Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Cytology images | Positive stains | Negative stains | Molecular / cytogenetics description | Molecular / cytogenetics images | Sample pathology report | Differential diagnosis | Additional references | Practice question #1 | Practice answer #1 | Practice question #2 | Practice answer #2 | Practice question #3 | Practice answer #3Cite this page: Huber AR, Bell PD. GIST. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/stomachgist.html. Accessed September 16th, 2025.
Definition / general
- Most common mesenchymal tumor of the gastrointestinal tract
- Differentiation towards the interstitial cells of Cajal within the myenteric plexus of the muscularis propria
Essential features
- Most common in stomach
- Most common mutation: KIT (exon 11), a proto-oncogene
- 3 histologic types: spindle, epithelioid and mixed
- Prognosis: depends on tumor size, mitotic rate and site of origin
- Treatment: surgical excision or tyrosine kinase inhibitors such as imatinib
Terminology
- Gastrointestinal stromal tumor (GIST)
- Historic terms
- Gastrointestinal smooth muscle tumor
- Gastrointestinal autonomic nerve tumor
- Leiomyoblastoma
- Smooth muscle tumor of uncertain malignant potential
- Gastrointestinal pacemaker cell tumor
ICD coding
- ICD-10
- C49.A0 - gastrointestinal stromal tumor, unspecified site
- C49.A1 - gastrointestinal stromal tumor of esophagus
- C49.A2 - gastrointestinal stromal tumor of stomach
- C49.A3 - gastrointestinal stromal tumor of small intestine
- C49.A4 - gastrointestinal stromal tumor of large intestine
- C49.A5 - gastrointestinal stromal tumor of rectum
- C49.A9 - gastrointestinal stromal tumor of other sites
Epidemiology
- M:F = 1:1; no clear sex predilection
- Mean age at diagnosis: 60 - 65 years old
- Annual incidence: 11 - 18 per million (World J Gastroenterol 2006;12:2223, Eur J Cancer 2005;41:2868, Cancer 2005;103:821, APMIS 2010;118:648)
Sites
- Can occur anywhere along the tubular gastrointestinal tract
- Stomach (60%) > jejunum and ileum (30%) > duodenum (4 - 5%) > rectum (4%) > colon and appendix (1 - 2%) > esophagus (< 1%) (Semin Diagn Pathol 2006;23:70)
