Table of Contents
Babesiosis | Malaria | Trypanosoma cruzi | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2 | Board review style question #3 | Board review style answer #3 | Board review style question #4 | Board review style answer #4 | Board review style question #5 | Board review style answer #5 | Board review style question #6 | Board review style answer #6Cite this page: Marrero-Rivera G, Bakhtary S. Parasites. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/transfusionmedparasites.html. Accessed April 20th, 2024.
Babesiosis
Definition / general
Essential features
Terminology
Pathophysiology
Clinical features
Transmission
Symptoms
Screening
Blood donor screening
Blood donor testing
Donor deferral
Diagnosis
Laboratory
Case reports
Treatment
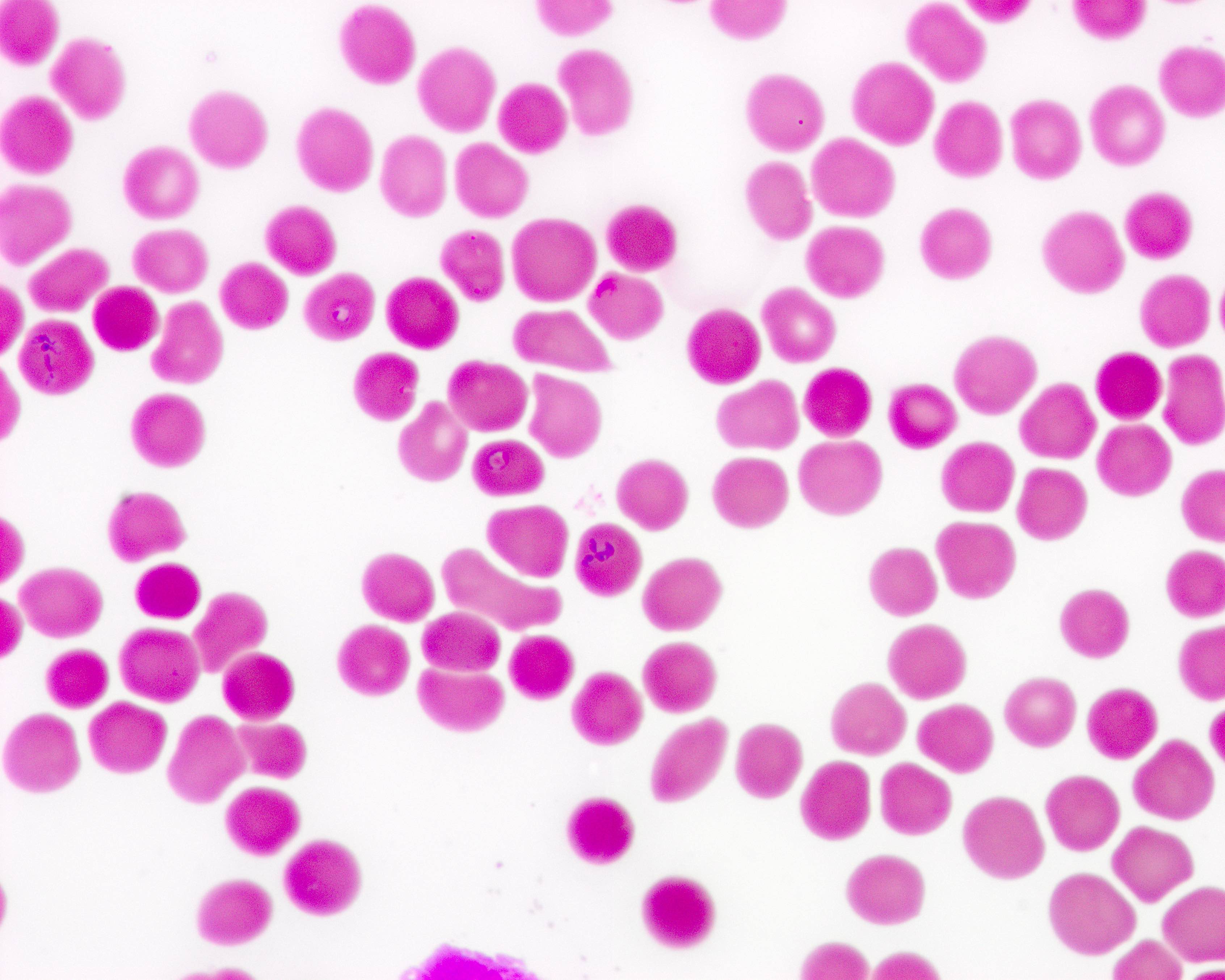
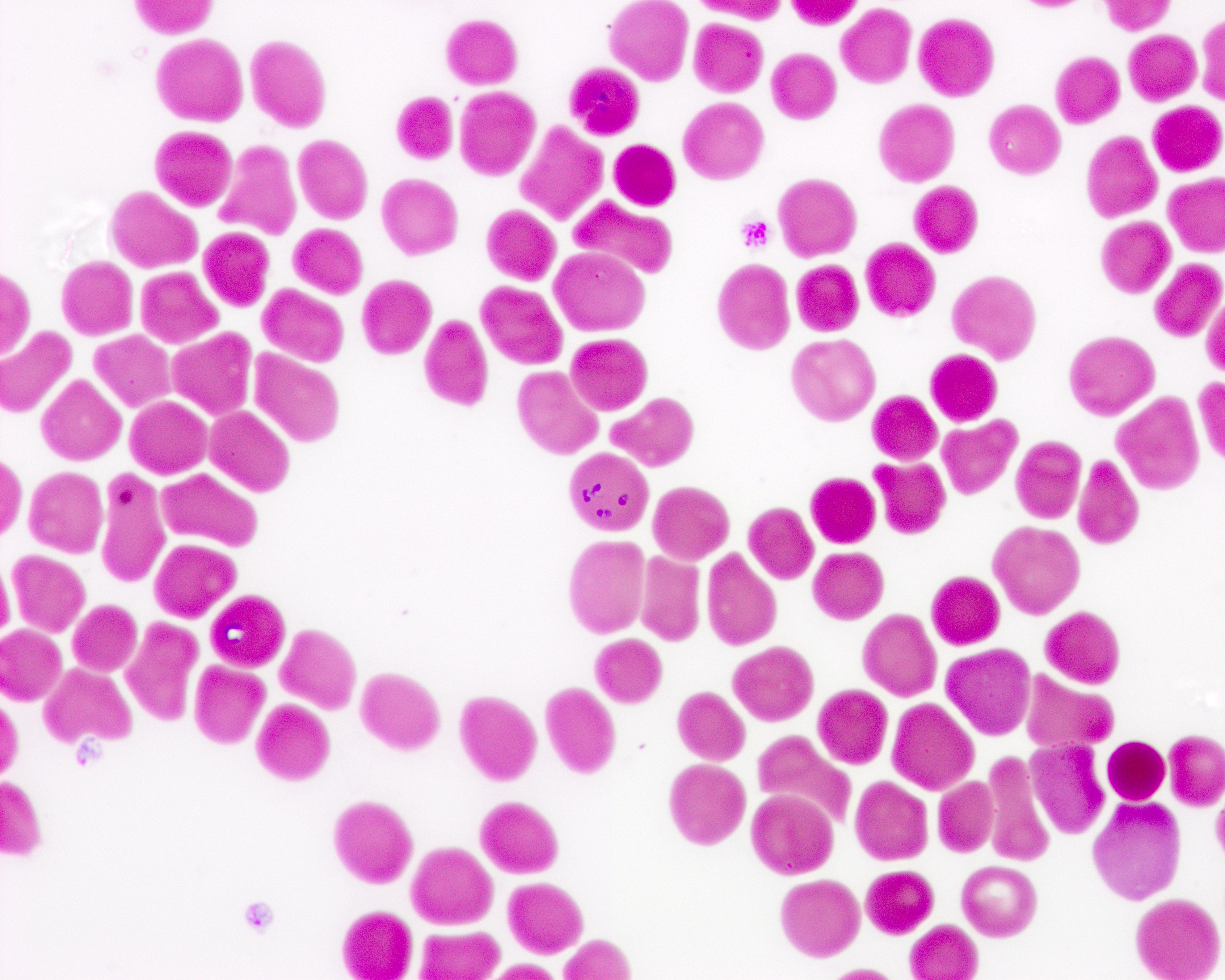
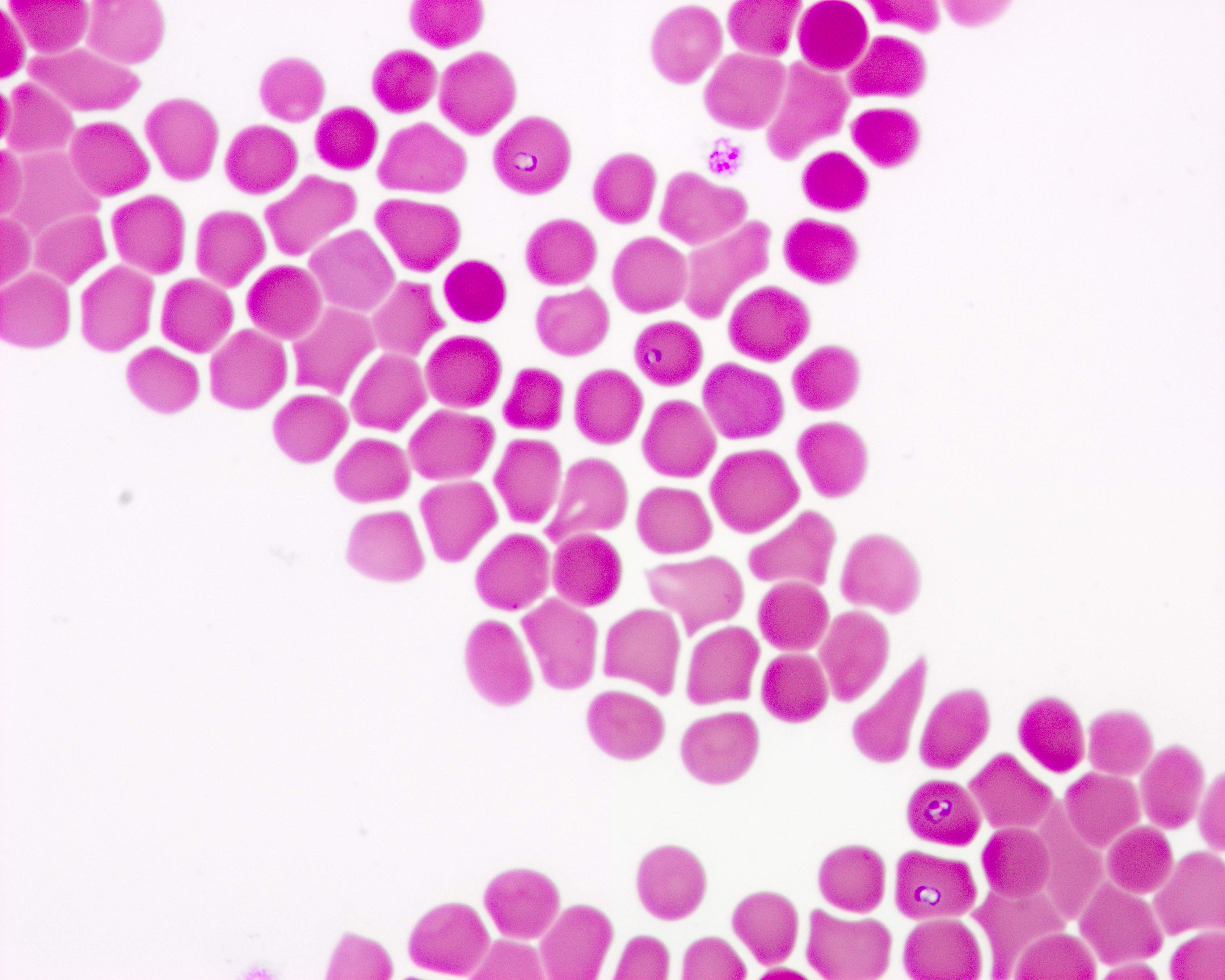
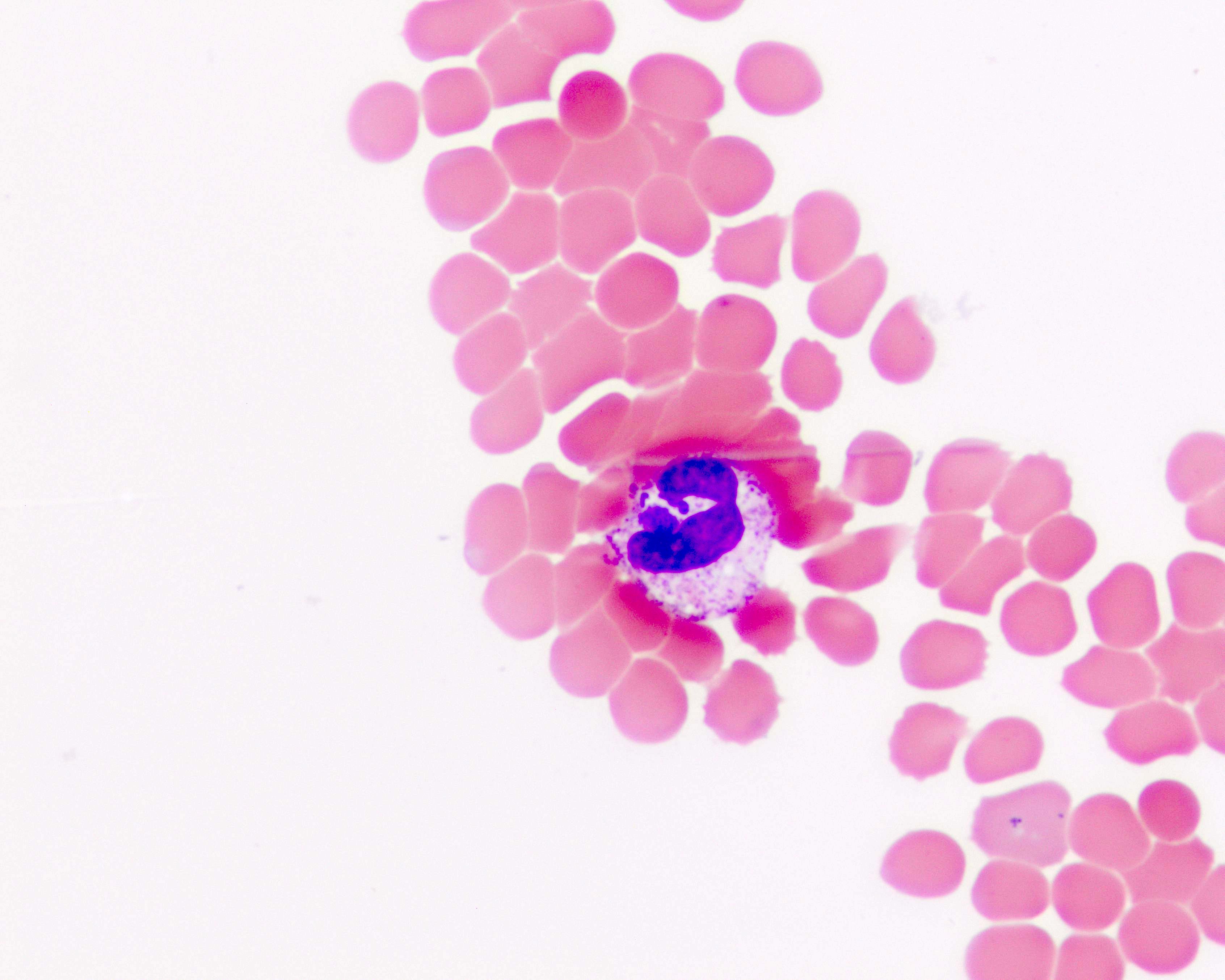
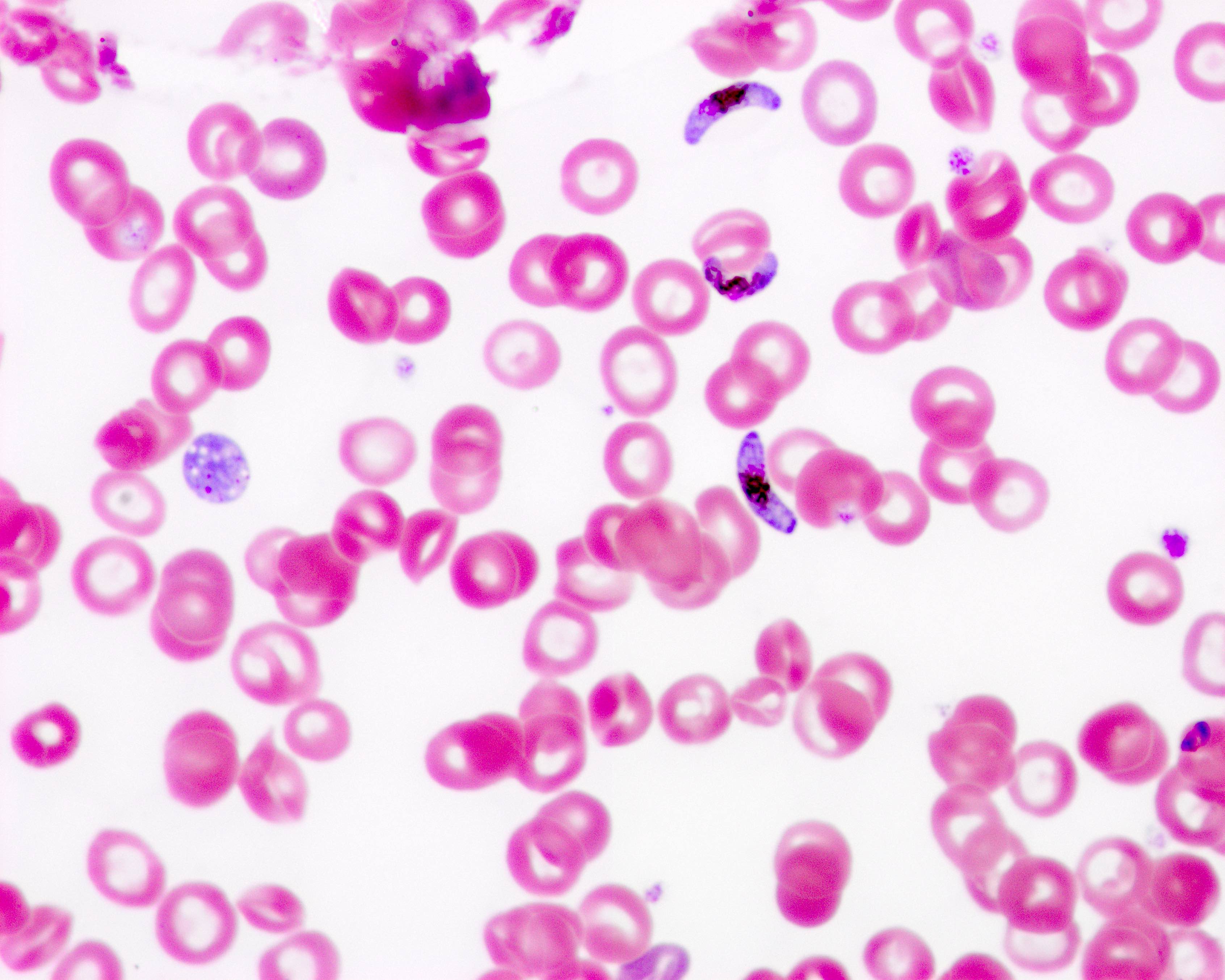
Peripheral smear images
Contributed by Melissa R. George, D.O.
Sample assessment & plan
Differential diagnosis
- Babesiosis is a disease caused by parasites that infect red blood cells (RBCs)
- Most human cases of Babesia infection in the United States are caused by the parasite Babesia microti, which is spread by ticks, primarily Ixodes scapularis ticks (MMWR Surveill Summ 2019;68:1)
Essential features
- The vast majority of babesiosis cases in the United States are caused by Babesia microti, the species that is prevalent in the Northeast and upper Midwest of the U.S.
