Table of Contents
Definition / general | Essential features | Terminology | Epidemiology | Pathophysiology | Diagrams / tables | Laboratory | Case reports | Prevention | Special considerations | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: George MR, Potochny EM. Blood donor testing. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/transmedblooddonortest.html. Accessed April 24th, 2024.
Definition / general
- Donor testing aims to:
- Detect transfusion transmissible disease in donors who have already been qualified through the donor history questionnaire
- Evaluate vital signs and hemoglobin
- Conduct a mini physical examination
Essential features
- Donor testing is skewed toward sensitivity over specificity
- Confirmatory testing (preferably FDA approved) often needed to diagnose infectious disease
Terminology
- Cytomegalovirus (CMV)
- Enzyme immunoassay (EIA)
- Enzyme linked immunosorbent assay (ELISA)
- Nucleic acid testing (NAT)
- Hepatitis B virus (HBV)
- Hepatitis B core antigen (HBc)
- Hepatitis B surface antigen (HBsAg)
- Hepatitis C virus (HCV)
- Human immunodeficiency virus (HIV)
- Human T cell lymphotropic virus (HTLV1 / HTLV2)
- Recombinant immunoblot assay (RIBA)
- West Nile virus (WNV)
- Zika virus (ZIKV)
- Chemiluminescence immunoassay (ChLIA)
Epidemiology
| Infectious disease | Frequency of detecting infection in donor (per screened donations) | Risk of transfusion transmission (per units screened) |
| Babesiosis (Babesia spp.) | ~1 in 250 in endemic state | ~1 in 100,000 in endemic* state, as high as 1 in 18,000 in endemic counties within endemic state |
| Chagas disease (Trypanosoma cruzi) | ~1 in 15,000 | Unknown |
| Hepatitis B (HBV) | ~1 in 12,000 | < 1 in 1 million |
| Hepatitis C (HCV) | ~1 in 5,000 | < 1 in 2 million |
| Human immunodeficiency virus (HIV) | HIV1: ~1 in 33,000 HIV2: ~1 in 57 million | < 1 in 2 million |
| HTLV1 / HTLV2 | ~1 in 27,000 | < 1 in 2 million |
| Treponema pallidum (syphilis) | Unknown | No cases recorded in > 50 years in the U.S. |
| West Nile virus (WNV) | Low: ~2,500 positives from 2003 - 2019 | 1 in 84 million; 1 in 35 million during summer (peak transmission season) |
| Zika virus** (ZIKV) | During the peak of the outbreak in 2016 - 2017, ~1 in 950,000 (all exposures were found to be outside the U.S. or in Florida) | Unknown |
- *14 states in the U.S. considered babesia endemic / contiguous to an endemic state: ME, VT, NH, CT, MA, NY, NJ, DE, RI, MD, PA, VA, MN, WI and the District of Columbia
- **Zika virus no longer considered threat to blood supply (FDA: Zika Virus Response Updates from FDA [Accessed 17 September 2021])
- Note: cytomegalovirus (CMV) not routinely tested
- Leukoreduced blood products = CMV safe
- Rare recipients may need serologically CMV negative units
- Residual risk of window period donation = length of window period x incidence of infections in repeat donors
- References: American Red Cross: Infectious Disease, HLA and ABO Donor Qualification Testing [Accessed 17 September 2021], Cohn: AABB Technical Manual, 20th Edition, 2020
Pathophysiology
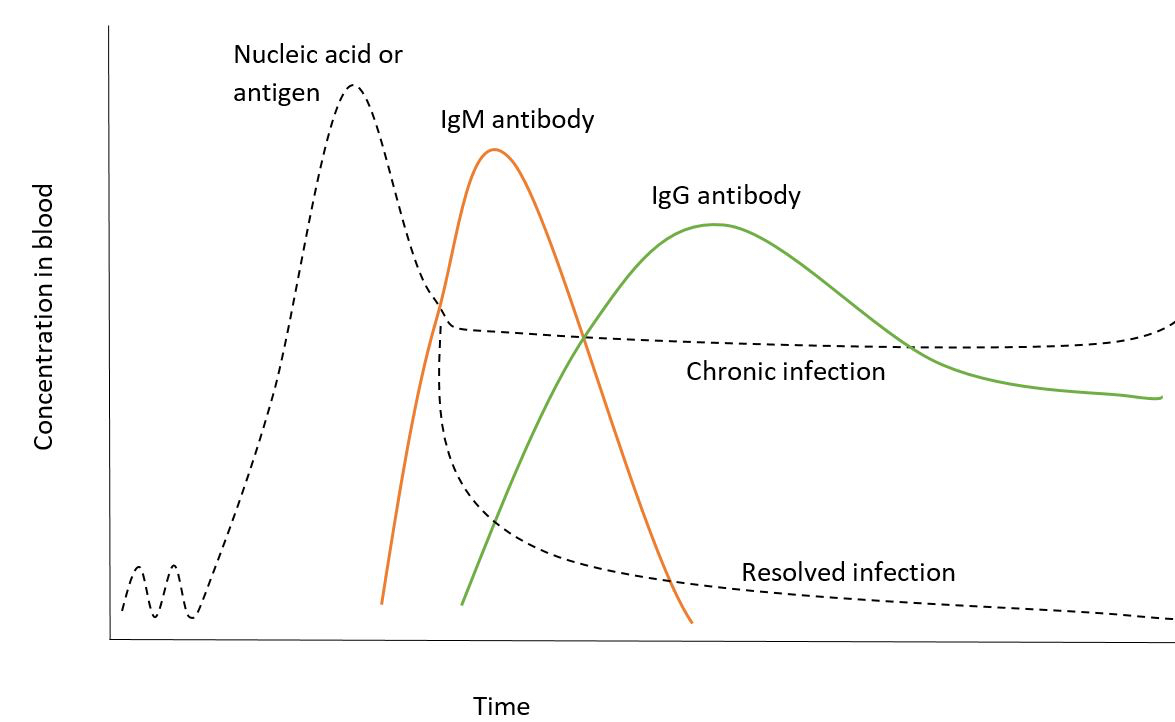
- Pattern by which serologic markers become positive is the basis of blood donor testing
