Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Diagrams / tables | Clinical features | Interpretation | Uses by pathologists | Prognostic factors | Microscopic (histologic) description | Microscopic (histologic) images | Molecular / cytogenetics description | Molecular / cytogenetics images | Sample pathology report | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Shafi S, Chen W. Colon cancer biomarker testing (including MSI / Lynch). PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/stainslynchcolorectal.html. Accessed April 18th, 2024.
Definition / general
- Colon cancer biomarker testing is essential for:
- Screening for Lynch syndrome: mismatch repair (MMR) by IHC or microsatellite instability (MSI) by PCR
- Therapeutic and prognostic predictions in advanced colorectal cancer (CRC): KRAS, NRAS, BRAF V600E, HER2
- Identification of other hereditary polyposis / cancer syndrome in indicated patients: multigene NGS panel
- Lynch syndrome (LS) is an autosomal dominant, highly penetrant inherited cancer predisposition syndrome, that arises due to germline pathogenic mutations in DNA mismatch repair genes
Essential features
- Lynch syndrome is the most common hereditary colorectal cancer syndrome:
- 2 - 5% of all colorectal cancers
- 1 in 25 unselected colorectal cancers
- Lifetime risk of colorectal and endometrial cancers in Lynch syndrome as high as 50% and 60%, respectively; there is also increased risk for cancers of ovary, small bowel, stomach, upper urinary tract, gallbladder, hepatobiliary tract, pancreas, kidney, prostate and brain, as well as sebaceous skin tumors
- Lynch syndrome screening is cost effective and saves lives (N Engl J Med 2005;352:1851)
Terminology
- Lynch syndrome was initially known as hereditary nonpolyposis colon cancer (HNPCC); however, this terminology is no longer recommended, as Lynch syndrome patients sometimes develop polyps and are at increased risk for cancers other than colorectal cancer (World J Gastroenterol 2006;12:4943)
Pathophysiology
- Lynch syndrome patients have a germline mutation affecting an MMR gene; subsequently, an additional somatic mutation or deletion must be acquired in the other allele of the gene (with the germline mutation) for MMR deficiency to manifest
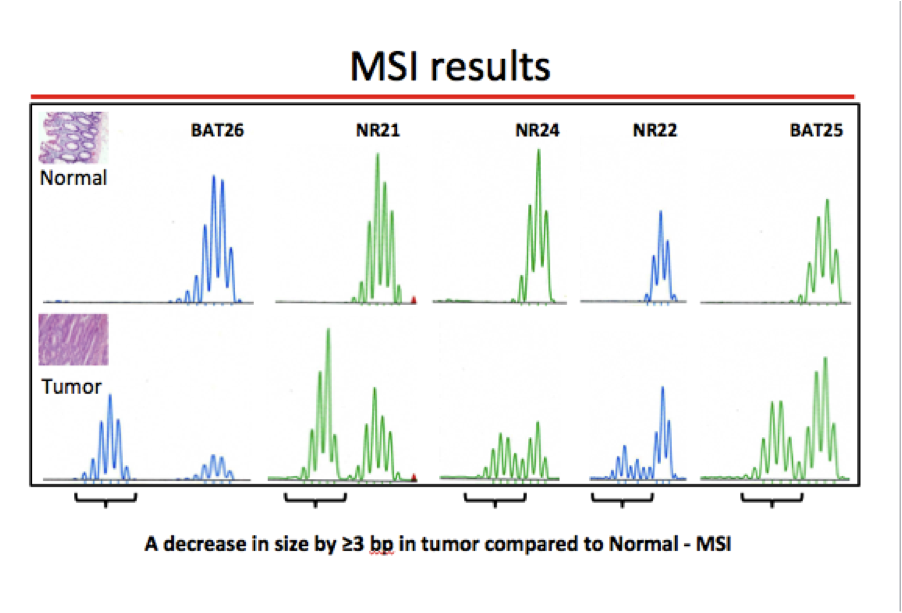
