Table of Contents
Definition / general | Essential features | Terminology | History | Procedure to create CAR T product | Pathophysiology | Types of CAR T cells | Diagrams / tables | Challenges | Adverse effects | Clinical features | Laboratory | Case reports | Treatment | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: George MR. CAR T cell therapy. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/transfusionmedcartcelltherapy.html. Accessed April 19th, 2024.
Definition / general
- Chimeric antigen receptor T cells (CAR T) are a form of cellular immunotherapy involving the genetic engineering of T cells to produce surface receptors targeted at specific cell surface receptors
- CAR T is primarily used to treat hematologic malignancies, with acute lymphoblastic leukemia (ALL) being the first disease targeted and diffuse large B cell lymphoma (DLBCL) being the second; its application to solid organ tumors is being studied
Essential features
- CAR T cell therapy was originally developed for B cell acute lymphoblastic leukemia
- It is also now approved for diffuse large B cell lymphoma (DLBCL)
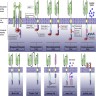
- Process starts with collecting autologous T cells via leukocytapheresis, shipping the cells to be engineered / manufactured, expanding the cell population to achieve appropriate dose, cryopreserving CAR T cells and shipping to the treating facility
- Patient is given lymphocyte depleting chemotherapy prior to receiving the treatment
- Key steps of CAR T therapy are trafficking, recognition, control, microenvironment, proliferation / persistence
- Safety measures are built in to allow ablation of CAR T cells if severe toxicity develops
- Severe toxicity can include cytokine release syndrome and a CAR T encephalopathy
Terminology
- CART-19: CAR T directed against CD19 antigen on B cells
- Tisagenlecleucel, CTL019, also known as Kymriah® (Novartis, Basel, Switzerland)
- Approved for relapsed acute lymphoblastic B cell leukemia / lymphomas
- Approved for patients with relapsed or refractory large B cell lymphoma including diffuse large B cell lymphoma, high grade B cell lymphoma and diffuse large B cell lymphoma arising from follicular lymphoma
- Axicabtagene ciloleucel, also known as Axi-cel, Yescarta® (Kite Pharma / Gilead, Los Angeles, CA)
- Approved for aggressive, relapsed or refractory diffuse large B cell lymphoma, primary mediastinal B cell lymphoma and transformed follicular lymphoma
- Tisagenlecleucel, CTL019, also known as Kymriah® (Novartis, Basel, Switzerland)
History
- 1989: Discovery that it was possible to redirect T cell signaling to an antigen of choice, independent of major histocompatibility complex (MHC) restrictions
- 2006: First results published of human clinical trials using chimeric antigen receptor T cell technology (J Clin Oncol 2006;24:e20, Clin Cancer Res 2006;12:6106)
- 2010: First use of CAR T was in a 5 year old girl with relapsed B cell acute lymphoblastic leukemia (ALL); received CAR T directed against CD19 antigen on B lymphoblasts (CART-19)
- Developed complications, treated with toclilizumab, an anti-IL6 monoclonal antibody
- Patient survived and became global ambassador for CAR T therapy
- 2014: CTL019 was granted breakthrough therapy designation by the United States Food and Drug Administration (Immunol Rev 2015;263:68)
Procedure to create CAR T product
- Collect autologous T cells via leukocytapheresis
- Obtain approximately 109 T cells
- Unknowns:
- Is there an optimal time window for collection?
- How to predict an adequate collection?
