Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Clinical features | Transmission / incidence | Symptoms | Screening | Laboratory | Case reports | Treatment | Peripheral smear images | Sample assessment & plan | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Potochny EM, Virk MS, George MR. Hemolytic disease of the fetus and newborn. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/transfusionmedfetalneonatal.html. Accessed April 25th, 2024.
Definition / general
- Hemolytic disease of the fetus and newborn (HDFN) is a complication of maternal alloantibodies directed at fetal / neonatal red blood cell antigens
- Symptoms can range from mild to severe or fatal for the fetus / neonate
Essential features
- Caused by maternal alloantibodies against fetal red blood cells
- May lead to fetal anemia and potentially hydrops fetalis and death in utero
- May lead to neonatal anemia, hyperbilirubinemia and kernicterus in the neonate
- Requires prenatal screening
- If clinically significant maternal antibodies detected, requires additional testing or monitoring prenatally / perinatally
Terminology
- Hemolytic disease of the fetus (HDF)
- Hemolytic disease of the newborn (HDN)
Pathophysiology
- Maternal IgG alloantibody(ies) cross the placenta, destroy fetal red blood cells
- May lead to extramedullary hematopoiesis
- Can manifest as splenomegaly, hepatomegaly
- May lead to portal hypertension, decreased albumin, ascites and edema
- Culminating in hydrops fetalis
- Potentially fatal from cardiac failure
- If not severe and fetus survives to delivery, neonatal anemia may be present
- Neonatal hyperbilirubinemia results from inability to adequately conjugate elevated unconjugated bilirubin levels
- Unaddressed, may result in kernicterus (bilirubin deposition in brain stem)
- In utero, bilirubin is cleared from fetal bloodstream via placenta / maternal circulation, protecting against kernicterus
- After delivery, maternal protection is lost and high bilirubin can accumulate in newborn's bloodstream and brain
- Reference: Simon: Rossi's Principles of Transfusion Medicine, 5th Edition, 2016, AABB: Technical Manual, 19th Edition, 2017
Clinical features
- Hemolytic disease of the fetus
- Fetal anemia
- Extramedullary hematopoiesis
- Splenomegaly, hepatomegaly
- Portal hypertension
- Cardiac failure
- Hydrops fetalis
- Hemolytic disease of the newborn
- Neonatal anemia
- Hyperbilirubinemia
- Kernicterus
- Reference: Simon: Rossi's Principles of Transfusion Medicine, 5th Edition, 2016
Transmission / incidence
- Maternal IgG mediated alloantibodies can cross the placenta and enter fetal circulation
- IgM antibodies cannot cross placenta
- Includes IgG anti-A, B (in blood type O mothers) attacking fetal red cells that are blood type A or B
- 1 - 4% incidence based on ethnicity (AABB: Technical Manual, 19th Edition, 2017)
- Includes IgG anti-D
- Without Rh immune globulin (RhIG) prophylaxis, roughly 16% incidence
- With RhIG prophylaxis, decreases risk to ≤ 2% (AABB: Technical Manual, 19th Edition, 2017)
- IgG antibodies also occur against non-ABO antigens and non-D antigens, such as other Rh blood group antigens, Duffy, Kidd and Kell antigens, etc.
