Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Chughtai A, Anjum S. Melanoma arising in giant congenital nevus. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/skintumormelanocyticmelanomagiantcongenitalnevus.html. Accessed April 25th, 2024.
Definition / general
- Malignant tumors arising from melanocytes in pre-existing, benign melanocytic nevi of skin (e.g., giant congenital melanocytic nevi) (Br J Dermatol 2017;176:1131)
Essential features
- Malignant tumors arising from melanocytes in pre-existing congenital nevus (Br J Dermatol 2017;176:1131)
- Presents in childhood or early adulthood (Br J Dermatol 2017;176:1131)
- Identification of benign melanocytic nevus, which is clinically present since birth, in the background (StatPearls: Congenital Melanocytic Nevi [Accessed 8 May 2023])
- Risk of development of melanoma depends largely on size of congenital nevus and is directly proportional to increase in size (StatPearls: Congenital Melanocytic Nevi [Accessed 8 May 2023], J Am Acad Dermatol 2013;68:441)
- Aggressive tumor contributing to the majority of deaths due to skin cancers (Br J Dermatol 2017;176:1131, Int J Mol Sci 2022;23:5911)
- Poor prognosis
Terminology
- Malignant melanoma in congenital nevus
- Historically called melanose and fungoid disease (Melanoma Res 2012;22:114)
ICD coding
Epidemiology
- Higher risk of malignant change is reported in women; F:M = 14.1:6.4
- Incidence of malignant change in large congenital nevi is reported to range from 3.8 to 18%
- Malignant change mostly occurs before puberty and has also been reported at birth
- Giant congenital nevi are associated with neurocutaneous melanosis in 3 - 15% of cases; this is associated with significantly higher risk of primary CNS melanoma