- Tickborne transmission usually peaks during warm months (N Engl J Med 2012;366:2397)
- Babesiosis is the most frequently reported tickborne pathogen disease transmitted by blood transfusion in the U.S. (Transfus Med Rev 2002;16:131)
- Babesia infection can range from asymptomatic to life threatening
- Risk factors for severe babesiosis include asplenia, advanced age (age > 50) and impaired immune function
- In 2019, the FDA licensed tests utilized for donor screening for detection of Babesia species and recommended year round regional testing for blood donations in areas endemic for babesiosis (FDA: Recommendations for Reducing the Risk of Transfusion-Transmitted Babesiosis [Accessed 22 December 2022])
Terminology
- See also: Babesia
Pathophysiology
- Nymphal stage of the Ixodes is the primary vector and requires attachment to a host for at least 36 to 72 hours to complete transmission of sporozoite forms
- These sporozoites attach and enter erythrocytes where they mature and divide to form merozoites; merozoites then rupture the host erythrocyte and continue infecting other erythrocytes, repeating the same cycle
- The spleen is essential in the host's ability to control this infection
- Erythrocytes infected with Babesia are recognized as abnormal as they pass through the spleen and are targeted for destruction by macrophages
- People with a history of splenectomy are at high risk for severe infection with high level parasitemia (Front Microbiol 2021;12:697669)
Clinical features
- Incubation period: 1 - 4 weeks following tick bite; 1 - 9 weeks after contaminated blood transfusion (up to 24 weeks)
- Asymptomatic infection has been reported in up to 20% of adults and 50% of children (StatPearls: Babesiosis [Accessed 22 December 2022])
- Symptomatic patients present with fever, general malaise, headache, hemolysis and rarely, renal failure and coagulopathy
Transmission
- Tickborne transmission babesiosis: 1 - 4 weeks following tick bite
- Transfusion transmitted babesiosis: 1 - 9 weeks after contaminated blood transfusion
- High risk patients include immunocompromised, elderly and asplenic patients (Transfusion 2000;40:285)
Symptoms
- Fever, chills, sweats
- Malaise, fatigue
- Myalgia, arthralgia, headache
- Gastrointestinal (GI) symptoms, such as anorexia and nausea (less common: abdominal pain, vomiting)
- Dark urine
- Less common: dry cough, sore throat, photophobia, conjunctival injection
- Mild splenomegaly, mild hepatomegaly or jaundice may occur in some patients
- Severe cases can be associated with marked thrombocytopenia, disseminated intravascular coagulation, hemodynamic instability, acute respiratory distress, renal failure, hepatic compromise, altered mental status and death
- Reference: MMWR Morb Mortal Wkly Rep 2012;61:505
Screening
- Screening for babesiosis is performed by donor questionnaire or by nucleic acid testing
- Babesia microti parasitemia (DNA) has been detected for > 1 year in immunocompetent patients treated with a standard course of antibiotic therapy and > 2 years in untreated individuals (N Engl J Med 2016;375:2236)
- Immunocompromised patients can experience persistent B. microti parasitemia and relapsing symptoms for > 2 years despite antimicrobial therapy
Blood donor screening
- Donor history questionnaire asking prospective donors if they have ever had a positive test result for Babesia, obtained from either a medical diagnosis or a reactive donor screening test
Blood donor testing
- Blood donor centers must test each donation for evidence of Babesia using a licensed nucleic acid testing (NAT) when collected in Connecticut, Delaware, Maine, Maryland, Massachusetts, Minnesota, New Hampshire, New Jersey, New York, Pennsylvania, Rhode Island, Vermont, Virginia, Wisconsin and Washington, D.C.
- Testing must be performed year round and in accordance with the instructions for use of the device (FDA: Recommendations for Reducing the Risk of Transfusion-Transmitted Babesiosis [Accessed 22 December 2022])
Donor deferral
- Blood donor centers must defer donors with a reactive nucleic acid test (NAT) result for Babesia for at least 2 years from the date of the reactive donation
- Blood donor centers must defer donors who report a history of a positive test result for Babesia, obtained from either a medical diagnosis or a reactive donor screening test result for 2 years
- Reference: FDA: Recommendations for Reducing the Risk of Transfusion-Transmitted Babesiosis [Accessed 22 December 2022]
Diagnosis
- Identification of intraerythrocytic Babesia parasites by light microscopic examination of a peripheral blood smear
- Ring forms are most commonly seen and can have multiple rings per cell
- Tetrad formations, also known as Maltese cross, are occasionally seen
- Positive Babesia (or B. microti) polymerase chain reaction (PCR) analysis
- Demonstration of a Babesia specific antibody titer by indirect fluorescent antibody (IFA) testing for total immunoglobulin (Ig) or IgG
- Reference: Clin Infect Dis 2021;72:e49
Laboratory
- Hemolytic anemia with decreased haptoglobin, elevated lactate dehydrogenase (LDH) values and reticulocytosis
- Thrombocytopenia
- Elevated creatinine and blood urea nitrogen (BUN) values
- Mildly elevated hepatic transaminase values
- Proteinuria
- Reference: CDC: Surveillance for Babesiosis [Accessed 25 January 2023]
Case reports
- Premature 1 month old infant with WA1 type Babesia (Transfusion 2002;42:1482)
- 40 year old man with fever, jaundice, greenish brown urine, loss of appetite and general malaise (Transfus Apher Sci 2020;59:102902)
- 45 year old man asymptomatic donor with multiple recipients (Transfusion 2002;42:1154)
- 46 year old healthy man with fever, myalgias and left sided abdominal pain radiating to the shoulder (J Gen Intern Med 2021;36:3869)
- 54 year old spleen intact man with transfusion associated Babesia microti infection after a heart transplant (Emerg Infect Dis 2003;9:116)
Treatment
- Treatment is indicated in symptomatic cases or in asymptomatic patients who have a positive blood smear or PCR for > 3 months
- Patients are usually treated for at least 7 - 10 days with a combination of medications, typically either clindamycin plus quinine or atovaquone plus azithromycin (Clin Infect Dis 2021;72:e49)
Peripheral smear images
Contributed by Melissa R. George, D.O.
Sample assessment & plan
- Assessment: Jane Doe is a 68 year old woman with a history of splenectomy who presented 14 days after packed red blood cell transfusion with fever, fatigue and abdominal pain. The patient's labs show anemia and impaired kidney function. Peripheral smear shows ring forms and some tetrad formations.
- Plan: The patient presentation and laboratory workup are consistent with a diagnosis of transfusion transmitted babesiosis.
