Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Molecular / cytogenetics images | Sample pathology report | Differential diagnosis | Additional references | Practice question #1 | Practice answer #1 | Practice question #2 | Practice answer #2Cite this page: Bennett JA. Inflammatory myofibroblastic tumor. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/uterusinflammpseudo.html. Accessed August 27th, 2025.
Definition / general
- Uncommon mesenchymal neoplasm of myofibroblastic / fibroblastic origin with variable amounts of myxoid stroma and lymphoplasmacytic inflammation; often characterized by ALK fusions
Essential features
- Best classified as a neoplasm of uncertain malignant potential, as histologically bland uterine confined tumors may recur
- Comprised predominantly of spindle cells with hypercellular (fascicular / storiform) and hypocellular (myxoid rich) areas, admixed with a variably prominent lymphoplasmacytic infiltrate
- Often positive for ALK and smooth muscle / stromal markers but staining is of variable intensity and distribution
- Most harbor ALK fusions but rearrangements involving ROS1, RET and ETV6::NTRK3 have also been reported
Terminology
- Inflammatory pseudotumor
- Plasma cell granuloma
ICD coding
Epidemiology
- Usually occur in premenopausal women but a broad age range has been reported (6 - 78 years) (Surg Pathol Clin 2022;15:315)
Sites
- Uterine corpus is the most common site in the gynecologic tract
- Rarely arises in the cervix, placenta, ovary, fallopian tube, cul de sac, broad ligament
Pathophysiology
- Fusion occurs between the promoter of the 5' partner gene and the 3' ALK kinase domain, resulting in ligand independent dimerization and constitutive activation of ALK (Nat Rev Cancer 2013;13:685)