- Transfusion transmissible illness antigens and nucleic acids are the first detectable markers
- Antibody response
- IgM antibodies
- First to form in immune response
- Taper to moderate levels in chronic infection
- Decrease to low levels or become undetectable in resolved infection
- IgG antibodies
- Begin to rise at peak levels of IgM antibodies
- Taper to moderate levels as part of sustained immune response
- Relationship of infectious disease marker concentration to timing of immune response (see Diagrams)
- IgM antibodies
- References: Cohn: AABB Technical Manual, 20th Edition, 2020, AABB: Standards for Blood Banks and Transfusion Services, 32nd Edition, 2020
Diagrams / tables
Laboratory
- First transfusion transmissible disease to be screened (1940 - 1950): syphilis (Treponema pallidum)
- In the 1960s, > 30% of transfusion recipients developed posttransfusion hepatitis (PTH)
- Hepatitis B accounted for about 25% of PTH
- Development of sensitive hepatitis B surface antigen test (HBsAg)
- Non-A, non-B hepatitis (NANB) accounted for the remainder
- Occurred more frequently in paid donors than volunteer donors
- No specific antigen test available in 1960 - 1970
- Implementation of surrogate marker test to identify donors at increased risk of NANB PTH
- Presence of antibody to hepatitis B core antigen (anti-HBc) or presence of elevated alanine aminotransferase
- Concern about nonspecific nature of tests led to delay in implementation
- Hepatitis B accounted for about 25% of PTH
- 1980s: concerns over transmission of acquired immunodeficiency syndrome (AIDS) by transfusion reinvigorated interest in surrogate testing
- These realizations led to expansion of donor screening beyond looking for known agents
- Donor history evaluation for increased risk of exposure to:
- Blood borne infections
- Sexually transmitted infections
- Donor history evaluation for increased risk of exposure to:
- Window period
- Transmission of HIV continued after antibody testing was initiated
- Time between infection and detectability of infection by testing used
- Including HIV, p24 antigen test can further reduce the serological window period by 3 to 7 days before antibodies are detectable
- Antibody levels: seroconversion - IgM antibodies form first, followed by IgG
- Lessons learned to reduce the risk of transfusion transmitted infection:
- Donor education and screening questions to exclude donors with high risk behaviors and risk of transfusion transmitted illnesses for which there is no test currently available
- Reducing the window period of tests and improving the sensitivity
- Adopting current good manufacturing practice (cGMP) regulations
- Surveillance for known and emergent transfusion transmissible illness
- Donor screening tests
- Required by FDA (FDA: Code of Federal Regulations Title 21 [Accessed 17 September 2021])
- Changes / updates provided in FDA guidance documents
- Guidance documents not technically legal requirement, however, are considered standard of care
- AABB (formerly American Association of Blood Bank) also issues recommendations via bulletins or the standards for blood bank and transfusion services (AABB: Standards for Blood Banks and Transfusion Services, 32nd Edition, 2020)