- Primary CNS melanoma accounts for ~33% of melanoma occurring in patients with giant congenital nevi (Br J Dermatol 2017;176:1131)
- Risk of melanoma development is exceedingly low in small to medium sized congenital nevi
- References: Elder: Lever's Histopathology of the Skin, 11th Edition, 2014, Calonje: McKee's Pathology of the Skin, 5th Edition, 2019
Sites
- Trunk and legs are the most common sites for congenital melanocytic nevi, followed by head and neck, feet and hands; melanoma can arise in any of these locations
- Risk of malignant transformation is strikingly higher in axial lesions
- Central nervous system (CNS) / leptomeningeal melanomas arise in settings of neurocutaneous melanosis / congenital nevus syndrome
- References: Elder: Lever's Histopathology of the Skin, 11th Edition, 2014, Calonje: McKee's Pathology of the Skin, 5th Edition, 2019
Pathophysiology
- Somatic gene mutations involving mitogen activated protein kinase (MAPK) pathways (mainly NRAS) lead to the formation of congenital melanocytic nevi during in vitro period (StatPearls: Congenital Melanocytic Nevi [Accessed 8 May 2023], StatPearls: Congenital Nevus [Accessed 8 May 2023])
- Additional mutations and copy number alterations lead to development of malignant change in congenital nevi (Br J Cancer 2017;116:990)
Etiology
- Melanoma can occur de novo or develop on a pre-existing nevus, known as melanoma arising in nevus
- Melanomas arise through either cumulative sun damage pathway or noncumulative sun damage pathway; melanomas arising in congenital nevi belong to the latter category (Annu Rev Pathol 2014;9:239, Arch Pathol Lab Med 2020;144:500)
Clinical features
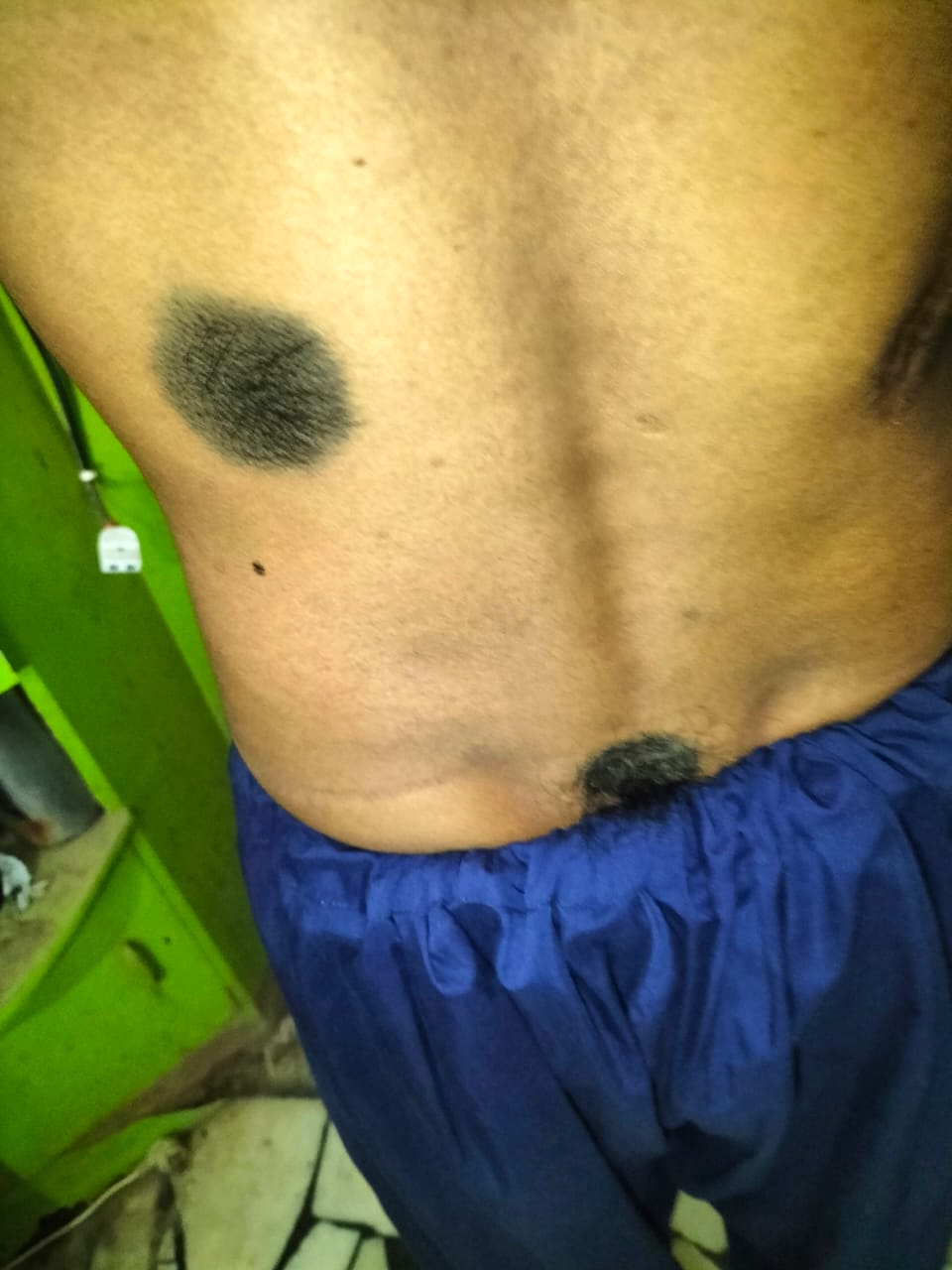
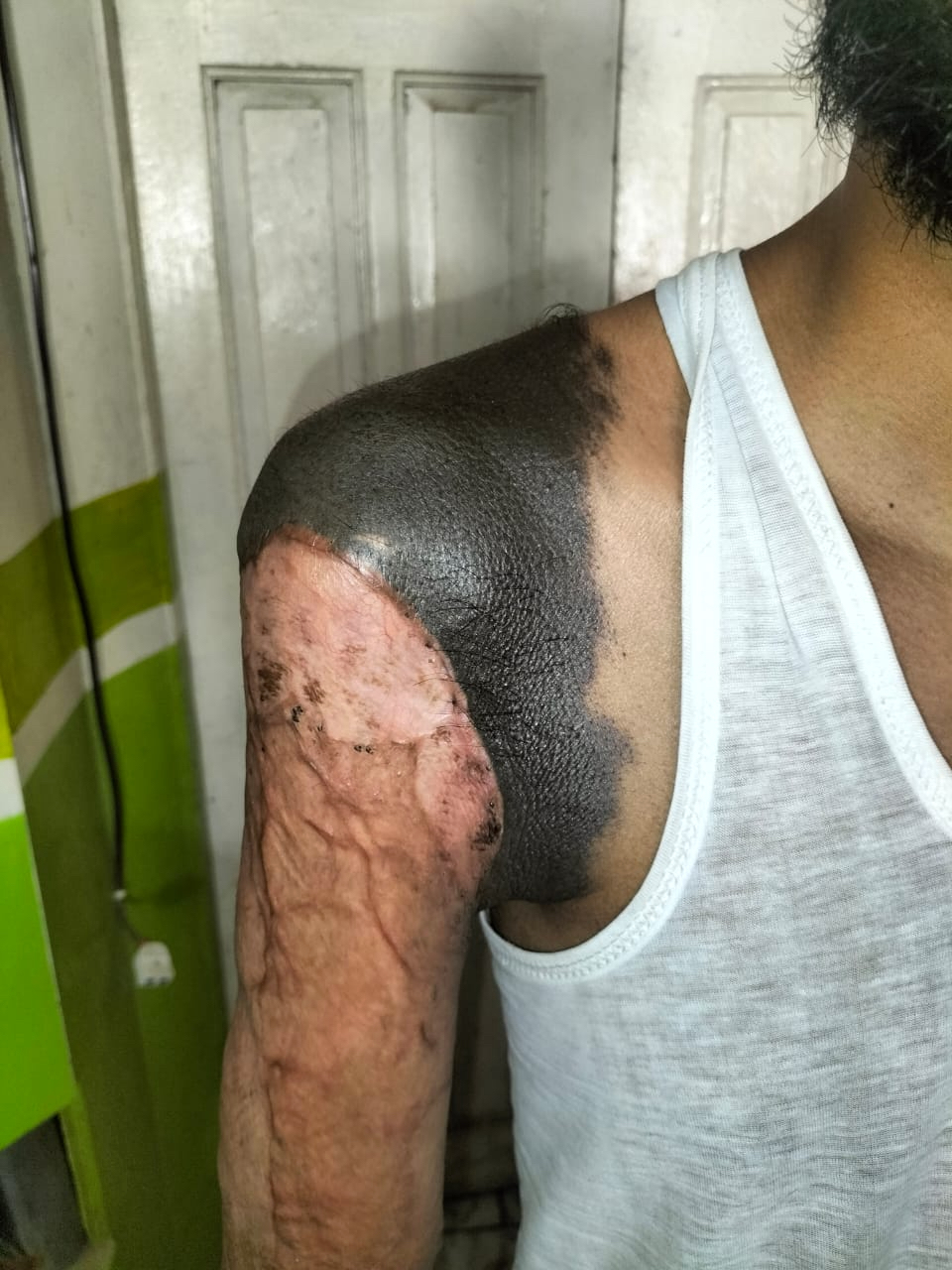