- Extragastrointestinal GIST: as the name implies, refers to GIST arising outside of the gastrointestinal tract in sites such as the omentum, mesentery, retroperitoneum or pleura
Pathophysiology
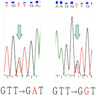
- Activating mutations in the proto-oncogene KIT (~75 - 80%) or platelet derived growth factor receptor α (PDGFRα) (~10%) (Science 1998;279:577, Diagnostics (Basel) 2021;11:194, Science 2003;299:708)
- Leads to constitutive phosphorylation of the receptor tyrosine kinase and activation of downstream pathways → cell proliferation and survival
- KIT and PDGFRA mutations are mutually exclusive in GIST
- Succinate dehydrogenase deficient GIST (Am J Surg Pathol 2010;34:636, Arch Pathol Lab Med 2020;144:655)
- Young adults (before age 40)
- 1 - 2% of all GIST in pediatric patients (J Pediatr Hematol Oncol 2005;27:179, Cancer Res 2007;67:9084)
- Female preponderance (> 2:1)
- Almost exclusively in stomach (predilection for distal stomach and antrum)
- Pathophysiology
- SDH is an enzyme complex in the electron transport chain and Krebs (citric acid) cycle, composed of 4 subunits (SDHA, SDHB, SDHC, SDHD)
- In the Krebs cycle, SDH catalyzes oxidation of succinate to fumarate
- Mutations in 1 of the subunits (most commonly SDHA) results in succinate accumulation, increased transcription of HIF1α regulated genes and decreased DNA methylation
- Loss of SDHB expression by immunohistochemistry
- Young adults (before age 40)
- Recently identified, less well understood mutations (Diagnostics (Basel) 2021;11:194)
- NF1 mutation
- Mutations in the RAS / RAF / MEK pathway (e.g., ETV1 transcription factor associated with GIST formation)
Etiology
- Unknown at this time
- Most are sporadic
- Small percentage are familial
Clinical features
- SDH deficient GIST
- Carney triad: GIST, pulmonary chondroma, paraganglioma
- Nonhereditary
- SDHC promoter hypermethylation
- Small percentage have germline SDH mutations
- Carney-Stratakis syndrome: GIST and paraganglioma
- Hereditary, autosomal dominant