- Recommend starting clindamycin and quinine for 7 - 10 days
- If patient's clinical status deteriorates, red blood cell exchange may be considered
- Partial or complete red blood cell exchange transfusion is indicated in patients presenting with a parasitemia of at least 10% and anemia with hemoglobin of < 10 g/dL
Differential diagnosis
- Lyme disease:
- Fever, headache, fatigue and a characteristic skin rash called erythema migrans
- Malaria:
- Fever and flu-like illness, including shaking chills, headache, muscle aches and fatigue
- Colorado tick fever:
- Biphasic fever, chills, headache, body aches and fatigue
- Anaplasmosis:
- Fever, chills, severe headache, muscle aches, nausea, vomiting, diarrhea, loss of appetite
Malaria
Definition / general
Essential features
Terminology
Pathophysiology
Clinical features
Transmission
Symptoms
Screening
Blood donor screening
Blood donor testing
Donor deferral
Laboratory
Case reports
Treatment
Peripheral smear images
Contributed by Melissa R. George, D.O.
Sample assessment & plan
Differential diagnosis
Additional references
- Malaria is a mosquito borne disease caused by a parasite
- There are 5 Plasmodium species that cause malaria in humans: P. falciparum, P. malariae, P. ovale, P. vivax, P. knowlesi (Malar J 2018;17:36)
- Common transfusion transmitted infection worldwide
Essential features
- In the U.S., there are 1,300 new cases of malaria each year but only 1 - 2 cases are due to transfusion transmission
- Risk of transfusion transmitted disease is estimated to be 0.25 cases per million transfusions and usually results from transmission of P. falciparum
- Symptoms can occur a week to several months after transfusion
- Rarely fatal
- P. vivax and P. ovale have a dormant stage that can persist in the liver (if untreated) and cause relapses by invading the bloodstream weeks or even years later
Terminology
- See also: Plasmodium falciparum
Pathophysiology
- Natural history of malaria involves cyclical infection of humans and female Anopheles mosquitoes
- In humans, the parasites grow and multiply first in the liver cells and then in the RBCs
- In the blood, successive broods of parasites grow inside the red cells and destroy them, releasing daughter parasites (merozoites) that continue the cycle by invading other RBCs (MMWR Surveill Summ 2019;68:1)
Clinical features
- Fever, chills, sweats, headaches, muscle pains, nausea and vomiting (Baron: Medical Microbiology, 4th Edition, 1996)
Transmission
- Mosquito borne malaria
- Airport malaria: malaria caused by infected mosquitoes that are transported rapidly by aircraft from a malaria endemic country to a nonendemic country
- Congenital malaria: infected mothers transmit parasites to their child during pregnancy before or during delivery
- Transfusion transmitted malaria
- Reference: WHO: Malaria [Accessed 25 January 2023]
Symptoms
- People with malaria often experience nonspecific symptoms such as fever, chills, muscle ache and flu-like illness
- In severe malaria (primarily caused by Plasmodium falciparum), clinical findings (confusion, coma, neurologic focal signs, severe anemia, respiratory difficulties) are more striking and may increase the index of suspicion for malaria
Screening
- Incubation period in most cases varies from 7 to 30 days
- Shorter incubation periods are observed most frequently with P. falciparum and the longer ones with P. malariae