- Other tyrosine kinase receptors (ROS, RET, ETV6::NTRK3) are less frequently involved
- TIMP3 and THBS1 are genes involved in endometrial remodeling during implantation and pregnancy (Am J Surg Pathol 2020;44:970)
- Common 5' partner genes in pregnancy associated tumors
Etiology
- Unknown
Clinical features
- Generally present with nonspecific gynecologic symptoms (abnormal uterine bleeding, abdominopelvic pain) or presumed fibroids
- May be incidentally discovered at cesarean section
Diagnosis
- Hysterectomy or myomectomy
- Rarely endometrial sampling
Radiology description
- No defining features to distinguish from other uterine mesenchymal neoplasms on imaging
Radiology images
Prognostic factors
- Not well defined since only a small subset have recurred or metastasized (Am J Surg Pathol 2022;46:105)
- Large size (> 7 cm), moderate to severe atypia, high mitotic activity (> 10 per 10 high power fields), tumor cell necrosis and infiltrative border have been hypothesized to be associated with aggressive behavior (Adv Anat Pathol 2017;24:354)
- Often fascicular / compact predominant, although one study showed myxoid dominant tumors had a worse outcome (Am J Surg Pathol 2015;39:157)
- Potential association between complete absence of p16 staining / CDKN2A deletions and aggressive behavior (Am J Surg Pathol 2020;44:1441, Am J Surg Pathol 2022;46:105)
- Can recur years after original diagnosis
Case reports
- 10 year old girl with menorrhagia and abdominal pain (J Clin Oncol 2015;33:e7)
- 30 year old woman with irregular menstruation (Gynecol Oncol Case Rep 2013;6:39)
- 36 year old woman with severe vaginal bleeding (Medicine (Baltimore) 2017;96:e8974)
- 44 year old woman with left uterine sidewall mass (J Int Med Res 2018;46:3498)
- 50 year old woman with increasing pelvic pain (J Hematol Oncol 2015;8:66)