- Germline mutations in SDHB, SDHC or SDHD subunit
- Carney triad: GIST, pulmonary chondroma, paraganglioma
- Neurofibromatosis (NF): 7% of patients with NF1 develop 1 or more GIST, usually in small bowel (Am J Surg Pathol 2005;29:1170, Am J Surg Pathol 2006;30:90, Hum Mol Genet 2006;15:1015)
- Familial: germline mutations in KIT or PDGFRα autosomal dominant
- Retained expression of SDHB by immunohistochemistry
Diagnosis
- Patients most commonly present with gastrointestinal bleeding or abdominal pain
- May be an incidental finding during radiologic or endoscopic workup for other clinical issues
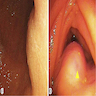
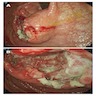
- Endoscopy: subepithelial lesion may be seen, which is nonspecific
- Definitive diagnosis cannot be made without histologic examination
- References: Int J Surg Pathol 2002;10:81, Arch Pathol Lab Med 2006;130:1466
Laboratory
- No significant laboratory findings
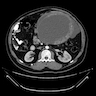
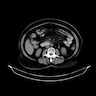
Radiology description
- Radiologic findings are variable, depending on size and time of presentation
- CT usually shows a solid, heterogeneous mass (reflecting the presence of hemorrhage or cystic degeneration)
- Endoscopic ultrasound reveals a hypoechoic solid mass
- Reference: Jpn J Radiol 2022;40:1105
Prognostic factors
- GIST may have overtly malignant behavior
- 25% of gastric GISTs act malignant versus 35 - 40% of small intestinal GISTs (Am J Surg Pathol 2005;29:52, Am J Surg Pathol 2006;30:477)
- Prognosis depends upon tumor size, mitotic rate and site of origin (Semin Diagn Pathol 2006;23:70)
- Intraoperative tumor rupture is also associated with poorer prognosis
- Compete surgical resection: improved local recurrence and overall survival rates
- Incomplete resection, particularly in the area of the rectum, is associated with a higher risk of recurrence
- 60 - 80% of patients with SDH deficient GIST developed distant metastasis; however, the NIH risk stratification criteria may not be appropriate for this subtype (Am J Surg Pathol 2016;40:1616)