- Required by FDA (FDA: Code of Federal Regulations Title 21 [Accessed 17 September 2021])
- Sample
- Generally about 5 test tubes drawn
- Nucleic acid testing (NAT)
- ABO / Rh testing and testing for syphilis antibodies
- Viral antibody markers
- Antibodies against T. cruzi (Chagas)
- Tube kept for retention in case repeat testing is necessary
- Generally about 5 test tubes drawn
- Serologic screening tests: immunoassays for antigens or antibodies (host response to infection)
- Enzyme linked immunosorbent assay (EIA or ELISA)
- Can detect antibodies:
- Anti-HIV1 / HIV2
- Anti-HTLV1 / HTLV2
- Anti-HCV
- Anti-HBc (hepatitis B core)
- Anti-T. cruzi
- Can detect antigens:
- Hepatitis B surface antigen (HBsAg)
- Methodology:
- Indirect antibody detection
- Antigen is bound to a microtiter plate well
- Patient sample is added
- Incubation
- Wash step to remove unbound antibody
- Antihuman antibody conjugated to enzyme is added
- Example enzyme is horseradish peroxidase
- Helps produce a color change
- Bound antihuman antibody binds to patient antibody in sample attached to antigen fixed in microtiter plate well
- Chromogen (color changing compound) causes visible color change in response to antibody detection
- Antigen detection
- Generally sandwich technique
- Antibody against a target is bound to a microtiter plate well
- Donor sample is added
- Incubation
- Wash step to remove unbound antigen
- Antibody conjugated to enzyme targeting different portion of antigen added
- Conjugated antibody binds to antigen / antibody complex bound in well
- Chromogen allows detection via visible color change
- Indirect antibody detection
- Can detect antibodies:
- Chemiluminescent immunoassay (ChLIA)
- Can detect antibodies
- Can detect antigens
- Methodology
- Similar to EIA or ELISA
- Uses chemical reaction that generates light rather than color change
- Interpretations for immunoassays are as follows:
- Readout below established cutoff = negative
- Readout exceeds established cutoff = reactive
- Reactive samples retested in duplicate
- Repeat reactive = positive
- Both repeat results negative = donor is eligible
- Aims to decrease issues from nonspecific binding in these tests
- Repeatedly positive results require further evaluation by FDA approved supplemental assay if such assay is available
- Still subject to serological limits of detection (window period)
- Currently available FDA approved supplemental assays include:
- HBsAg neutralization
- HIV type 1 (HIV1) antibodies
- HCV antibodies
- HTLV1 / HTLV2 antibodies
- T. cruzi antibodies
- Confirmatory testing
- Specific antigen neutralization for HBsAg reactive confirmation
- HBsAg reactive samples that are HBV DNA reactive do not require further testing by neutralization
- FDA licensed enzyme strip immunoassay (ESA)
- Recombinant proteins containing antigens targeted by antibodies from those with T. cruzi infection
- Placed on lines on nitrocellulose membrane strips and incubated
- Antibodies, if present, bind the test strip
- Involves a conjugate antibody addition prior to second incubation and color intensity interpretation at each antigen site on the strip