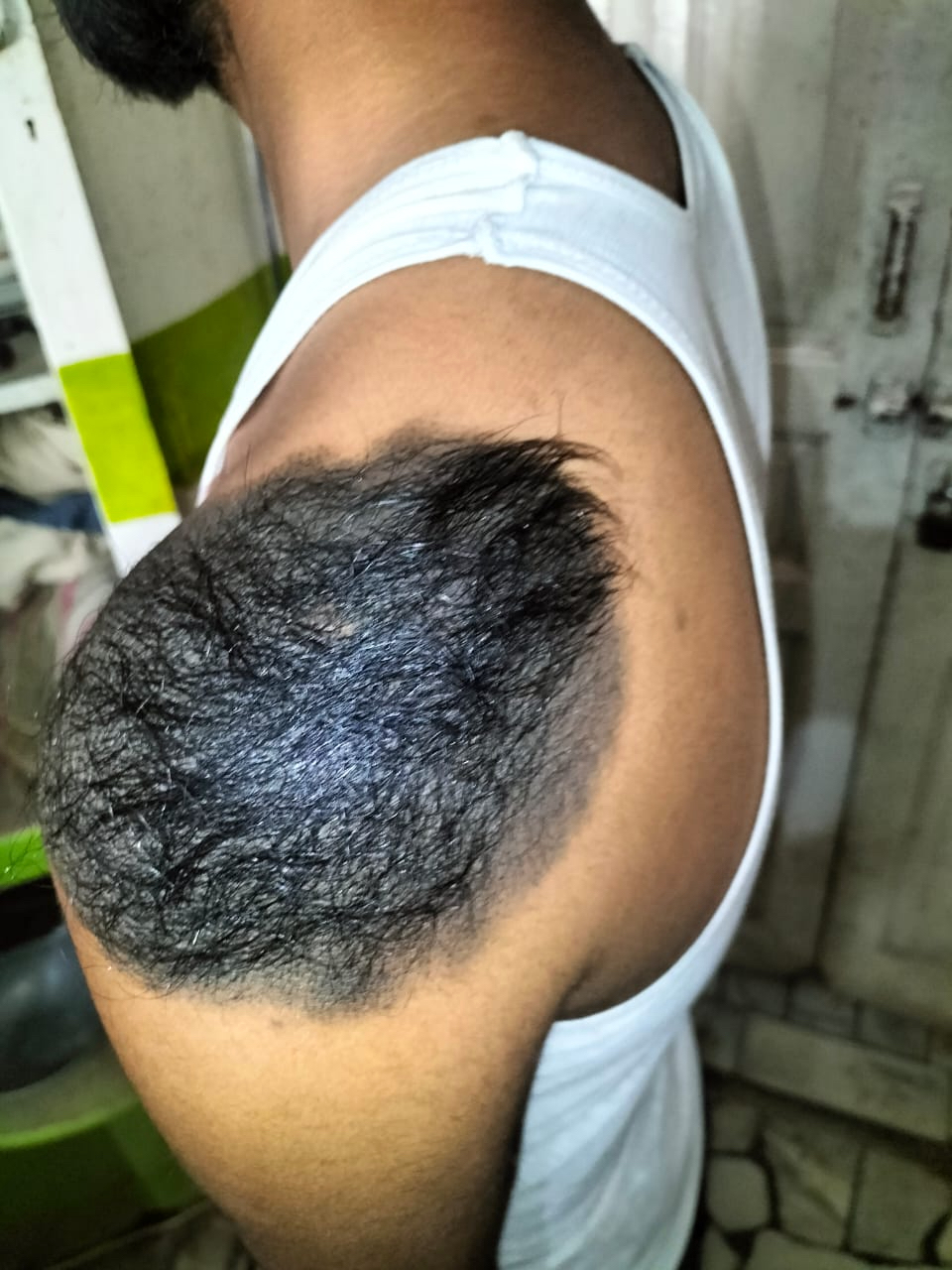
- Malignant change is usually associated with congenital nevi, which are giant / large or multiple; congenital nevi are present since birth and are usually heavily pigmented, hairy and cover significant surface area of skin (StatPearls: Congenital Nevus [Accessed 8 May 2023])
- The following changes are possible indications of the malignant change in congenital nevi
- Appearance of new nodule in pre-existing congenital nevus (Br J Dermatol 2017;176:1131)
- Surface ulceration
- Variation in pigmentation
- Rapid increase in size of the lesion
- Local lymphadenopathy due to metastasis (Br J Dermatol 2017;176:1131)
- Signs of neurocutaneous melanosis include seizures, cranial nerve dysfunctions, hydrocephalus and endocrine abnormalities (StatPearls: Congenital Nevus [Accessed 8 May 2023])
- References: Elder: Lever's Histopathology of the Skin, 11th Edition, 2014, Calonje: McKee's Pathology of the Skin, 5th Edition, 2019
Diagnosis
- Histopathological diagnosis following surgical excision is the gold standard (Br J Dermatol 2017;176:1131)
- Molecular testing may aid in diagnosis (Br J Dermatol 2017;176:1131)
Radiology description
- MRI is the modality of choice for CNS screening in settings of congenital nevus syndrome / neurocutaneous melanosis and is currently the best predictor of all adverse outcomes in children
- Those with a normal scan are in a low risk group for all complications independent of the rest of their clinical phenotype (Br J Dermatol 2017;176:1131)
- Abnormal MRI is an indicator of a higher burden of mutated cells in the body (Br J Dermatol 2017;176:1131)
Prognostic factors
- Prognosis largely depends upon stage of tumor at the time of diagnosis (Int J Mol Sci 2022;23:5911)