- How to separate T cells from collected mononuclear cell apheresis product? (Maitta: Immunologic Concepts in Transfusion Medicine, 1st Edition, 2019)
- Ship T cells to manufacturing site
- CAR structure is composed of an extracellular antigen recognition domain fused to intracellular TCR signaling domains (CD3z) and costimulatory domains such as CD28
- Manufacture CAR T cells by CD3 / CD28 bead stimulation and lentiviral transduction (Tisagenlecleucel)
- Manufacture CAR T cells by CD3 antibody / IL2 stimulation and retroviral transduction (Axicabtagene)
- Expand cell population over 6 - 10 days
- Achieve patient dose of 2 - 4 x 106 CAR expressing T cells / kg patient body weight
- Cryopreserve CAR T cells
- Ship to treating facility
- Patient is given lymphodepleting chemotherapy (usually fludarabine and cyclophosphamide)
- Deplete endogenous T cells that might reject the CAR T cells
- Increase likelihood of expanding CAR T population in recipient
- Enhance antigen presentation capabilities
Pathophysiology
- Structure:
- Receptor: combines facets of normal T cell activation into single protein
- Links extracellular antigen recognition domain to an intracellular signaling domain to activate the T cell when an antigen is bound
- 4 parts:
- Antigen recognition domain
- Exposed to the outside of the cell in the ectodomain portion of the receptor
- Allows the CAR T cell to attack any cell that expresses the matching molecule
- Derived from variable regions of a monoclonal antibody linked together as a single chain variable fragment (scFv)
- A scFv is a chimeric protein made up of the light (VL) and heavy (VH) chains of immunoglobulins connected with a short linker peptide
- VL and VH regions are selected to bind the target antigen like CD19
- Extracellular hinge region
- Structural domain between the antigen recognition region and the cell's outer membrane
- Optimizes flexibility of the scFv receptor head to promote antigen binding between CAR T and target antigen
- Transmembrane domain
- Anchors the CAR to the plasma membrane, links the extracellular hinge and antigen recognition domains with the intracellular signaling region
- Stabilizes the entire structure
- CD28 transmembrane domain often used and is very stable
- Should not use CD3-zeta transmembrane domain because it can cause incorporation of the artificial TCR into the native T cell receptor
- Intracellular T cell signaling domain
- Receives and perpetuates signal after antigen binds external recognition portion
- Cytoplasmic domain of CD3-zeta is often used to mimic the normal T cell activation dependent on phosphorylation of immunoreceptor tyrosine-based activation motifs
- Includes one or more chimeric domains from costimulatory proteins
- Antigen recognition domain
- Function of CAR T cells
- Trafficking
- Engineered T cell must be able to get to site of tumor cells
- Target tumor cells to be killed
- Possibility of introducing chemokine receptors into CAR T cells to improve trafficking to tumors that produce cognate chemokines
- Recognition
- Recognize target tumor cells
- Discriminate and ignore bystander tissues
- Control
- T cells are relatively autonomous once they are infused
- New work on regulatory processes to modulate the survival of T cells, timing, strength and location of their activity
- Microenvironment
- Resist immunosuppression
- Prime / mobilize endogenous immunity
- Proliferation / persistence
- Expand the population of CAR T cells to optimize activity (Cell 2017;168:724)
- Trafficking
- Safety measures: allow ablation of CAR T cells if severe toxicity develops
- Inclusion of suicide gene, iCaspase9
- Surface tag such as epidermal growth factor receptor
Types of CAR T cells
- First generation: contained only TCR complex CD3-ξ chain domain with additional costimulatory domains
- Second generation: incorporated costimulatory domains like CD28 or CD137 to boost survival, proliferation and antitumor activity
- Third generation: combined CD3-ξ chain domain with additional costimulatory domains (Cell 2016;164:780)
- Fourth generation: T cells redirected for universal cytokine killings (TRUCKS)
- Improve tumor microenvironment
- May have potential for solid tumors
- Newer generation: additional gene editing such as CRISPR-Cas9
- Armored CARs: CAR T cells modified to express cytokines, ligands or scFv that help turn suppressive tumor environment into pro-inflammatory to better fight tumor
- Tandem CARs: CAR molecule engineered to recognize multiple antigens via two binders on a single molecule
- Designer CARs: modified by CRISPR-Cas9 to edit CAR T genomes for advantageous features, such as being less susceptible to immunosuppressive effects
- Smart CARs: logic gated, regulated
- Have new receptors that function independently of CAR / TCR pathways but interfere with CAR activity in controlled way
- Use of modular receptors called synthetic Notch (synNotch) receptors
- Use extracellular domain like scFv to recognize target antigen, without triggering T cell activation
- Ligand engagement results in cleavage of receptor and subsequent release of transcriptional activator domain, which enters nucleus and drives expression of user specified target genes (Maitta: Immunologic Concepts in Transfusion Medicine, 1st Edition, 2019, Cell 2016;164:780)
- Split, universal and programmable (SUPRA) CARs
- Universal receptor found on T cells and tumor targeting scFv adaptor molecule
- Two component receptor system with universal receptor (zipCAR) expressed on T cells and tumor targeting scFv adaptor (zipFv)
- Fusion of intracellular signaling domains and leucine zipper as extracellular domain
- scFv of zipFv binds tumor antigen
- Leucine zipper binds and activates zipCAR on T cells
- Fusion of intracellular signaling domains and leucine zipper as extracellular domain
- Regulate activity to limit over activation, decrease cytokine secretion and improve tumor targeting (Maitta: Immunologic Concepts in Transfusion Medicine, 1st Edition, 2019, Cell 2018;173:1426)
Challenges
- Tumor specific antigens
- CD19 is a great target, since a loss of normal B cells can be compensated for by replacement antibody therapy (IVIG)
- How specific must they be?