- Mutation in a MMR gene results in defective repair of DNA sequence mismatches, which most frequently occurs in long, repetitive DNA sequences (e.g., in microsatellite regions, hence the term microsatellite instability [MSI])
- Accumulation of DNA mismatches leads to increased risk of developing malignant neoplasms
- References: Oncol Lett 2019;17:3048, Gastroenterology 2010;138:2073
Diagrams / tables
Clinical features
- Clinical presentation of Lynch syndrome can vary depending on the MMR gene affected (Diagn Pathol 2017;12:24):
- MLH1: typically presents with classic Lynch syndrome (colorectal cancer as the first presenting disease at 43 - 46 years)
- MSH2: classic Lynch syndrome presentation as MLH1, in addition to increased risk of extracolonic cancers (such as Muir-Torre syndrome)
- MSH6: more likely to develop endometrial cancer
- PMS2: lower risk and later onset to development of colorectal cancer, compared to the other 3 MMR genes
- Rare biallelic mutation (constitutional MMR deficiency syndrome):
- Multiple adenomas at a very young age
- Very early onset (pediatric) hematological, colorectal, urinary tract and brain cancers and neurofibromatosis type 1-like skin features
- Muir-Torre syndrome: concurrence of a sebaceous skin tumor with any internal cancer
- Turcot syndrome:
- Coexistence of a hereditary colorectal cancer syndrome and central nervous system (CNS) tumors
- In Lynch syndrome, the most common primary CNS tumor is glioblastoma multiforme
Interpretation
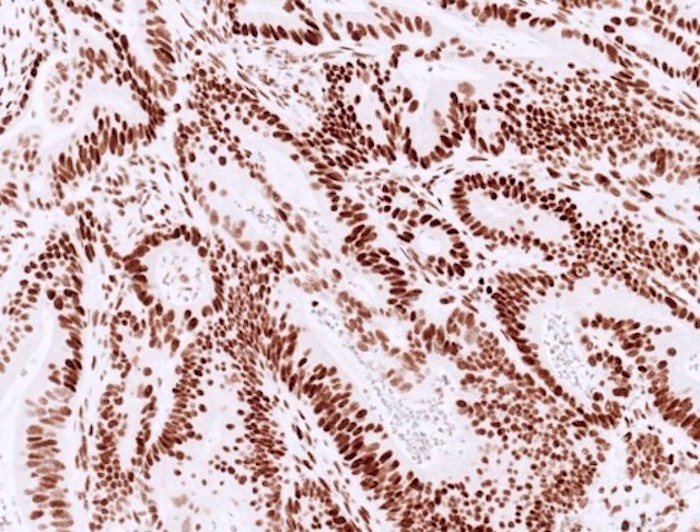
- MMR IHC:
- Control is the key (Appl Immunohistochem Mol Morphol 2015;23:1, Pathologica 2016;108:104, Surg Pathol Clin 2017;10:977):
- Staining in neoplastic cells requires a similar or higher intensity than nuclear immunoreactivity in internal positive control (normal basal crypt epithelium, lymphocytes, fibroblasts and endothelium)
- Loss of tumor staining in areas without internal control staining is not interpretable
- Cutoff for normal staining: not well studied, evidence based exact cutoff has been established; ranges from any positive reaction in the nuclei of tumor cells (CAP) to 1%, 5% or 10% by different authors (Mod Pathol 2019;32:1, Am J Surg Pathol 2016;40:e17)
- Reporting terminology (Arch Pathol Lab Med 2014;138:166):