- Younger age, female gender, extremities as primary site, early tumor stage and brisk tumor infiltrating lymphocytes are linked with favorable prognosis (Elder: Lever's Histopathology of the Skin, 11th Edition, 2014, Calonje: McKee's Pathology of the Skin, 5th Edition, 2019)
- Older age, male gender, high risk sites (head and neck, trunk and acral sites), high tumor stage, increased Breslow thickness, nodal metastasis, absent or nonbrisk tumor infiltrating lymphocytes, surface ulceration, neurotropism, microsatellite nodules and high mitotic rate are associated with poor prognosis (An Bras Dermatol 2018;93:19, Elder: Lever's Histopathology of the Skin, 11th Edition, 2014, Calonje: McKee's Pathology of the Skin, 5th Edition, 2019)
Case reports
- 19 month old boy and 57 month old girl with neurocutaneous melanosis in association with large congenital nevi (Front Neurol 2019;10:79)
- 3 year old boy with melanoma arising in giant congenital nevus (J Craniofac Surg 2021;32:e342)
- 12 year old girl with melanoma arising in giant congenital melanocytic nevus (Diagn Cytopathol 2020;48:564)
- 34 year old man with a giant congenital nevus of the axilla (J Cutan Pathol 2020;47:1164)
- 46 year old man with multicentric melanoma in giant congenital melanocytic nevus (Dermatol Surg 2003;29:99)
Treatment
- Surgical excision is the standard treatment approach for cutaneous disease; sentinel lymph node assessment for staging and prognostic purposes is usually carried out (Br J Dermatol 2017;176:1131)
- Management of leptomeningeal melanoma involving the spine is mainly palliative as excision is not possible in these cases (Br J Dermatol 2017;176:1131)
- Chemotherapy
- Radiotherapy
