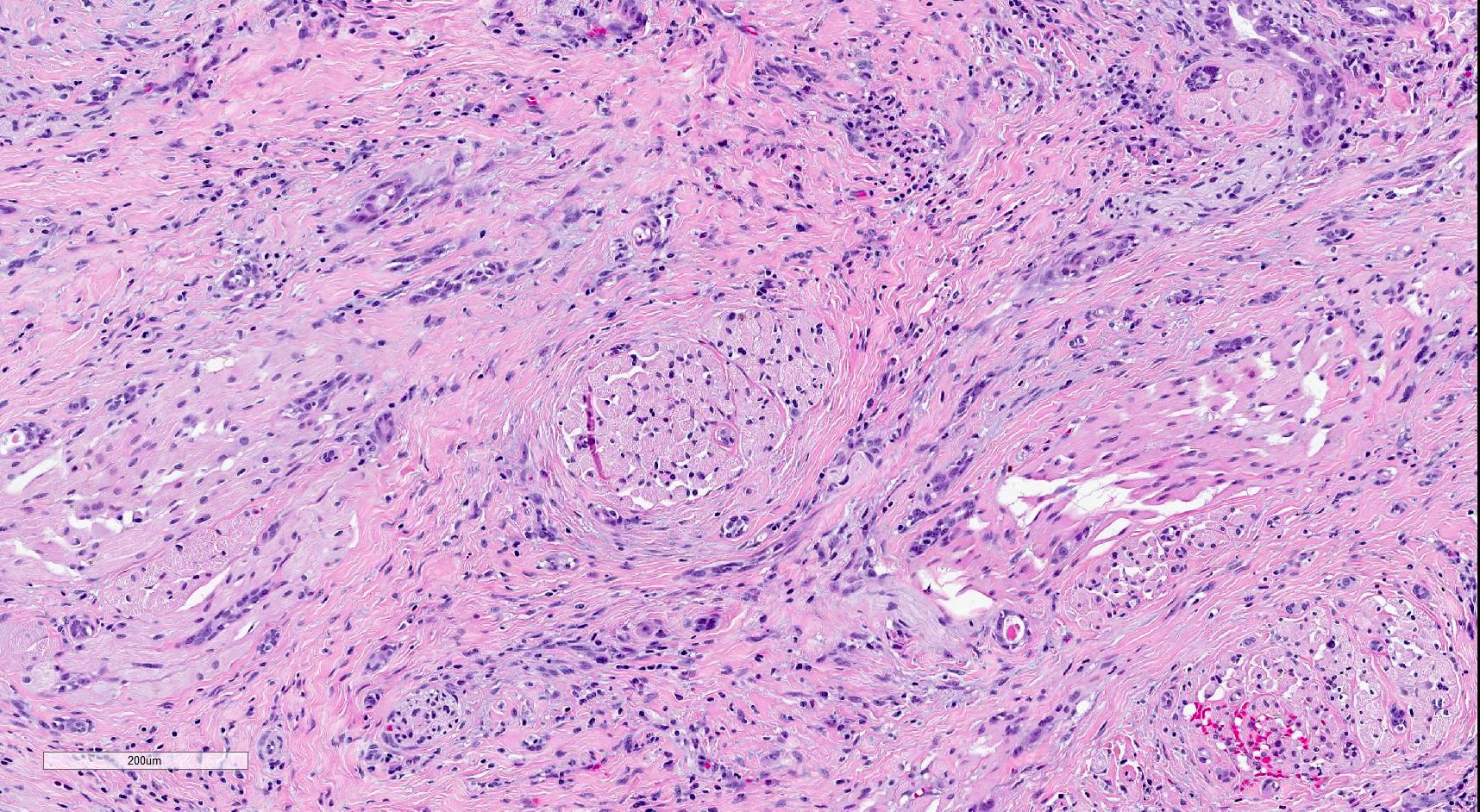
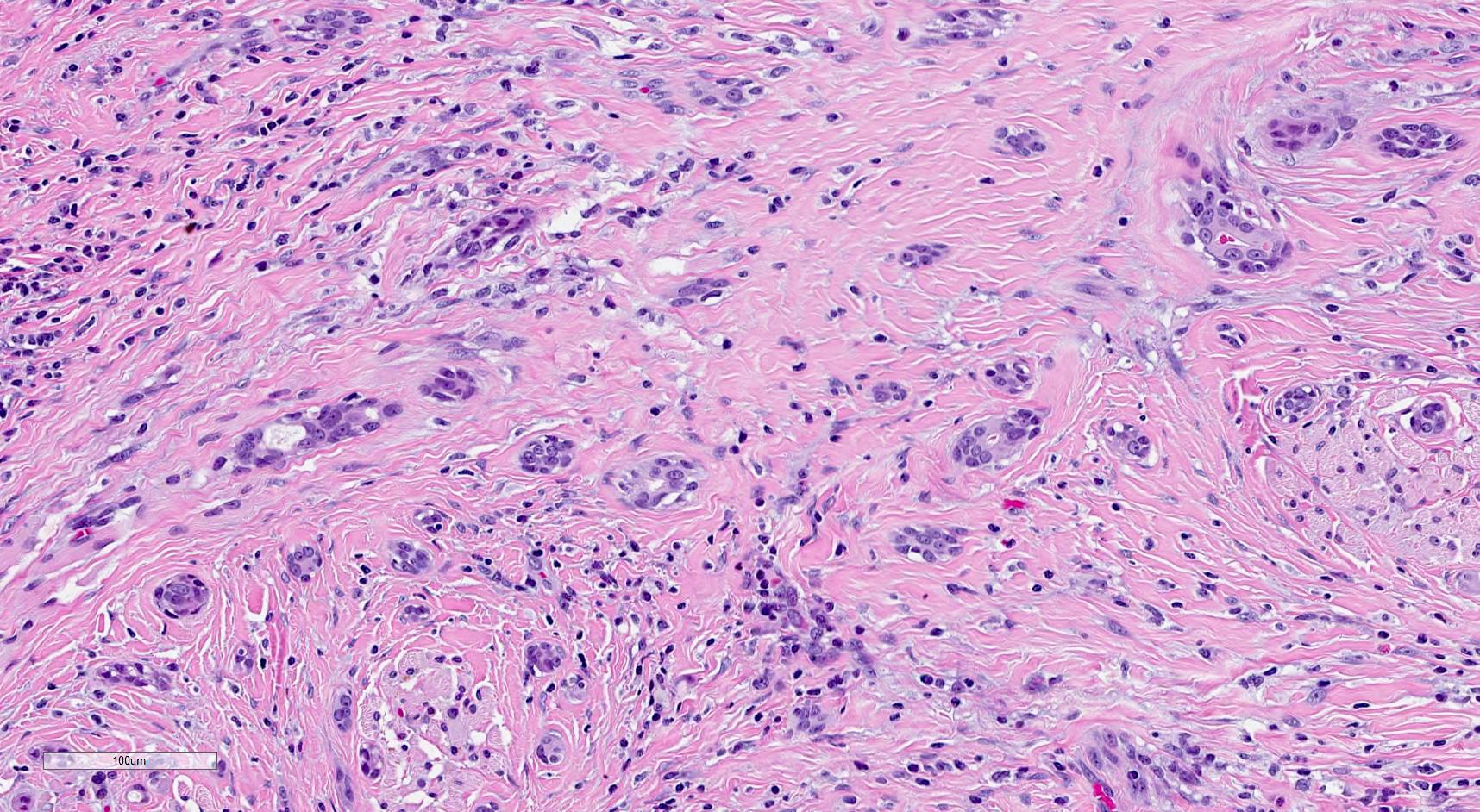
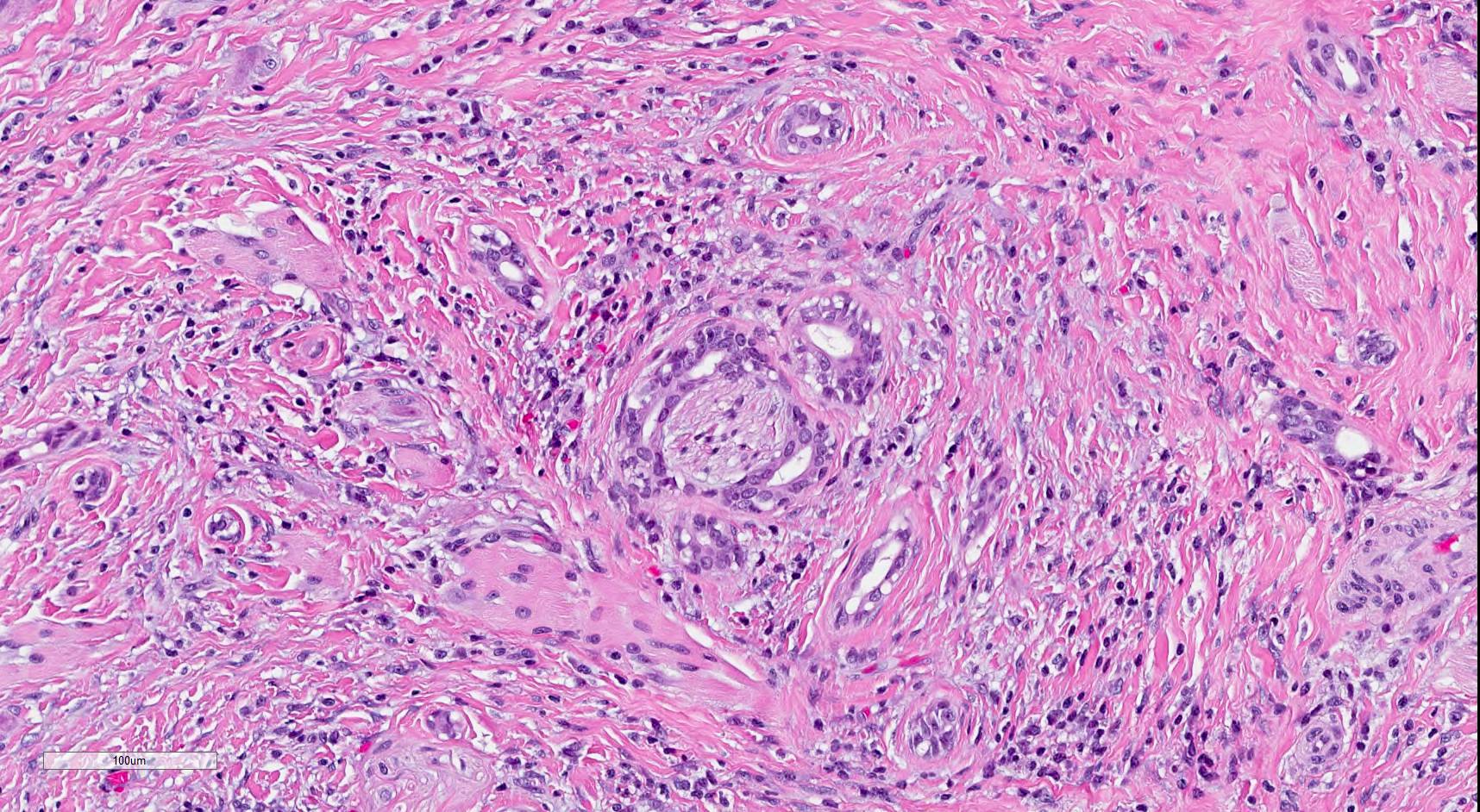
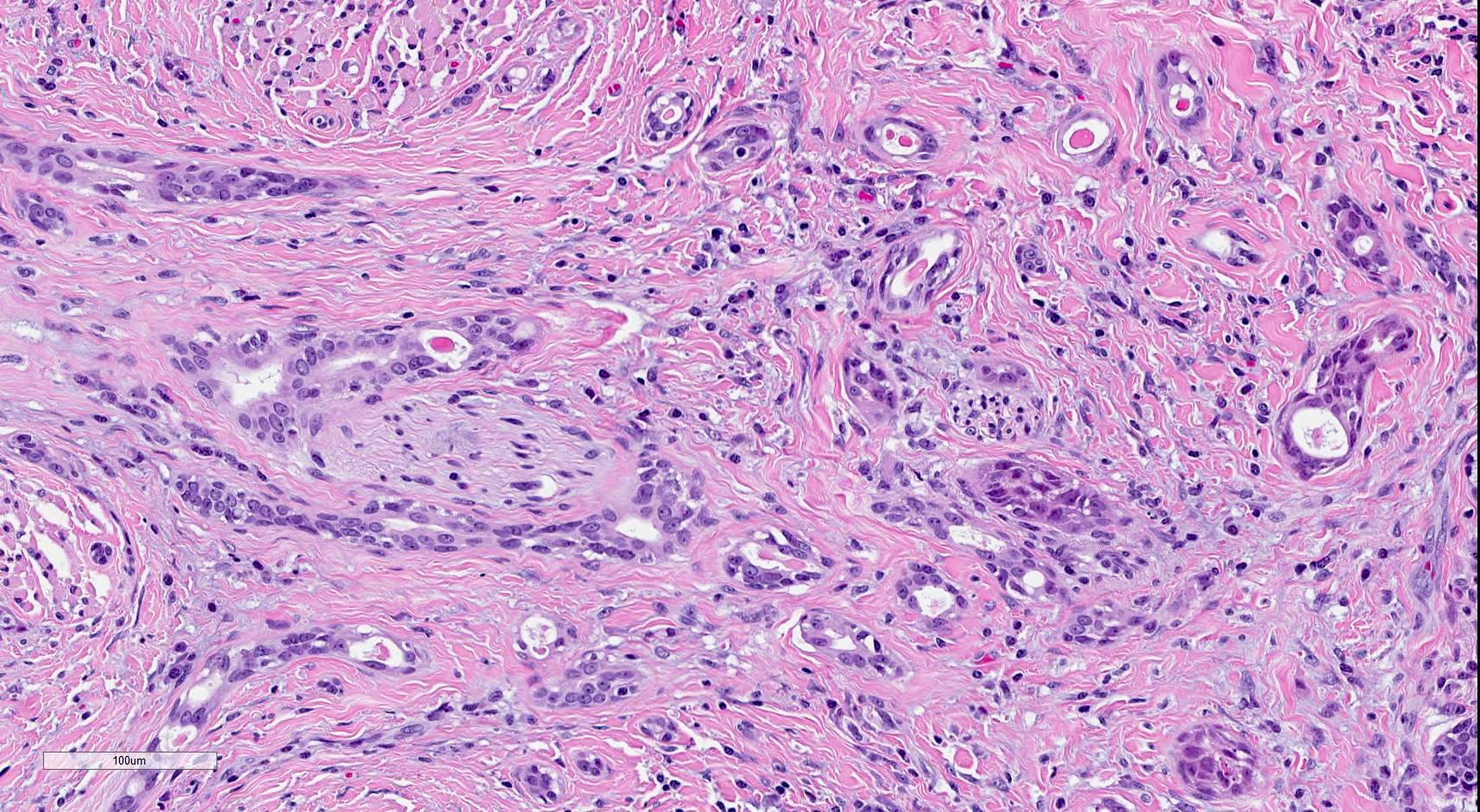
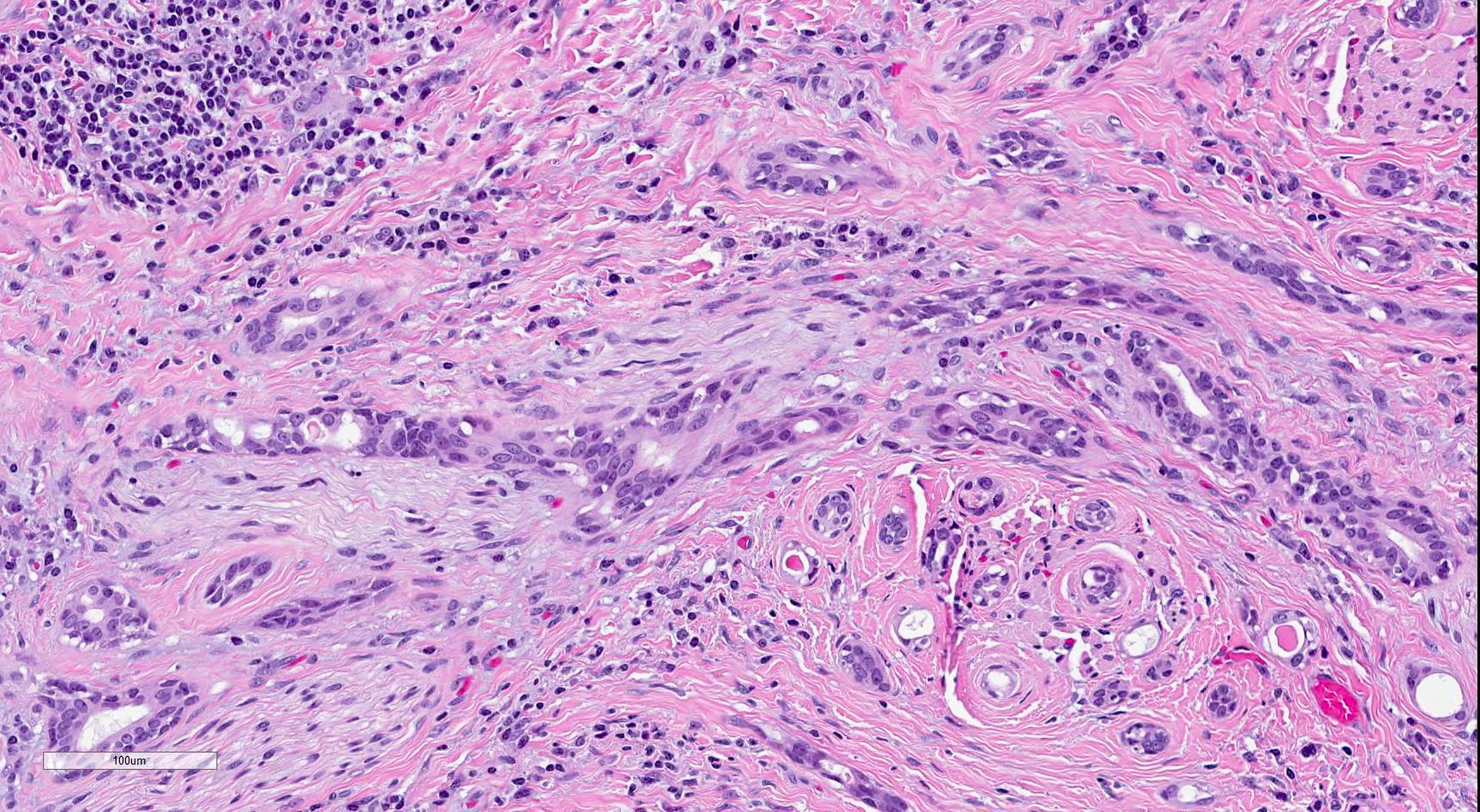
Microscopic (histologic) description
- SMA has a distinctive low power appearance of a relatively paucicellular tumor with clearly invasive variably sized ducts, tubules, cords and nests within densely sclerotic or desmoplastic stroma
- Invasive component is composed of a biphasic population of inner cuboidal and outer myoepithelial cells
- Myoepithelial cells are often attenuated and flattened but are occasionally more prominent and clear
- Cuboidal cells are relatively monomorphic with eosinophilic cytoplasm and round nuclei with even chromatin, occasional nucleoli and no increase in mitosis
- No significant nuclear atypia or necrosis (Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2011;112:284)
- No dysplasia is noted in the overlying mucosa (Diagnostics (Basel) 2022;12:1288)
- Ducts and tubules are frequently filled with eosinophilic secretions that stain positively with mucicarmine (Surg Pathol Clin 2021;14:137)
- Tumor infiltrates skeletal muscle (Head Neck Pathol 2019;13:215)
- Perineural invasion is common (AJSP: Reviews & Reports 2021;26:329)
Microscopic (histologic) images