- Use intact or lost, not positive or negative
- Positive or negative could be misinterpreted as positive or negative for MMR deficiency rather than positive or negative nuclear staining
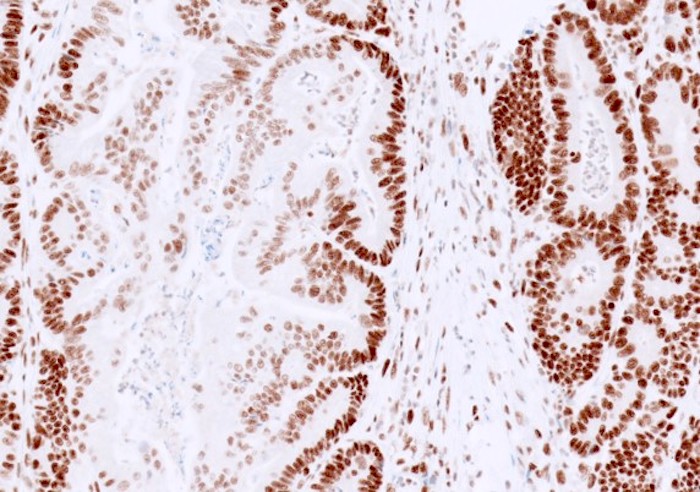
- Normal / intact staining:
- Typically diffuse, strong staining is present in most tumor nuclei of colorectal cancer
- Can be patchy or show variable staining intensity within one case, due to antibody diffusion, fixation and tissue hypoxia
- Absent / lost staining:
- Complete absence of nuclear immunoreactivity within neoplastic cells in the presence of positive internal control (Am J Clin Pathol 2004;122:389, N Engl J Med 2005;352:1851)
- Loss of only PMS2 staining is suggestive of PMS2 mutation
- Loss of only MSH6 staining is suggestive of MSH6 mutation
- Loss of MLH1 and PMS2 staining may be seen in MLH1 associated Lynch syndrome or in sporadic colorectal cancer due to MLH1 promoter hypermethylation (BRAF V600E mutation analysis or MLH1 hypermethylation studies warranted)
- Loss of MSH2 and MSH6 staining is suggestive of MSH2 mutation
- If MLH1 or MSH2 is lost, its partner becomes unstable, will be degraded and show loss of protein expression; however, the opposite is not true (the absence of PMS2 or MSH6 does not affect the stability of MLH1 and MSH2 since they can be stabilized by binding to other molecules) (Mod Pathol 2018;31:1891)
- Note: if most of the tumor shows absent staining but focal tumor staining is present with staining intensity weaker than control, then repeat stain or MSI by PCR is warranted; this pattern most likely represents MMR deficiency
- Complete absence of nuclear immunoreactivity within neoplastic cells in the presence of positive internal control (Am J Clin Pathol 2004;122:389, N Engl J Med 2005;352:1851)
- Interpretation pitfalls:
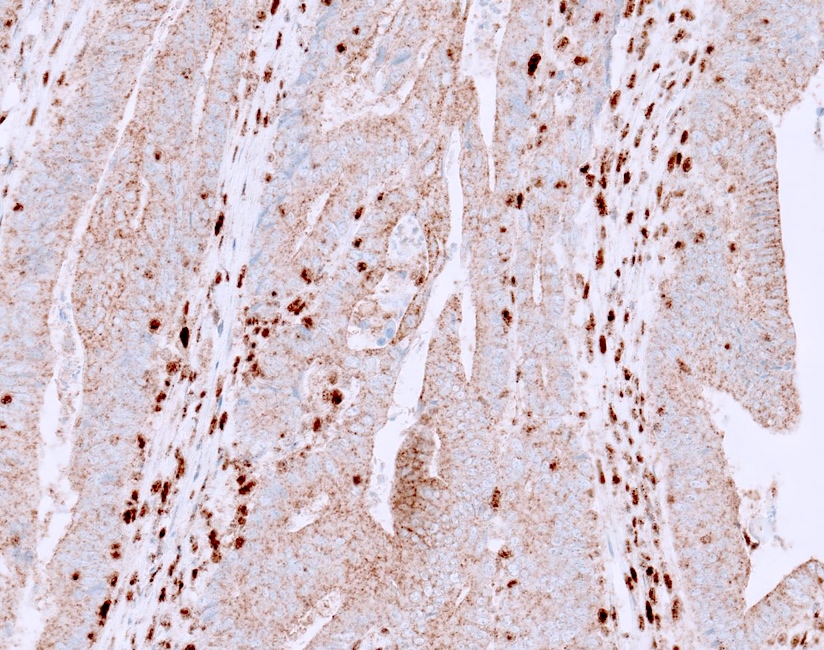
- Cytoplasmic staining: if only cytoplasmic staining is present, it should not be misinterpreted as normal as it is an abnormal pattern; it can be seen in Lynch syndrome with EPCAM-MSH2 fusion and others (Histopathology 2017;70:664)
- Punctate, granular or dot-like staining pattern is most often observed with the MLH1 M1 clone; if there is concurrent PMS2 loss, this pattern most likely represents MLH1 deficiency and should not be reported as isolated PMS2 loss (Pathol Res Pract 2020;216:152581, Histopathology 2018;73:703, Histopathology 2019;74:795)