- Targeted therapy includes BRAF inhibitors, MEK inhibitors, KIT inhibitors, PD-1 / PDL1 inhibitors and CTLA4 inhibitors
- References: Elder: Lever's Histopathology of the Skin, 11th Edition, 2014, Calonje: McKee's Pathology of the Skin, 5th Edition, 2019
Clinical images
Gross description
- Skin ellipse / excision specimens with pigmented lesion on the surface
- Surface ulceration is usually present
- Lesion can be of variable sizes
- References: Elder: Lever's Histopathology of the Skin, 11th Edition, 2014, Calonje: McKee's Pathology of the Skin, 5th Edition, 2019
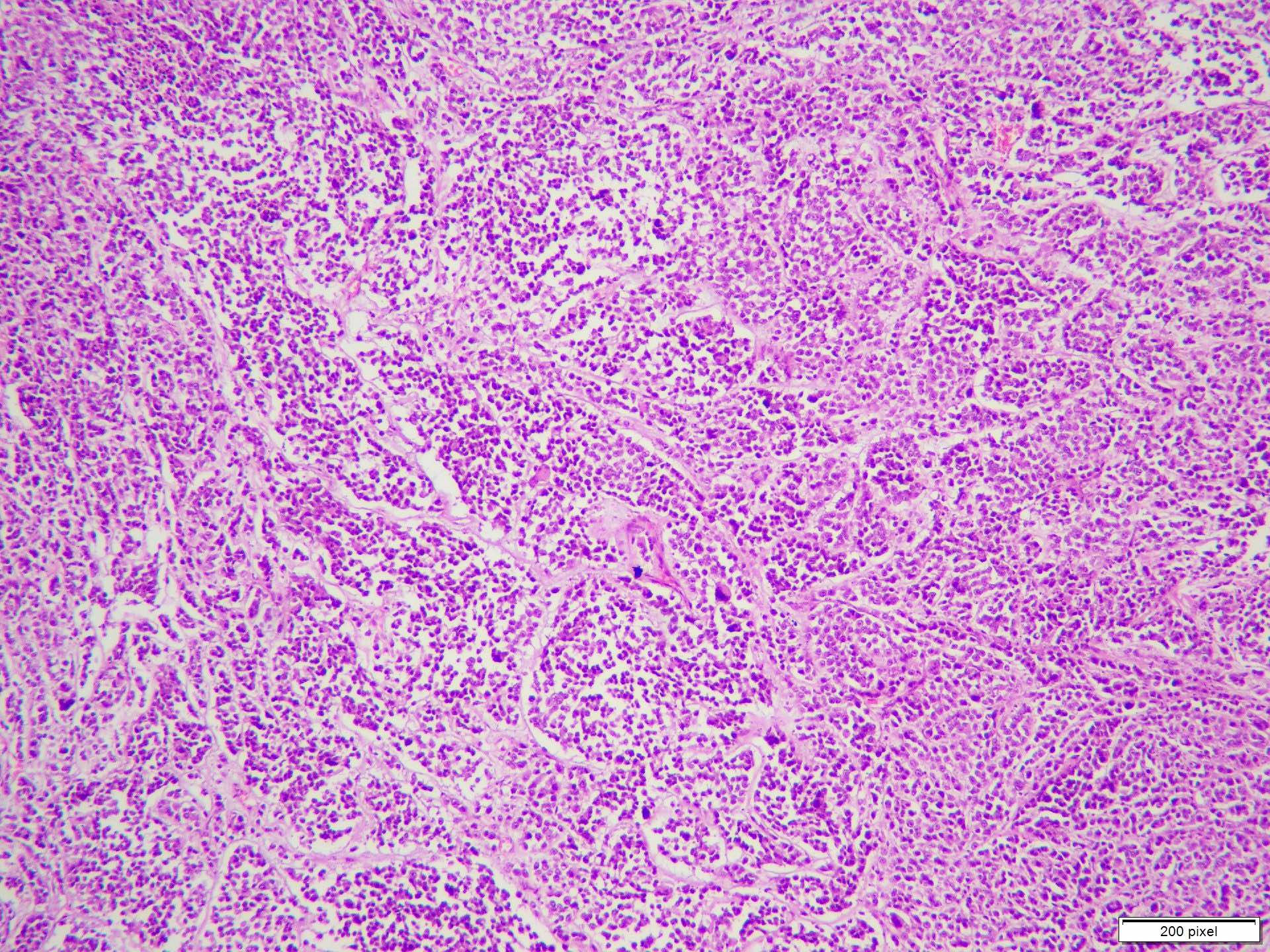
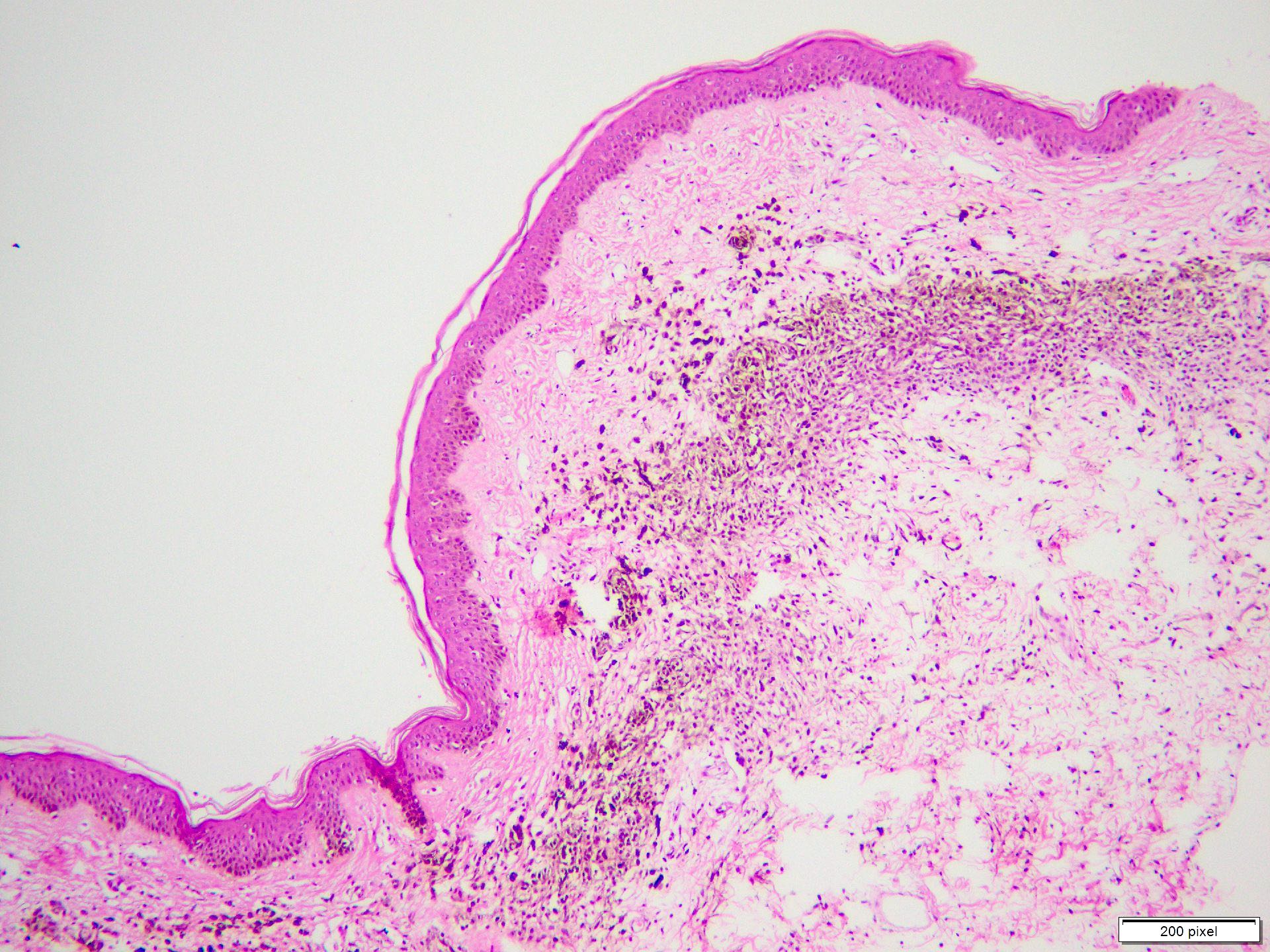
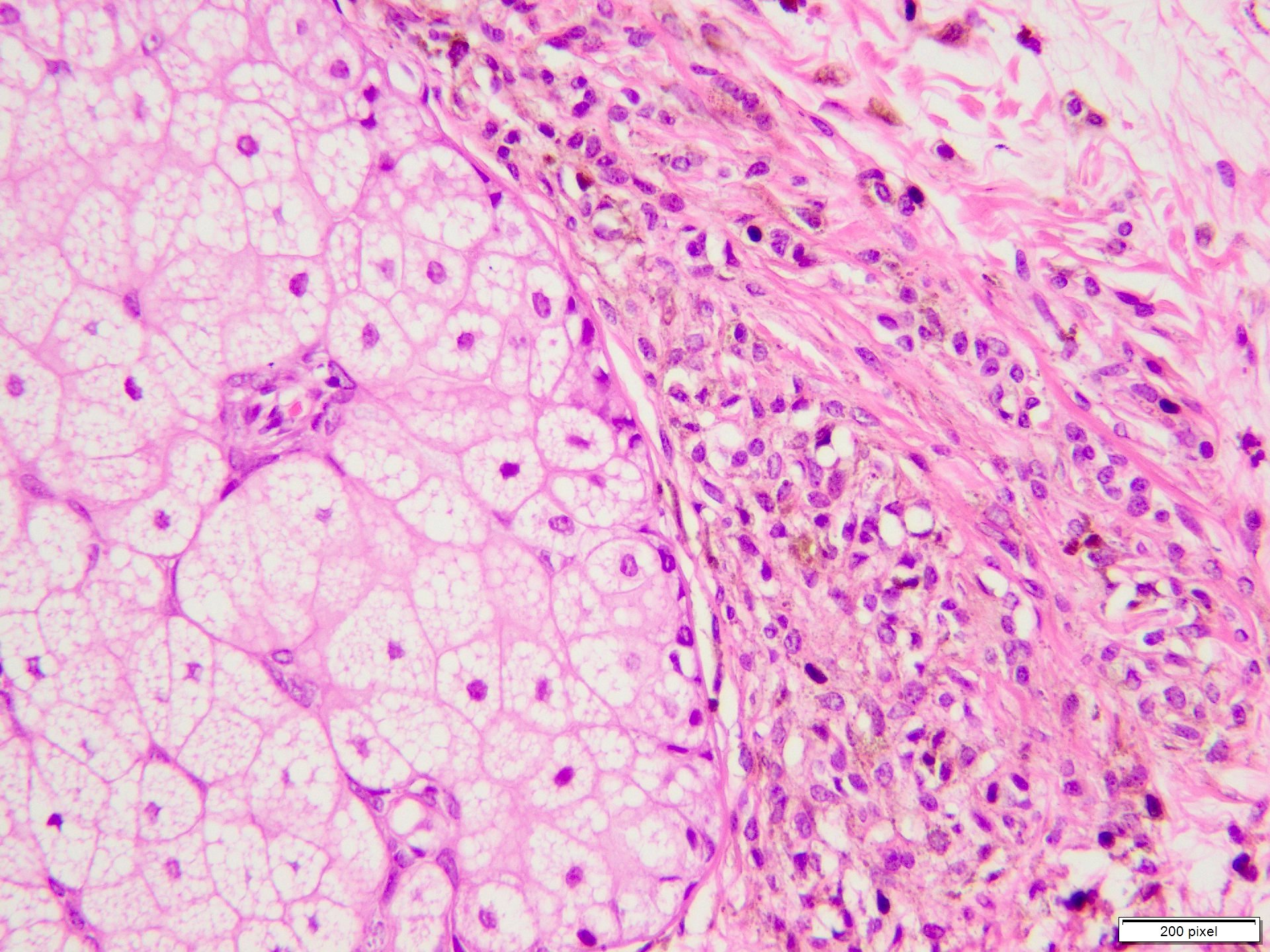
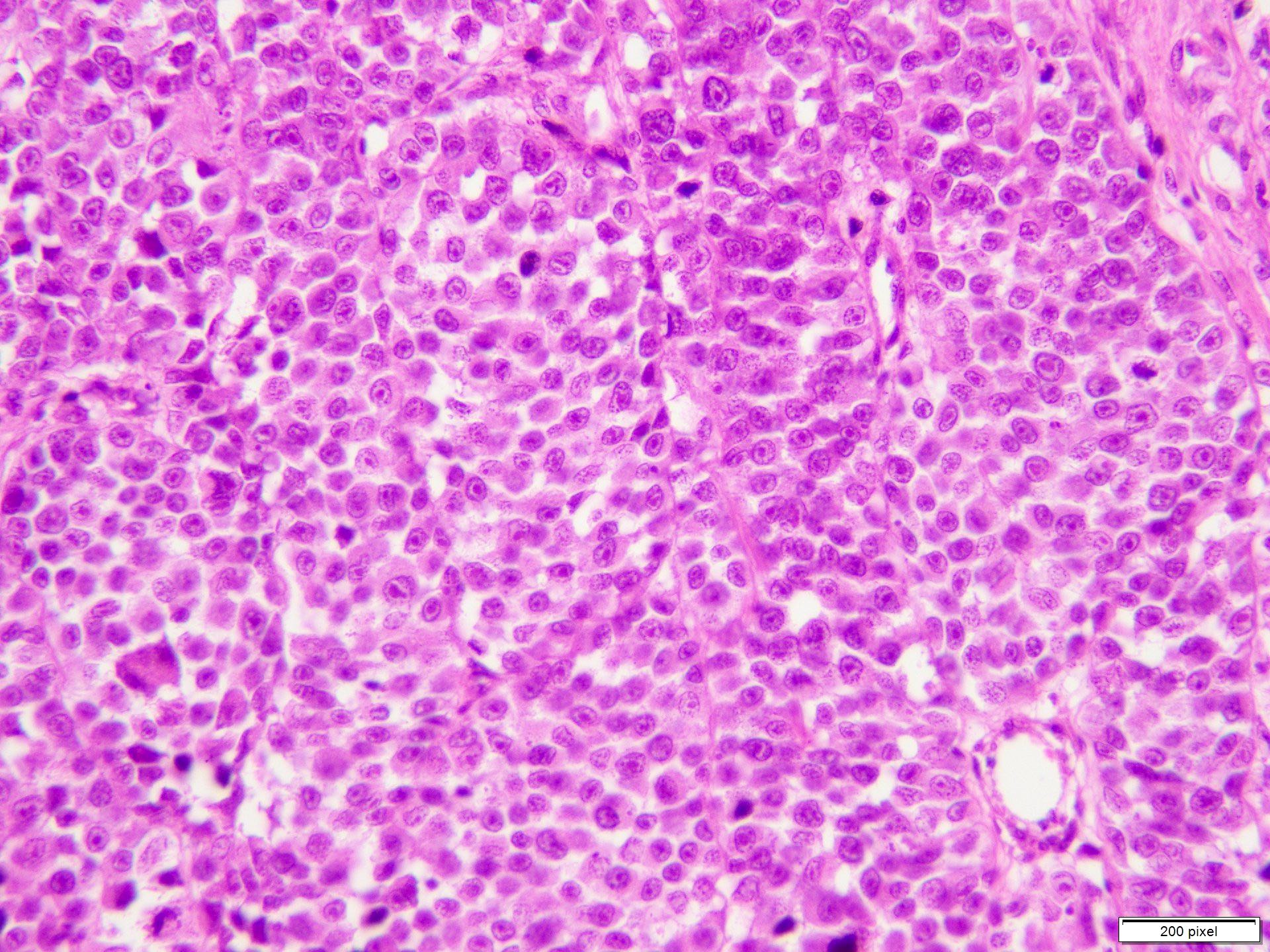
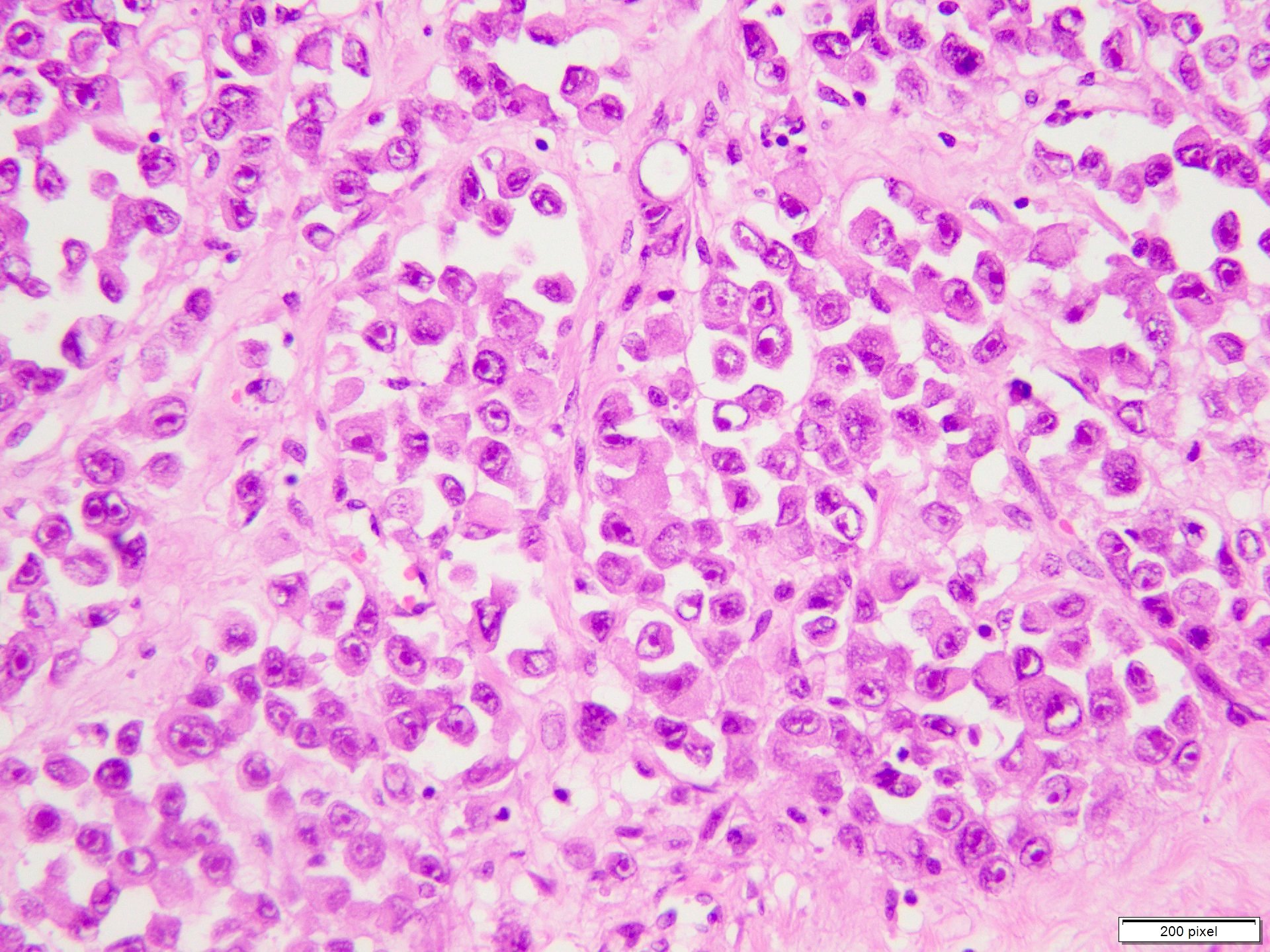
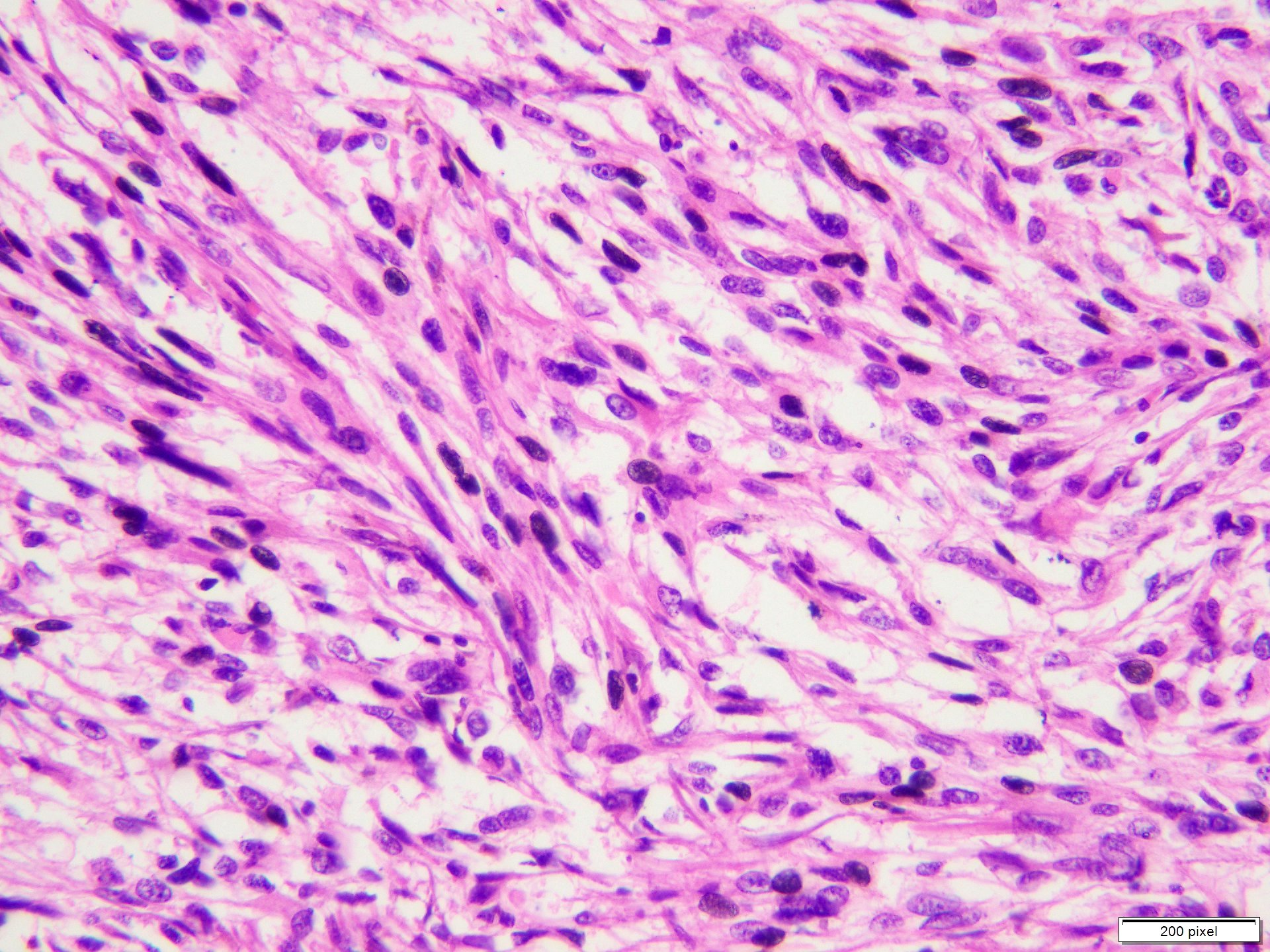
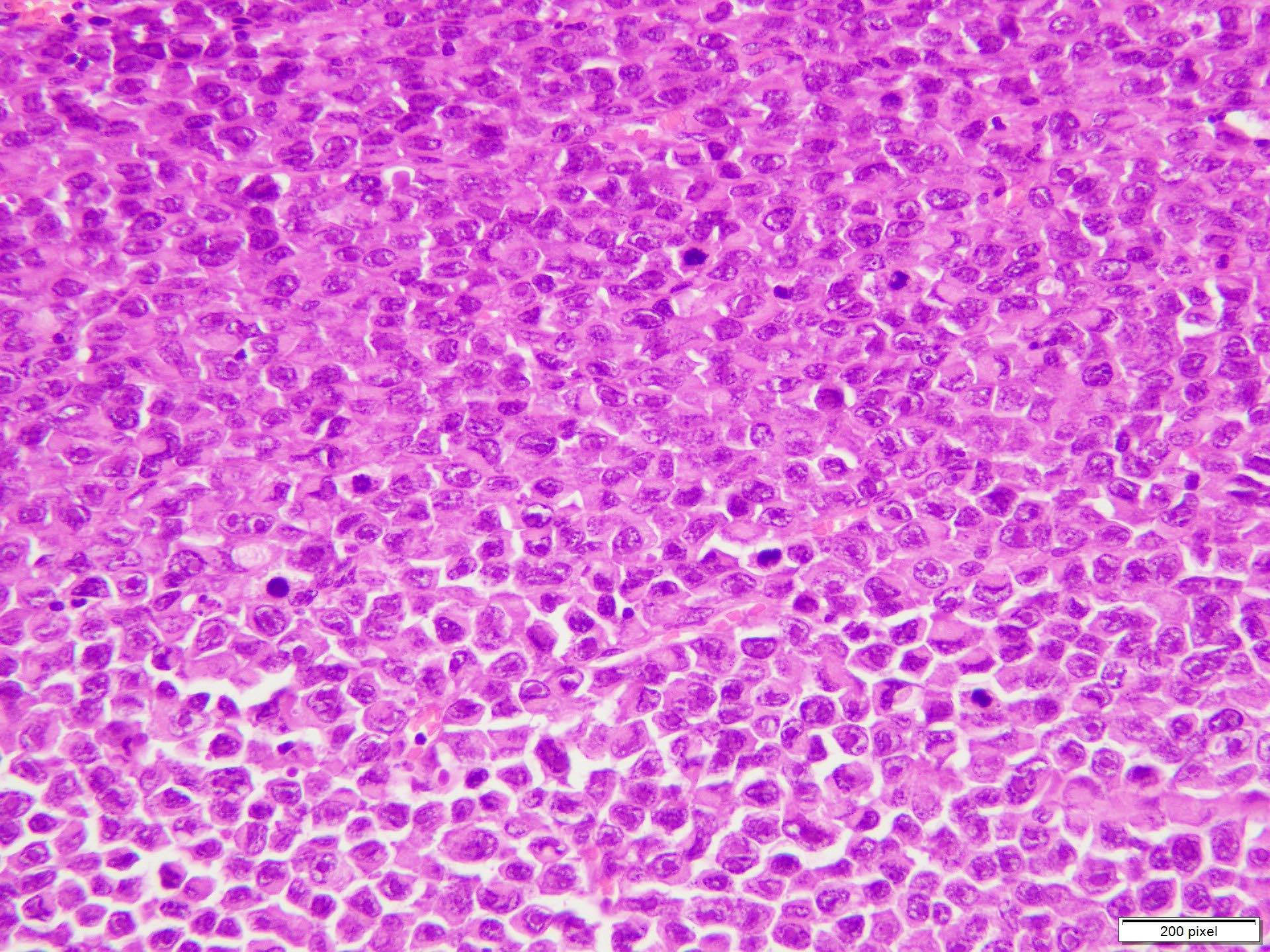
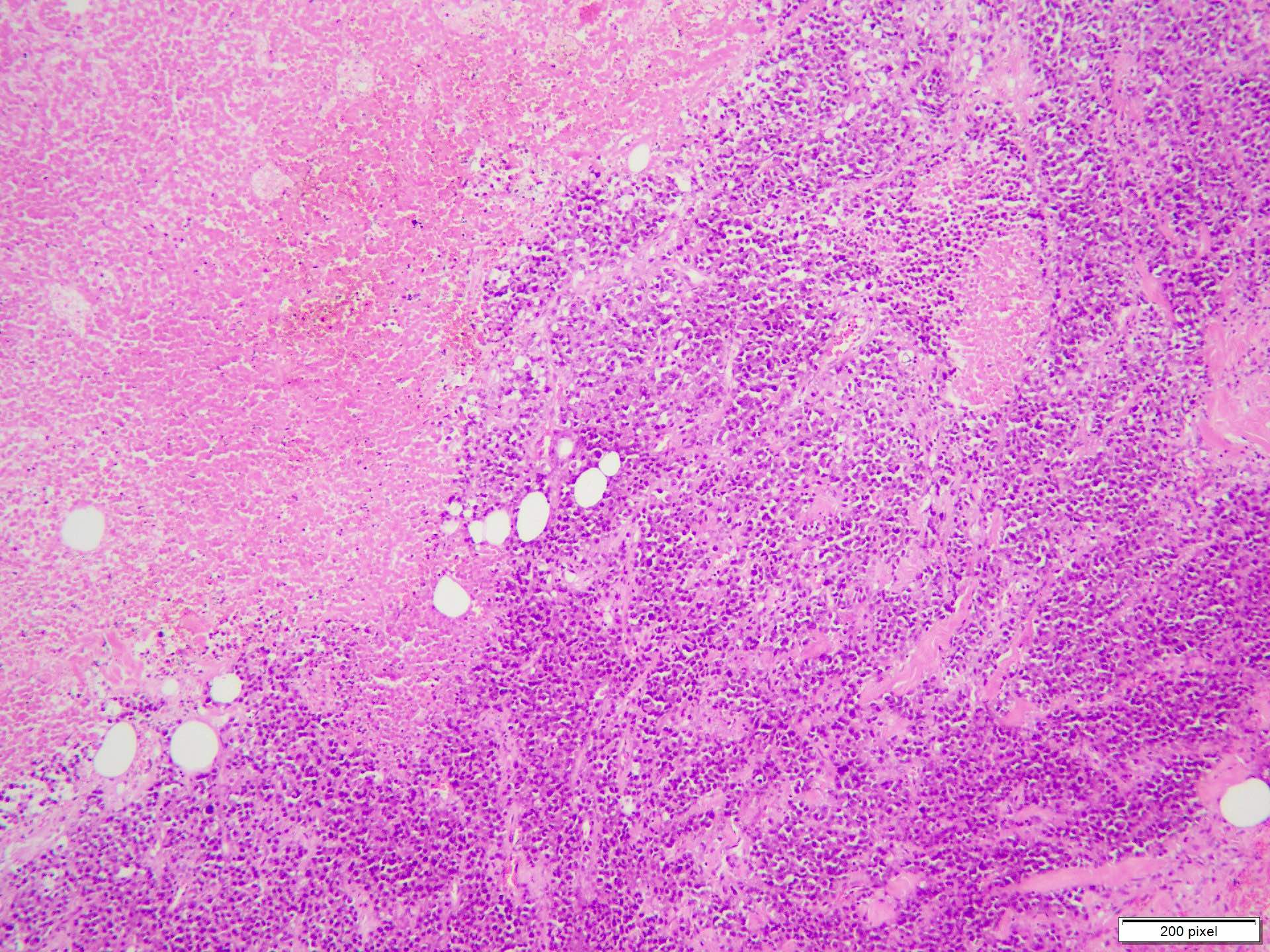
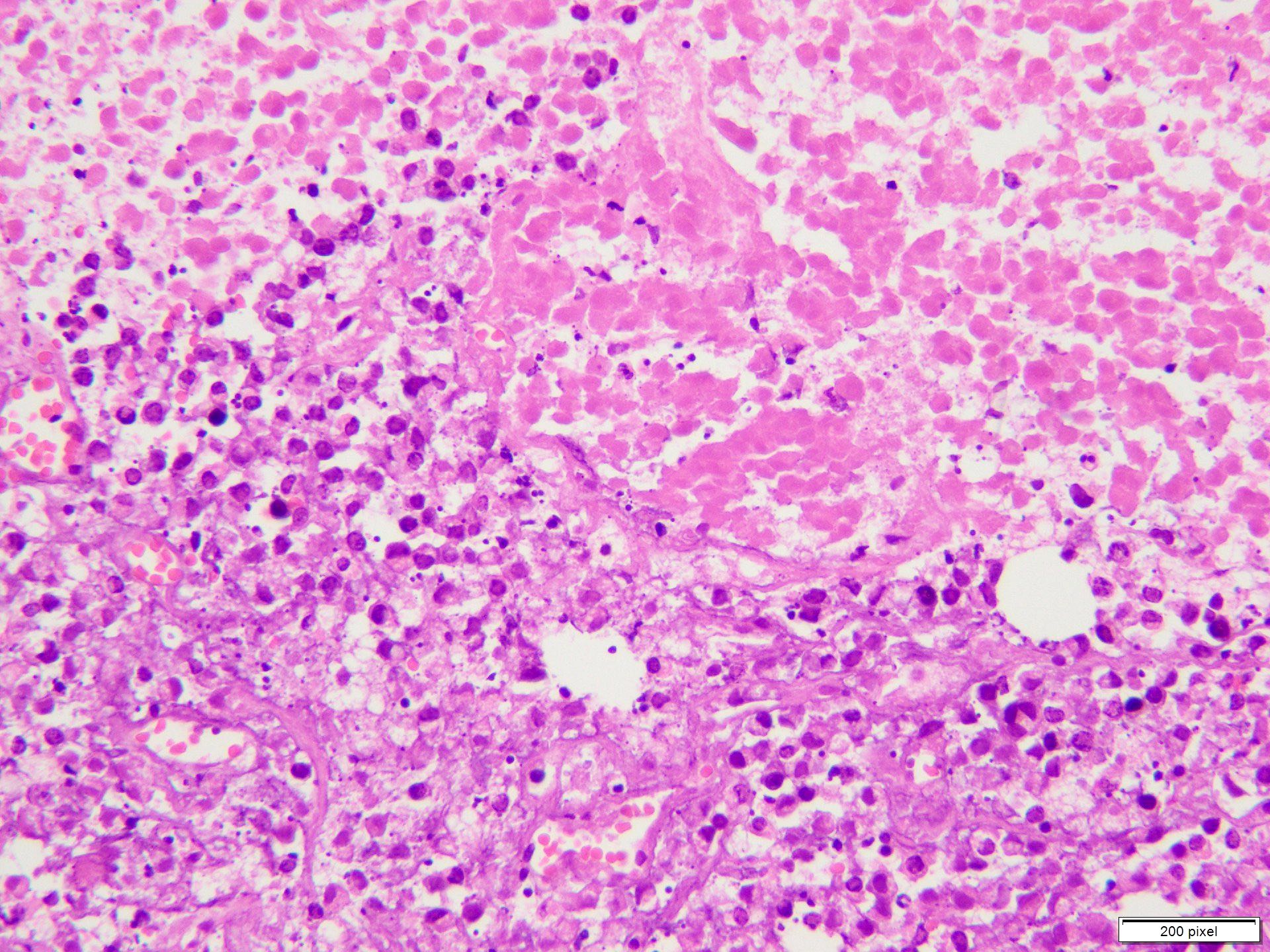
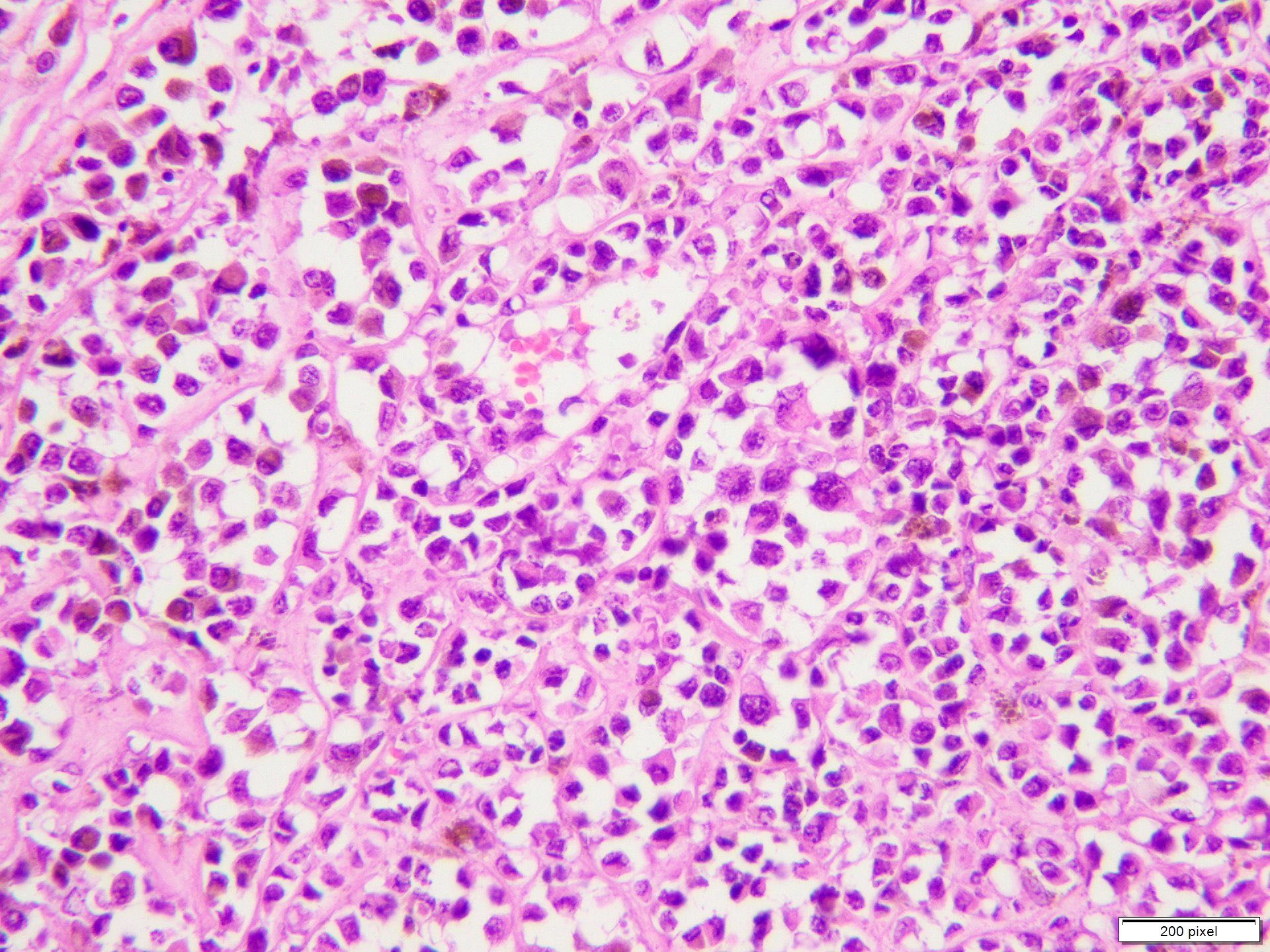
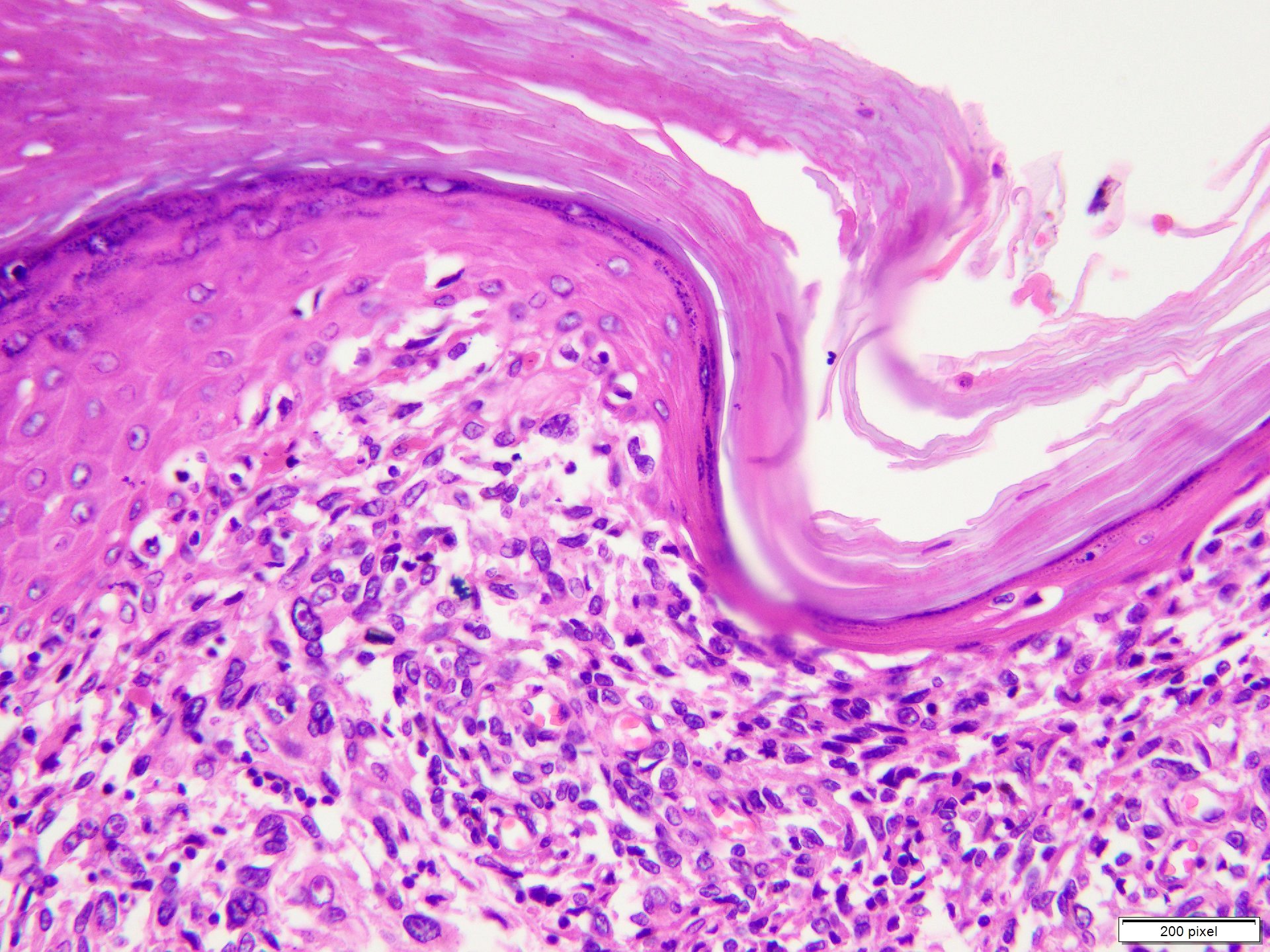
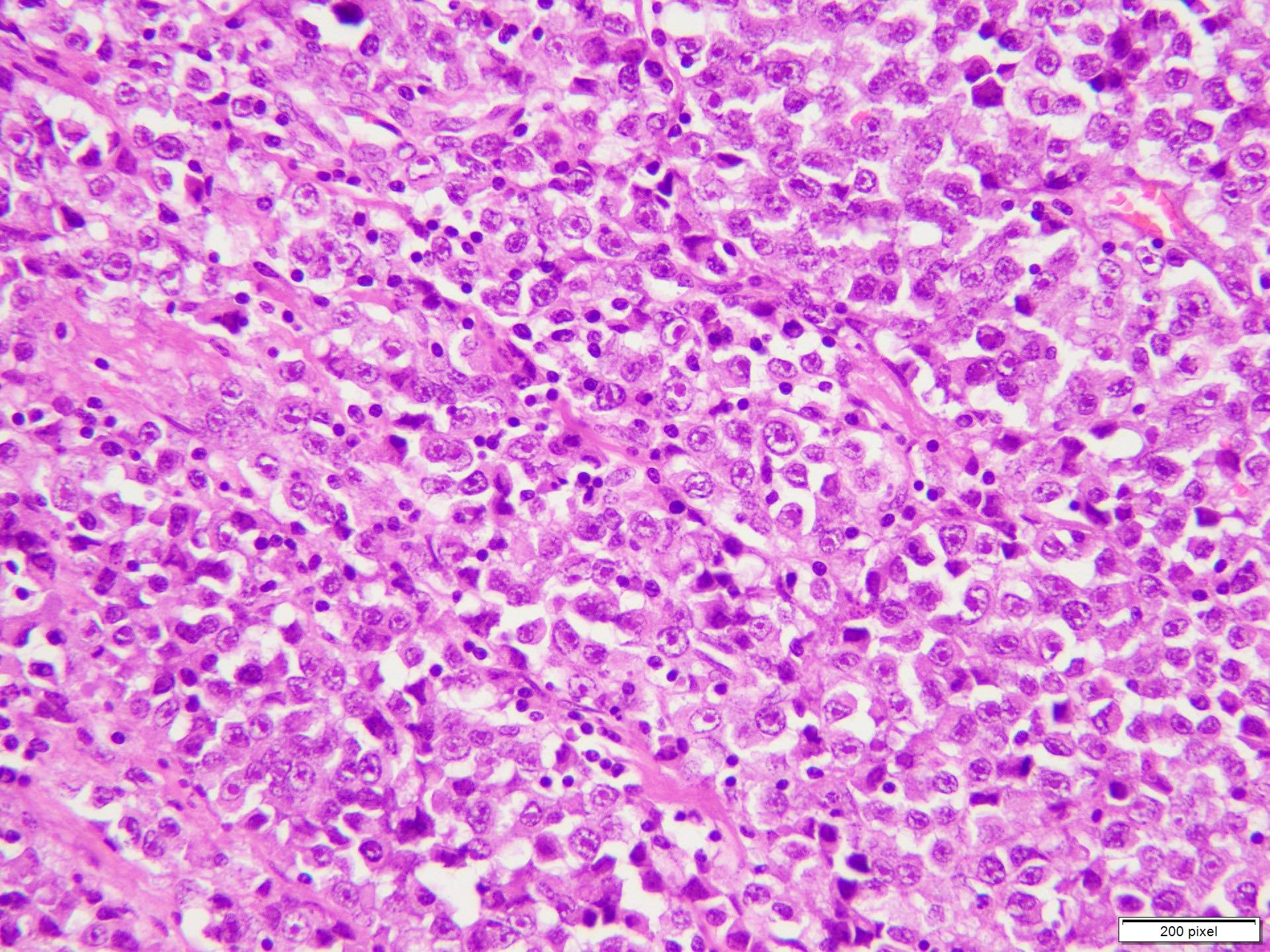
Microscopic (histologic) description
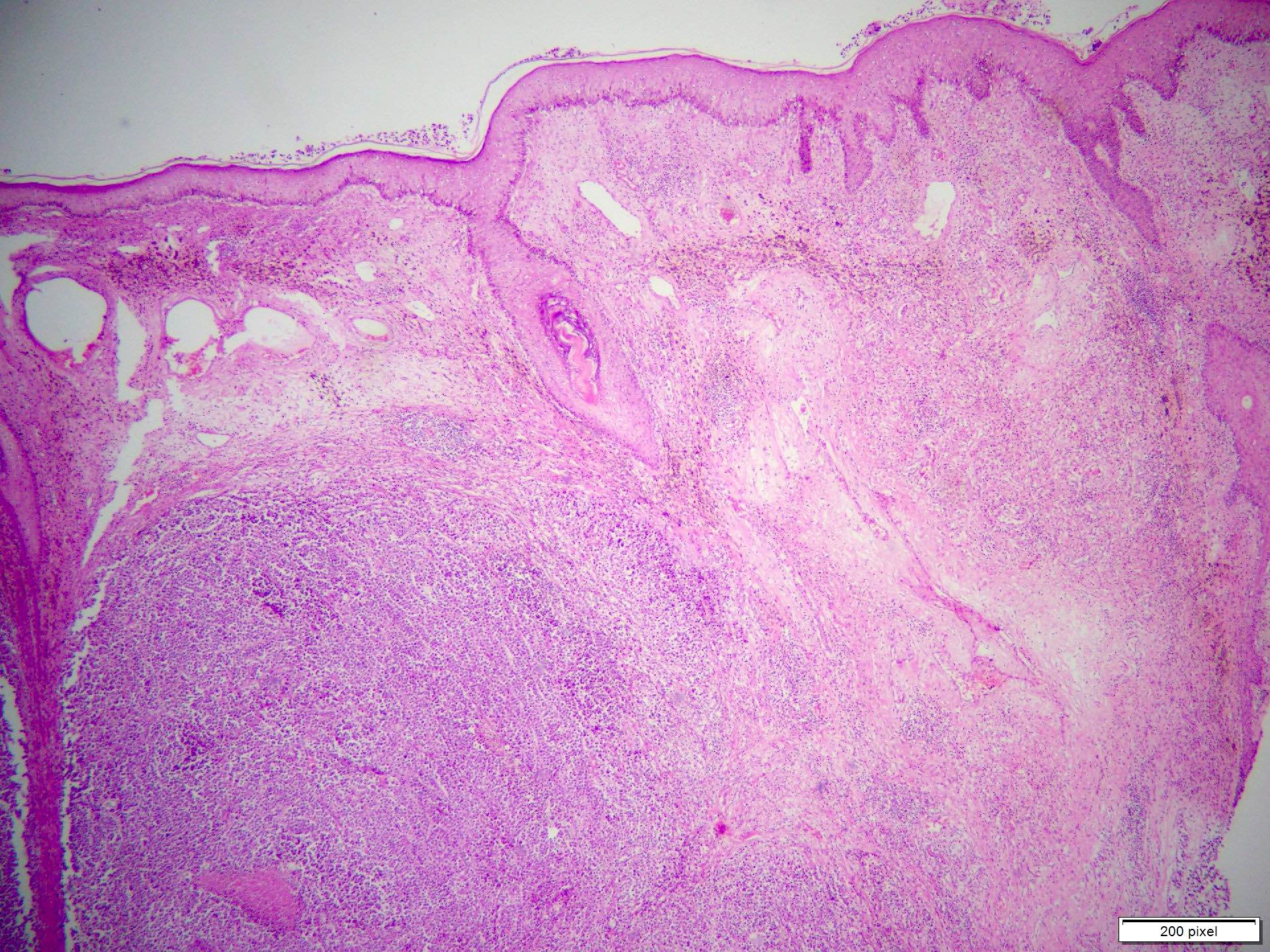
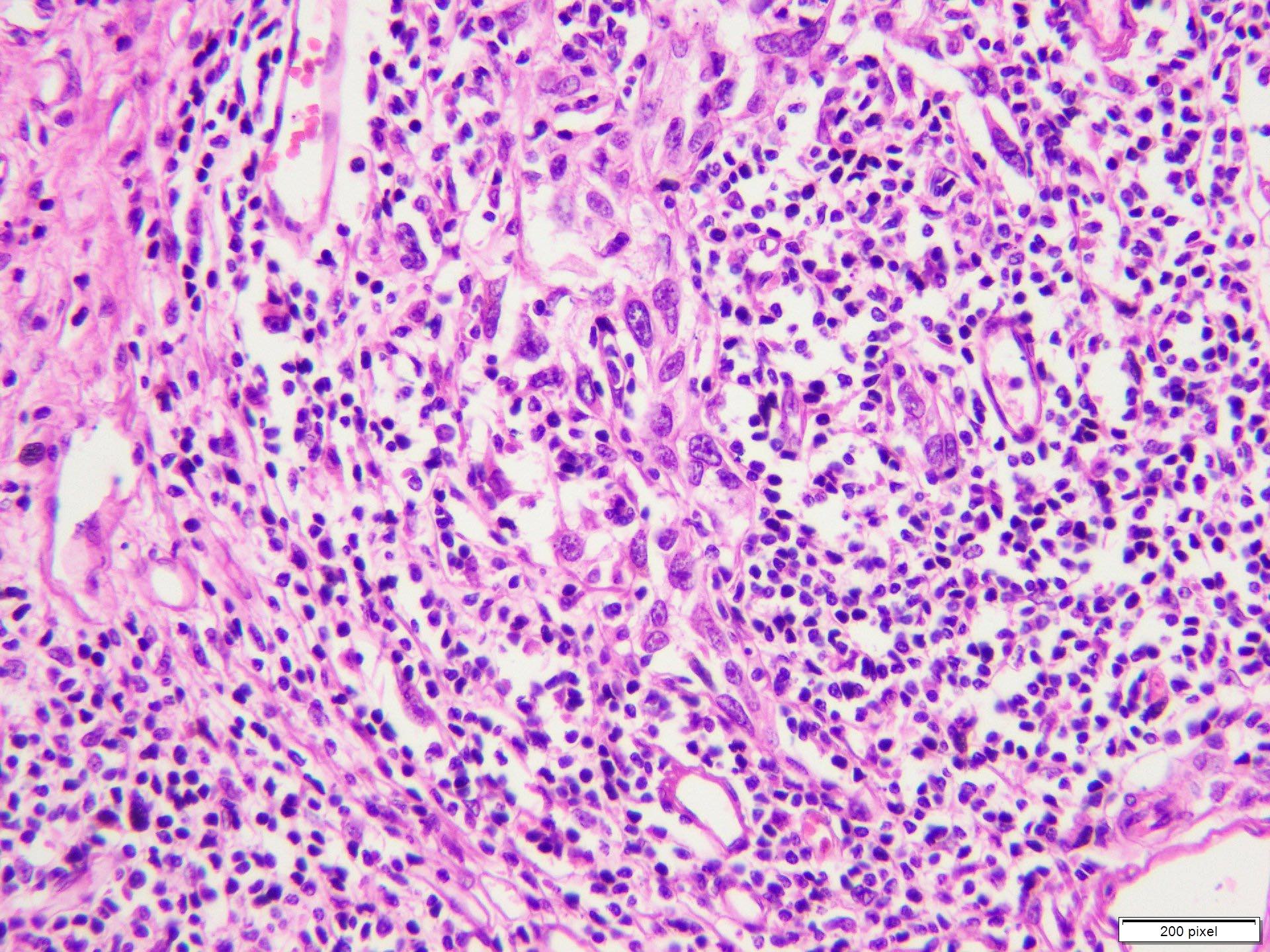
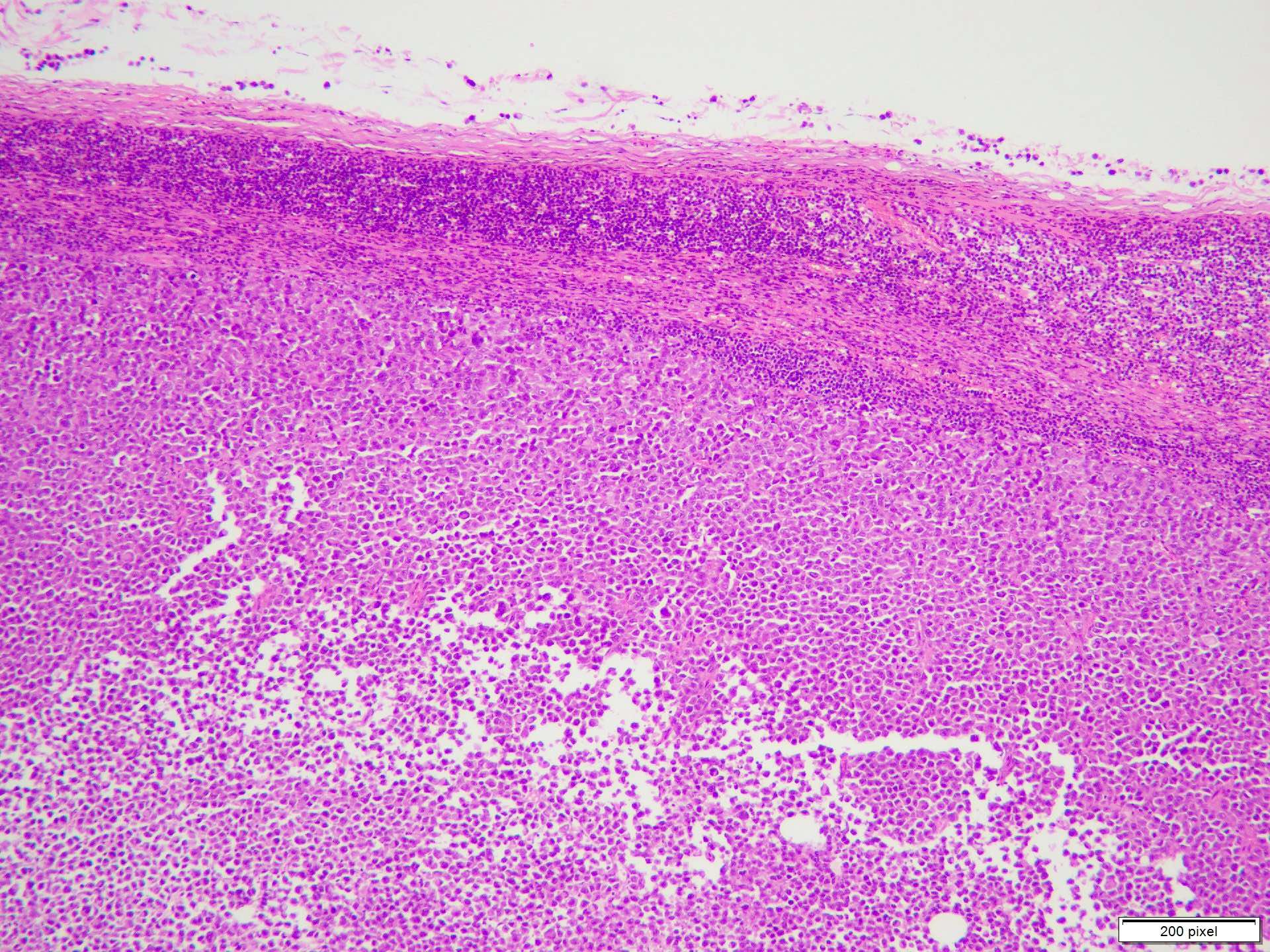
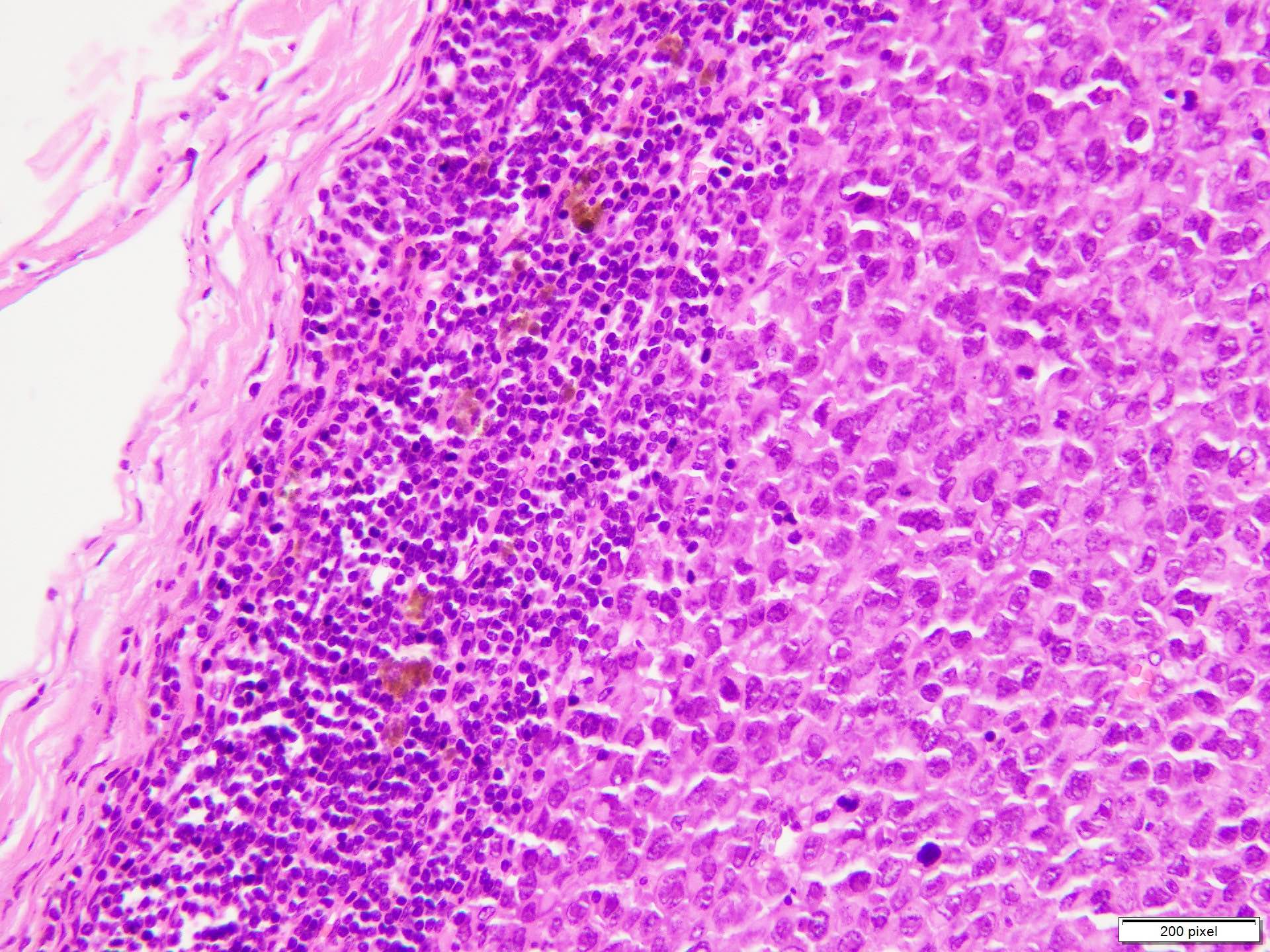
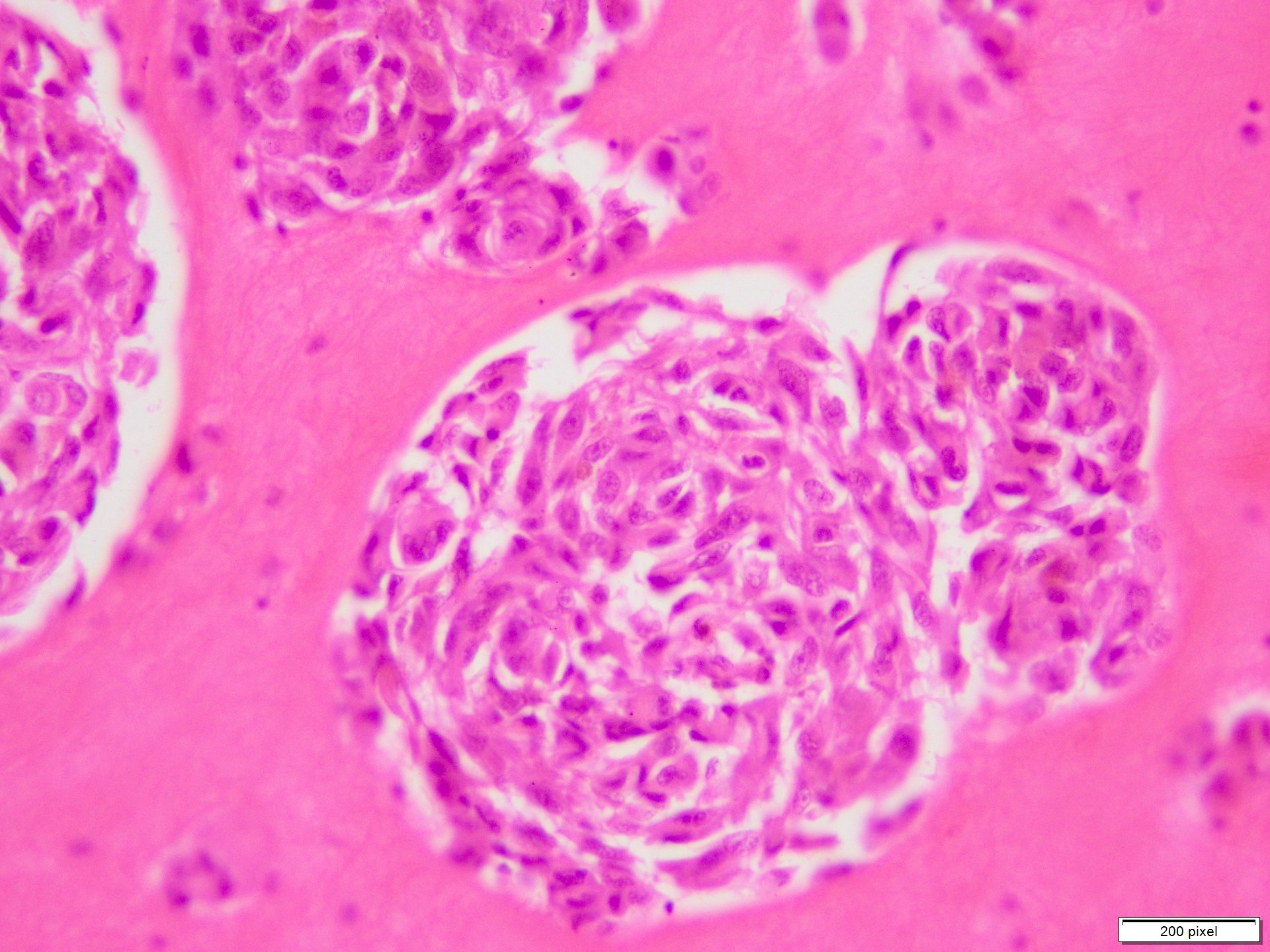
- Benign melanocytic nevus can be identified in background of malignant proliferation
- Malignant proliferation / melanoma exhibits the following morphological features
- Epidermal ulceration is commonly present
- Architectural asymmetry is evident with either radial growth phase or vertical growth phase
- Radial growth phase comprises growth predominantly along the dermoepidermal junction with lateral spread and only small nests or individual cells in superficial dermis