Cytology description
- Distinct population of basaloid epithelial cells arranged in branching sheets and clusters with minimal nuclear pleomorphism
- Biphasic appearance is apparent
- Some of the cell clusters are bordered by a layer of flattened cells with ovoid bland nuclei
- Occasional intranuclear inclusions are seen
- Hyaline globular matrix, fibrillary metachromatic stroma and mucin are not identified
- Mitotic activity, significant nuclear atypia and necrosis are absent (Ann Diagn Pathol 2021;54:151806)
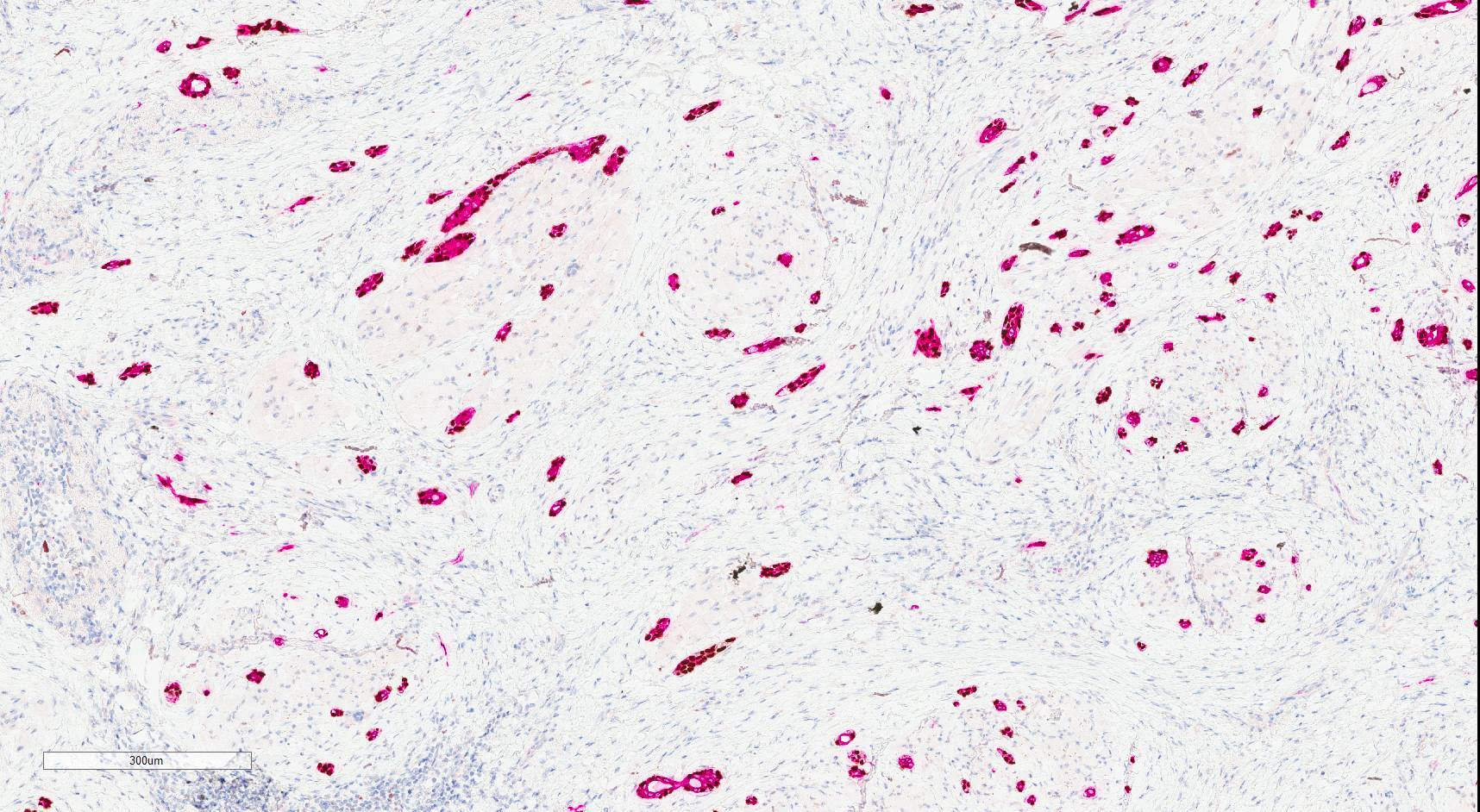
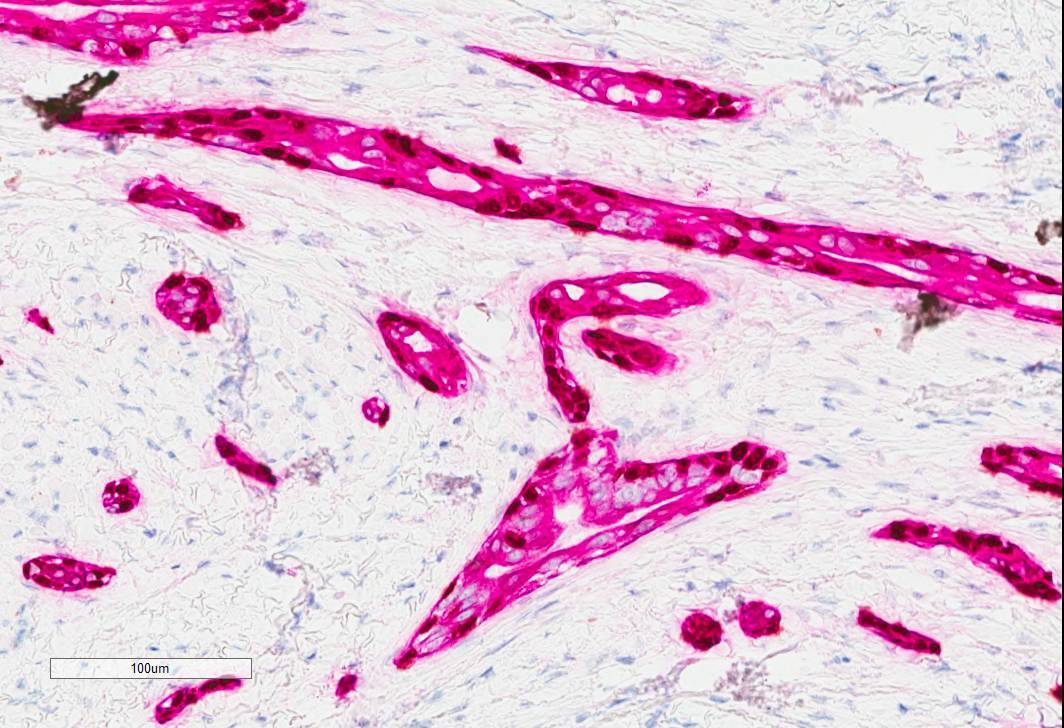
Positive stains
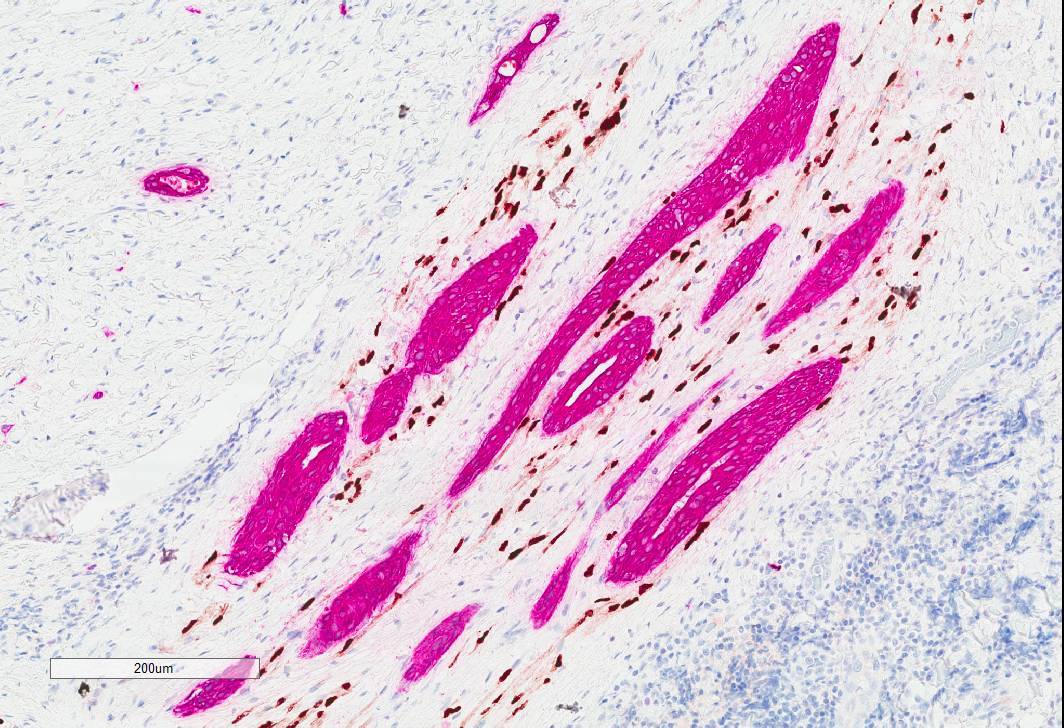
- Epithelial cells: positive for CK7, carcinoembryogenic antigen (CEA), epithelial membrane antigen (EMA), AE1 / AE3, CAM5.2, CK5/6 (Surg Pathol Clin 2021;14:137, Head Neck Pathol 2019;13:215, AJSP: Reviews & Reports 2021;26:329)
- Myoepithelial cells: typically positive for myoepithelial markers, such as p40, p63, S100 and SMA (Surg Pathol Clin 2021;14:137, Head Neck Pathol 2019;13:215, AJSP: Reviews & Reports 2021;26:329)
- Luminal secretions are positive for mucicarmine (Surg Pathol Clin 2021;14:137)
- Ki67 proliferation index is low (< 5%) (Diagnostics (Basel) 2022;12:1288, Oral Surg Oral Med Oral Pathol Oral Radiol 2018;125:e94)