- Postneoadjuvant therapy: treated colorectal cancer may show decreased or absent MMR protein expression, especially with MSH6 and PMS2; further investigation using pretreatment sample can be helpful (Am J Surg Pathol 2010;34:1798, Hum Pathol 2011;42:1247, Hum Pathol 2014;45:2029, Hum Pathol 2017;63:33)
- MSH6 heterogenous staining: may be seen as a secondary change in colorectal cancer with MLH1 / PMS2 deficiency due to somatic mutation of an unstable mononucleotide tract in MSH6; MSH6 germline mutation has not been observed in such cases (Hum Pathol 2020;96:104, Am J Surg Pathol 2015;39:1370, Mod Pathol 2013;26:131)
- Missense mutation with retained protein antigenicity:
- Presence of MMR staining does not unequivocally exclude the possibility of Lynch syndrome, as certain mutations (especially missense) can produce defective protein that still retains its antibody binding site for IHC (Hum Pathol 2020;103:34)
- High clinical suspicion for Lynch syndrome and attention to the staining pattern of the partner gene may be helpful
- Tumor weaker than control: it is necessary to repeat the stain; if it is still weaker than internal positive control, then it should be interpreted as abnormal and additional studies are warranted (Mod Pathol 2019;32:1)
- Control is the key (Appl Immunohistochem Mol Morphol 2015;23:1, Pathologica 2016;108:104, Surg Pathol Clin 2017;10:977):
- MSI by PCR (see microsatellite instability pathway)
- HER IHC interpretation (see HER2 colon)
- Patients with constitutional MMR deficiency will show complete loss of staining in both the tumor and normal tissue
- This can falsely be interpreted as failed staining
- External controls should be verified
- This pattern of staining, especially in a child, should raise concern for constitutional MMR deficiency (N Engl J Med 2016;374:772)
Uses by pathologists
- MMR IHC or MSI by PCR are the recommended tests for Lynch syndrome screening
Prognostic factors
- Patients with MMR deficient colorectal cancer have a better prognosis than those with stage matched MMR proficient colorectal cancer
- BRAF mutation is associated with poor prognosis