- Vertical growth phase comprises growth predominantly occupying dermis in form of large dermal nest and expansile sheet-like areas
- Atypical melanocytes show irregular distribution at dermoepidermal junction (confluent growth alternating with skip area)
- Junctional melanocytic nests show significant variation in size
- Singly scattered melanocytes in the superficial part of the epidermis (pagetoid spread)
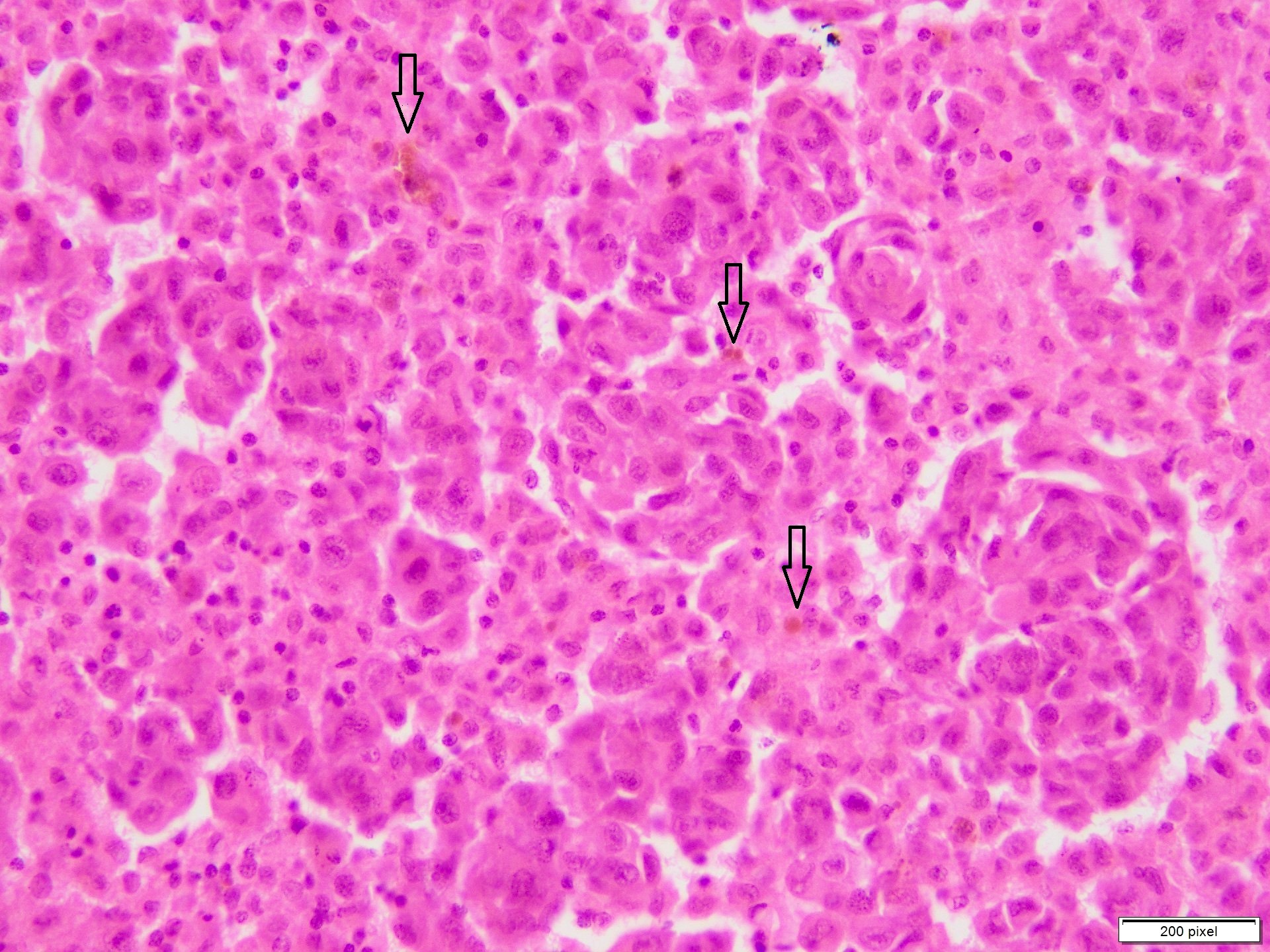
- Atypical melanocytes can be round, epithelioid or spindle shaped with prominent nuclear pleomorphism, nuclear hyperchromasia, prominent nucleoli and irregular distribution of melanin pigment
- Absence of maturation
- Increased dermal mitotic activity (> 1/mm2) with atypical mitotic figures
- Tumor necrosis might be present
- Tumor infiltrating lymphocytes can be seen (brisk / nonbrisk / absent)
- Evidence of lymph node metastasis is usually present at the time of first clinical presentation
- Primary CNS melanoma in settings of congenital melanocytic nevi (CMN) can present either as solid tumors within the brain parenchyma or as leptomeningeal lesions (Br J Dermatol 2017;176:1131)
- Leptomeningeal melanosis shows diffuse proliferation of melanin producing cells in leptomeninges which exhibits minimal cellular atypia and usually no brain parenchymal invasion
- Transformation to leptomeningeal melanoma is documented on the basis of unequivocal brain parenchymal invasion or cytological atypia (Br J Dermatol 2017;176:1131)
- References: Elder: Lever's Histopathology of the Skin, 11th Edition, 2014, Calonje: McKee's Pathology of the Skin, 5th Edition, 2019
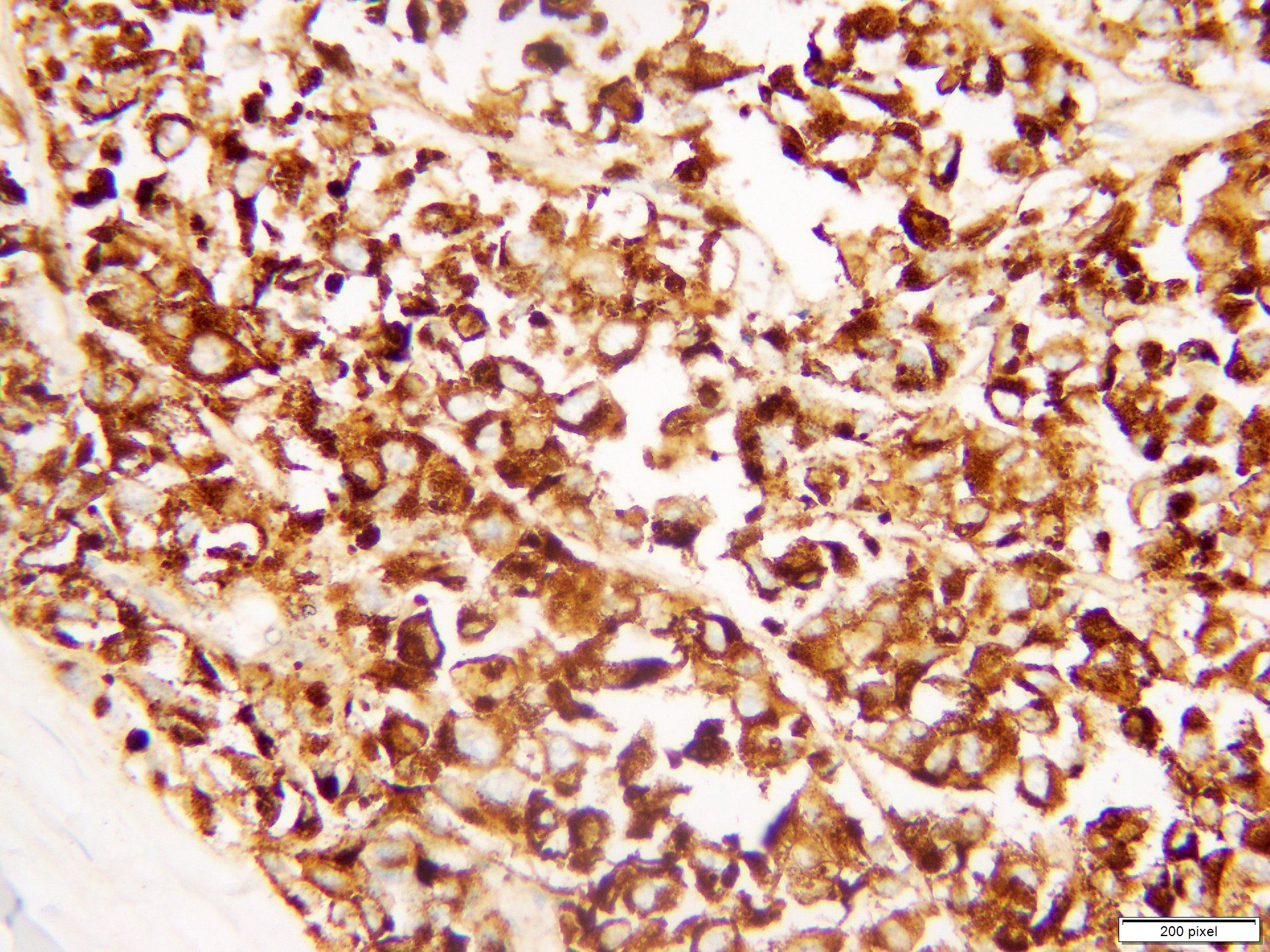
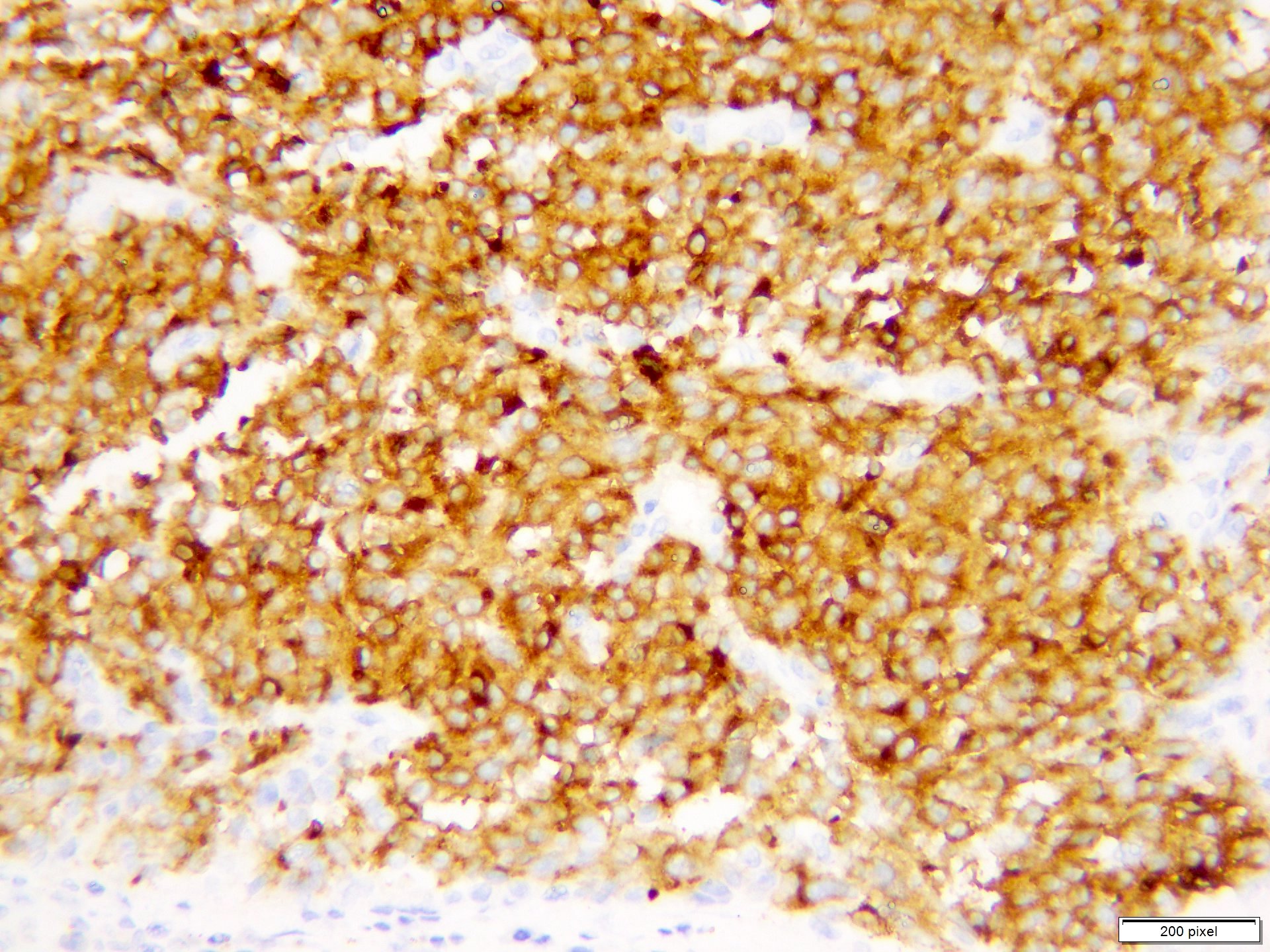
Microscopic (histologic) images
Contributed by Anila Chughtai, M.B.B.S.