- Overall, the tumor cells show a similar staining pattern to MAC (Head Neck Pathol 2019;13:215)
Negative stains
Molecular / cytogenetics description
- Recently, whole exome sequencing of a single case revealed a moderate tumor mutational burden, a putative loss of function mutation in CDK11B and no molecular overlap with mutations previously identified in MAC (Anticancer Res 2020;40:6375)
- However, the molecular landscape of SMA is largely unknown at this point in time
Sample pathology report
- Tongue, partial glossectomy:
- Sclerosing microcystic adenocarcinoma (2.4 cm), margins uninvolved by carcinoma (see synoptic report and comment)
- Comment: Sections show deeply infiltrative nests, cords and tubules of tumor cells embedded in prominent desmoplastic to densely collagenous stroma. The tumor is composed of a biphasic population of peripheral, flattened myoepithelial cells with central, bland cuboidal ductal cells with round nuclei, evenly dispersed chromatin and occasional nucleoli. Significant atypia and necrosis are not identified and mitotic figures are not conspicuous. Lumens of various sizes containing occasional dense, globular eosinophilic secretory material are present. The tumor infiltrated into skeletal muscles and multiple foci of perineural invasion are identified. On immunohistochemistry, the outer myoepithelial population stains positively for p63 and SMA while the inner cuboidal ductal cells stain positively for CK7. This immunohistochemical staining pattern supports the diagnosis.
Differential diagnosis
- Squamous cell carcinoma (SCC):
- Single cell population
- Presence of overt keratinization or associated squamous dysplasia (Surg Pathol Clin 2021;14:137)
- High grade and significantly more cytologic atypia (Surg Pathol Clin 2021;14:137)
- Prominent mitotic figures (Surg Pathol Clin 2021;14:137)
- Tubular variant of adenoid cystic carcinoma:
- Cribriform architecture (Ann Diagn Pathol 2021;54:151806)
- Hyalinized / myxoid globules (Ann Diagn Pathol 2021;54:151806)
- CD117 is expressed by the luminal cells in virtually all cases of tubular adenoid cystic carcinoma but is consistently negative in SMA (Ann Diagn Pathol 2021;54:151806)
- More pronounced cytologic atypia with hyperchromatic, angulated nuclei (Surg Pathol Clin 2021;14:137)
- Detection of MYB or MYBL1 rearrangements can also confirm the diagnosis of adenoid cystic carcinoma in 50 - 80% of cases (Surg Pathol Clin 2021;14:137)
- Epithelial myoepithelial carcinoma:
- Biphasic phenotype with a bilayered nature and arranged in tubular structures
- EMC is usually multinodular and surrounded by a thick capsule