- References: Oncologist 2016;21:618, Front Oncol 2020;10:563407
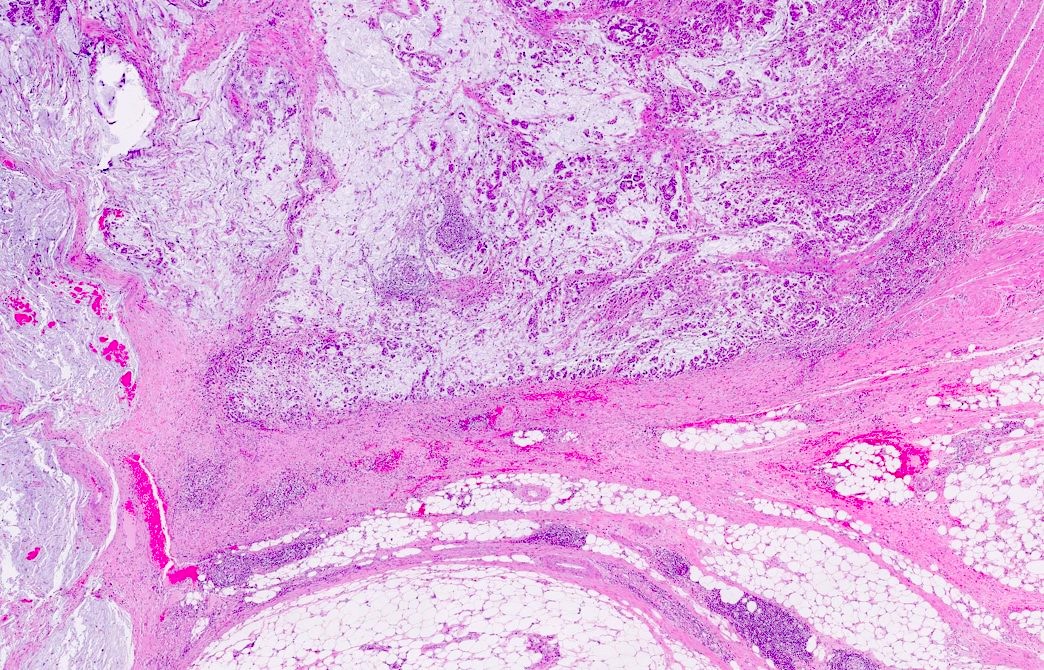
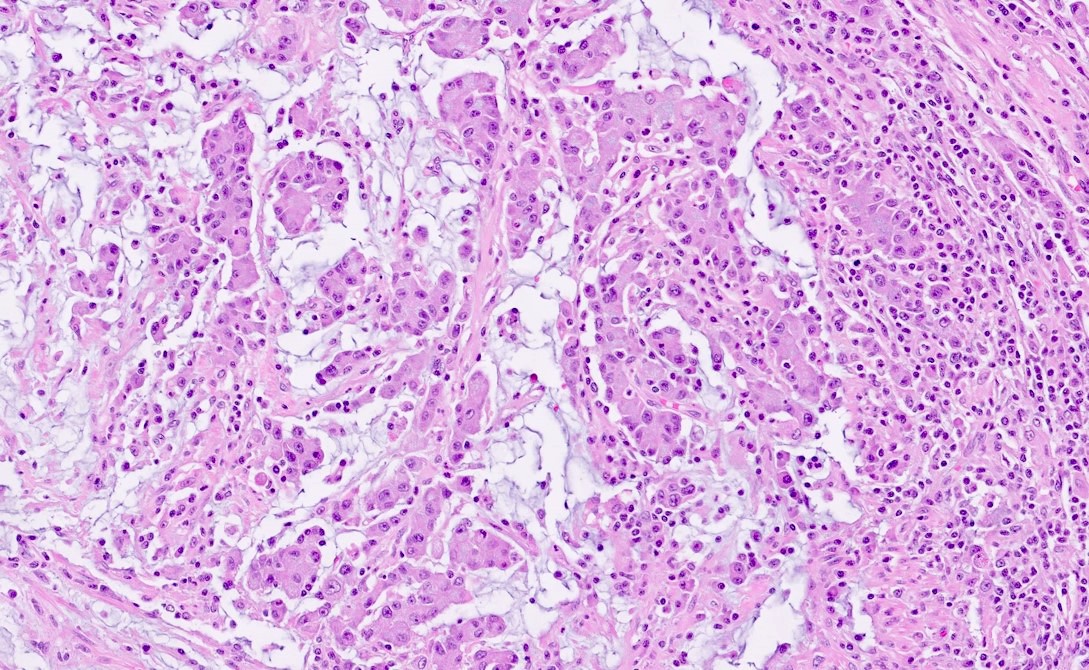
Microscopic (histologic) description
- Histological features suggestive of MMR deficiency include:
- Tumor infiltrating lymphocytes
- Crohn's-like peritumoral lymphocytic reaction
- Poor differentiation / medullary growth pattern
- Mucinous and signet ring cell features
- Reference: Gastroenterology 2007;133:48
Microscopic (histologic) images
Molecular / cytogenetics description
- Pathogenic variant or epigenetic alteration of MMR genes:
- MLH1 / PMS2 and MSH2 / MSH6 form 2 functional pairs (MutLα and MutSα respectively)
- If MLH1 or MSH2 is defective, its partner becomes unstable but not vice versa
- This pair relationship helps with MMR IHC interpretation
- Rarely due to EPCAM (TACSTD1) mutation and germline promoter hypermethylation of MLH1
- Microsatellites are dinucleotide repeat sequences, such as [CA]n, normally present in human genome; microsatellite instability (MSI) is the hallmark of Lynch syndrome associated colorectal cancer (World J Gastroenterol 2006;12:4745)
- MLH1 / PMS2 and MSH2 / MSH6 form 2 functional pairs (MutLα and MutSα respectively)
- Emerging trends for colorectal cancer biomarker testing:
- Upfront tumor sequencing in colorectal cancer is simpler and has superior sensitivity to current multitest approaches to Lynch syndrome screening, while simultaneously providing critical information for treatment selection; it may eventually replace MMR IHC or PCR MSI testing (JAMA Oncol 2018;4:806)
- Multigene next generation sequencing panels that include assessment of somatic and germline mutations in the syndromic genes, are most useful in colorectal cancer patients under the age of 50 or those with high suspicion for hereditary cancer / polyposis syndromes (JAMA Oncol 2017;3:464, Fam Cancer 2020;19:223)
Sample pathology report
- Mismatch repair protein (MMR) nuclear expression by IHC: (present / intact or absent / lost)
- MLH1: ***
- PMS2: ***
- MSH2: ***
- MSH6: ***
- IHC interpretation:
- No loss of nuclear expression of MMR proteins: low probability of microsatellite instability high (MSI-H)*