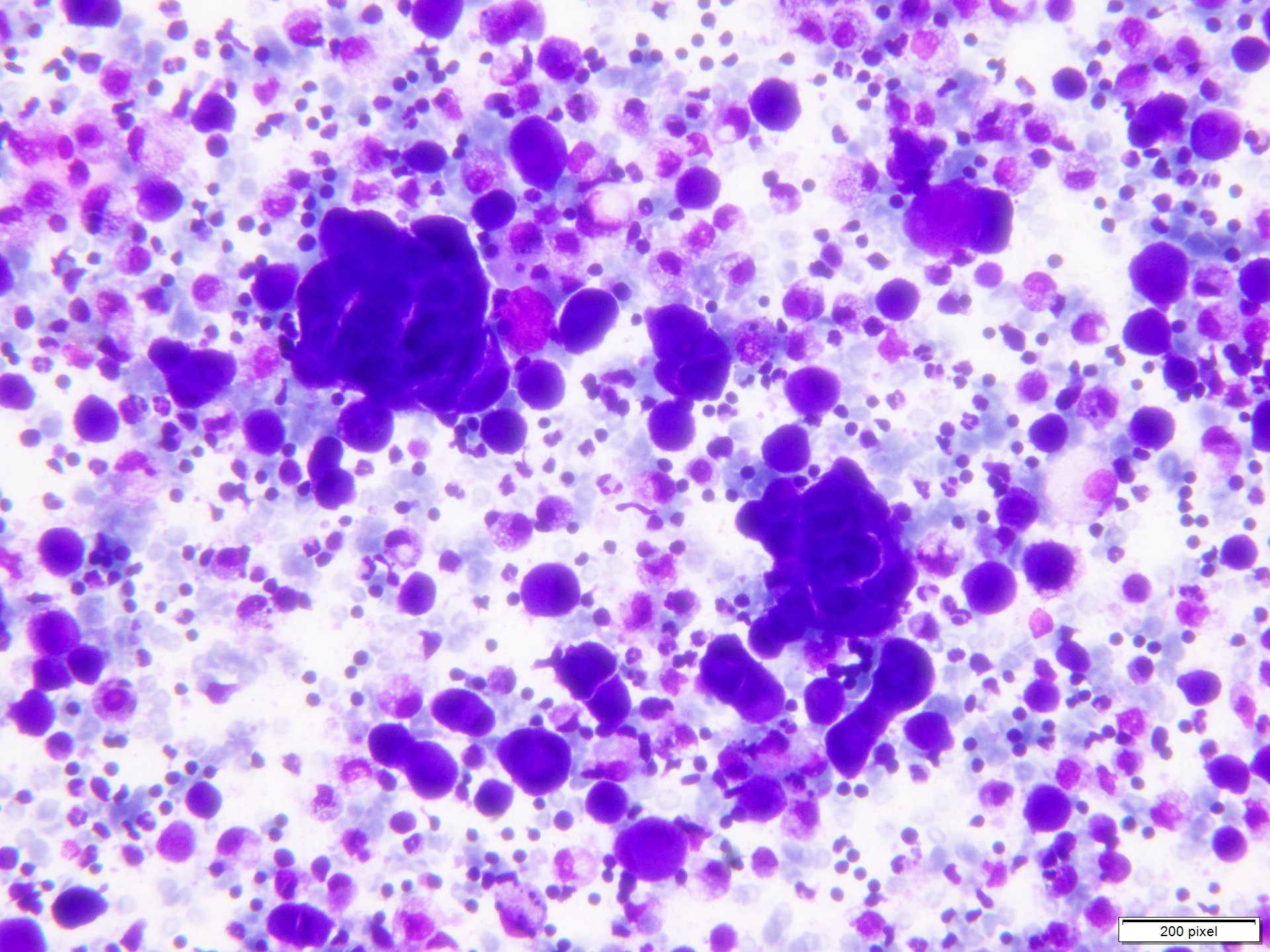
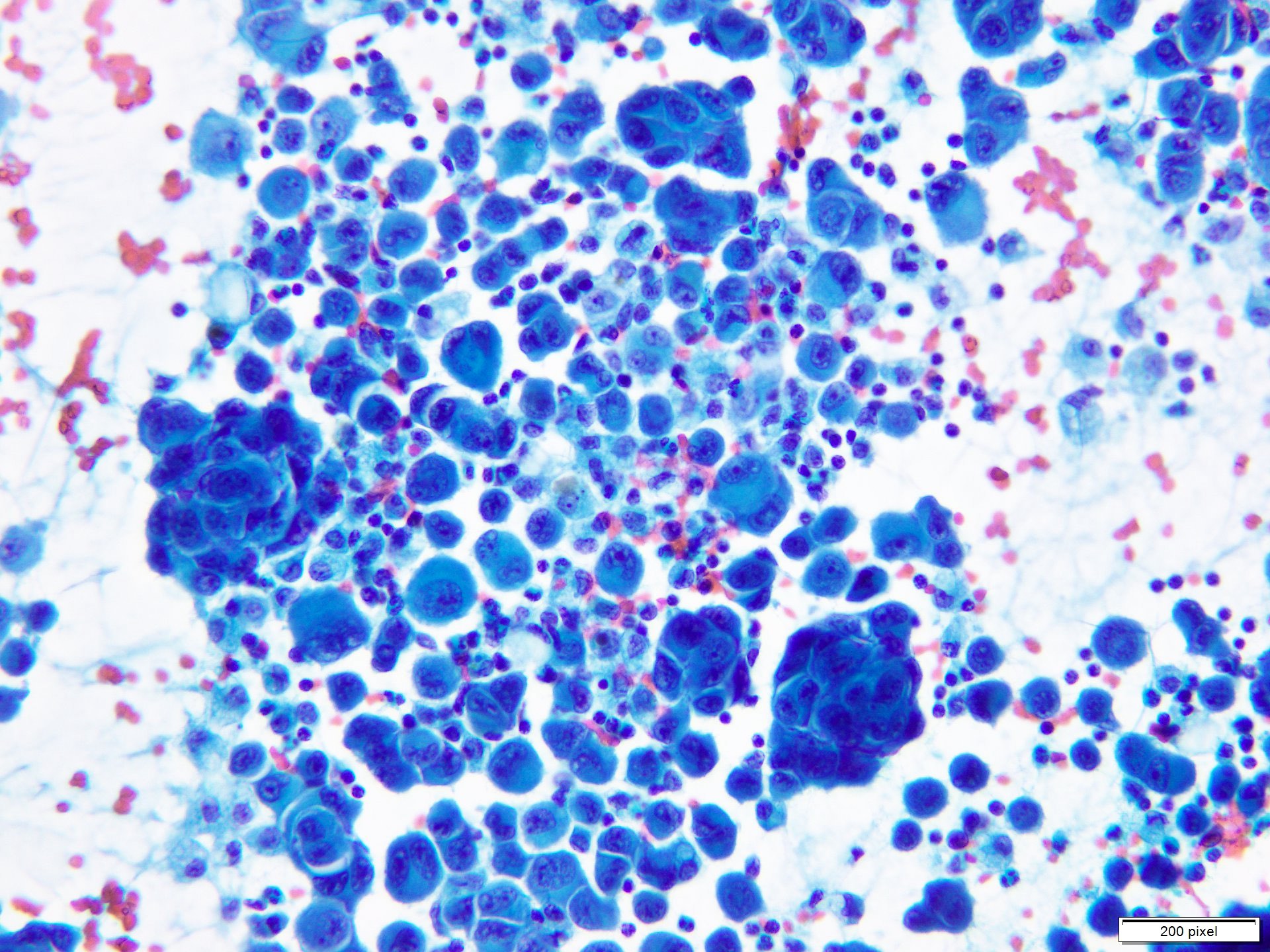
Cytology description
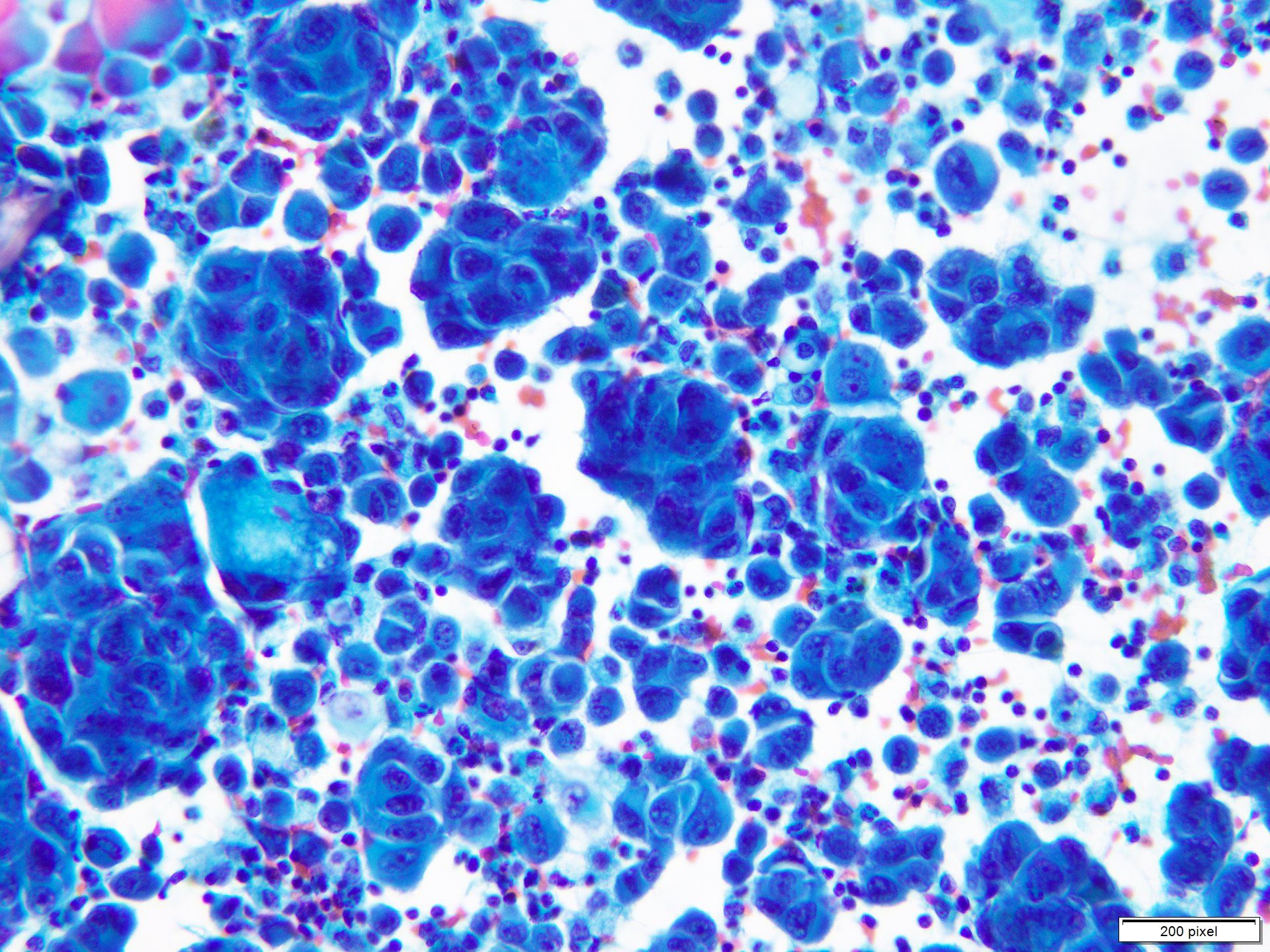
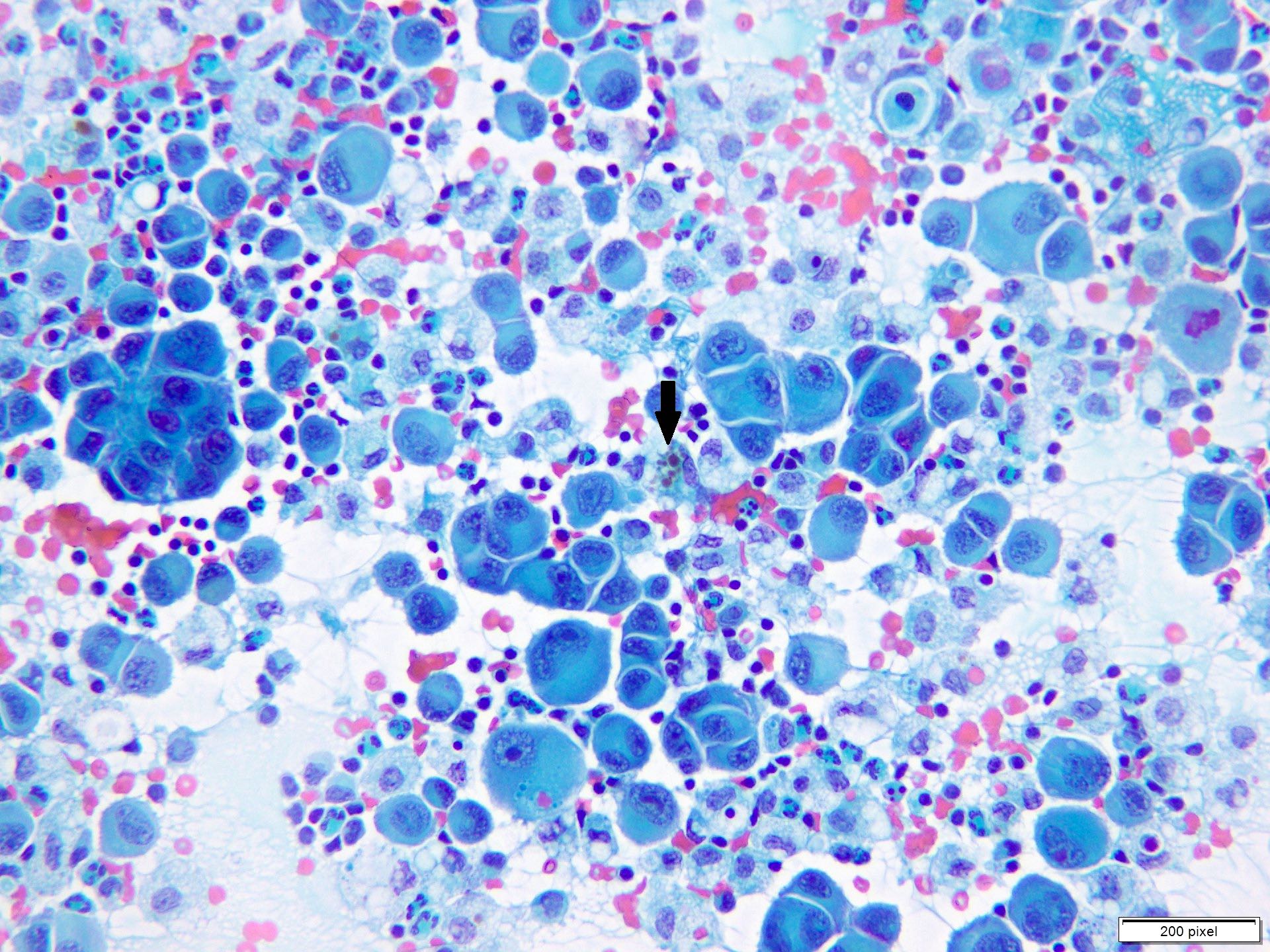
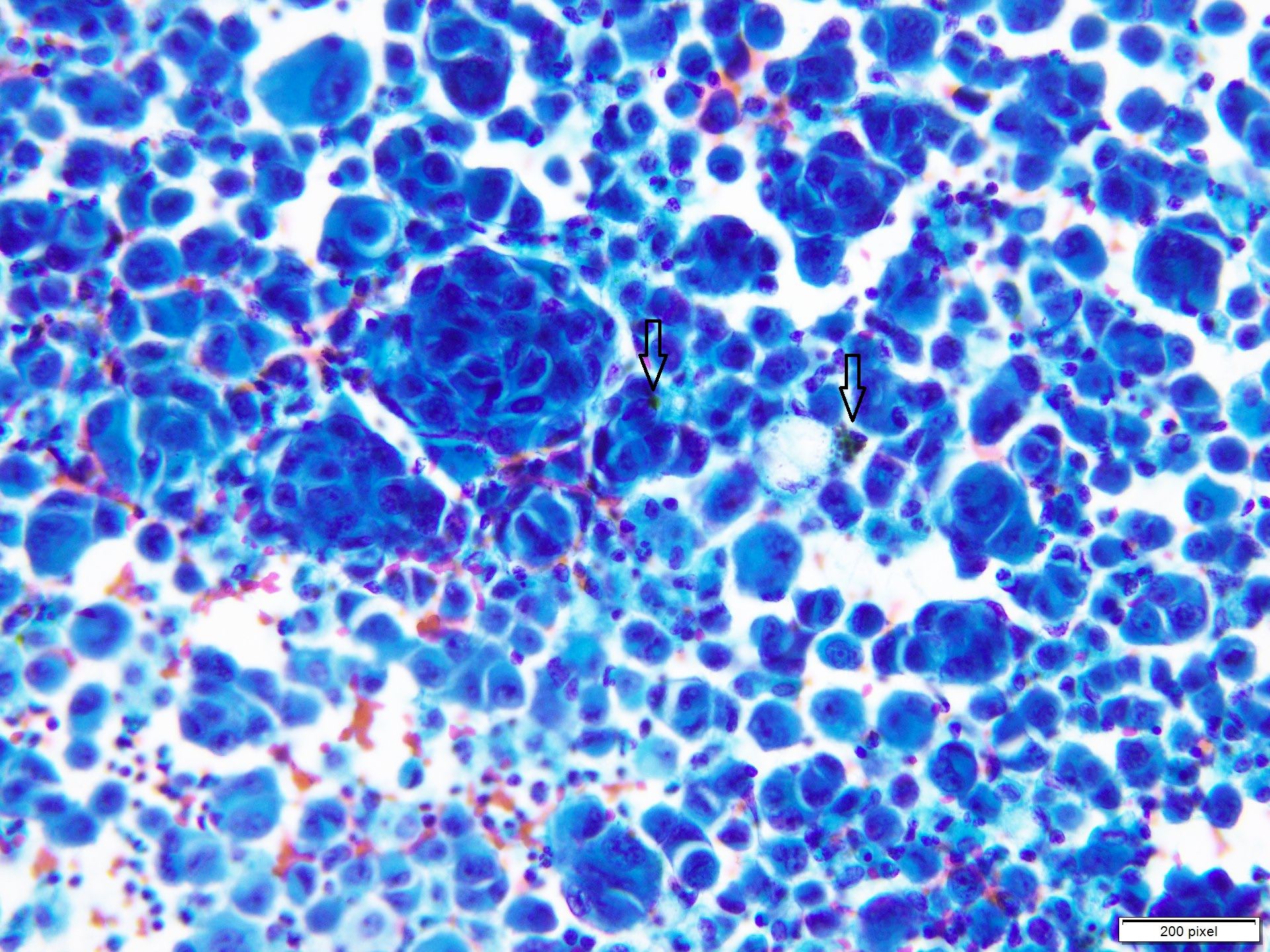
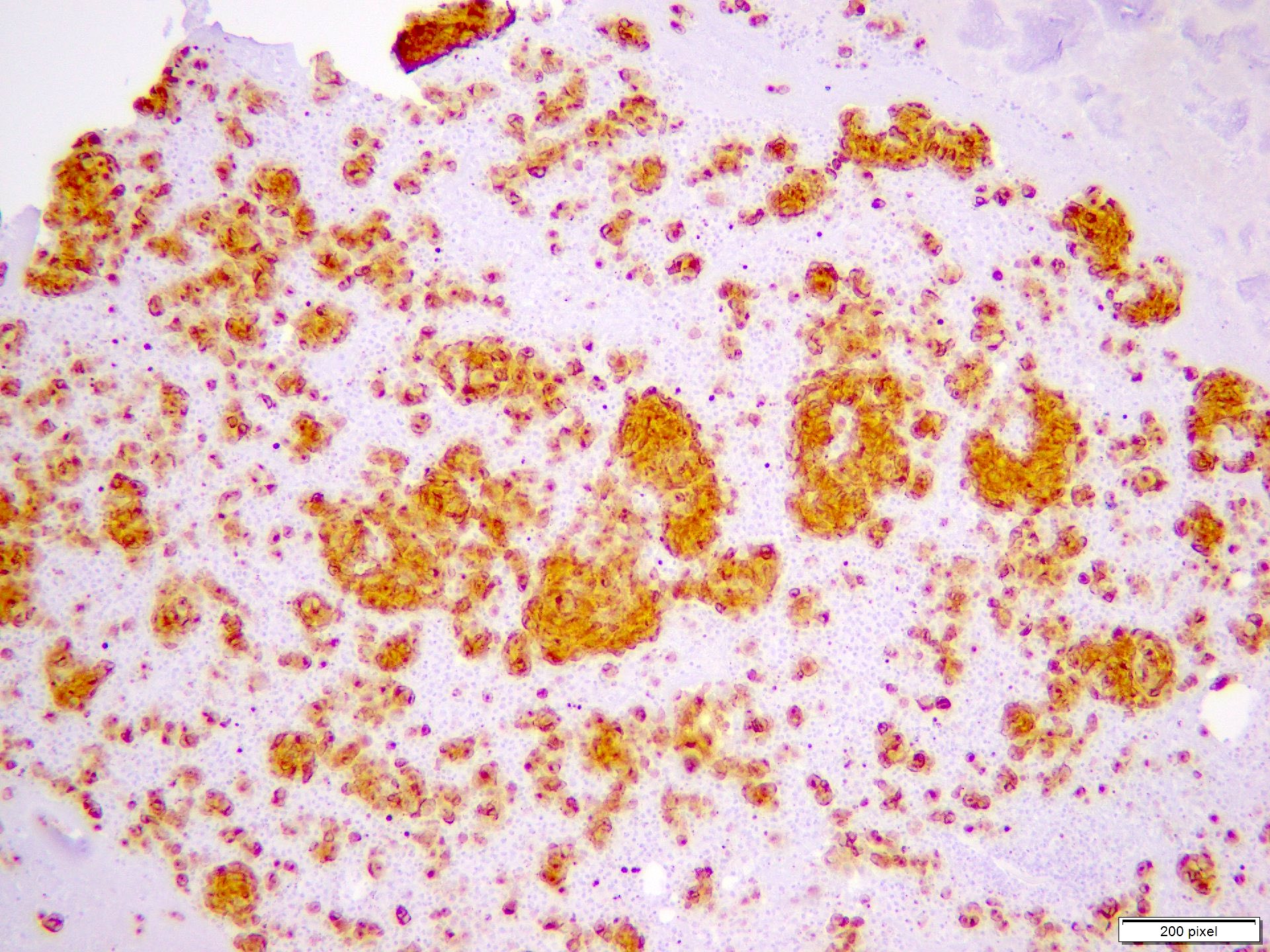
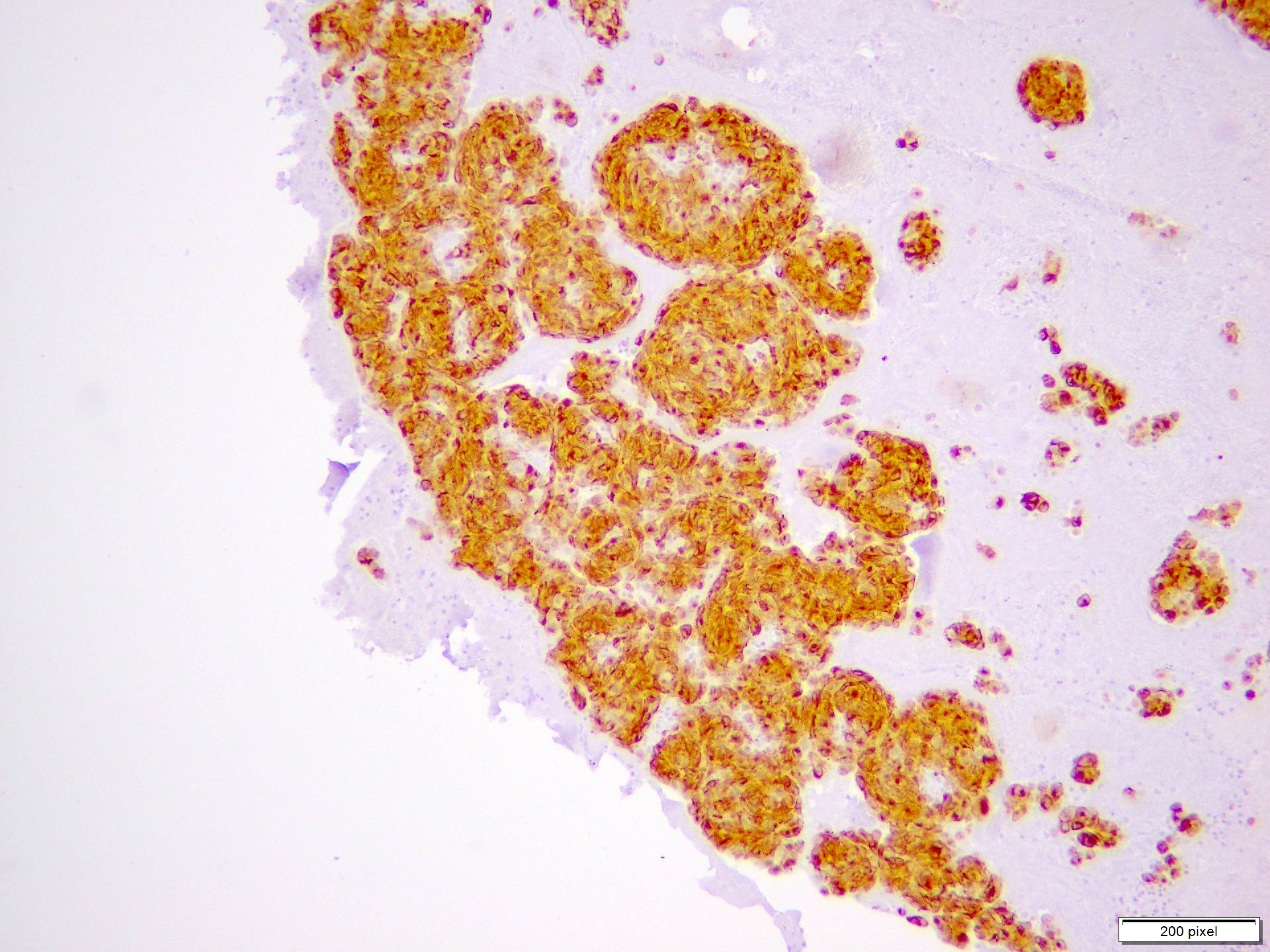
- Clusters and individually scattered cells with nuclear pleomorphism, prominent nucleoli, nuclear pseudoinclusions and coarse cytoplasmic blackish brown pigment (variable, can be absent)
- Limitation: cytological findings can be subtle and can mimic other malignant tumors
- Reference: Cibas: Cytology - Diagnostic Principles and Clinical Correlates, 5th Edition, 2020
Cytology images
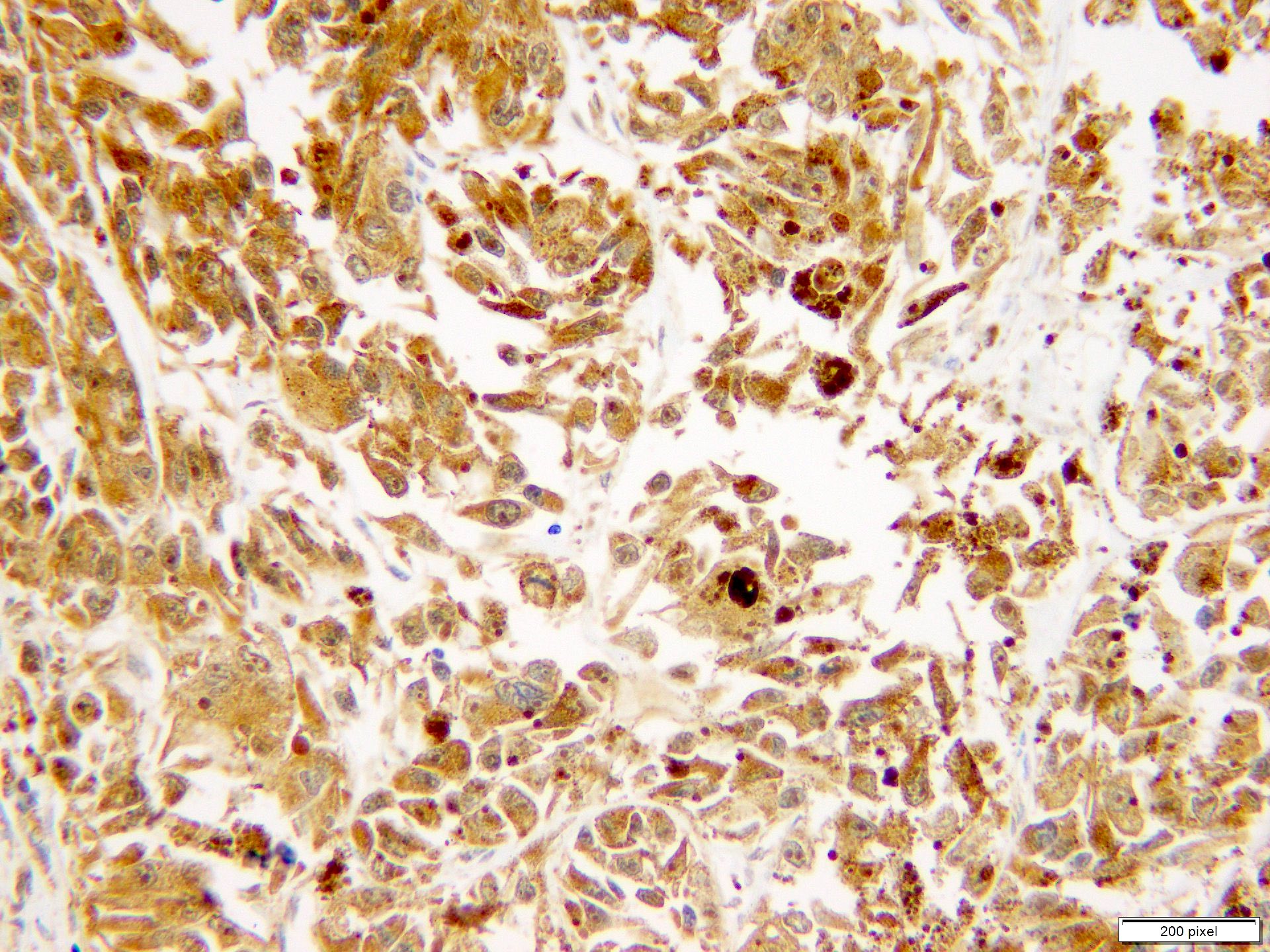
Positive stains
- S100
- SOX10
- MITF
- MelanA / MART1
- HMB45
- Tyrosinase
- PRAME
- PD-1 / PDL1
- Ki67 / MIB1 > 10% (benign melanocytic nevi < 2%)
- References: Am J Surg Pathol 2018;42:1456, Int J Mol Sci 2022;23:5911, Elder: Lever's Histopathology of the Skin, 11th Edition, 2014, Calonje: McKee's Pathology of the Skin, 5th Edition, 2019
Negative stains
- H3K27me3 (nuclear loss in up to 50 - 80% of tumor cells)
- p16 (nuclear loss in melanoma)
- Cytokeratin
- CD34
- CD45
- References: Int J Mol Sci 2022;23:5911, Elder: Lever's Histopathology of the Skin, 11th Edition, 2014, Calonje: McKee's Pathology of the Skin, 5th Edition, 2019
Electron microscopy description
- Intracytoplasmic melanosomes (ORL J Otorhinolaryngol Relat Spec 1975;37:233)
Molecular / cytogenetics description
- Melanoma harbors KIT, PTEN, TERT-p, CDKN2A, TP53 and NRAS mutations (Cell 2015;161:1681)
- BRAF mutations have not been described in melanoma arising in congenital nevi (Br J Dermatol 2017;176:1131)
- Copy number abnormalities (loss or gain of part of or whole chromosome) are commonly documented in melanoma arising in settings of congenital nevi (Br J Cancer 2017;116:990)
Videos
Melanocytic dermpath basics: melanoma
Sample pathology report
- Lesion, right arm, excisional biopsy:
- Malignant melanoma arising in background of congenital nevus
- Macroscopic:
- Received elliptical skin excision specimen (4.3 x 3.7 x 2.4 cm) with ulcerated pigmented nodular lesion (3.1 x 2.5 cm). Representative sections of the lesion are taken along with margins.
- Microscopy:
- Sheet and confluent nests of atypical melanocytes with enlarged pleomorphic nuclei having prominent eosinophilic nucleoli, eosinophilic cytoplasm, brisk and abnormal mitotic activity. Cytoplasmic melanin pigment is identified. Necrosis and epidermal ulceration are seen. Pagetoid spread to epidermis is noted. Benign melanocytic nevus is seen at the periphery of malignant proliferation. Tumor infiltrating lymphocytes are noted and are brisk. Resection margins are free.
- Details of morphological prognostic parameters are as follows
- Ulceration: present
- Growth phase: vertical
- Breslow depth: 3.8 mm
- Dermal mitotic rate (mitoses/mm2): 3
- Microsatellites: absent
- Regression: absent
- Neurotropism: absent
- Lymphatic invasion: absent