- Used for T. cruzi supplemental testing (FDA: ABBOTT ESA Chagas [Accessed 17 September 2021])
- FDA licensed western blot
- Also known as a protein immunoblot
- First step is to separate proteins by the length of the polypeptide using gel electrophoresis
- Electrophoretic transfer onto a membrane, such as nitrocellulose
- Immunostaining to visualize a certain protein on the blot membrane
- Used for HTLV1 and HTLV2
- Specific antigen neutralization for HBsAg reactive confirmation
- Nucleic acid testing (NAT)
- Detects deoxyribonucleic acid (DNA) or ribonucleic acid (RNA)
- Extraction of nucleic acid from donor plasma or serum
- Nucleic acid amplification
- Detection of genetic sequences of viruses
- Techniques include:
- Polymerase chain reaction (PCR)
- Permits synthesis of large amounts of genetic material for analysis
- Components include target DNA, oligonucleotide primers, DNA polymerase, deoxyribonucleotide triphosphate base pairs, electrolyte solutions and buffers
- 3 steps:
- Denaturation: reaction mixture is heated to separate strands of DNA
- Annealing: cool to allow primers to bind specifically with a target DNA
- Extension: DNA polymerase initiates extension of primers (strands of DNA)
- Primary extension products dissociate from target DNA through heating
- Each extension fragments can serve as a template for additional rounds of kneeling and extension
- Many variants of this process possible
- Transcription mediated amplification (TMA)
- Isothermal nucleic acid amplification technique
- Begins with RNA target, usually single stranded and therefore no need for heat denaturation step
- Sequence specific DNA primer binds RNA target
- Reverse transcriptase extends primer creates DNA-RNA hetero duplex
- 5' end of sequence specific primer contains promoter for T7 bacteriophage polymerase
- Synthesizes DNA strand complementary to initial RNA target containing T7 promoter at 5' end
- Reverse transcriptase enzyme degrades initial RNA template while synthesizing complementary DNA
- Advantages include no need for denaturation step and use of isothermal processes that do not require thermocyclers
- Polymerase chain reaction (PCR)
- Reduces window period from weeks to days
- Performed in mini pools (MP) of 6 - 16 samples (cost and labor savings)
- If pool is reactive, component samples tested individually
- Fully automated NAT systems are available
- Followed by virus specific testing for donor notification / counseling
- Positive donor is deferred
- Used for:
- Babesia
- HBV
- HCV
- HIV
- West Nile virus (WNV)
- Zika virus (ZIKV; no longer considered a threat per May 12, 2021 FDA guidance) (FDA: Zika Virus Response Updates from FDA [Accessed 17 September 2021], McPherson: Henry's Clinical Diagnosis and Management by Laboratory Methods, 23rd Edition, 2016, Cohn: AABB Technical Manual, 20th Edition, 2020)
- Detects deoxyribonucleic acid (DNA) or ribonucleic acid (RNA)
- Enzyme linked immunosorbent assay (EIA or ELISA)
- Basic screening tests (Cohn: AABB Technical Manual, 20th Edition, 2020):
- Current FDA licensed blood donor screening tests (U.S.)
- Babesia spp.