Gastric GIST: risk of disease progression (Semin Diagn Pathol 2006;23:70)
| Size | ≤ 5 mitoses per 5 mm2 | > 5 mitoses per 5 mm2 |
| > 10 cm | Moderate | High |
| > 5 to ≤ 10 cm | Low | High |
| > 2 to ≤ 5 cm | Very low | Moderate |
| ≤ 2 cm | No | No |
Case reports
- 18 year old man with a large gastric wall mass (Pediatr Dev Pathol 2019;22:492)
- 45 year old man with weight loss and anorexia (Cureus 2024;16:e55655)
- 50 year old woman with rupture of large gastric mass (Case Rep Surg 2023;2023:2733295)
- 57 year old man with wild type gastrointestinal stromal tumor with unexpected response to imatinib (Front Oncol 2024;13:1334784)
- 79 year old man status postgastrectomy for gastric carcinoma with incidentally discovered nodules on gross examination (Gastrointest Tumors 2019;5:63)
Treatment
- Most GIST are treated with surgical resection
- Imatinib mesylate (Gleevec): tyrosine kinase inhibitor of KIT and PDGFRα
- Metastatic / recurrent GIST
- Sunitinib malate (Sutent): a tyrosine kinase inhibitor of KIT, PDGFRα, VEGFR
- Imatinib resistant GIST
- SDH deficient tumors are less responsive to tyrosine kinase inhibitors
- Reference: Arch Pathol Lab Med 2006;130:1466
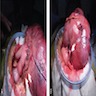
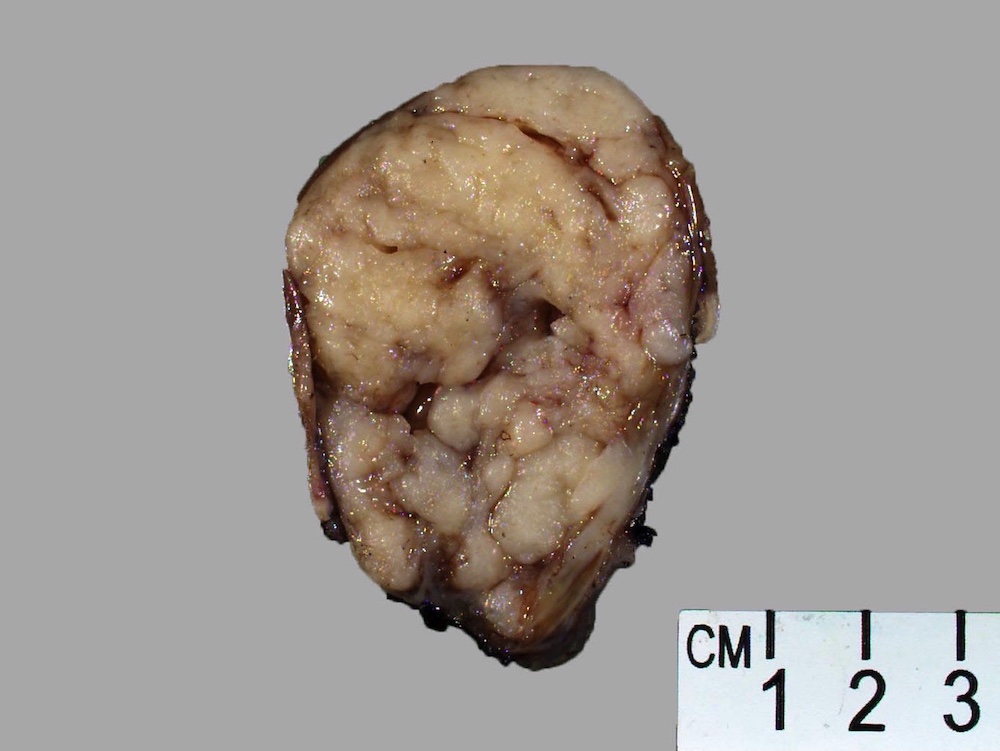
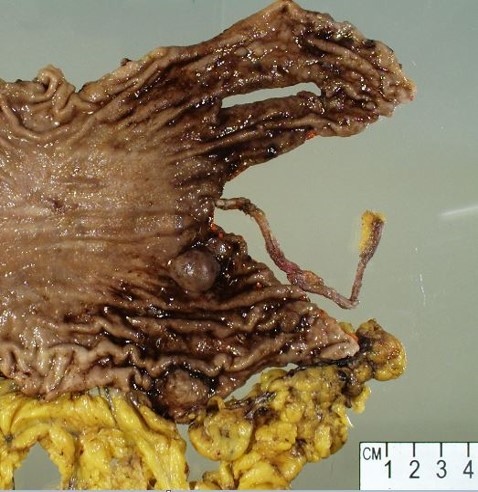
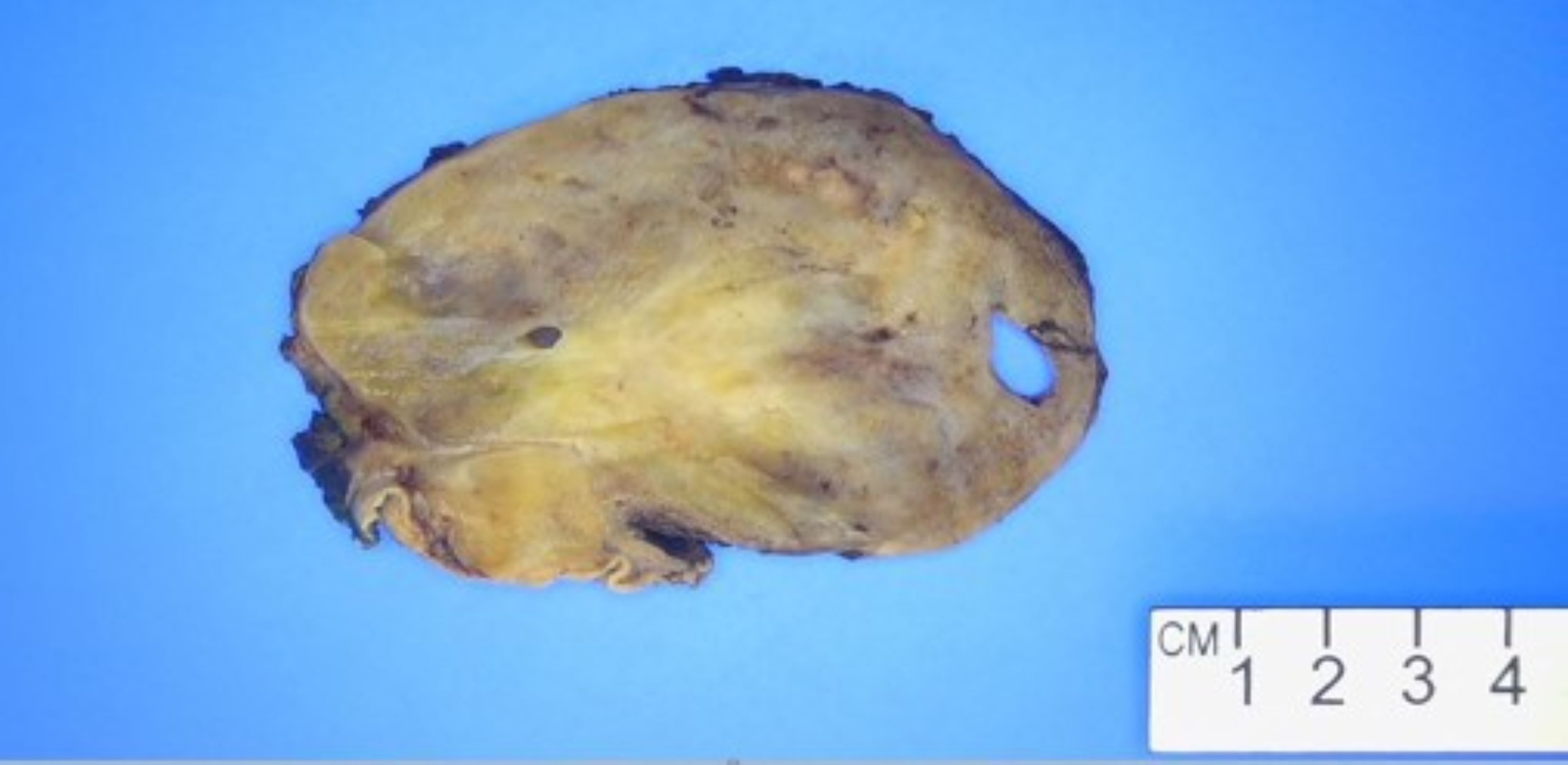
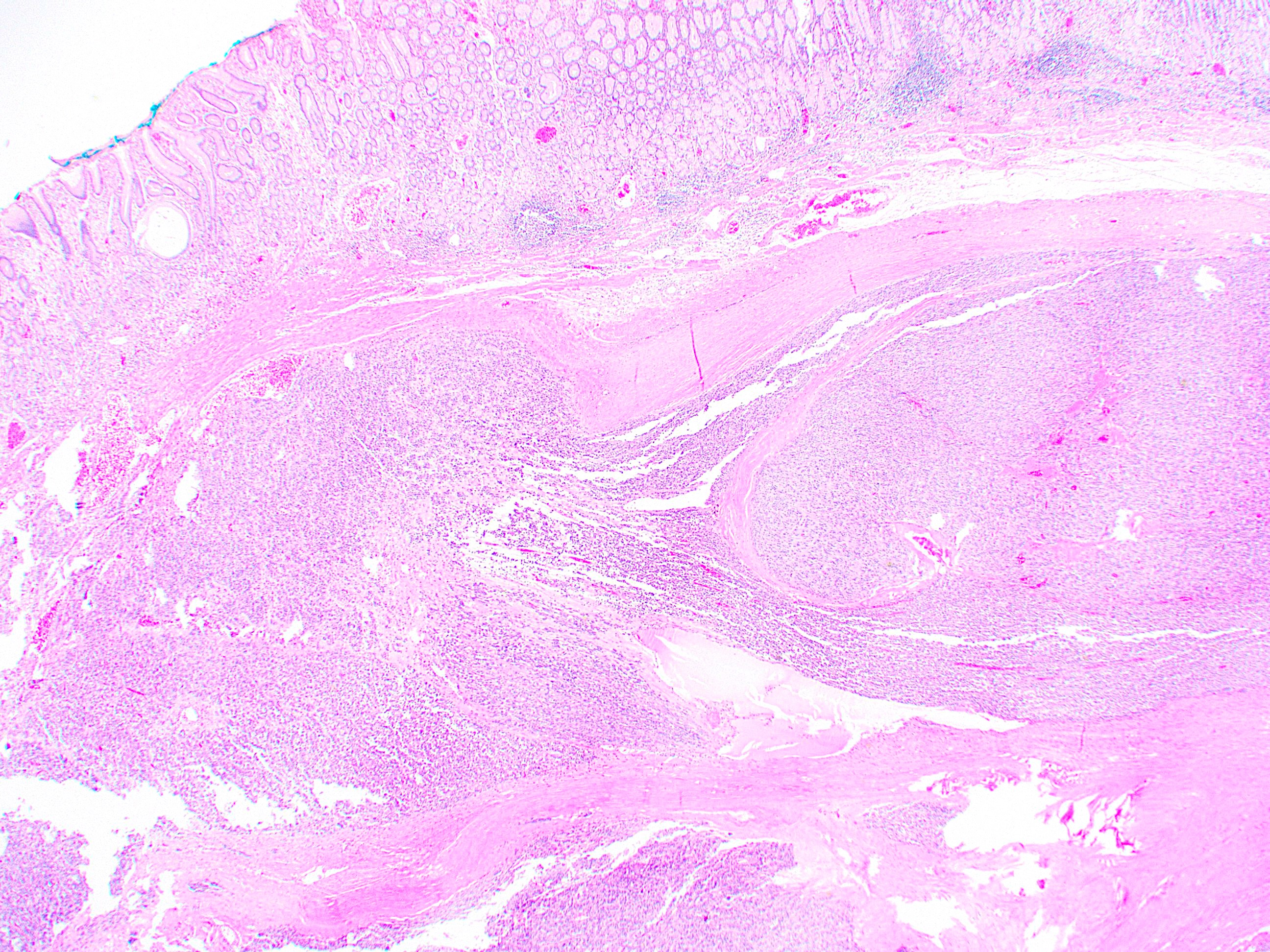
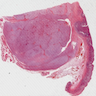
Gross description
- Well circumscribed, intramural lesion, centered within the muscularis propria
- Fleshy, tan-pink cut surfaces, which may show hemorrhage or cystic degeneration
- Mean size: 6 cm (0.4 - 40 cm) (Am J Surg Pathol 2005;29:52)
Gross images
Frozen section description
- Spindle cell neoplasm
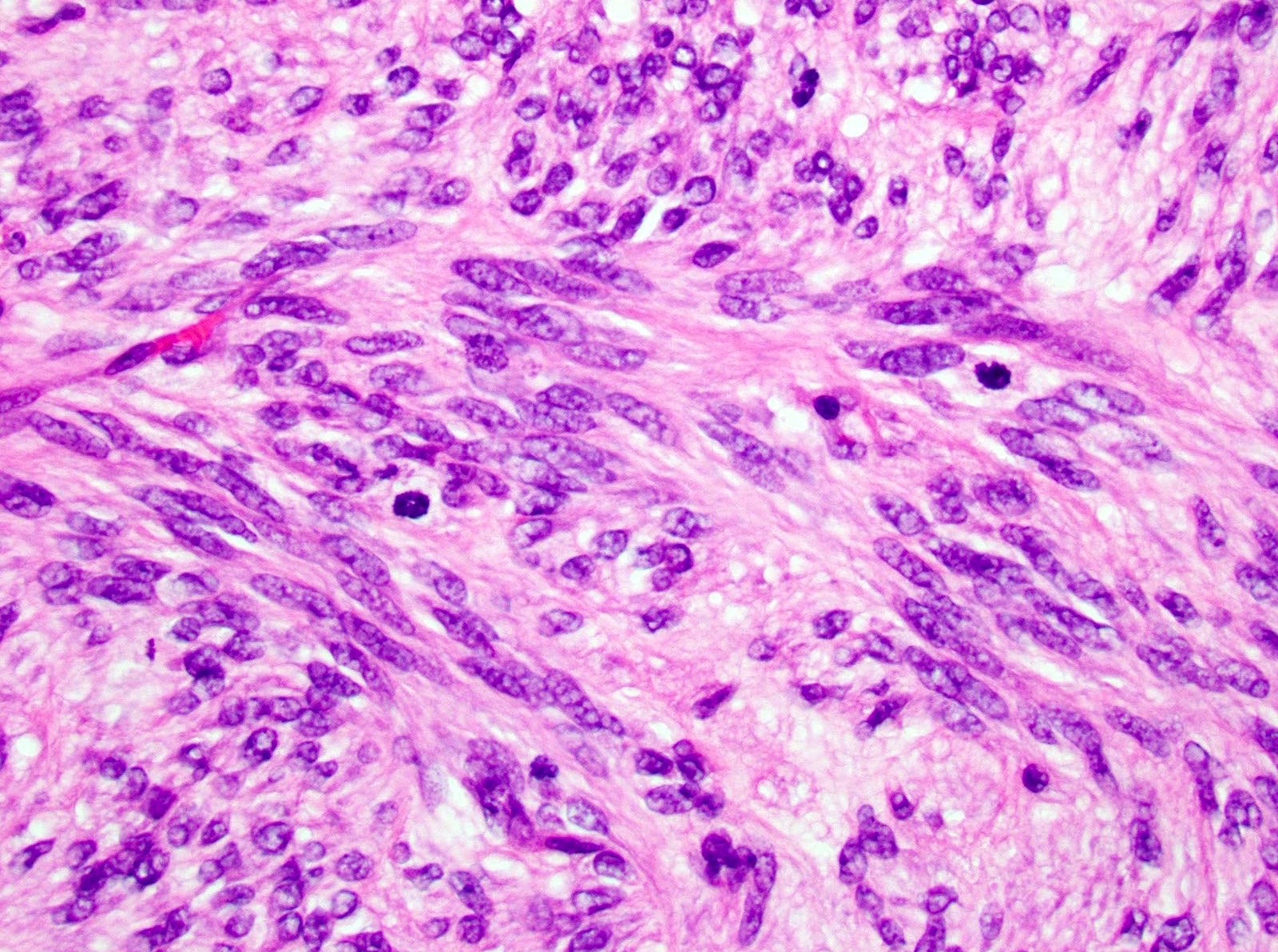
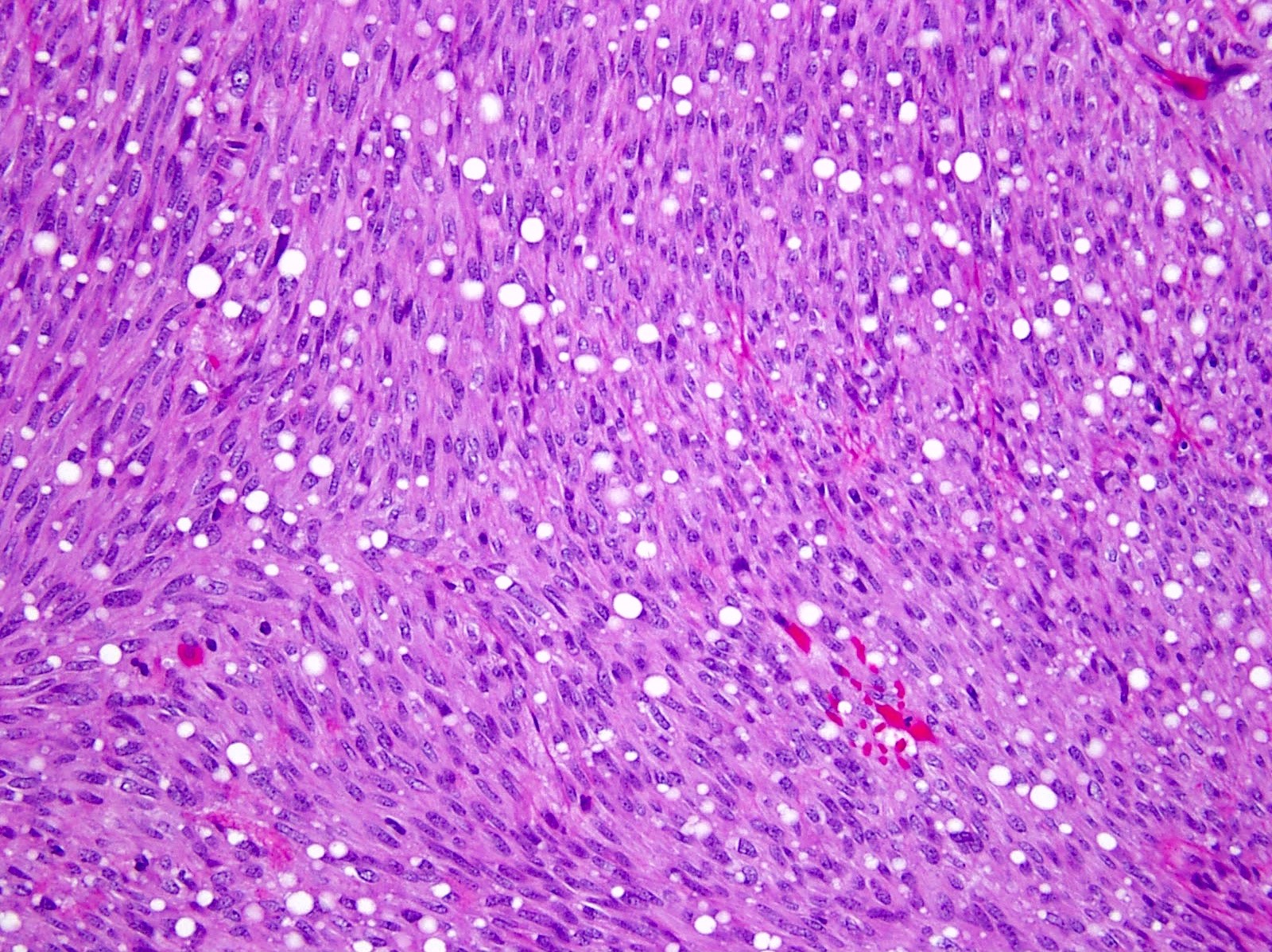
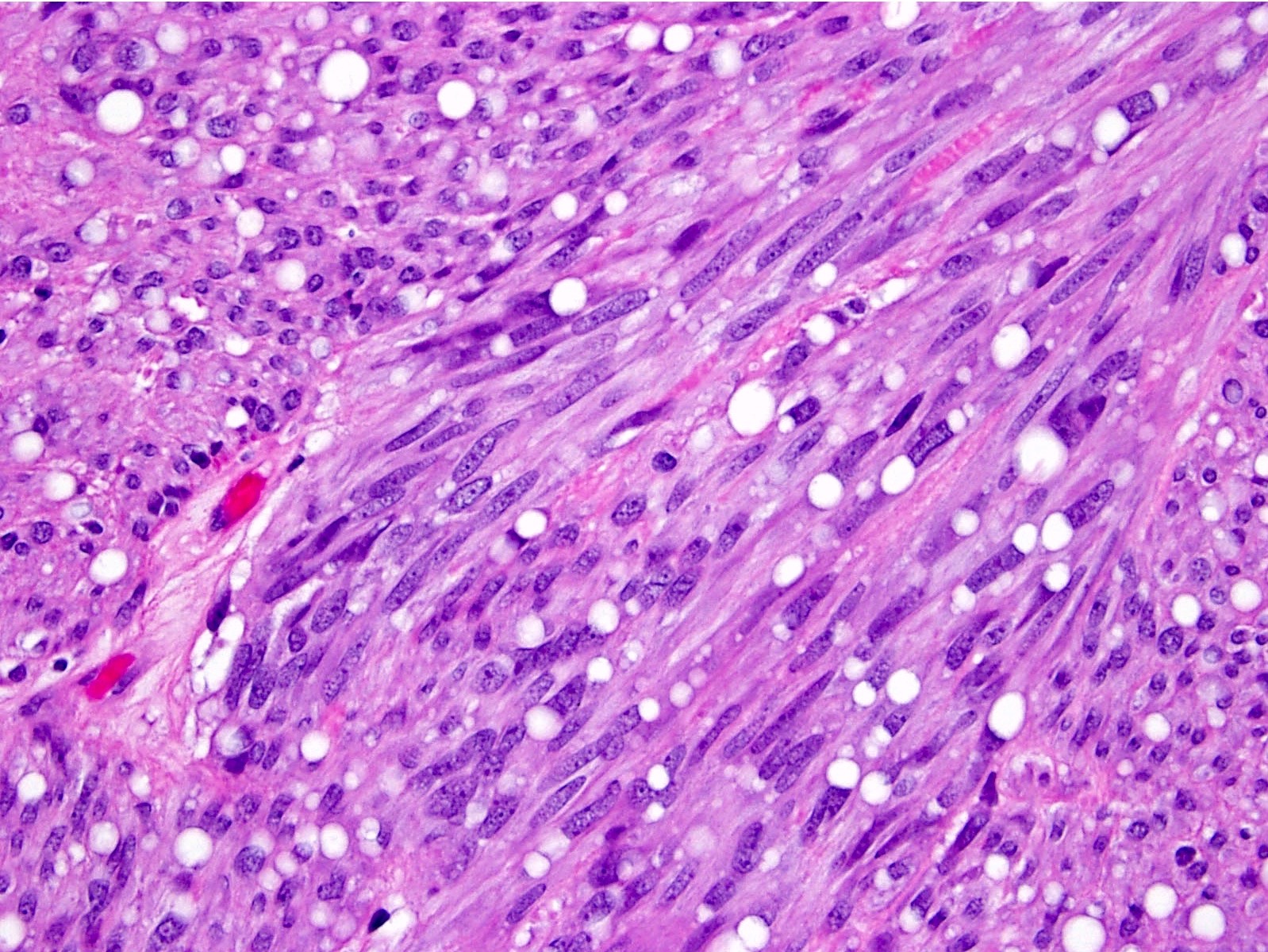
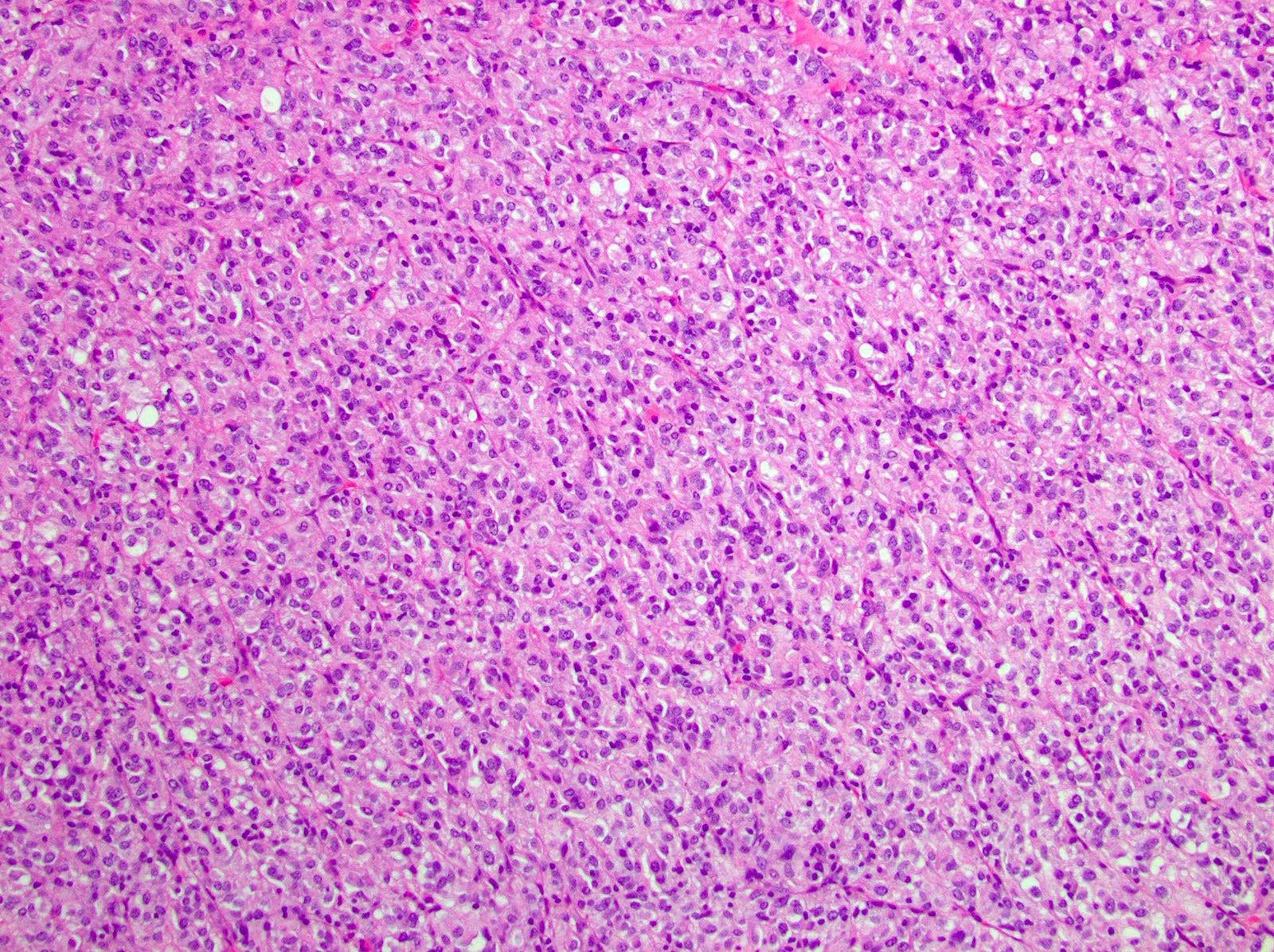
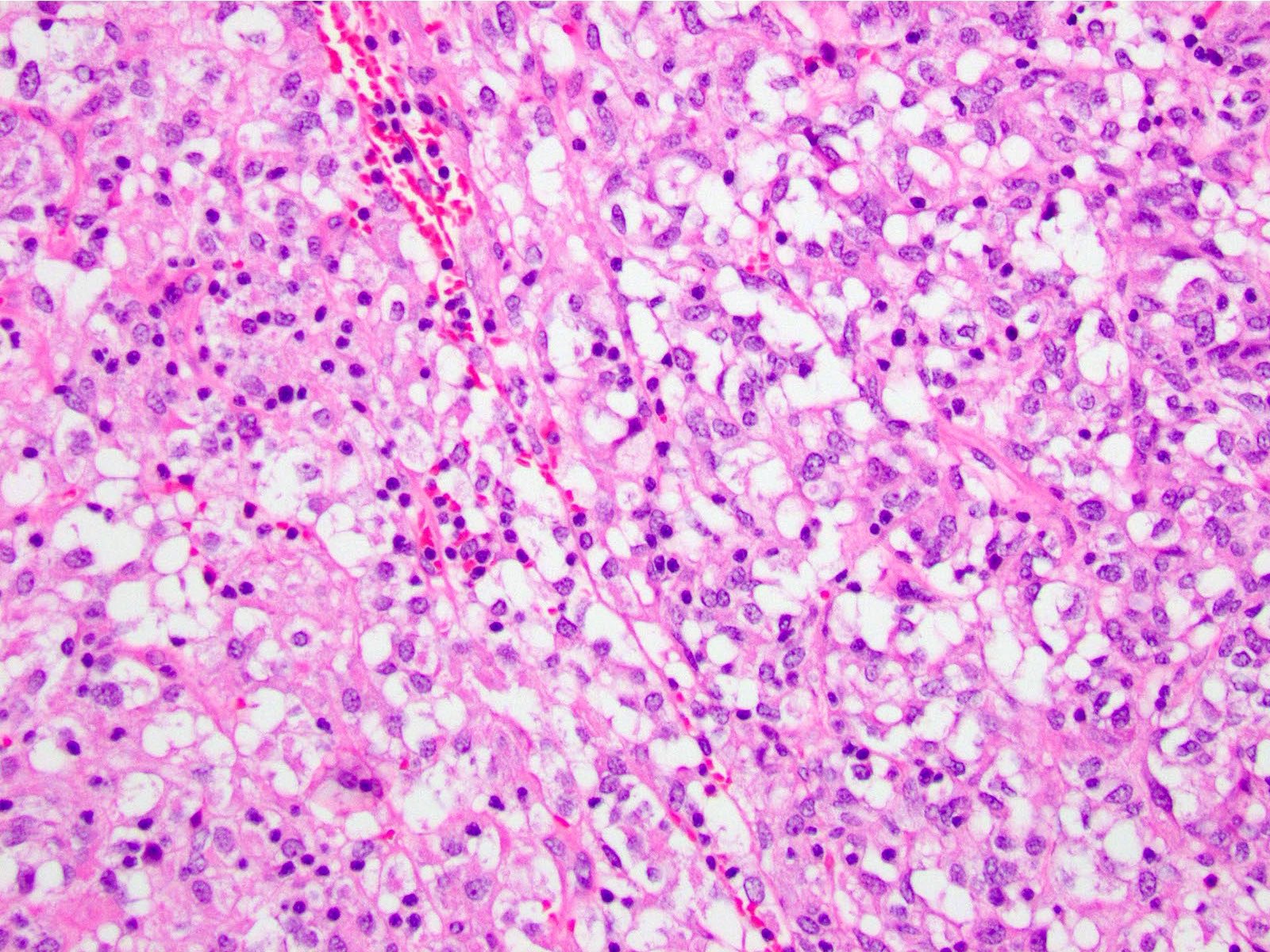
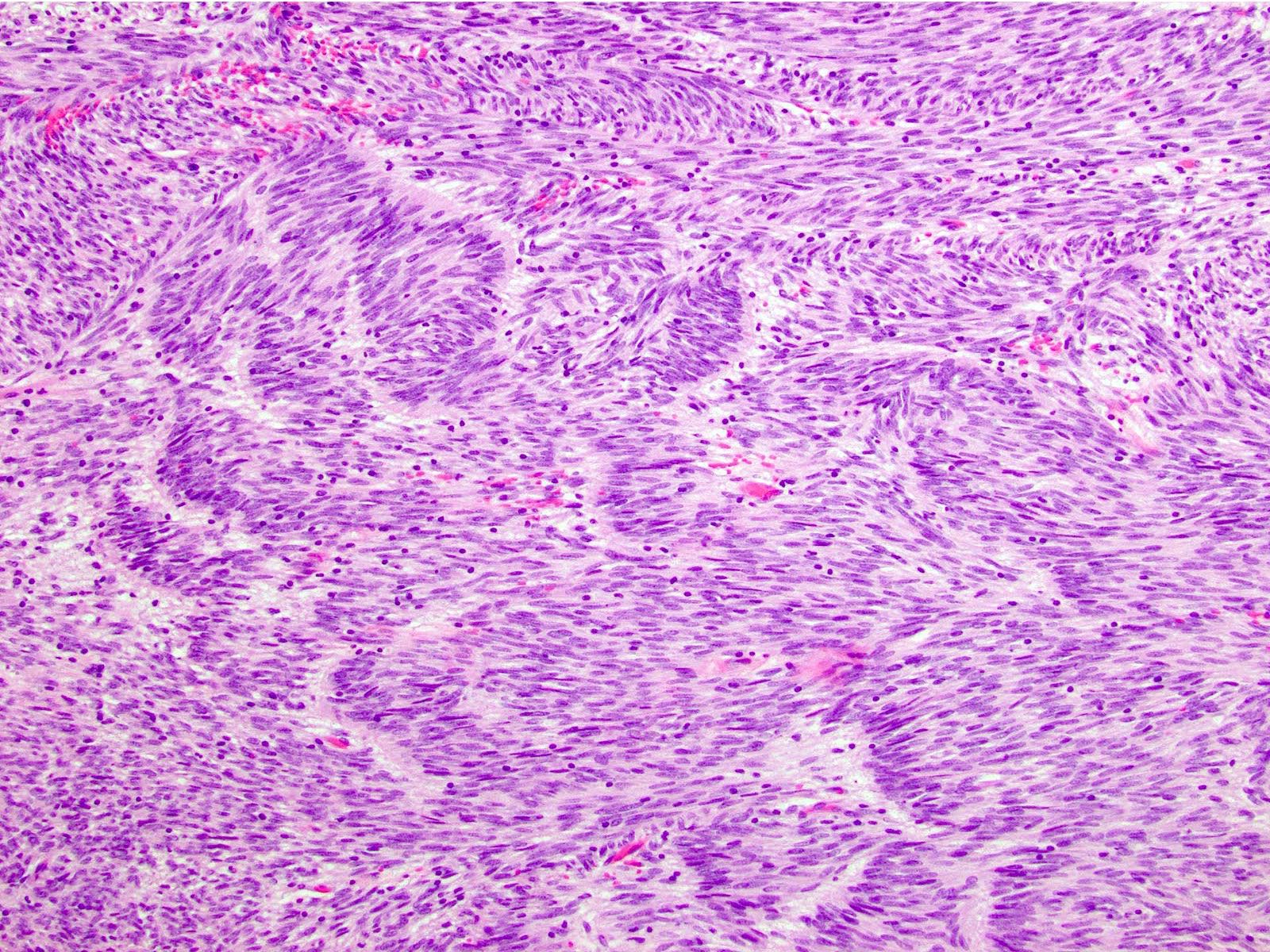
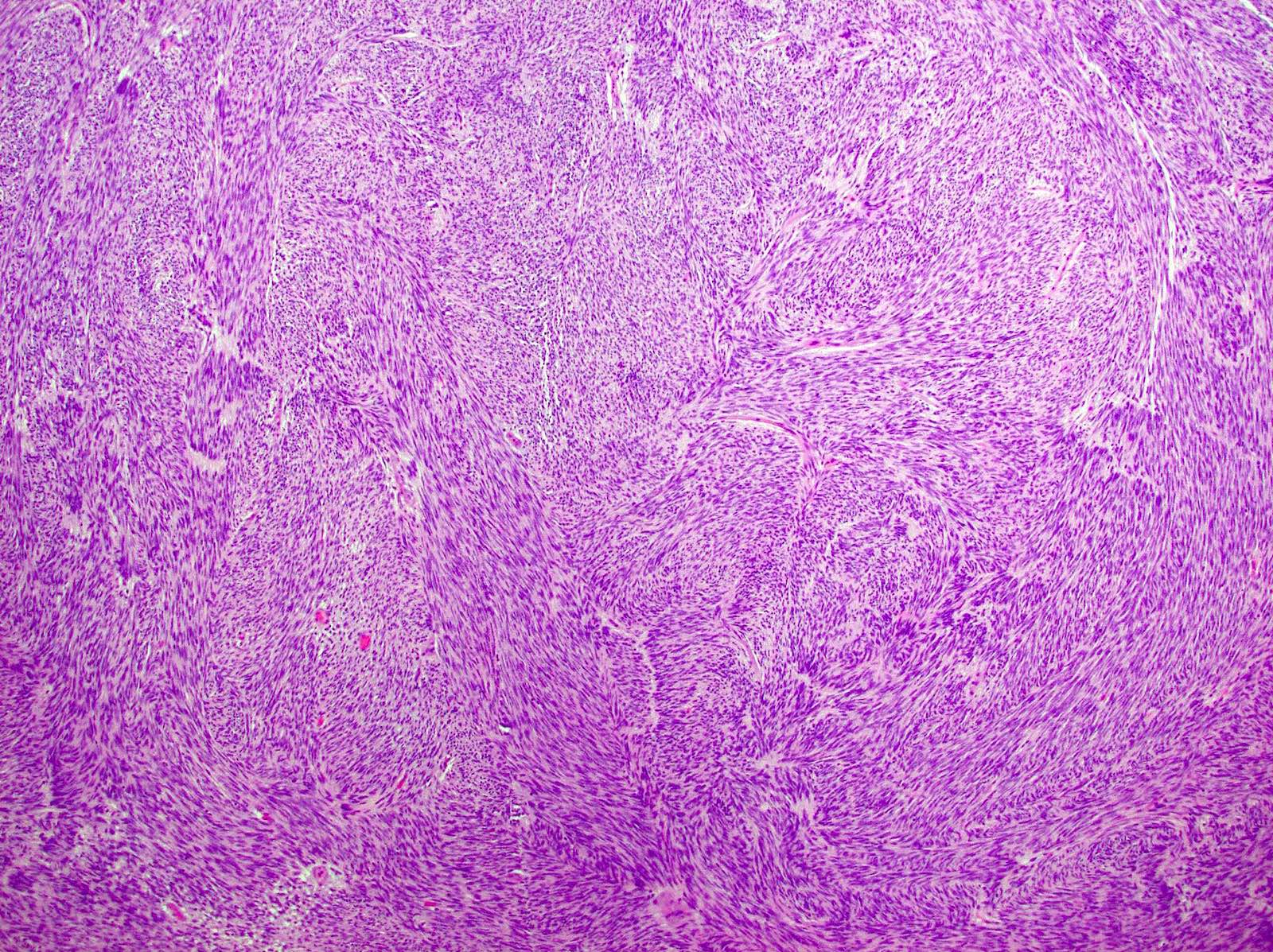
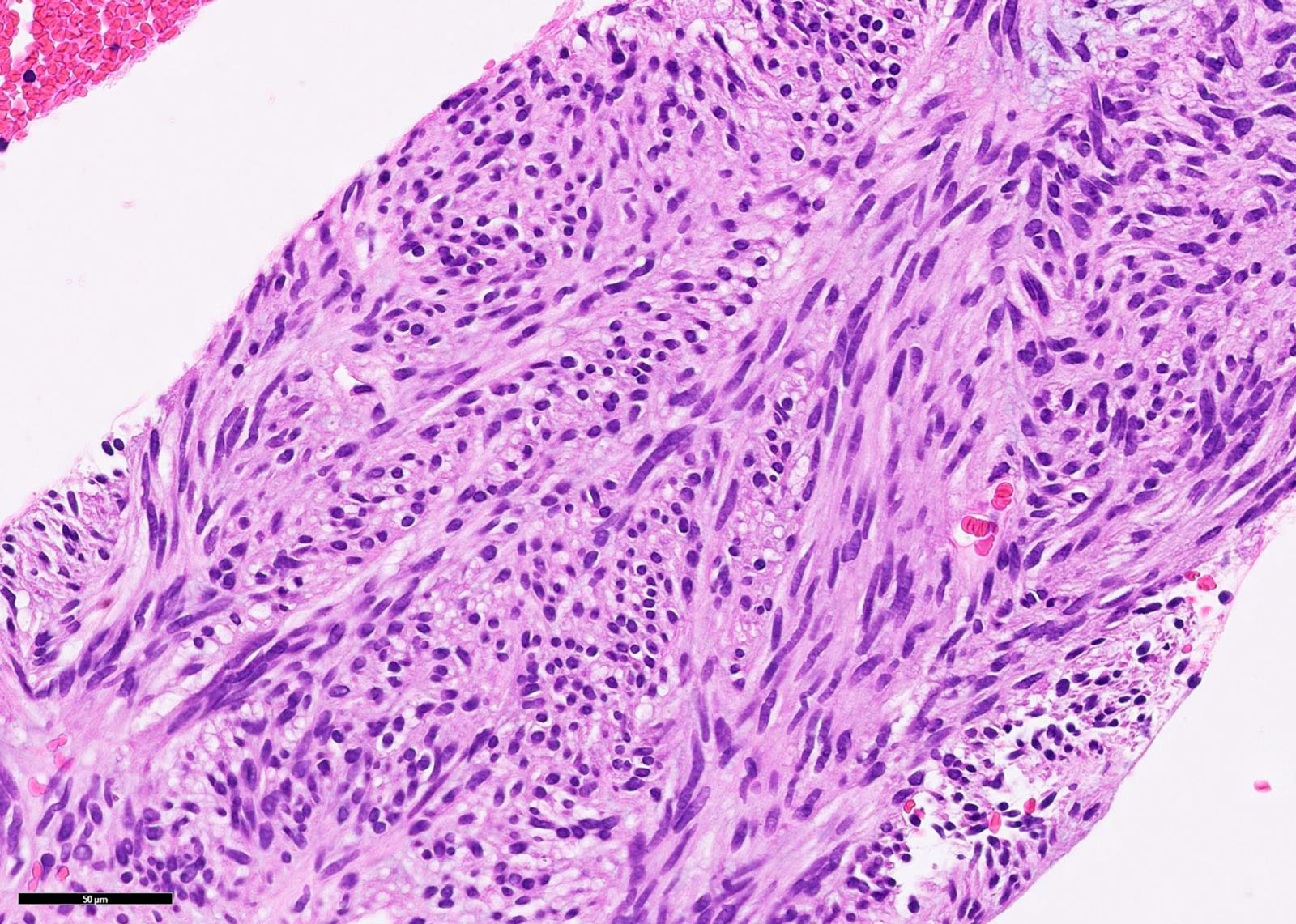
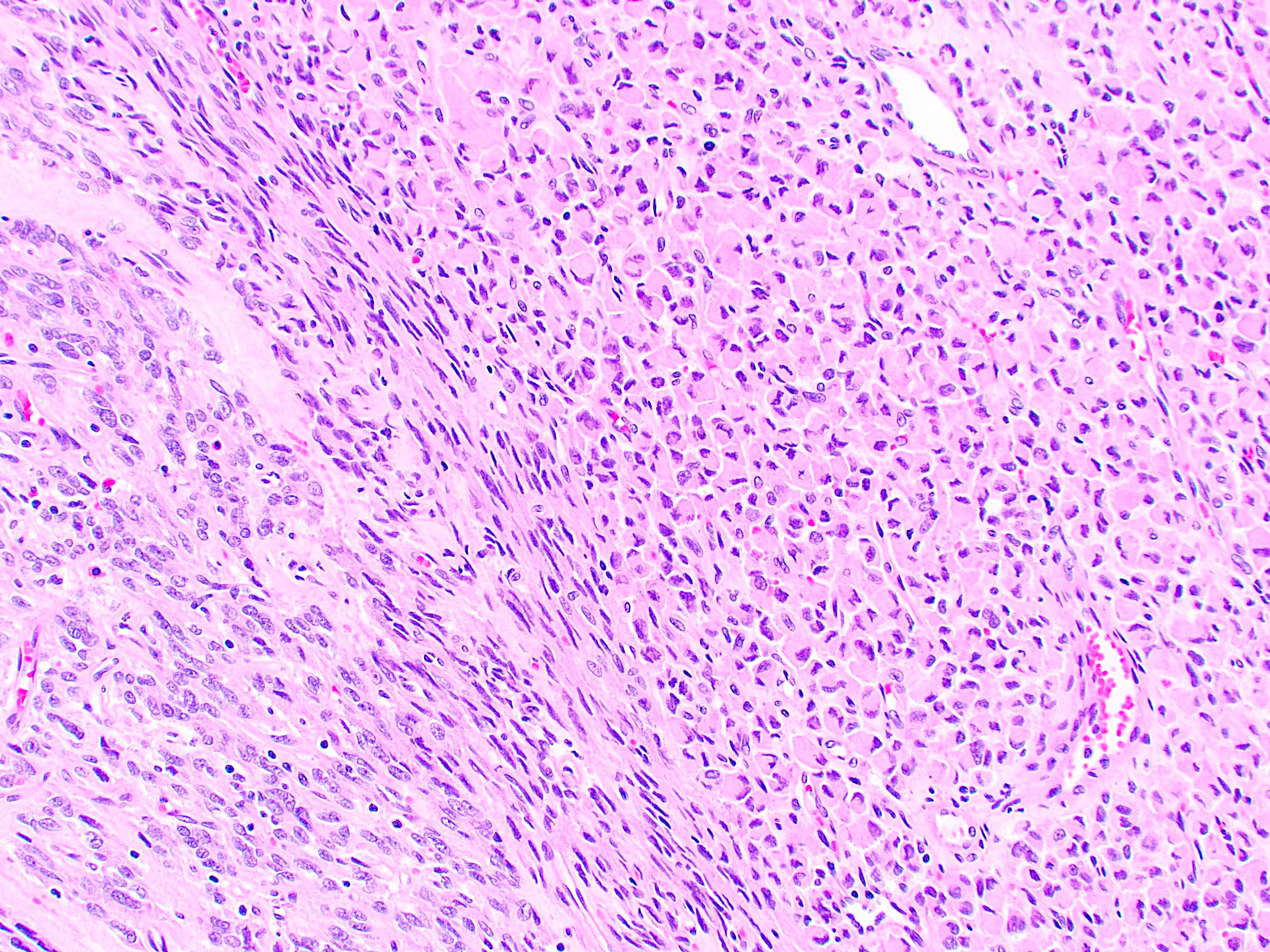
Microscopic (histologic) description