- Tumor infiltrating lymphocytes (TILs): present (brisk)
- Margin: all margins are free with closest being free by 2.0 cm
- TNM staging: pT3; pNx; pMx
Differential diagnosis
- Proliferating nodule (Elder: Lever's Histopathology of the Skin, 11th Edition, 2014, Calonje: McKee's Pathology of the Skin, 5th Edition, 2019):
- Proliferating nodules can mimic melanoma arising in congenital melanocytic nevus
- Proliferating nodules exhibit large melanocytes with conspicuous nucleoli (however, nucleoli are not prominent), rare mitotic activity (< 1/mm2), no abnormal mitosis, no necrosis, no pagetoid spread, rare epidermal involvement
- Proliferating nodules merge with adjacent nevus cells while malignant melanoma usually exhibits sharp delineation
- Melanocytes in proliferation nodules retain nuclear expression of H3K27me3 stain while melanoma cells show nuclear loss
- PRAME stain is positive in melanoma
- Proliferating nodules exhibit HMB45 expression in only the superficial part of the lesion while melanoma shows its expression in the superficial as well as in the deeper part of the lesion
- Melanoma harbors BRAF, KIT, PTEN, TERT-p, CDKN2A, TP53 mutations in addition to the NRAS mutation, which is commonly seen in proliferating nodules (Cell 2015;161:1681)
Board review style question #1
A 21 year old man presented with a new lump that appeared in previously known congenital nevus. The histopathology features of the nodule are shown in the photograph. Which of the following immunohistochemical stains would help differentiate proliferating nodule from melanoma?
- H3K27me3
- HMB45
- MelanA / MART1
- S100
- SOX10
Board review style answer #1
A. H3K27me3. The majority of melanoma cells show nuclear loss of H3Kme3 IHC stain while it is retained in proliferating nodule as well as in background nevus cells. Answers B, C, D and E are incorrect because HMB45, MelanA, S100 and SOX10 IHC stains will be positive in both benign proliferating nodules and melanoma; therefore, these will not help in differentiation.
Comment Here
Reference: Melanoma arising in giant congenital nevus
Comment Here
Reference: Melanoma arising in giant congenital nevus
Board review style question #2
Which of the following morphological features is the most important in determining prognosis of malignant melanoma arising in congenital nevi?
- Breslow thickness
- Necrosis
- Pagetoid spread to epidermis
- Surface ulceration
- Tumor regression
Board review style answer #2
A. Breslow thickness. Breslow thickness is the most important morphological parameter that helps determine pathological stage of the tumor and ultimately prognosis. Answers B, C, D and E are incorrect because while tumor necrosis, pagetoid spread, surface ulceration and tumor regression are all important parameters and add significant prognostic value, Breslow thickness is the most important parameter out of all others.
Comment Here
Reference: Melanoma arising in giant congenital nevus
Comment Here
Reference: Melanoma arising in giant congenital nevus