- Loss of nuclear expression of MLH1 and PMS2: testing for methylation of the MLH1 promoter or mutation of BRAF is indicated (the presence of a BRAF V600E mutation or MLH1 methylation suggests that the tumor is sporadic and germline evaluation is probably not indicated; absence of both MLH1 methylation and of BRAF V600E mutation suggests the possibility of Lynch syndrome, sequencing or large deletion / duplication testing of germline MLH1 may be indicated)I*
- Loss of nuclear expression of MSH2 and MSH6: high probability of Lynch syndrome (sequencing or large deletion / duplication testing of germline MSH2 may be indicated and if negative, sequencing or large deletion / duplication testing of germline MSH6 may be indicated)*
- Loss of nuclear expression of MSH6 only: high probability of Lynch syndrome (sequencing or large deletion / duplication testing of germline MSH6 may be indicated)*
- Loss of nuclear expression of PMS2 only: high probability of Lynch syndrome (sequencing or large deletion / duplication testing of germline PMS2 may be indicated)*
- *There are exceptions to the above IHC interpretations. These results should not be considered in isolation and clinical correlation with genetic counseling is recommended to assess the need for germline testing.
Additional references
Board review style question #1
Board review style answer #1
A. Abnormal staining. The stain shows adequate staining in the positive internal control cells (lymphocytes and stromal cells); however, the staining in the neoplastic cells is that of cytoplasmic / membranous without nuclear staining. Therefore, it is considered abnormal / lost staining.
Comment Here
Reference: Colon cancer biomarker testing (including MSI / Lynch)
Comment Here
Reference: Colon cancer biomarker testing (including MSI / Lynch)
Board review style question #2
Which of the following histologic features is suggestive of mismatch repair deficiency in colorectal cancer?
- Abundant tumor infiltrating lymphocytes
- Cribriform gland formation
- Extensive tumor necrosis
- Lymphovascular invasion
Board review style answer #2
A. Abundant tumor infiltrating lymphocytes. All other choices are nonspecific features that can also be seen with mismatch repair proficient colorectal cancers.
Comment Here
Reference: Colon cancer biomarker testing (including MSI / Lynch)
Comment Here
Reference: Colon cancer biomarker testing (including MSI / Lynch)