- Tumor cells that do not express the target antigen may evade therapy
- Tumor cells with splice variants, lacking a specific epitope may also escape targeting (Blood 2018;131:2621)
- Can we pinpoint antigens expressed by tumors versus normal cells reliably?
- Do all tumor cells in a given tumor express the same antigens?
- Can a tumor be effectively treated even if only some of the cells are susceptible to targeting?
- How significant is bystander toxicity? (Blood 2018;131:2621)
- Rare chance of accidentally introducing CAR gene into a tumor cell during manufacture
- Proliferation of tumor cells
- Tumor cells escape detection by CAR T cells
- Response in non-Hodgkin lymphoma (NHL) worse than in ALL
- CD19 loss variants
- Microenvironment factors that limit proliferation and effect of CAR T cells
- Remission rates 70 - 80%
- Solid tumors
- Lack of suitable cell surface molecules for the CAR T cells to target
- Attempts to engineer T cells with T cell receptors capable of recognizing tumor specific antigens from intracellular proteins
- Modify tumor microenvironment to be more hospitable to CAR T cells
Adverse effects
- Toxicity
- On target effects: reversible when target cells are eliminated or CAR T cell engraftment is terminated
- B cell aplasia
- More severe than that caused by anti-CD20 monoclonal antibody rituximab
- Rapidly reversed after ablation of CAR T cells
- May require immunoglobulin therapy
- Cytokine release syndrome
- Initial flu-like presentation
- Fevers, hypotension, hypoxia, neurologic changes
- Can progress to capillary leak
- T cell activation and high levels of cytokines, IL6 and interferon-γ
- Neurotoxicity: CAR T related encephalopathy syndrome (Blood 2014;123:2625, Maitta: Immunologic Concepts in Transfusion Medicine, 1st Edition, 2019)
Clinical features
- Patient selection considerations may differ from autologous stem cell transplant (ASCT) and take into account previous therapies, upper age limit, severity of comorbidities and resistance to chemotherapy
- Some clinical trials may include transplant ineligible patients
- Other clinical trials are studying CAR T cell therapy as second-line treatment and for both transplant eligible and ineligible patients
- Other factors that may be considered in therapy include:
- Performance status
- Organ function
- T cell count
- B-acute lymphoblastic leukemia (ALL), target antigen is CD19
- CART-19: CAR T directed against CD19 antigen on B lymphoblasts
- Tisagenlecleucel, CTL019, also known as Kymriah® (Novartis, Basel, Switzerland)
- Axicabtagene ciloleucel, also known as Axi-cel, Yescarta® (Kite Pharma / Gilead, Los Angeles, CA)
- Diffuse large B cell lymphoma, target antigen is CD19, no specific age limit
- Tisagenlecleucel, CTL019, also known as Kymriah® (Novartis, Basel, Switzerland)
- Axicabtagene ciloleucel, also known as Axi-cel, Yescarta® (Kite Pharma / Gilead, Los Angeles, CA)
- Other diagnoses under investigation for possible CAR T therapies include:
- Hodgkin lymphoma (HL), target antigen is CD30
- Anaplastic large cell lymphoma, target antigen is CD30
- Myeloma, target antigens: SLAMF7, B cell maturation antigen (BCMA)
- Acute myeloid leukemia (AML), target antigens are CD123, CD33, Lewis Y and FOLR2
- Other potential costimulatory domains are PD1 / CD28 and CD200R / CD28
- T cell malignancies are a challenge since candidate target antigens are found on normal T cells
- CARs post transplant: maximize graft versus tumor effect while minimizing graft versus host disease (GVHD)
- CARs in donor leukocyte infusions (DLI): may help improve survival in relapses of hematologic malignancy
- CARS in virus specific T cells
- Chimeric autoantibody receptor T cells (CAARs): autoimmune disease such as pemphigus vulgaris (Immunol Rev 2015;263:68, Clin Cancer Res 2016;22:1875)
Laboratory
- Polymerase chain reaction (PCR) molecular assays are available for all CARs produced, however, such testing might not accurately reflect if the CAR is actually expressed on the cell surface and is generally performed retrospectively