- Severity depends on how well developed target antigen is on fetal red blood cells
- More than one antibody may occur (e.g. anti D and anti C)
Symptoms
- Discussed under Clinical features
Screening
- Type and screen of mother at prenatal examination
- Obtain serial titers of an alloantibody in the mother associated with HDFN
- Invasive cordocentesis / amniocentesis may be considered to assess fetal anemia via amniotic bilirubin levels
- Carries risk of fetal maternal hemorrhage
- Carries risk of spontaneous abortion
- Consider paternal zygosity testing to determine likelihood the fetus inherited the antigen from the father
- Cell free fetal DNA testing of maternal plasma may be used in some circumstances to determine antigen status of fetal cells
- Ultrasound Doppler studies of the middle cerebral artery (MCA) of the fetus
- MCA Doppler studies may be used to surveil for fetal anemia noninvasively
- Enhanced peak systolic velocity > 1.50 multiples of median (MoM) identifies moderate to severe fetal anemia
- Reference: Cunningham: Williams Obstetrics, 25th Edition, 2018
- To prevent HDFN from anti-D alloimmunization in Rh(D) negative of partial D phenotype women
- Give Rh immune globulin (RhIG) typically at 28 weeks gestational age (U.S. practice)
- Give RhIG within 72 hours of delivery if the neonate is Rh(D) positive
- Perform rosette test (or other fetal blood screening test) to determine if significant fetal maternal hemorrhage may have occurred
- Demonstrates presence of D positive cells in a D negative suspension using anti-D reagent
- Anti-D binds to D positive fetal RBCs → indicator D positive RBCs are added → rosettes are formed (see Peripheral smear images)
- If positive, perform quantification test to dose additional vials of RhIG
- Quantify the percent of fetal RBCs in maternal blood by flow cytometry or Kleihauer-Betke testing (see details in Laboratory)
- Perinatal fetomaternal hemorrhage (FMH)
- RhIG can be administered for any event associated with FMH during the perinatal period
- Events associated with perinatal FMH: abortion, ectopic pregnancy, amniocentesis, chorionic villus sampling, external cephalic version, abdominal trauma
- Reference: AABB: Technical Manual, 19th Edition, 2017
Laboratory
- ABO mediated hemolytic disease of the fetus and newborn
- Antibody screen may be negative
- Mother's blood group is generally type O
- Fetus / neonate's blood group is A or B
- Non-ABO mediated hemolytic disease of the fetus and newborn
- Prenatally, the mother's type and screen may reveal a known or new antibody
- Serial titers are often performed during pregnancy
- Clinically significant antibodies other than anti-K are critical ≥ 16
- Anti-K is significant at lower titers; 8 or even lower
- K is present on red cell and platelet precursors
- Anti-K causes suppression of erythropoiesis and even thrombopoiesis in addition to hemolysis
- Once a critical titer is reached, ultrasound Doppler studies are performed on the fetus' middle cerebral artery
- > 1.5 MoM (multiples of the median) may require intrauterine transfusion or delivery