- Prevalence in donors (in endemic regions):
- Brazil: 1 - 3% (Rev Inst Med Trop Sao Paulo 2007;49:1)
- Kenya: 9% (East Afr Med J 2005;82:565)
- Nigeria: 10% (Trop Doct 2007;37:32)
- Countries with high donor infection rates may process donor blood with quinine or sulfadoxine pyrimethamine to kill parasites (Saudi Med J 2006;27:986, Am J Trop Med Hyg 2005;73:1119)
Blood donor screening
- Donor history questionnaire asking prospective donors if they have ever had malaria or have been in malaria endemic areas
Blood donor testing
- In the U.S., there is no FDA licensed test to detect the presence of malaria parasites in blood donors
- FDA strategy for reducing the risk of transfusion transmitted malaria (TTM) is to defer potential blood donors based on their travel or residence history in malaria endemic countries
Donor deferral
- A resident of a country nonendemic for malaria who has traveled to a malaria endemic area may be accepted as a donor 3 months after their return to the nonendemic country (irrespective of the use of chemoprophylaxis) if they have been free from malaria symptoms
- An immigrant or visitor from a malaria endemic country may be accepted as a donor 3 years after departure from the malaria endemic area if they have been free from malaria
- Former residents of malaria endemic countries who have lived in the United States for < 3 consecutive years and who return to visit a malaria endemic area may be accepted as donors 3 years after their most recent visit if they have been free from malaria
- Former residents of malaria endemic countries who have lived in the United States for ≥ 3 consecutive years and who return to visit a malaria endemic area may be accepted as donors 3 months after their most recent visit if they have been free from malaria
- Persons who have had a diagnosis of malaria should be deferred for 3 years after becoming asymptomatic
- References: CDC: Malaria - Blood Donor Screening [Accessed 25 January 2023], FDA: Recommendations to Reduce the Risk of Transfusion-Transmitted Malaria [Accessed 25 January 2023]
Laboratory
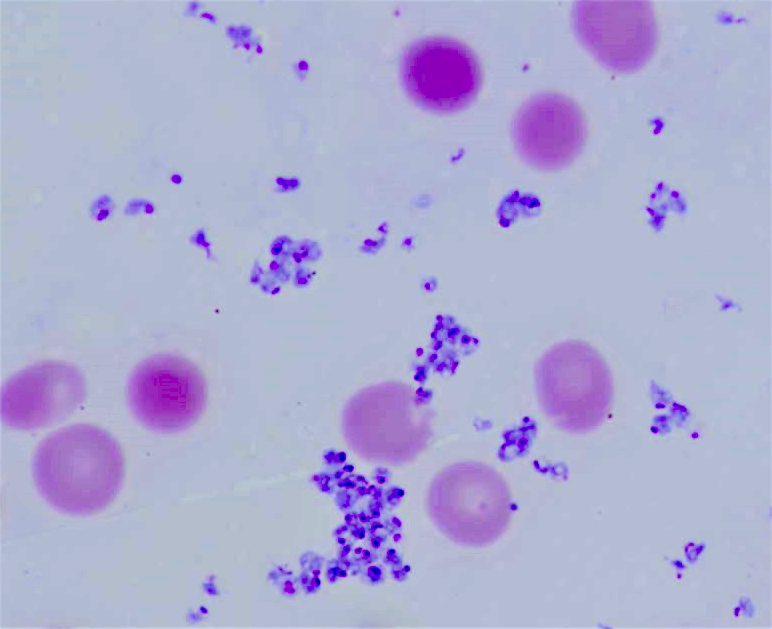
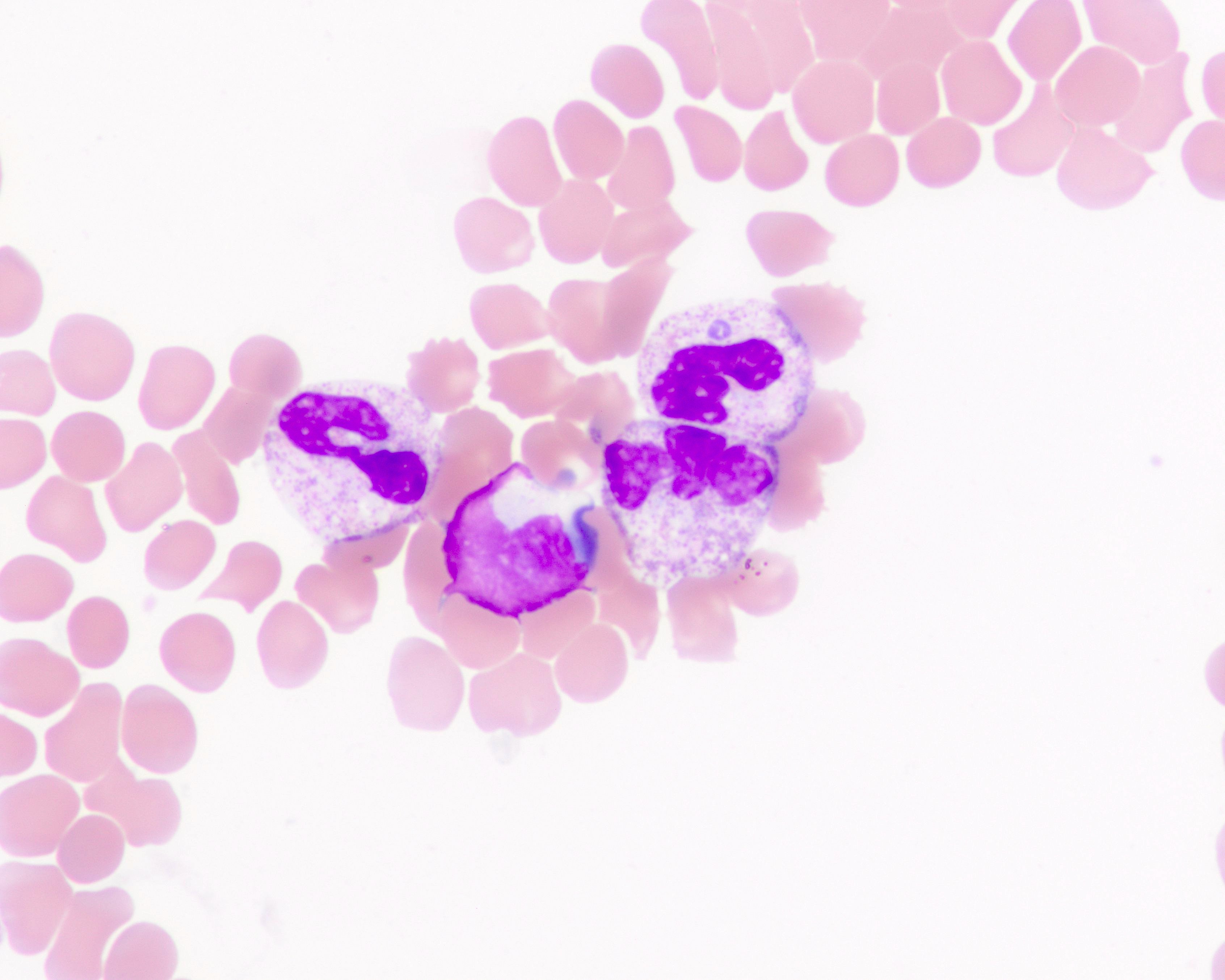
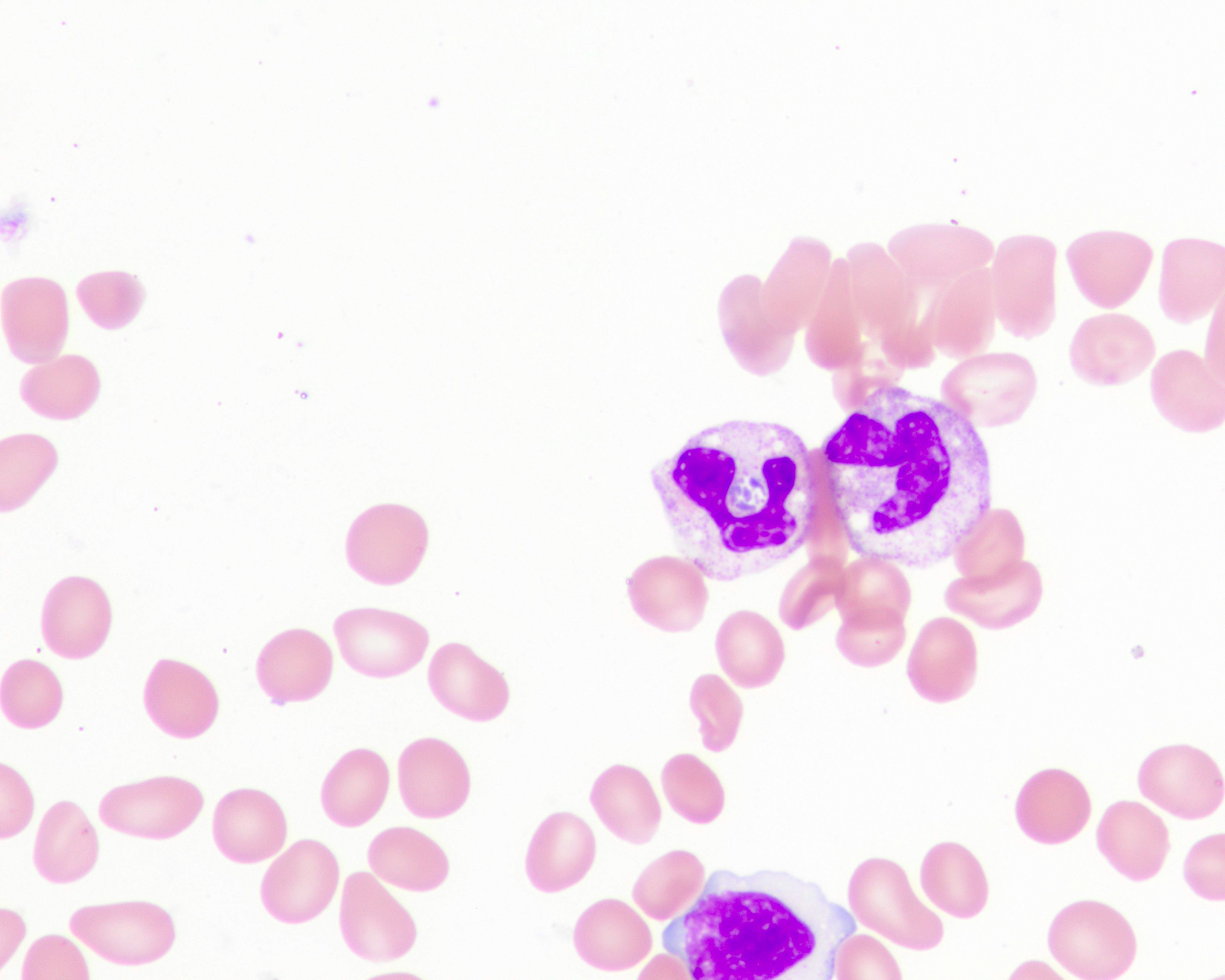
- Blood smear remains the gold standard for laboratory confirmation of malaria with parasites seen within RBCs
- Antigen detection test was approved by FDA in 2007 to detect antigens derived from malaria parasites
- PCR: parasite nucleic acids may be detected; this is most useful for confirming the species of malarial parasite after the diagnosis has been established by either smear microscopy or antigen detection test
- Serology does not detect current infection but rather measures past exposure
- Reference: CDC: Malaria - Disease [Accessed 25 January 2023]
Case reports
- Patients between 2 days and 85 years old with transfusion transmitted malaria (N Engl J Med 2001;344:1973)
- 47 year old woman with striking fever (Mikrobiyol Bul 2005;39:101)
- 69 year old man in Texas with blood donor from Ghana (MMWR Morb Mortal Wkly Rep 2003;52:1075)
- 70 year old man with donor from Cameroon (Swiss Med Wkly 2001;131:320)
Treatment
- Depends on many factors including disease severity, the species of malaria parasite causing the infection and the part of the world in which the infection was acquired
Peripheral smear images
Contributed by Melissa R. George, D.O.