- DNA / RNA test using TMA or PCR for screening test; supplemental test via research antibody and PCR
- T. cruzi (Chagas)
- IgG antibody (once in lifetime), ChLIA or EIA; supplemental ESA
- HBV
- HBsAg by ChLIA or EIA; supplemental via neutralization assay, positive HBV DNA
- HBc antigen by ChLIA or EIA
- HBV DNA by TMA or PCR
- HCV
- IgG antibody via ChLIA or EIA; supplemental via positive HCV RNA
- HIV1 / HIV2
- IgM, IgG antibody via ChLIA or EIA; supplemental via positive HIV RNA, HIV1 IFA or western blot, HIV2 EIA
- HIV1 RNA by TMA or PCR
- HTLV I - II
- IgG antibody via ChLIA or EIA; supplemental via western blot
- T. pallidum (syphilis)
- IgG or IgG / IgM screening via microhemagglutination, particle agglutination, EIA or immunofluorescence; supplemental via T. pallidum screening test
- WNV
- RNA by TMA or PCR; supplemental repeat or alternate NAT test or antibody (IgG, IgM)
- ZIKV
- RNA TMA or PCR; supplemental repeat or alternate NAT and antibody (IgG, IgM)
- Babesia spp.
- False positives
- Nonspecific reactivity or crossreactivity to antigens and antibodies may occur
- False positive nucleic acid testing is possible as well
- Donors believed to demonstrate false positive results may be entered into a donor eligibility reentry algorithm
- Reentry algorithm is complex and features many decision points
- Reentry criteria is best accessed from current FDA guidance
- Current FDA licensed blood donor screening tests (U.S.)
- References: FDA: Requalification Method for Reentry of Blood Donors Deferred Because of Reactive Test Results for Antibody to Hepatitis B Core Antigen (Anti-HBc) [Accessed 17 September 2021], FDA: "Lookback" for Hepatitis C Virus (HCV) [Accessed 17 September 2021]
Case reports
- 62 year old asymptomatic man apheresis platelet donor with intermittent E. coli bacteremia (Transfusion 2015;55:2606)
- 69 year old man with West Nile disease following apheresis platelet transfusion (Transfusion 2020;60:424)
- Cases of transfusion transmitted hepatitis B virus prior to nucleic acid testing (Transfusion 2013;53:291)
- Cases of transfusion transmitted babesiosis prior to nucleic acid testing (Transfusion 2017;57:2348)
Prevention
| Transfusion transmissible illness (TTI) | Window period by serological testing in days | Window period by NAT in days |
| HBV HBsAg | 30 - 38 | 18.5 - 26.5 |
| HCV | 45 - 60 | 7.4 |
| HIV (CDC: Types of HIV Tests [Accessed 17 September 2021]) | Antigen test: 18 - 45 Antibody test: 18 - 90 | 9.1 |
| HTLV1 / HTLV2 | Unknown | No test available |
- Whole blood donation NAT for HIV and HCV costs millions of U.S. dollars per year to avert small numbers of HIV, HCV and HBV infections
- High cost to benefit ratio
- Ethical standards of protecting recipients outweigh pure material costs
- References: American Red Cross: Infectious Disease, HLA and ABO Donor Qualification Testing [Accessed 17 September 2021], Transfusion 2003;43:721
Special considerations
- Human cells, tissues and cellular and tissue based products (HCT / P)
- Requirements outlined in 21 Code of Federal Regulations 1271 and FDA guidance documents (FDA: Code of Federal Regulations Title 21 [Accessed 17 September 2021], FDA: Eligibility Determination for Donors of Human Cells, Tissues and Cellular and Tissue Based Products [Accessed 17 September 2021])
- Autologous donations
- FDA requires infectious disease testing of autologous donations being shipped from one facility to another
- If receiving facility does not permit autologous donations to enter into general inventory if not used for donor, FDA requires testing of only the first donation each 30 day period (FDA: Code of Federal Regulations Title 21 [Accessed 17 September 2021])
- Cytomegalovirus (CMV) testing for immunocompromised recipients (Cohn: AABB Technical Manual, 20th Edition, 2020):
- CMV is lipid enveloped Herpesviridae DNA virus
- Causes lifelong infection
- Latent phase and white blood cells
- Potential for reactivation
- Primary infection in immunocompetent host is usually minor
- Immunocompromised patients are in danger of severe or even fatal primary or reactivated disease
- Fetuses
- Low birth weight premature infants of CMV negative mothers
- CMV seronegative recipients of solid organ or hematopoietic stem cell transplants from seronegative donors pathogen reduced products