- Characteristic abluminal myoepithelial cells with abundant clear cytoplasm
- Infiltration and perineural invasion that is usually accompanied by high grade transformation or dedifferentiation (Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2011;112:284)
- Lacks the characteristic sclerotic / hyalinized stroma observed in SMA
- Cribriform adenocarcinoma of the tongue and minor salivary glands:
- Consists of variably solid and cribriform areas containing bland, overlapping, pale nuclei with a distinct similarity to papillary thyroid carcinoma
- Lesion can have a fibromyxoid stroma and a tubular architecture
- Tubules have a single cell layer
- Margin of the tumor may be infiltrative and lymphovascular invasion may be present
- Positivity with antibodies to S100, cytokeratins and KIT has been recorded (Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2011;112:284)
- Polymorphous adenocarcinoma:
- Greater architectural variability, including tubules, fascicles and solid, cribriform and small papillary areas
- Most typical diagnostic features are concentric layers or whirl-like structures of neoplastic cells around the central nidus
- Lacks the densely sclerotic stroma and bilayered ducts of SMA
- Typically takes the form of a cellular solid, unencapsulated neoplasm that is infiltrative at the periphery
- Uniform positivity with antibodies to S100 (Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2011;112:284, Diagnostics (Basel) 2022;12:1288)
- PRKD1, PRKD2 or PRKD3 gene rearrangements as well as PRKD1 point mutations can help confirm the diagnosis (Nat Genet 2014;46:1166, Genes Chromosomes Cancer 2014;53:845)
- Low grade mucoepidermoid carcinoma:
- Cystic with admixture of intermediate cells, epidermoid cells and scattered mucocytes or nests of mucocytes in varying proportions
- Lacks the characteristic sclerotic / hyalinized stroma observed in SMA
- One exception is an uncommon eosinophilic variant of MEC with areas of hyalinization
- However, this rare variant manifests in major salivary glands, mainly the parotid gland and prominent eosinophilia is not observed in SMA (Diagnostics (Basel) 2022;12:1288)
- Detection of MAML2 gene fusions can confirm the diagnosis
- Mammary analog secretory carcinoma:
- Cells of the microcysts are dramatically different from those of SMA, showing abundant foamy cytoplasm
- They are much more richly cellular than SMA without a considerable stromal contribution
- ETV6 fusions can confirm the diagnosis (ETV6::NTRK3 most common) (Head Neck Pathol 2016;10:501)
- Low grade adenocarcinoma, nonintestinal type:
- Tumors are composed of tubules lined by bland cuboidal to columnar cells
- They can be fairly mitotically quiescent
- Cytoplasm often contains pale basophilic mucinous material
- Lack the dense hyalinized stroma, instead showing more crowded glandular configurations
- Perineural invasion is rare (Head Neck Pathol 2016;10:501)
- Chronic sclerosing sialadenitis (Küttner tumor):
- Extensive periductal and perivascular fibrosis and sclerosis
- Foci of hyalinosis
- Infiltration by mononuclear cells (mostly lymphocytes and plasma cells)
- Acinar atrophy and obliterative phlebitis (Clin Case Rep 2019;7:1600)
- Lacks perineural invasion
- Sclerosing sialometaplasia:
- Benign, reactive lesion
- Lacks the diffusely infiltrative growths beyond the extent of minor salivary glands
- Lobular configuration without perineural invasion (Diagnostics (Basel) 2022;12:1288)
Additional references
Practice question #1
Which of the following immunohistochemical staining patterns support a diagnosis of sclerosing microcystic adenocarcinoma?
- CK7 in central ductal / epithelial component; p63 in peripheral myoepithelial cell population
- p63 in central ductal / epithelial component; CK7 in peripheral myoepithelial cell population
- S100 in central ductal / epithelial component; pancytokeratin in peripheral myoepithelial cell population
- SMA in central ductal / epithelial component; pancytokeratin in peripheral myoepithelial cell population
Practice answer #1
A. CK7 in central ductal / epithelial component; p63 in peripheral myoepithelial cell population. The central ductal component showing positivity for pancytokeratin and CK7 and the peripheral myoepithelial cell population expressing smooth muscle actin, S100, p63 and p40 (Surg Pathol Clin 2021;14:137). Answers B - D are incorrect because these are not the immunohistochemical staining patterns for sclerosing microcystic adenocarcinoma.
Comment Here
Reference: Sclerosing microcystic adenocarcinoma
Comment Here
Reference: Sclerosing microcystic adenocarcinoma
Practice question #2
Sclerosing microcystic adenocarcinoma (SMA) has been seen in association with which of the following?
- Alcohol
- HPV infection
- Immunosuppression
- Smoking
Practice answer #2
C. Immunosuppression. SMA frequently arises in the context of immunosuppression (Anticancer Res 2020;40:6375). Answers A, B and D are incorrect because alcohol, HPV infection and smoking have not been reported as associations with this tumor.
Comment Here
Reference: Sclerosing microcystic adenocarcinoma
Comment Here
Reference: Sclerosing microcystic adenocarcinoma