Sample assessment & plan
- Assessment: Joe Doe is a 60 year old man who presented with fatigue, pallor and headaches. Patient has a history of traveling all around the world in the last 6 months. Lab peripheral blood smear shows a parasite inside a red blood cell. Malaria is confirmed with an antigen detection test.
- Plan: The patient presentation and laboratory workup are consistent with a diagnosis of malaria.
- Recommend starting antimalarial therapy
- Patient will be deferred from blood donation for 3 years
Differential diagnosis
- Typhoid fever:
- Weakness, stomach pain, headache, diarrhea or constipation, cough and loss of appetite
- Influenza:
- Fever, cough, sore throat, congestion, headaches, body aches and fatigue
- Dengue fever:
- Fever, rash, eye pain, muscle pain, headache, nausea or vomiting
- Babesiosis:
- Fever, chills, dark urine, arthralgia, general malaise, loss of appetite
Additional references
Trypanosoma cruzi
Definition / general
Essential features
Terminology
Pathophysiology
Clinical features
Transmission
Symptoms
Screening
Blood donor screening
Blood donor testing
Donor deferral
Laboratory
Case reports
Treatment
Peripheral smear images
Contributed by Melissa R. George, D.O.
Sample assessment & plan
Differential diagnosis
Additional references
- ~6 - 7 million people worldwide, mostly in Latin America, are estimated to be infected with Trypanosoma cruzi (T. cruzi), the parasite that causes Chagas disease
- Trypanosoma cruzi infection is curable if treatment is initiated soon after infection
- Blood screening is vital to prevent infection through transfusion and organ transplantation all over the world (WHO: Chagas Disease (American Trypanosomiasis) [Accessed 23 December 2022])
Essential features
- Triatoma infestans, Rhodnius prolixus and Triatoma dimidiata are the 3 most important insect vector in the transmission of T. cruzi to humans (WHO: Control of Chagas Disease - Second Report of the WHO Expert Committee [Accessed 23 December 2022])
- A large proportion of patients exhibit an acute phase characterized by flu-like symptoms followed by an asymptomatic phase
- Infected individuals may remain asymptomatic for years but they may have circulating parasites that are potentially transferable to an eventual recipient of a transfusion (Hematol Transfus Cell Ther 2019;41:262)
- FDA licensed a screening test for antibodies to T. cruzi in blood donors in 2006, which blood collection centers are currently using in the U.S.
- Photoinactivation may be useful for red cell suspensions, platelets or plasma (Transfus Apher Sci 2007;37:23, Transfusion 2007;47:434, Vox Sang 2006;91:285)
Terminology
- See also: Chagas disease (trypanosomiasis)
Pathophysiology
- In Latin America, T. cruzi parasites are mainly transmitted by contact with feces / urine of infected blood sucking triatomine bugs
- After the parasite enters through an open wound or mucous membrane, the infectious trypomastigote is found in the plasma
- There is a predilection for the myocardium or myenteric plexus of the GI tract, where the parasite replicates by binary fission (StatPearls: Chagas Disease [Accessed 23 December 2022])
Clinical features
- Acute phase: flu-like symptoms, unilateral palpebral edema (Romana sign) or local swelling at the inoculation site (chagoma)
- Chronic phase: megacolon, megaesophagus and cardiomyopathy
- 30 - 40% of infected patients will develop cardiomyopathy or digestive mega syndromes such as megacolon or megaesophagus
- Reference: MMWR Morb Mortal Wkly Rep 2002;51:210
Transmission
- T. cruzi is transmitted by the triatomine bug (vector borne), as well as orally (food borne), through blood / blood products, mother to child (congenital) transmission, via organ transplantation and by laboratory accidents
Symptoms
- Unilateral palpebral edema (Romana sign)
- Fever
- Lymphadenopathy
- Hepatosplenomegaly
- Myocarditis
- Megacolon / megaesophagus
- Meningoencephalitis
- References: MMWR Morb Mortal Wkly Rep 2002;51:210, Circulation 2018;138:e169
Screening
- Primary infection is asymptomatic in ~70% of people and occurs most frequently in children
- Incubation period is ~14 days and the clinical manifestations, when present, can last for 6 - 8 weeks
- Donor / control positive rates:
- Brazil: 2% (Rev Soc Bras Med Trop 2006;39:530)
- Columbia: 0.4% (Biomedica 2005;25:527)
- Mexico: 0.5% (Salud Publica Mex 2006;48:13)
- U.S. high risk regions: 1 per 5,000 (MMWR Morb Mortal Wkly Rep 2007;56:141)
Blood donor screening
- No specific questions on donor history questionnaire regarding T. cruzi or Chagas disease
Blood donor testing
- Donor centers must test donations for evidence of T. cruzi using a licensed screening test for antibodies to T. cruzi
- FDA recommends one time testing of each donor using a licensed test for antibodies to T. cruzi
Donor deferral
- Donors who test repeatedly reactive on a licensed screening test for T. cruzi antibody must be deferred
- Donors whose blood tests positive or indeterminate on the licensed supplemental test should be deferred permanently and informed of the likelihood and medical significance of infection with T. cruzi
- Reference: MMWR Morb Mortal Wkly Rep 2007;56:141
Laboratory
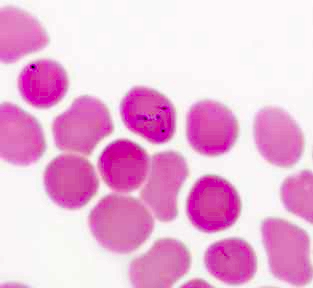
- Diagnosis of T. cruzi infection relies on serological or parasitological techniques
- Available serological tests include hemagglutination, indirect immunofluorescence or enzyme linked immunosorbent assay (ELISA)
- One of the most significant problems in the serological diagnosis of T. cruzi infection is the absence of a gold standard test
- Reference: Adv Parasitol 2011;75:19
Case reports
- 9 month old boy infant born to a seropositive mother to chronic Chagas in Mexico (Iran J Parasitol 2021;16:697)
- 3.5 year old girl with stage 4 neuroblastoma received multiple blood components and had transfusion acquired T. cruzi infection from Bolivian blood donor in the U.S. (Transfusion 2007;47:540)
- 86 year old woman with autoimmune rheumatic disease presents with reactivation of Chagas disease (Open Forum Infect Dis 2021;8:ofaa642)
Treatment
- Chagas disease can be treated with benznidazole or nifurtimox; both medicines are nearly 100% effective in curing the disease if given soon after infection, at the onset of the acute phase
- During the chronic phase of Chagas disease, most people are asymptomatic and unaware of their infection
- References: WHO: Chagas Disease (American Trypanosomiasis) [Accessed 23 December 2022], J Pediatric Infect Dis Soc 2019;8:461
Peripheral smear images
Contributed by Melissa R. George, D.O.