- Live, intact white blood cells in cellular blood products can transmit virus
- Frozen or thawed plasma products do not appear to transmit CMV
- Most blood donors have had exposure to CMV reduce risk options for vulnerable recipients include:
- Leukoreduction
- CMV seronegative donors
- Pathogen inactivation (platelets only in United States at present)
- Pathogen inactivation
- Donor screening cannot fully eliminate risk
- Not possible to test for every potentially transmissible illness
- Window periods
- Sensitivity / specificity of testing available
- Developing test takes time and resources
- As of yet, unknown pathogens cannot be tested for
- Reduces infectivity of residual pathogens and blood products
- Methods include:
- Solvent detergent (SD) treated pooled plasma products (Octaplas) (FDA: Octaplas [Accessed 17 September 2021])
- SD and methylene blue / visible light treatment works in plasma but damages cellular membranes and cannot be used for red blood cell or platelet products
- Plasma to be used in these products are prescreened for parvovirus B19, hepatitis E virus (HEV) and hepatitis A virus (HAV) (nonenveloped)
- Amotosalen / psoralen ultraviolet A (UVA) light treated products (Cerus: How INTERCEPT Works [Accessed 17 September 2021])
- Amotosalen / psoralen intercalates between nucleic acid base pairs
- UVA illumination activates amotosalen and causes permanent cross links between nucleic acid strands
- Prevents further replication and inactivates bacteria, viruses and leukocytes
- Available in U.S. for plasma and platelets
- Significant activity against HIV, HBV, HCV, HTLV, WNV, CMV, Zika virus, Babesia and parasites, syphilis and many bacteria sp.
- Solvent detergent (SD) treated pooled plasma products (Octaplas) (FDA: Octaplas [Accessed 17 September 2021])
- Donor screening cannot fully eliminate risk
Additional references
Board review style question #1
Which of the following disease markers are part of the panel of those required to be tested for during voluntary whole blood and apheresis donations in the United States?
- Cytomegalovirus nucleic acid test, hepatitis B virus surface antigen, hepatitis B virus nucleic acid test
- Hepatitis B virus surface antigen, HIV nucleic acid test, West Nile virus nucleic acid test
- HIV1, HIV2 antibody testing, syphilis, dengue nucleic acid test
- West Nile virus nucleic acid test, syphilis nucleic acid test, hepatitis C virus nucleic acid test
Board review style answer #1
B. Hepatitis B virus surface antigen, HIV nucleic acid test, West Nile virus nucleic acid test. Answer A is incorrect because CMV is not a required infectious disease marker and its residual risk is reduced by leukoreduction of cellular blood products or pathogen reduction technology. Answer C is incorrect because dengue is not currently one of the required infectious disease markers. Answer D is incorrect because syphilis (T. pallidum) is not screened for by nucleic acid testing.
Comment Here
Reference: Blood donor testing
Comment Here
Reference: Blood donor testing
Board review style question #2
A premature low birthweight infant needs red blood cell transfusion. What additional intervention might be recommended to lower the risk of transfusion transmissible infection?
- Irradiation
- Leukoreduction
- Volume reduction
- Washing of the unit / aliquot
Board review style answer #2
B. Leukoreduction is considered cytomegalovirus (CMV) safe because CMV tends to reside in white blood cells where it can cause latent infection or pose risk of new infection in a transfusion recipient. Answer A, irradiation, reduces the risk of transfusion associated graft versus host disease (TA-GVHD), however, it generally is not considered protective against infectious diseases. Answer C, volume reduction, may be helpful for reducing the amount of potentially incompatible plasma if no better alternative product is available, especially in the case of platelets whereby ABO identical products may not always be available. Answer D, washing of a unit or aliquot, is helpful to prevent potassium overload or recurrence allergic reaction in susceptible patients, however, it is not indicated for the prevention of any transfusion transmissible illness.
Comment Here
Reference: Blood donor testing
Comment Here
Reference: Blood donor testing