- Positive direct antiglobulin test (DAT) on newborn blood may indicate HDFN
- DAT usually positive for IgG if the mother has non-ABO antibody
- Elution would be consistent with the mother's known antibody
- DAT may be positive for complement (C3d or C3b) if ABO related HDFN
- DAT usually positive for IgG if the mother has non-ABO antibody
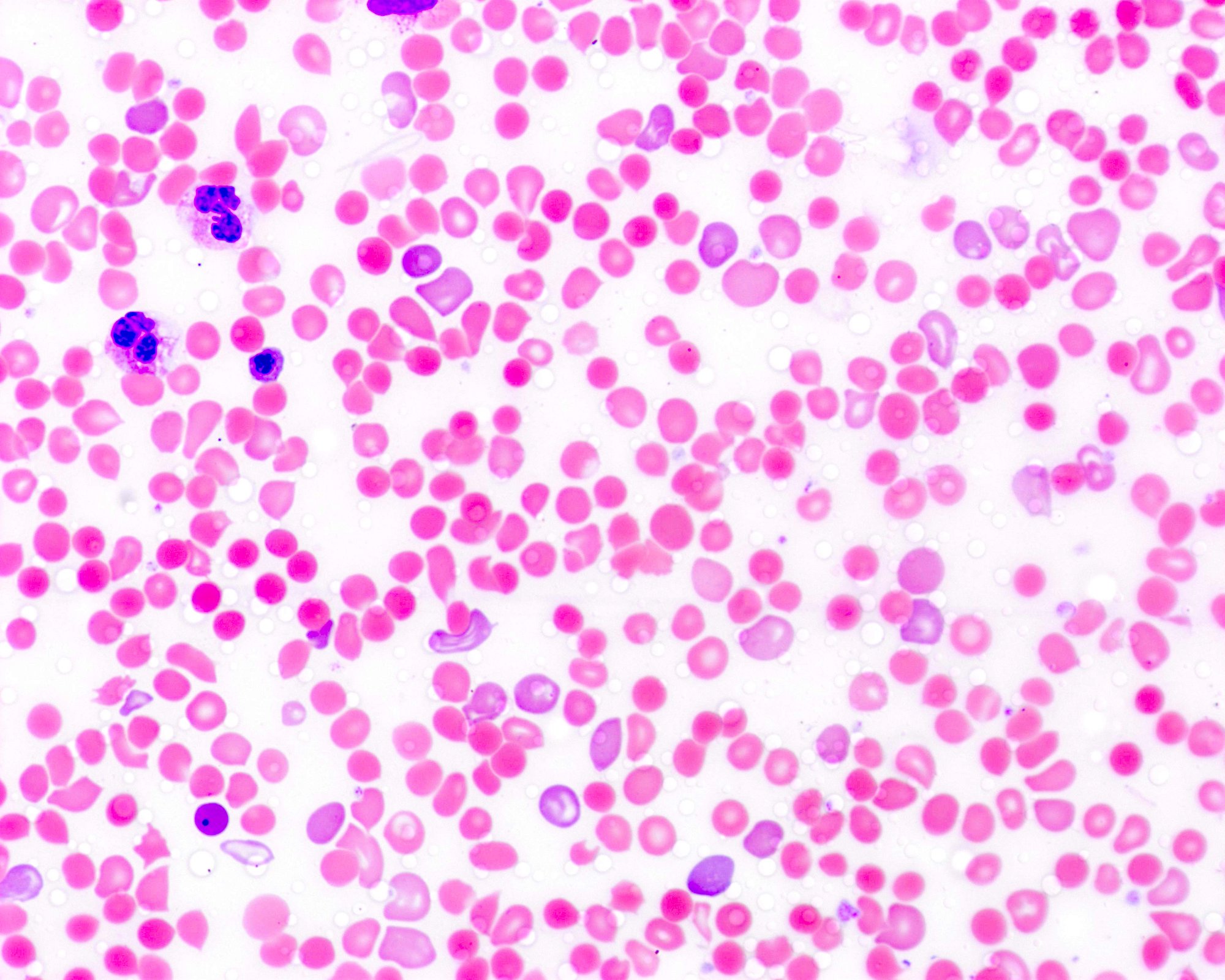
- A peripheral blood smear may show findings of increased spherocytes, polychromasia, and sometimes normoblastemia (nucleated RBCs)
- For a peripheral blood smear from a 38 week gestation group A positive baby with mild HDFN born to a mother who was group O positive (see Peripheral smear images)
- Rosette screening test
- Screening test for FMH that detects fetal RhD positive red cells in maternal RhD negative blood (Transfusion 1991;31:303)
- Anti-D IgG added to maternal RBC suspension, which will bind to any fetal RhD positive RBCs, if present
- Indicator cells added that will bind to antibody coated fetal RBCs and form rosettes visible on microscopy
- A manufacturer specified number of rosettes (e.g., 3 or more in 10 fields) designates a positive result and suggests a significant FMH (> 30 mL), requiring quantification
- Acid elution quantification
- Commonly referred to as the Kleihauer-Betke test
- Based on the principle that red cells containing fetal hemoglobin (HbF) are less susceptible to acid elution than cells containing adult hemoglobin (HbA)
- Maternal peripheral smear is acid treated, washed and stained
- Pink stained fetal RBCs counted as a percentage of maternal RBCs, which will appear as ghost cells due to elution of hemoglobin
- Fetal RBC % used to calculate volume of hemorrhage and calculate dose of RhIG
- Reference: Cunningham: Williams Obstetrics, 25th Edition, 2018
Case reports
- 32 year old woman with HDFN from anti-D, anti-G with passive anti-D in breast milk (Transfusion 2017;57:2121)
- 41 year old woman with HDFN due to partial D (Transfusion 2018;58:2260)
- 45 year old type O woman with ABO HDFN in type AB twins (Transfusion 2010;50:2102)
- 4 women with HDFN due to Jk3 alloantibodies (Transfusion 2018;58:1157)
- 5 women with HDFN requiring intrauterine transfusion, therapeutic apheresis and IVIG (Transfusion 2018;58:677)
Treatment
- Prenatal treatments for hemolytic disease of the fetus include:
- Intrauterine transfusion
- Therapeutic plasma exchange
- Intravenous immune globulin (IVIG)
- Postnatal treatments for hemolytic disease of the neonate include:
- Phototherapy (ultraviolet light to convert serum unconjugated bilirubin to the water soluble form)
- Double volume exchange transfusion
- Reference: Simon: Rossi's Principles of Transfusion Medicine, 5th Edition, 2016
Peripheral smear images
Sample assessment & plan
- Assessment: Jane Doe is a 26 year old G2P1 woman with O positive blood type who presented for prenatal intake examination. A type and screen identified an anti-E alloantibody. The current titer is 2. Anti-E is capable of causing hemolytic disease of the fetus / newborn and hemolytic transfusion reactions.