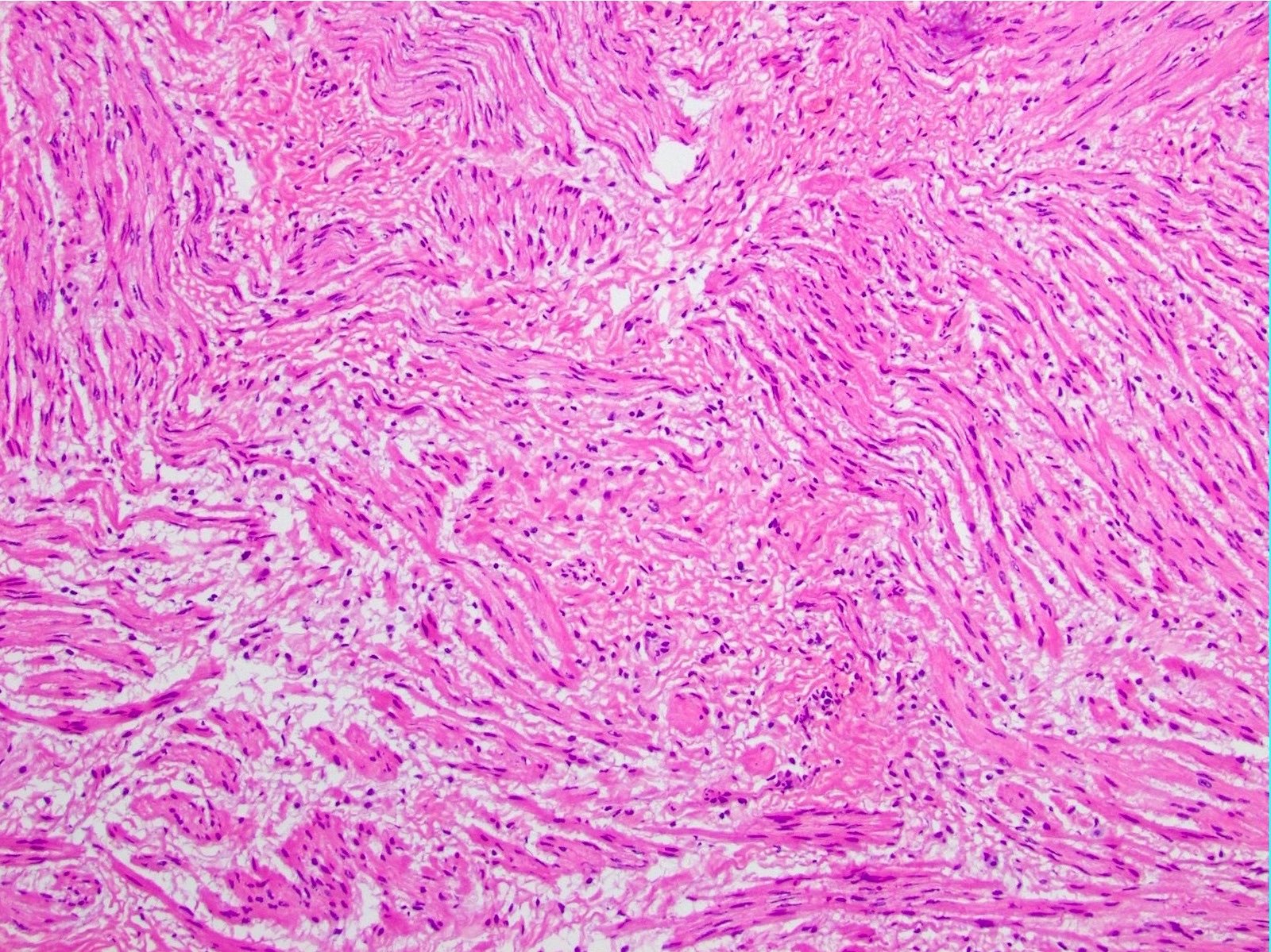
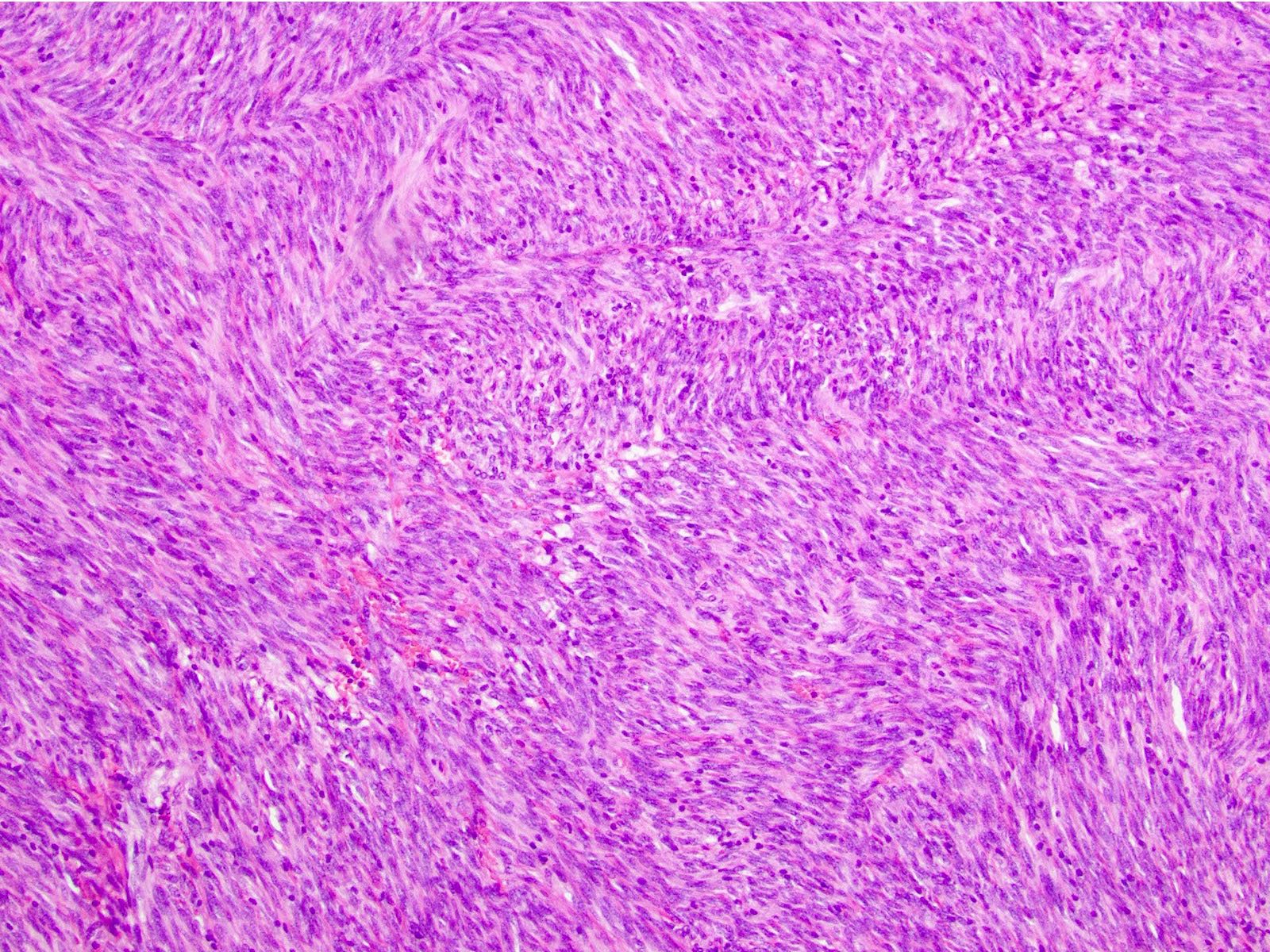
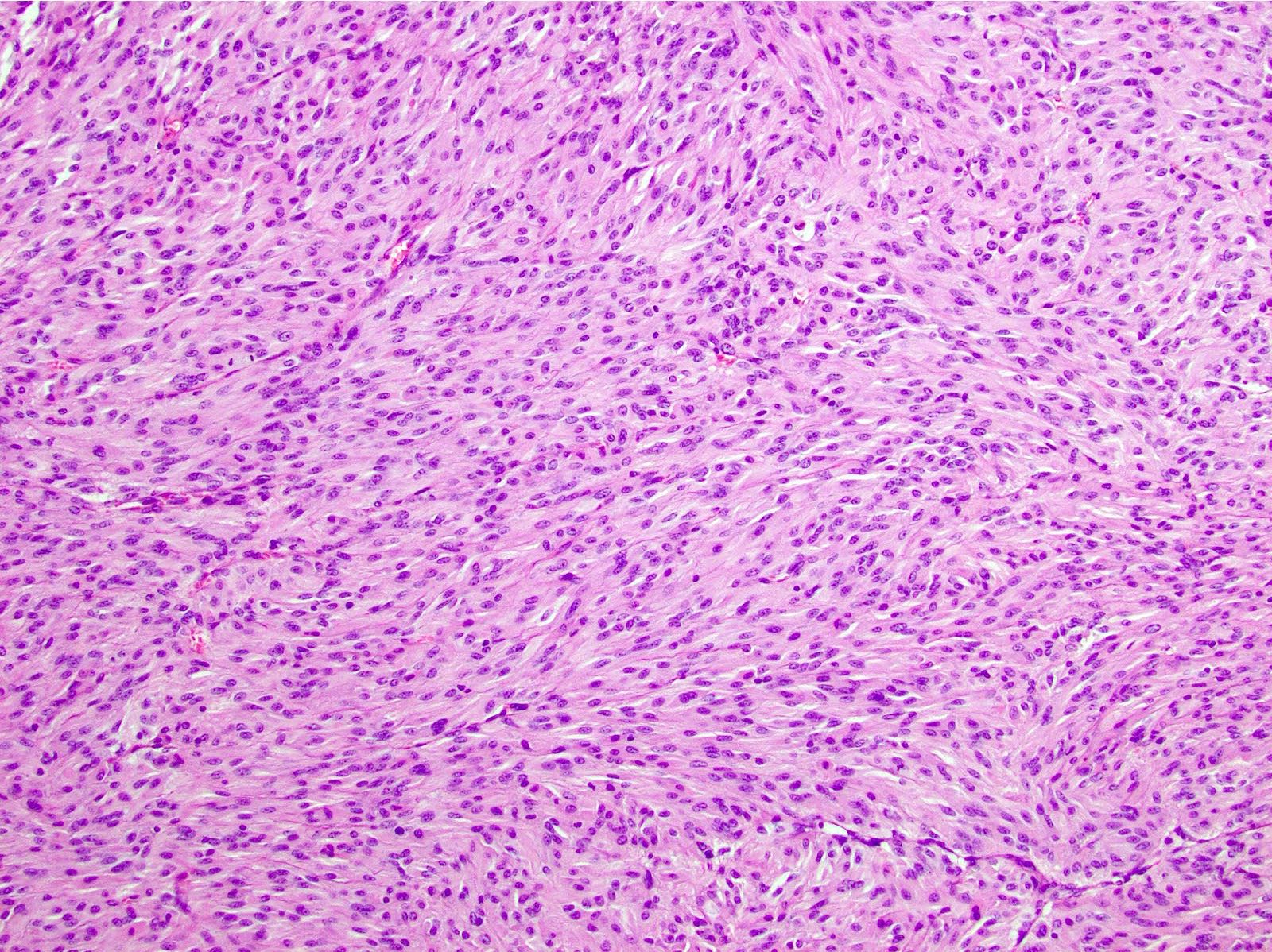
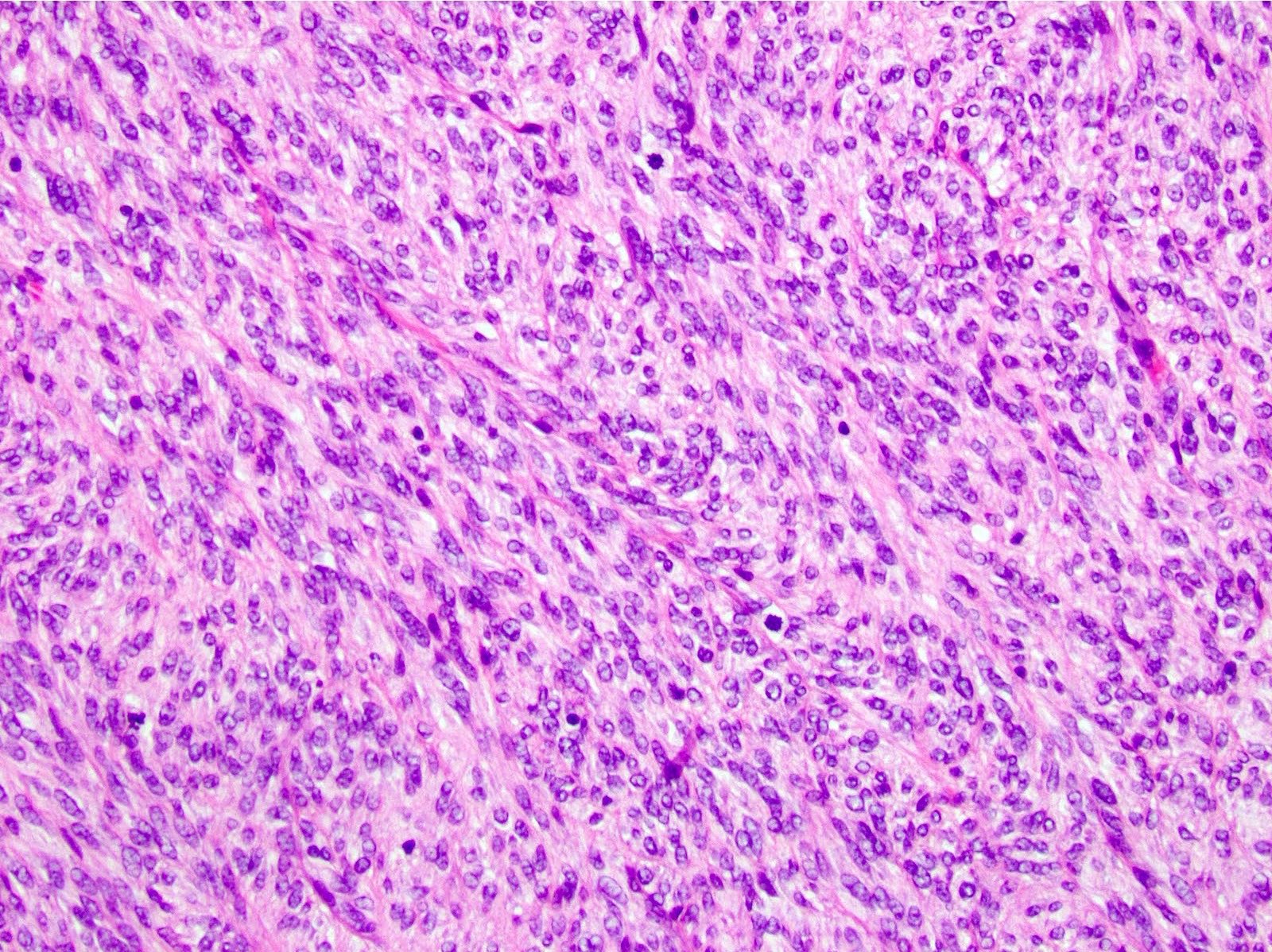
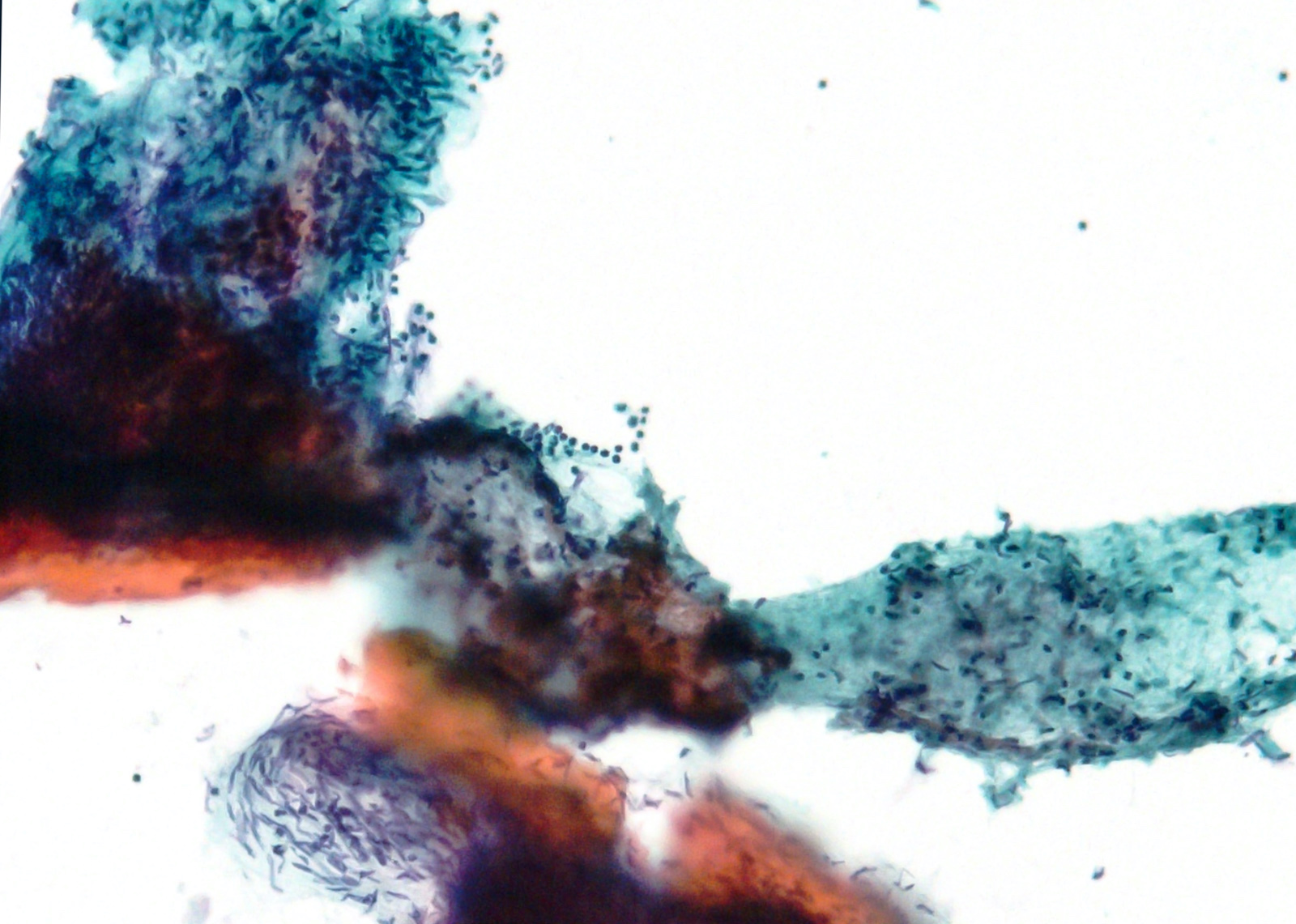
- 3 morphologic types: spindle (70%), epithelioid (20%), mixed (10%)
- Spindle
- Bland spindle cells with faintly eosinophilic cytoplasm in a syncytial pattern
- Elongated nuclei with inconspicuous nucleoli
- Artifactual paranuclear vacuoles common in stomach GIST
- Subtypes: sclerosing, palisaded, vacuolated, diffuse hypercellular, sarcomatoid features with significant nuclear atypia and mitotic activity
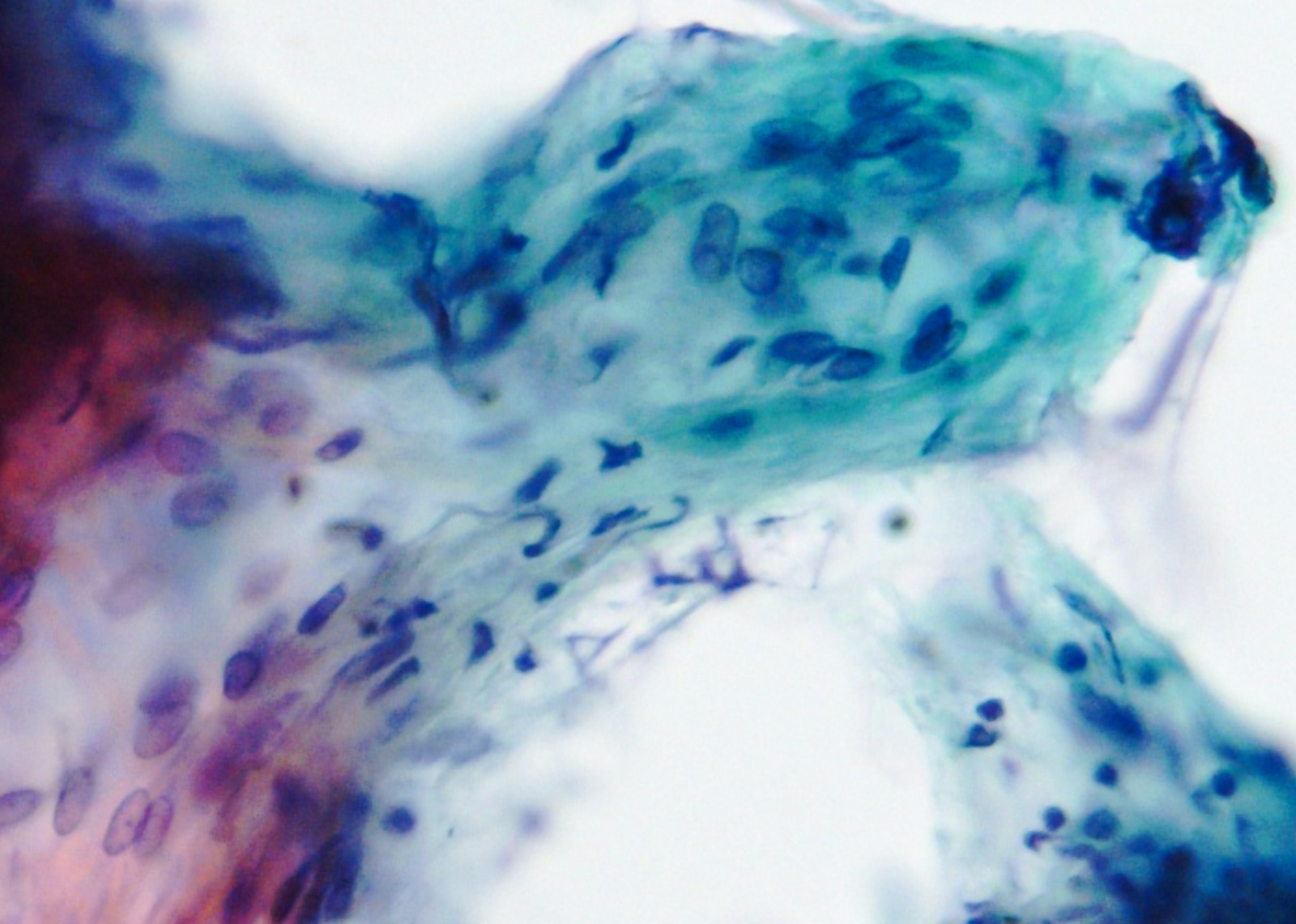
- Epithelioid
- Round cells with clear to eosinophilic cytoplasm in sheets or nests
- Increased tendency for pleomorphism versus spindle type
- Subtypes: sclerosing, discohesive, hypercellular, sarcomatous with significant atypia and mitotic activity
- Mixed
- Tumor is composed of cells with spindle and epithelioid morphology
- Spindle
- SDH deficient: epithelioid or mixed epithelioid / spindle cell morphology, multinodular pattern, minimal nuclear pleomorphism, occasional atypical mitoses
- Dedifferentiated: anaplastic appearance with an unusual phenotype (may lose expression of KIT or may aberrantly express other markers such as cytokeratin and desmin) and may occur de novo or following treatment with tyrosine kinase inhibitors (Ann Diagn Pathol 2019;39:118)
- Morphology of GIST treated with tyrosine kinase inhibitors (Adv Anat Pathol 2024;31:354)
- Histologic response varies: may see decreased cellularity, decreased proliferative index, myxoid change, cystic change, stromal hyalinization or necrosis
- Many of these changes such as stromal hyalinization, cystic change or myxoid change can also be seen in untreated GIST, complicating assessment
- Per the current CAP protocol, "as a practical compromise, it is best to report the percentage of viable tumor after treatment" (CAP: Protocol for the Examination of Resection Specimens From Patients With Gastrointestinal Stromal Tumor (GIST) [Accessed July 10, 2025])