Sample assessment & plan
- Assessment: Jane Doe is a 50 year old woman who moved from Mexico 5 years ago. Patient was recently diagnosed with rheumatoid arthritis and is currently on immunosuppression therapy. She presented with obstructive megacolon. Due to high suspicion of parasitemia, serological test was done and confirmed Chagas disease.
- Plan: The patient presentation and laboratory workup are consistent with a diagnosis of Chagas disease.
- Recommend starting antiparasitic treatment with benznidazole or nifurtimox
Differential diagnosis
- Malaria:
- Fever and flu-like illness, including shaking chills, headache, muscle aches and fatigue
- Toxic megacolon:
- Pain, distention of the abdomen, fever, rapid heart rate and dehydration
- Myocarditis:
- Chest pain, fatigue, rapid or irregular heartbeat, shortness of breath, flu-like symptoms, light headedness
- Achalasia:
- Dysphagia, regurgitating food or saliva, heartburn, belching, chest pain, coughing at night, vomiting
Additional references
Board review style question #1
A 40 year old man with no significant medical history presented to the emergency room due to 1 week of generalized weakness, fatigue, myalgia, lightheadedness, achiness and sporadic fevers. Patient also has a history of a tick bite on the anterior abdomen 4 months prior to presentation. What pathognomonic presentation on peripheral smear would confirm a diagnosis of babesiosis?
- Crescent shaped gametocytes
- Ring forms
- Schistocytes
- Tetrad formations
Board review style answer #1
D. Tetrad formations. The pathognomonic presentation of babesiosis on peripheral smear is a tetrad formation within the red blood cell, also called a Maltese cross. Ring forms can be seen in babesiosis but are not pathognomonic for it as they are commonly seen in malaria. Crescent shaped gametocytes are also present in malaria disease. Gametocytes of Plasmodium falciparum are crescent or sausage shaped, and are usually ~1.5 times the diameter of a red blood cell in length. Schistocytes are red blood cell fragments indicative of intravascular hemolysis.
Comment Here
Reference: Parasites
Comment Here
Reference: Parasites
Board review style question #2
A 40 year old man with no significant medical history presented to the emergency room due to 1 week of generalized weakness, fatigue, myalgia, lightheadedness, achiness and sporadic fevers. Patient also has a history of a tick bite on the anterior abdomen 4 months prior to presentation. If babesiosis is confirmed after a positive nucleic acid test, how long should this patient be deferred for blood donation?
- 1 year
- 2 years
- 3 years
- 4 years
Board review style answer #2
B. 2 years. A positive nucleic acid test for babesiosis requires a blood donation deferral for 2 years.
Comment Here
Reference: Parasites
Comment Here
Reference: Parasites
Board review style question #3
A 25 year old woman presents with symptoms of fatigue, nausea, vomiting, headache and general malaise. Patient returned 1 month ago from studying abroad in Kenya. Laboratory testing shows anemia and a blood smear shows a parasite inside a red blood cell. What is the most common infectious pathogen to cause these symptoms and test results?
- P. falciparum
- P. malaria
- P. ovale
- P. vivax
Board review style answer #3
A. P. falciparum. The most common infectious pathogen of malaria is P. falciparum. P. vivax and P. ovale generally cause less serious illnesses but the parasites can remain dormant in the liver for many months, causing a reappearance of symptoms months or even years later.
Comment Here
Reference: Parasites
Comment Here
Reference: Parasites
Board review style question #4
A 25 year old woman presents with symptoms of fatigue, nausea, vomiting, headache and general malaise. Patient returned 1 month ago from studying abroad in Kenya. Laboratory testing shows anemia and a blood smear shows a parasite inside a red blood cell. For how long should this patient be deferred from donating blood?
- 3 months
- 1 year
- 3 years
- 5 years
Board review style answer #4
C. 3 years. A patient diagnosed with malaria is deferred for 3 years after becoming asymptomatic.
Comment Here
Reference: Parasites
Comment Here
Reference: Parasites
Board review style question #5
An 8 year old boy who recently moved from South America (Brazil) presents to the ER with left eyelid swelling associated with a history of general malaise and flu-like symptoms. His family states they have a farm and the boy has been in contact with several animals. The physician is concerned about a parasite induced disease. Considering the presentation, what would be the most likely area of entry of the parasite?
- Blood
- Ear
- Eye
- Mouth
Board review style answer #5
C. Eye. The most common area of entry when a patient presents with eyelid swelling (Romina sign) is the eye, which usually presents during the acute phase in Chagas disease.
Comment Here
Reference: Parasites
Comment Here
Reference: Parasites
Board review style question #6
An 8 year old boy who recently moved from South America (Brazil) presents to the ER with left eyelid swelling associated with a history of general malaise and flu-like symptoms. His family states they have a farm and the boy has been in contact with several animals. The physician is concerned about a parasite induced disease. What is a common complication in chronic carriers of this disease?
- Anemia
- Diarrhea
- Fatigue
- Megacolon
Board review style answer #6
D. Megacolon. The most common complication of chronic phase of Chagas disease is megacolon. Most infected people develop a prolonged asymptomatic form of disease called chronic indeterminate, during which few or no parasites are found in the blood. ~20 - 30% of infected individuals will later develop severe and sometimes life threatening medical problems over the course of their lives, such as megacolon, cardiomyopathy or arrhythmias.
Comment Here
Reference: Parasites
Comment Here
Reference: Parasites