- Flow cytometry may be a useful modality to monitor real time expansion and response
- CD19 CARs are known to expand rapidly, clear target tumor cells, then contract
- CARs have single chain variable fragments (scFv) for specificity against a target antigen and have little else on the cell surface
- There are three potential ways to detect the scFv using flow cytometry
- Develop an anti-idiotype antibody to the scFv
- Use Fc-conjugated soluble antigen such as Fc-CD22 that interacts with CD22 CAR T cells followed by a secondary detection antibody (anti-Fc)
- Use biotinylated protein L followed by a streptavidin-conjugated fluorophore
- There are benefits and deficits to each method and the process is labor intensive
- A well constructed assay provides meaningful information for individual patient care and a better understanding of CARs as a whole
- Additionally, there is need for standardized methods to profile memory phenotype of CAR Ts to evaluate quality and promote manufacturing improvements
- Use of a standardized memory T cell panel can help evaluate how T cell phenotype impact the efficacy and longevity of response in patients receiving CAR T therapies
- One attempt at this includes a dried memory T cell panel containing a pre-validated mixture of 7 antibodies for the identification of naïve, stem cell memory, central memory and effector memory CD4+ and CD8+ T cell subsets (BD Biosciences)
- One study used this pre-validated mix and additional drop in antibodies can complement the panel and enable more in depth evaluation of the T cell phenotype and monitor changes in expression of PD-1, TIM-3, LAG-3, HLA-DR, CD45RO and CXCR3 on T cells transduced to express a novel anti-CD37 CAR (Transfus Med Hemother 2019;46:15, Blood 2019;134:5626)
Case reports
- 18 month old girl and 52 year old woman treated with CAR T for B-ALL with mixed lineage leukemia gene (MLL) mutations developed AML clonally related to their B-ALL, suggesting CD19-negative immune escape (Blood 2016;127:2406)
- 20 year old man relapsing 9 months after CD19-targeted CAR T cell (CTL019) therapy (Nat Med 2018;24:1499)
- Mini review of control mechanisms for future CAR T products (Front Immunol 2020;11:326)
- Advancements in safety for new CAR T models (Mol Cancer 2019;18:125)
- Applications of CAR T for community oncology physicians (Oncologist 2016;21:608)
Treatment
- Cytokine release syndrome: treated with anti-IL6 monoclonal antibody, tocilizumab
Board review style question #1
- A 10 year old boy diagnosed with acute lymphoblastic leukemia with several relapses is on a clinical trial for CD19 CAR T therapy. He develops the rapid onset of flu-like symptoms, including fever, and progresses with mental status change and clinical concern for cytokine release syndrome. What would be an appropriate treatment for suspected cytokine release syndrome?
- Anti-CD20 (rituximab)
- Anti-CD38 (daratumumab)
- Anti-IL6 (tocilizumab)
- Azathioprine
- Interferon-γ
Board review style answer #1
C. Anti-IL6 (tocilizumab). Cytokine release syndrome is likely due to high levels of IL6 and Interferon-γ. Anti-IL6 (tocilizumab) has mainly been used in inflammatory conditions such as juvenile rheumatoid arthritis and has been effective in decreasing the IL6 levels involved in cytokine release syndrome.
Comment Here
Reference: CAR T cell therapy
Comment Here
Reference: CAR T cell therapy
Board review style question #2
- In which function of CAR T cells does the engineered T cell travel to the site of tumor cells targeted for destruction?
- Control
- Microenvironment
- Proliferation / persistence
- Recognition
- Trafficking
Board review style answer #2
E. Trafficking is the first function of a CAR T cell. The engineered cell must be able to travel to the site of the target tumor cells.
Comment Here
Reference: CAR T cell therapy
Comment Here
Reference: CAR T cell therapy