- Histologic changes after treatment that may be indicative of disease progression: increased mitoses, pleomorphic nuclei, phenotypic changes (spindled to epithelioid or pseudopapillary morphology or aberrant expression of desmin and loss of KIT expression) or KIT expression that persists
- Histologic response varies: may see decreased cellularity, decreased proliferative index, myxoid change, cystic change, stromal hyalinization or necrosis
- Micro / mini / subclinical GIST: minute growths (1 - 10 mm) of interstitial cells of Cajal / GIST-like cells (Am J Pathol 2002;160:1567)
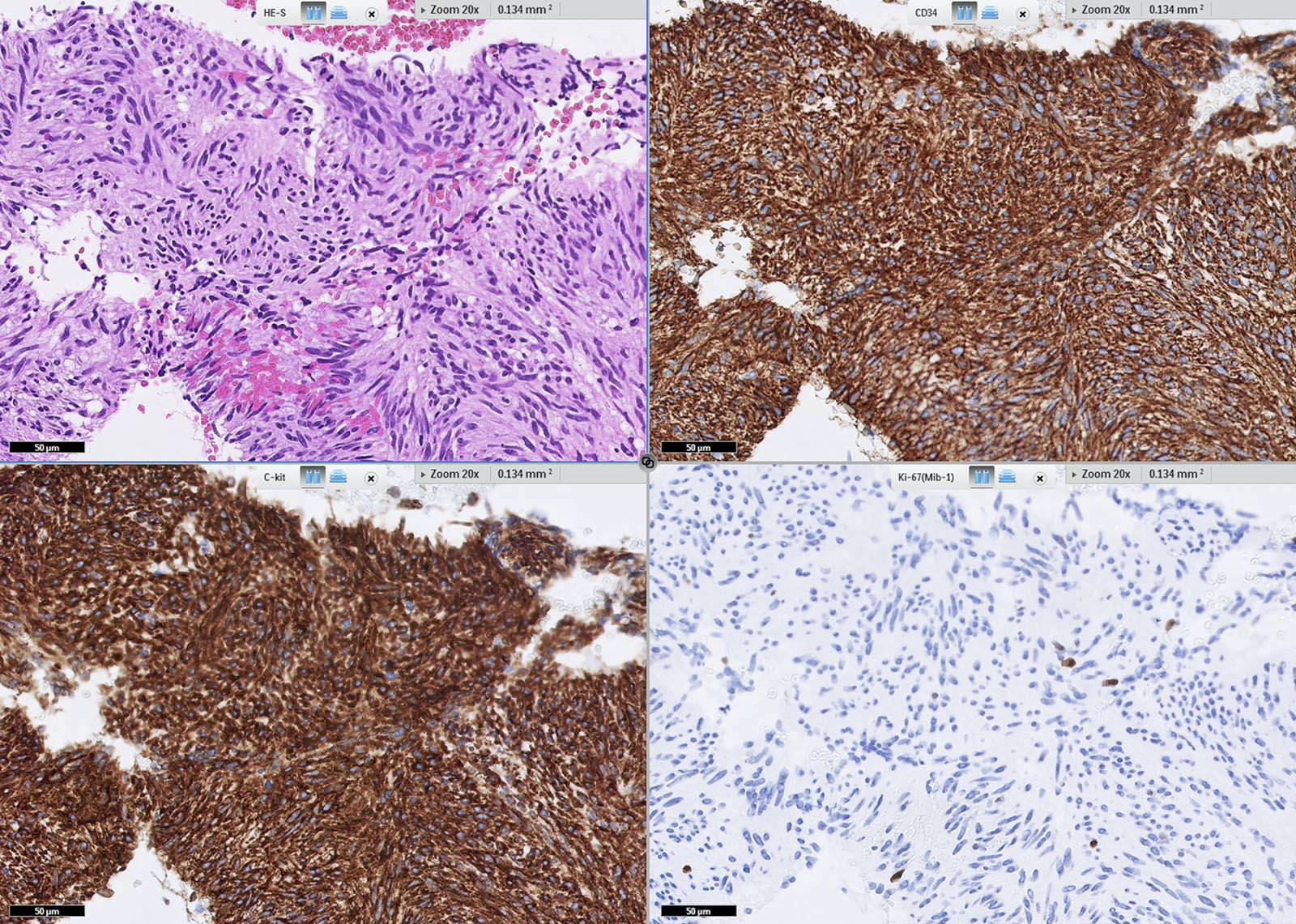
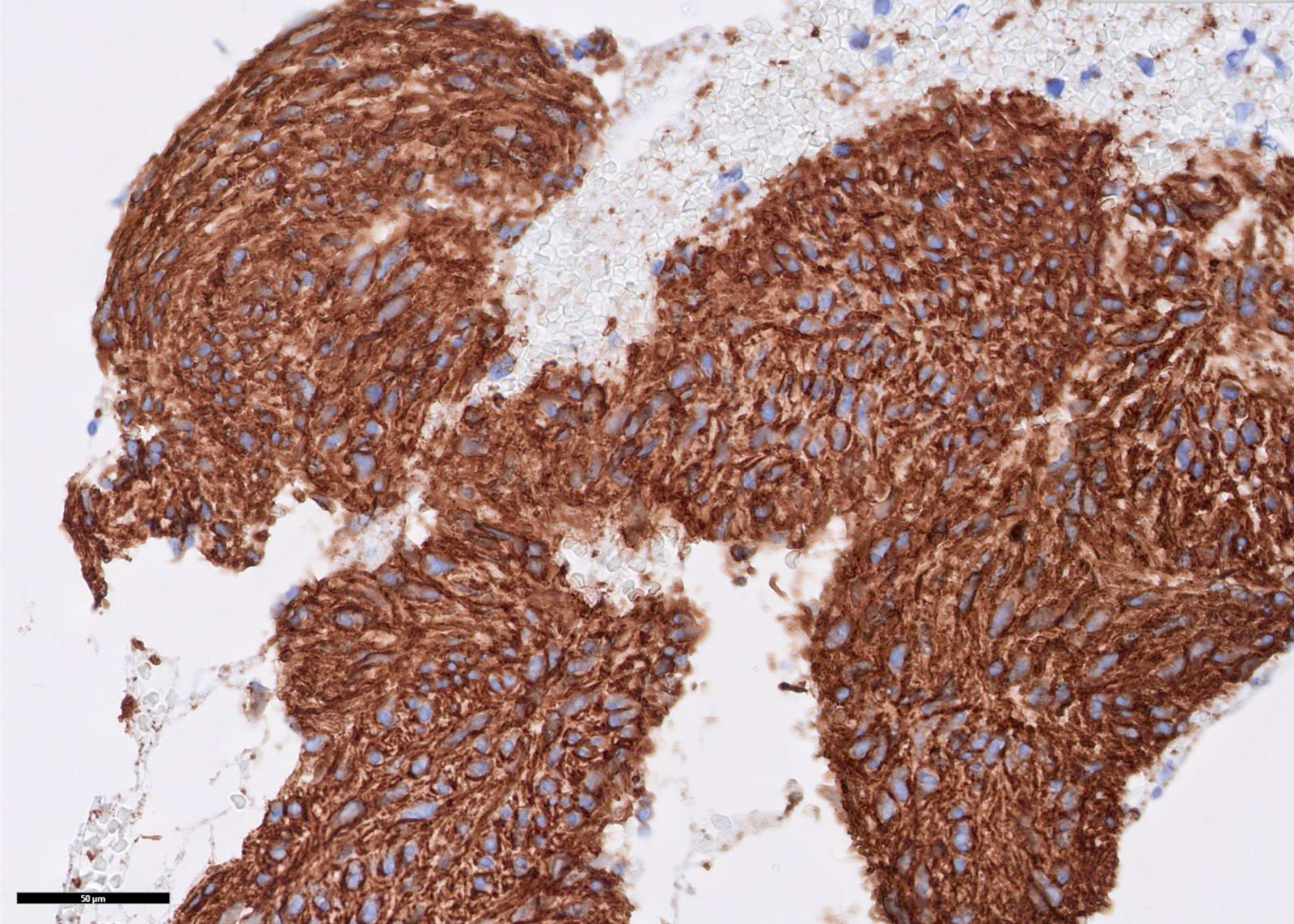
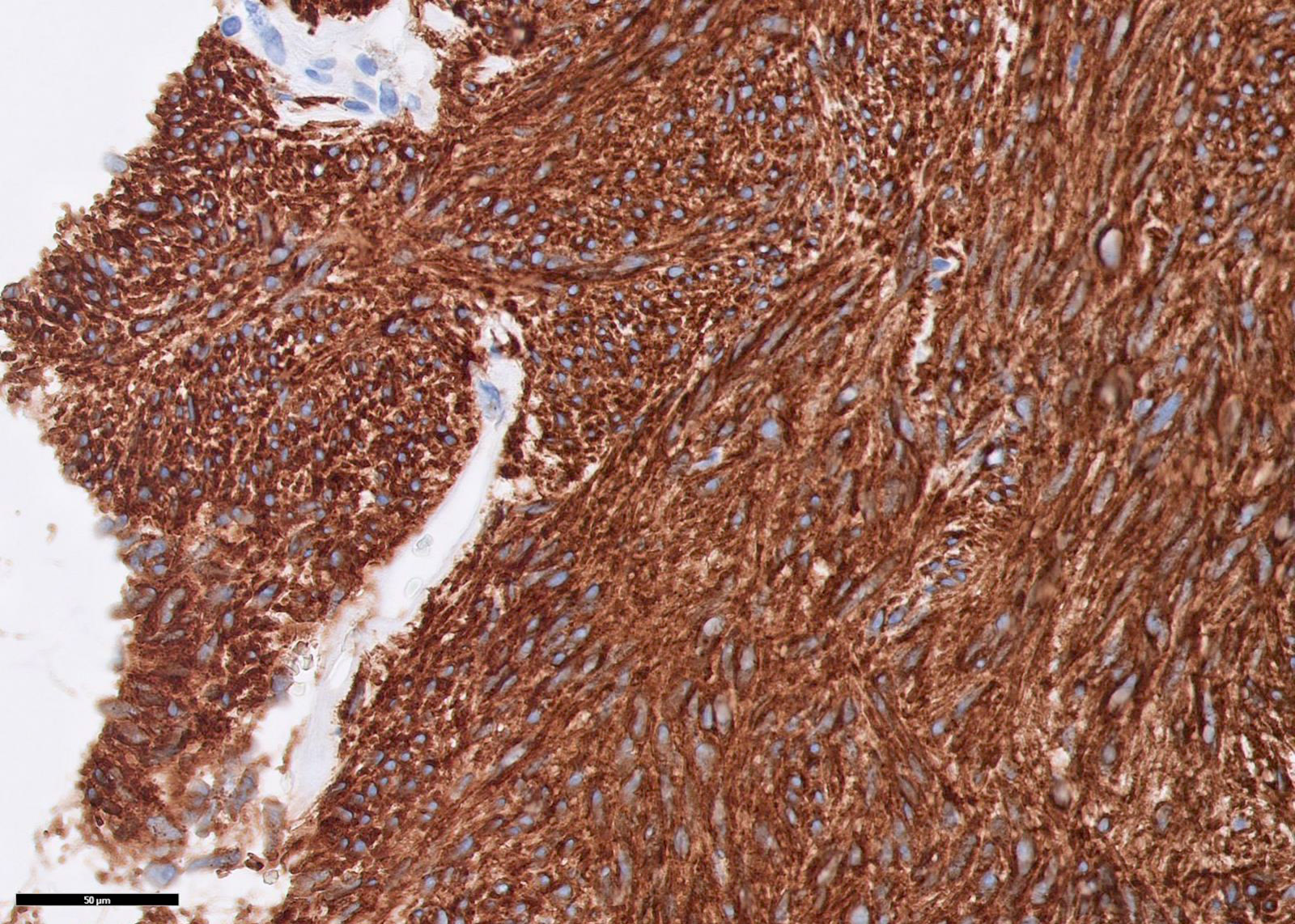
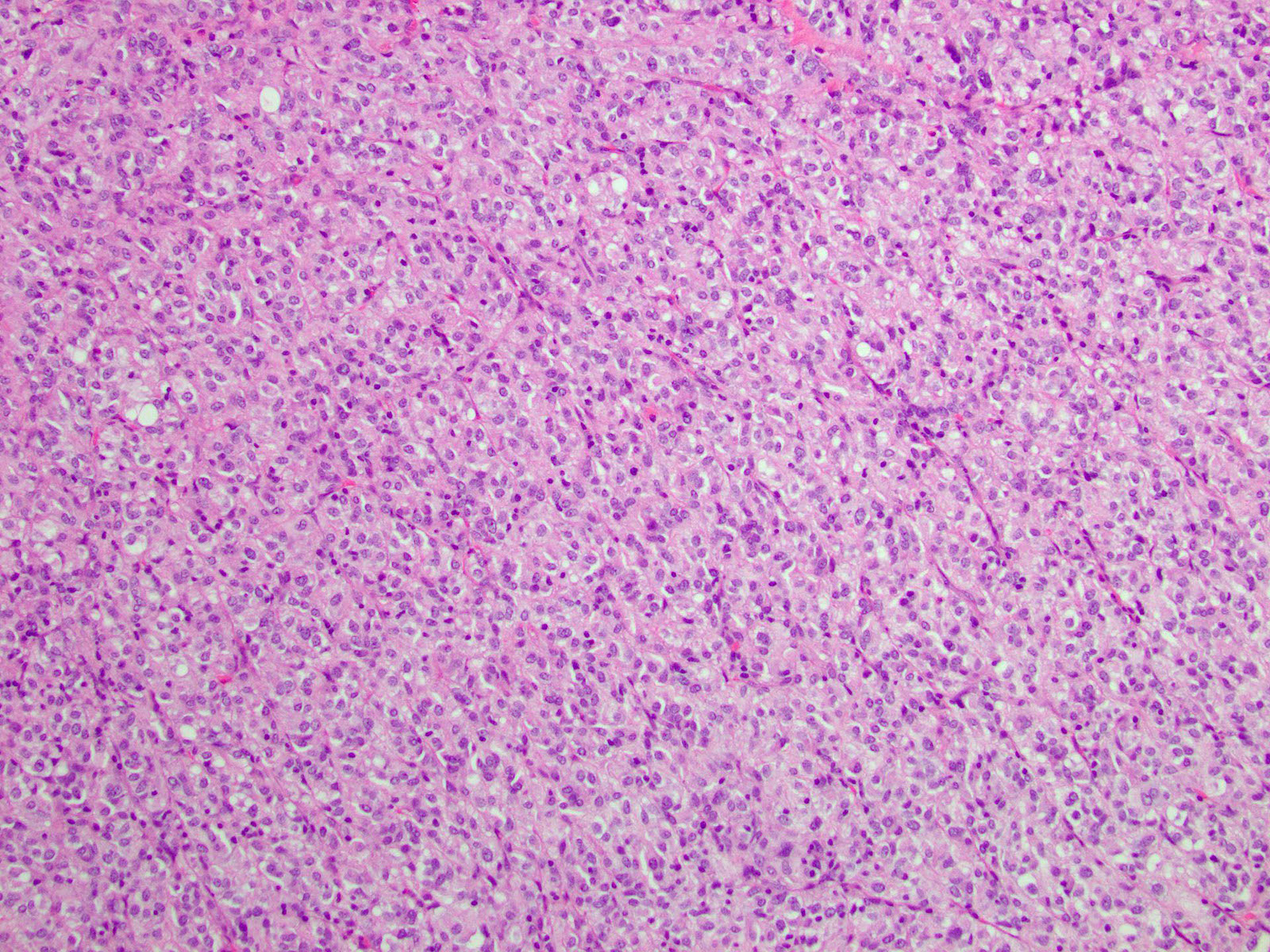
Microscopic (histologic) images
Contributed by Phoenix D. Bell, M.S., M.D., Jennifer Findeis-Hosey, M.D., Andrey Bychkov, M.D., Ph.D. and Raul S. Gonzalez, M.D. (Case #523)
Cytology description
- Endoscopic ultrasound guided fine needle aspiration may lend a preliminary diagnosis
- Bland spindle to epithelioid cells arranged in fascicles
Positive stains
- DOG1 / ANO1 (98%) (Am J Pathol 2004;165:107)
- KIT / CD117 (95%) (Am J Surg Pathol 2009;33:1401)
- Membranous, diffusely cytoplasmic or concentrated in a dot-like perinuclear pattern
- CD34 (82%, versus 40% in small intestinal GIST) (Am J Surg Pathol 2005;29:52, Am J Surg Pathol 2006;30:477)
- SMA positive in only 18% of gastric tumors versus 34% in small intestinal GIST (Am J Surg Pathol 2005;29:52, Am J Surg Pathol 2006;30:477)
- Desmin positivity may also be seen (~5%); this is typically focal and more often seen in epithelioid GISTs (Am J Surg Pathol 2005;29:52)
Negative stains
Molecular / cytogenetics description
- See Pathophysiology
Sample pathology report
- Stomach, fundus, biopsy:
- Gastrointestinal stromal tumor (GIST), mixed spindle cell and epithelioid types
Differential diagnosis
Additional references
Practice question #1
Practice answer #1