- Plan:
- If red cell transfusion is required, O, Rh(D) positive or negative, E antigen negative, full crossmatch compatible units that are irradiated and leukocyte reduced will be issued.
- Recommend maternal-fetal medicine consultation.
- Zygosity testing of the father can be obtained by antigen typing the father for E, e antigens to determine inheritance risk for fetus.
- If father carries E antigen, consider monitoring the antibody via serial titrations.
- A fourfold increase is a clinically significant rise in titer. An absolute titer of ≥ 16 is considered a critical titer. If reached, recommend following with ultrasound middle cerebral artery Doppler flow velocity studies.
Differential diagnosis
- Infection / sepsis:
- White blood cell count may be elevated
- Pathogen specific testing may confirm
- Red blood cell enzymopathies:
- May be identified during newborn screening
- Peripheral blood smear may reveal red cell morphologic abnormalities
- Glucose-6-phosphate-dehydrogenase (G6PD) deficiency most common
- Thalassemia:
- Hemoglobin electrophoresis may identify
- Iron studies may be needed to exclude iron deficiency anemia
- Genetic studies can confirm
- Hemoglobinopathy:
- Hemoglobin electrophoresis to confirm
- Physiologic jaundice:
- Maternal antibody screen negative
- Direct antiglobulin test (DAT) negative
- Neonatal antibody screen (if performed) negative
- More likely if above are true and ABO related HDFN unlikely (i.e., maternal blood type not O and neonate blood type not A or B)
- Breast milk jaundice:
- Exact etiology unknown
- Consider when:
- Breastfeeding exclusively
- Nursing not going well or newborn weight loss > 8 - 10%
- Largely diagnosis of exclusion
- References: Am Fam Physician 2018;98:354, Semin Fetal Neonatal Med 2010;15:129
Board review style question #1
A 25 year old G2P1 woman has an anti-E alloantibody detected on her prenatal examination. During a serial titration with the current pregnancy, the titer rose from 8 to 64 one month later. Fetal middle cerebral artery (MCA) Doppler flow studies were initiated. What is true about these studies?
- Decreased flow rates may be indicative of fetal hyperbilirubinemia
- Increased flow rates may be indicative of fetal anemia
- It is an invasive technique that increases alloimmunization risk to the mother
- It is most useful for monitoring ABO mediated hemolytic disease of the fetus
Board review style answer #1
B. Increased flow rates suggest fetal anemia. Hemolytic disease of the newborn (but not fetus) is associated with hyperbilirubinemia, since the fetus has assistance of the mother to conjugate and excrete any indirect bilirubin. It is a noninvasive technique; therefore, it does not further increase risk of bleeding and alloimmunization for the mother. It is most useful for non-ABO alloantibodies, as the ABO type of the fetus is generally unknown until birth and ABO mediated hemolytic disease of the newborn is usually mild (full expression of ABO antigens occurs at 2 - 4 years old).
Comment Here
Reference: Hemolytic disease of the fetus and newborn
Comment Here
Reference: Hemolytic disease of the fetus and newborn
Board review style question #2
A 30 year old woman with A negative blood type gives birth to a healthy male neonate who is blood type O positive. The mother received Rh immune globulin (RhIG) at 28 weeks estimated gestational age. What is proper management regarding RhIG administration and prevention of anti-D alloimmunization to the mother?
- 1 vial of 300 μg RhIG should be administered and a fetal blood screen should be performed on the mother
- 1 vial of 300 μg RhIG should be administered; no additional testing required
- RhIG is not indicated because it was given at 28 weeks
- RhIG is not indicated because the infant is Rh positive
Board review style answer #2
A. 1 vial of 300 μg RhIG should be given and a fetal blood screen / rosette test should be performed. If positive, a quantitative method for fetal to maternal hemorrhage, such as flow cytometry or Kleihauer-Betke (semiquantitative) study should be done to determine whether more than 1 vial of RhIG is required. The half life of RhIG is approximately 3 weeks, so dosing after delivery (when the infant is Rh positive) would be required even if given at 28 weeks estimated gestational age. RhIG is required following delivery when the mother is either Rh negative or a partial D phenotype and capable of forming an anti-D alloantibody and the neonate is Rh positive.
Comment Here
Reference: Hemolytic disease of the fetus and newborn
Comment Here
Reference: Hemolytic disease of the fetus and newborn