D. SDHA. The image shows cells with round nuclei, abundant eosinophilic cytoplasm, inconspicuous nucleoli and minimal pleomorphism, representative of an epithelioid type GIST. This subtype is commonly seen in patients with an SDH deficient GIST, which is most often due to mutations in the SDHA subunit; however, rare mutations in the other subunits have also been identified. Answer E is incorrect because while SDHC mutations can also result in SDH deficient GISTs, they are much less common than SDHA mutations. Thus, SDHC is not the most likely mutation in this scenario. The majority of GISTs result from mutations in the KIT (75%) or PDGFRα (10%) proto-oncogenes. They are usually found in the stomach and are composed of spindle (versus epithelioid) cells. Answer A is incorrect because although KIT mutations account for ~75% of GISTs overall, they are not commonly seen in SDH deficient, epithelioid type GISTs. Answer C is incorrect because while PDGFRA mutations are found in ~10% of GISTs, they typically present with spindled morphology and occur in older adults. They are not the most common mutations seen in epithelioid GISTs in young individuals. More recently, there have been reports suggesting patients with neurofibromatosis 1 (NF1 mutations) are predisposed to GIST development. However, answer B is incorrect because GIST in patients with NF1 mutations occur more commonly in the small intestine and will show spindled morphology.
Comment Here
Reference: Stomach - GIST
Comment Here
Reference: Stomach - GIST
Practice question #2
- A 60 year old man presents with abdominal pain. A CT scan reveals a mass within the gastrointestinal system, which is resected. Histopathologic examination reveals a submucosal mass composed of spindle cells with lightly eosinophilic cytoplasm arranged in a syncytial pattern. The pathologic diagnosis is a GIST, spindle cell type. Which of the following answer choices correlates with the most favorable prognosis for this patient?
- KIT mutation present
- Mass is 11 cm in greatest dimension
- Mass is located in the jejunum
- Mitotic rate ≥ 5 per 5 mm2
- R1 resection
Practice answer #2
A. KIT mutation present. The main prognostic factors associated with GIST are mitotic rate, tumor size and tumor location. The extent of surgical resection and the presence of a KIT mutation also affect prognosis. GIST findings suggesting a more favorable prognosis include stomach location, tumor size ≤ 2 cm, mitotic rate < 5 per 5 mm2, R0 (a resection with negative margins) and the presence of a KIT mutation. Therefore, the best answer, of the given choices, is the presence of a KIT mutation. Answers B, C, D and E are incorrect because a mass over 11 cm, location in the jejunum, mitotic rate ≥ 5 mitoses per 5 mm2 and R1 resection (microscopic residual disease) are not associated with a favorable prognosis. It should be noted that mitotic counts should be counted in 5 mm2. The original studies based the risk of progressive disease nomogram on counts of 50 high power fields, which utilized older microscopes. Today, with modern microscopes, 5 mm2 correlates with ~20 - 25 high power fields but if necessary, the pathologist should determine their actual field of view and the number of fields required to achieve 5 mm2.
Comment Here
Reference: Stomach - GIST
Comment Here
Reference: Stomach - GIST
Practice question #3
Which of the following is true about succinate dehydrogenase deficient gastrointestinal stromal tumors (SDH deficient GISTs)?
- They almost always arise in the colon
- They are negative for KIT by immunohistochemistry
- They have a very poor prognosis
- They sometimes metastasize to lymph nodes
Practice answer #3
D. They sometimes metastasize to lymph nodes. SDH deficient GISTs may metastasize to lymph nodes, which almost never happens in GISTs that arise through other molecular pathways. Answer C is incorrect because despite this behavior, they have a relatively good prognosis. Answer A is incorrect because SDH deficient GISTs almost always arise in the stomach, not the colon. Answer B is incorrect because they are positive for KIT and DOG1 by immunohistochemistry, facilitating the diagnosis of GIST. They have a moderate prognosis and patients often have a protracted clinical course with metastases to lymph nodes and distant organs.
Comment Here
Reference: Stomach - GIST
Comment Here
Reference: Stomach - GIST