Treatment
- Surgery (hysterectomy, myomectomy) is first line therapy, as many are presumed leiomyomas
- Tyrosine kinase inhibitors (i.e., crizotinib) have shown durable responses of at least 12 months in one study (Gynecol Oncol Rep 2021;37:100852)
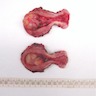
Gross description
- Most are < 10 cm (mean: 7.5 cm) (Surg Pathol Clin 2022;15:315)
- Typically intramural but may be polypoid
- Pregnancy associated tumors may be attached to the placental disc or extraplacental fetal membranes (Hum Pathol 2020;97:29, Am J Surg Pathol 2020;44:970, Hum Pathol 2020;106:62)
- Tan-white and whorled, with a gelatinous or myxoid cut surface
- Hemorrhage or necrosis may be present
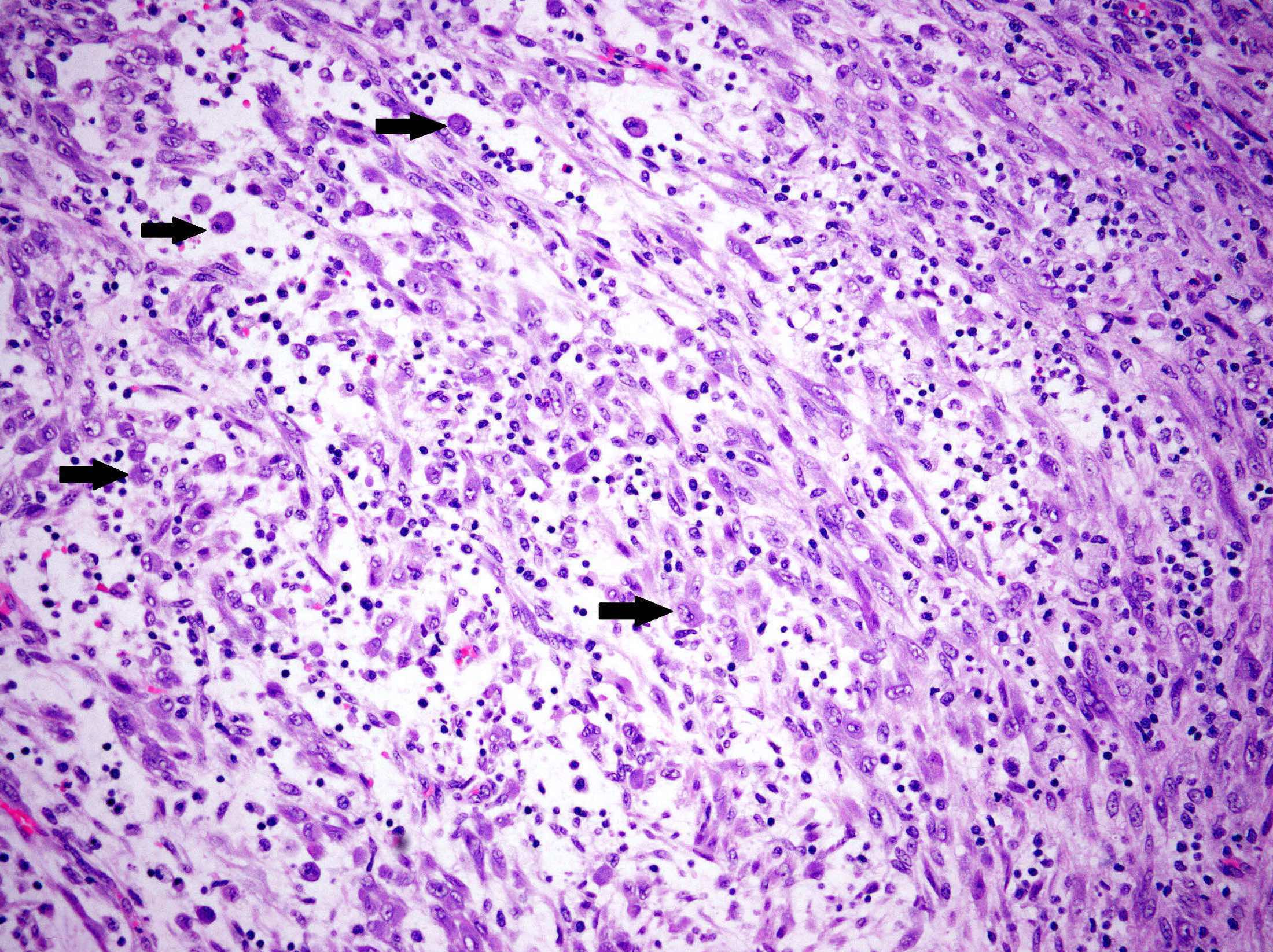
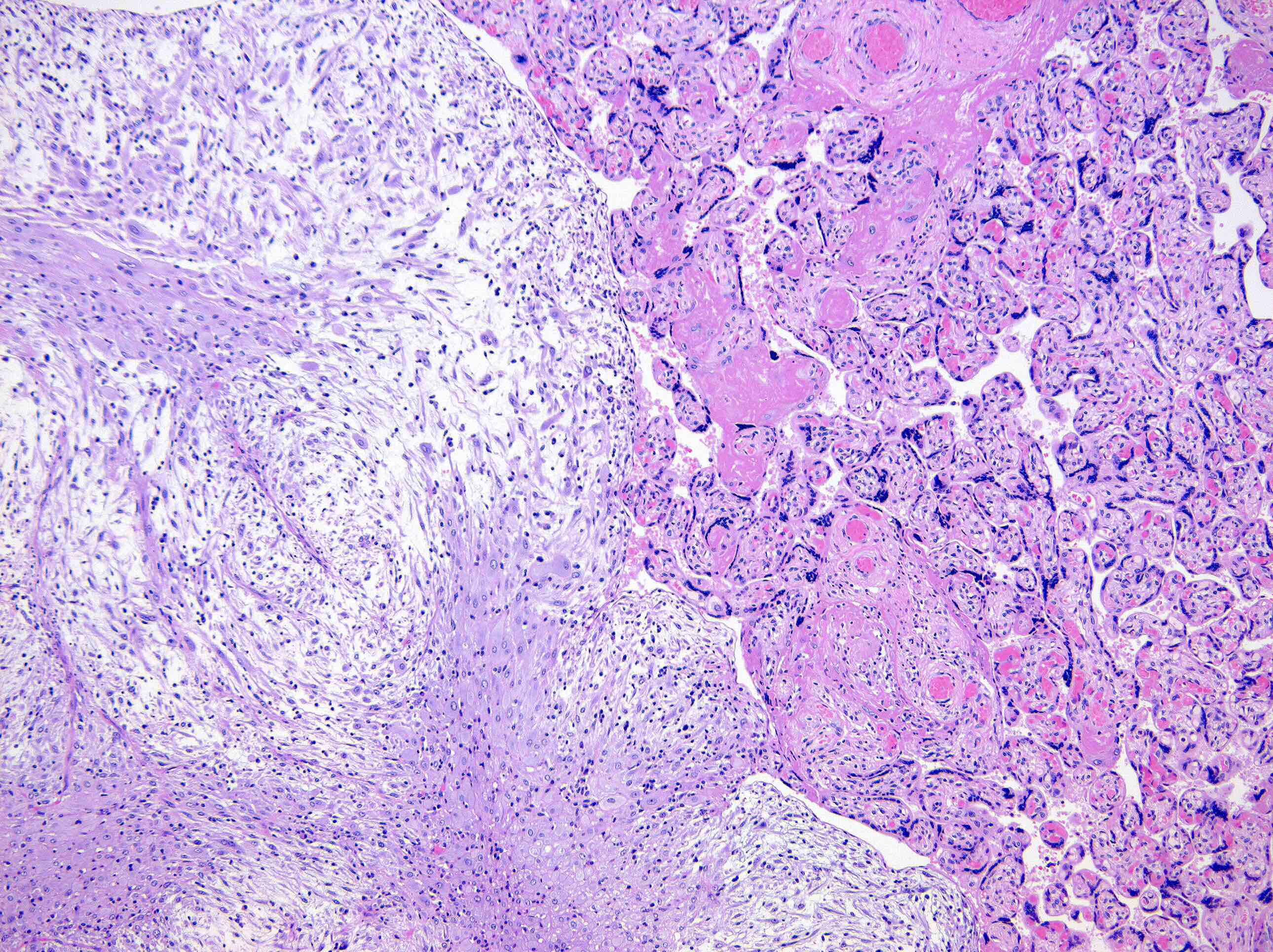
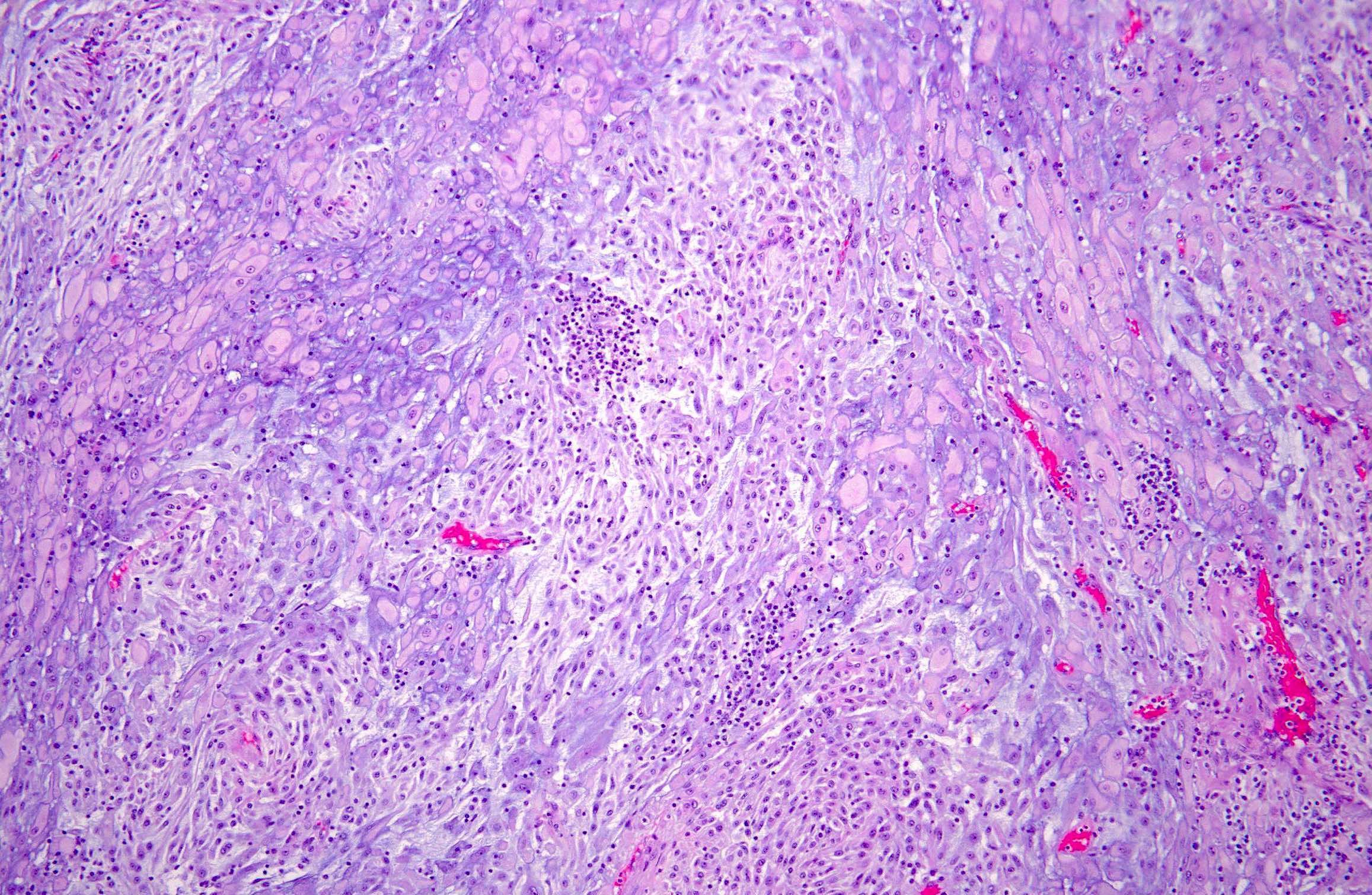
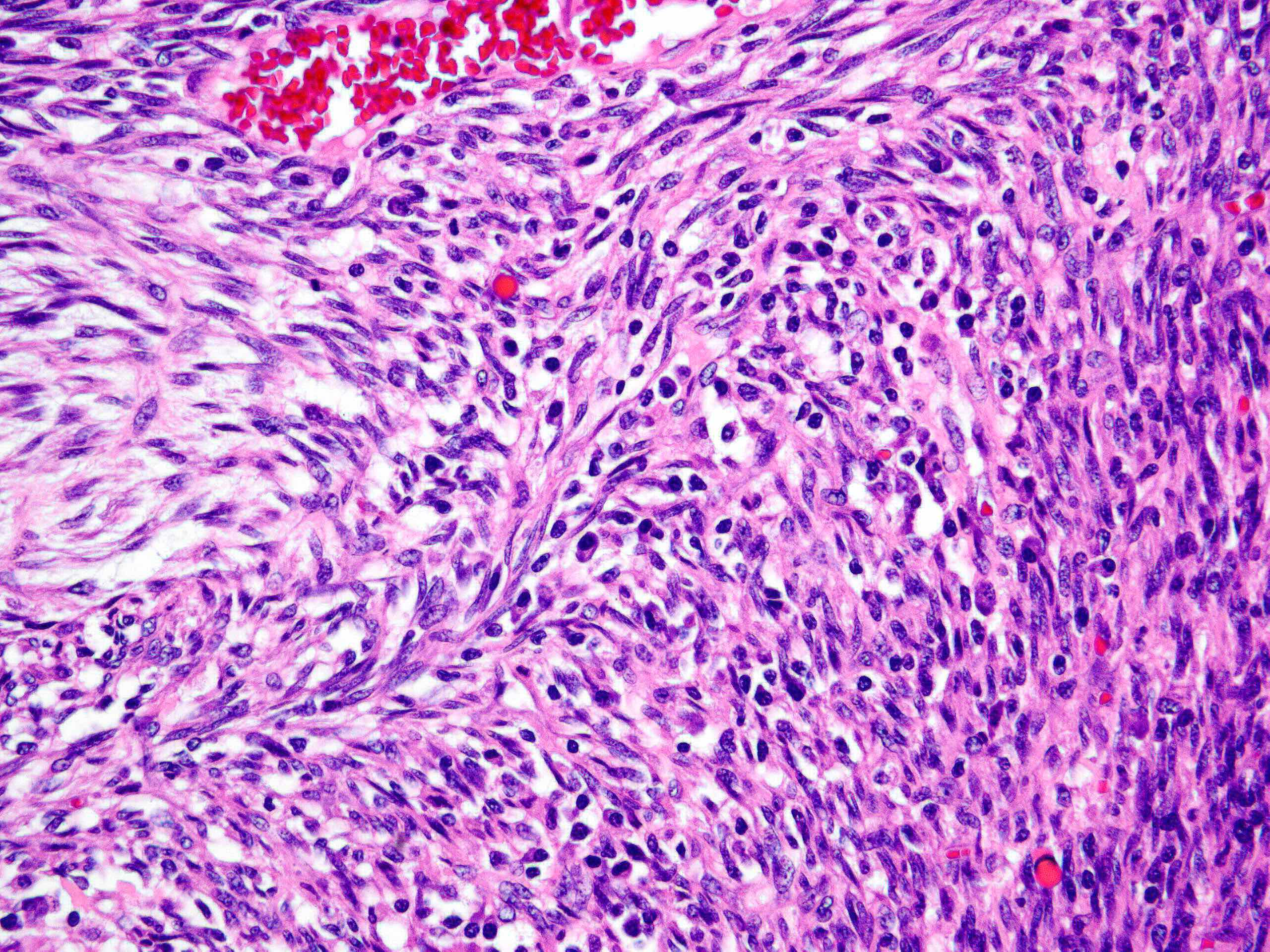
Microscopic (histologic) description
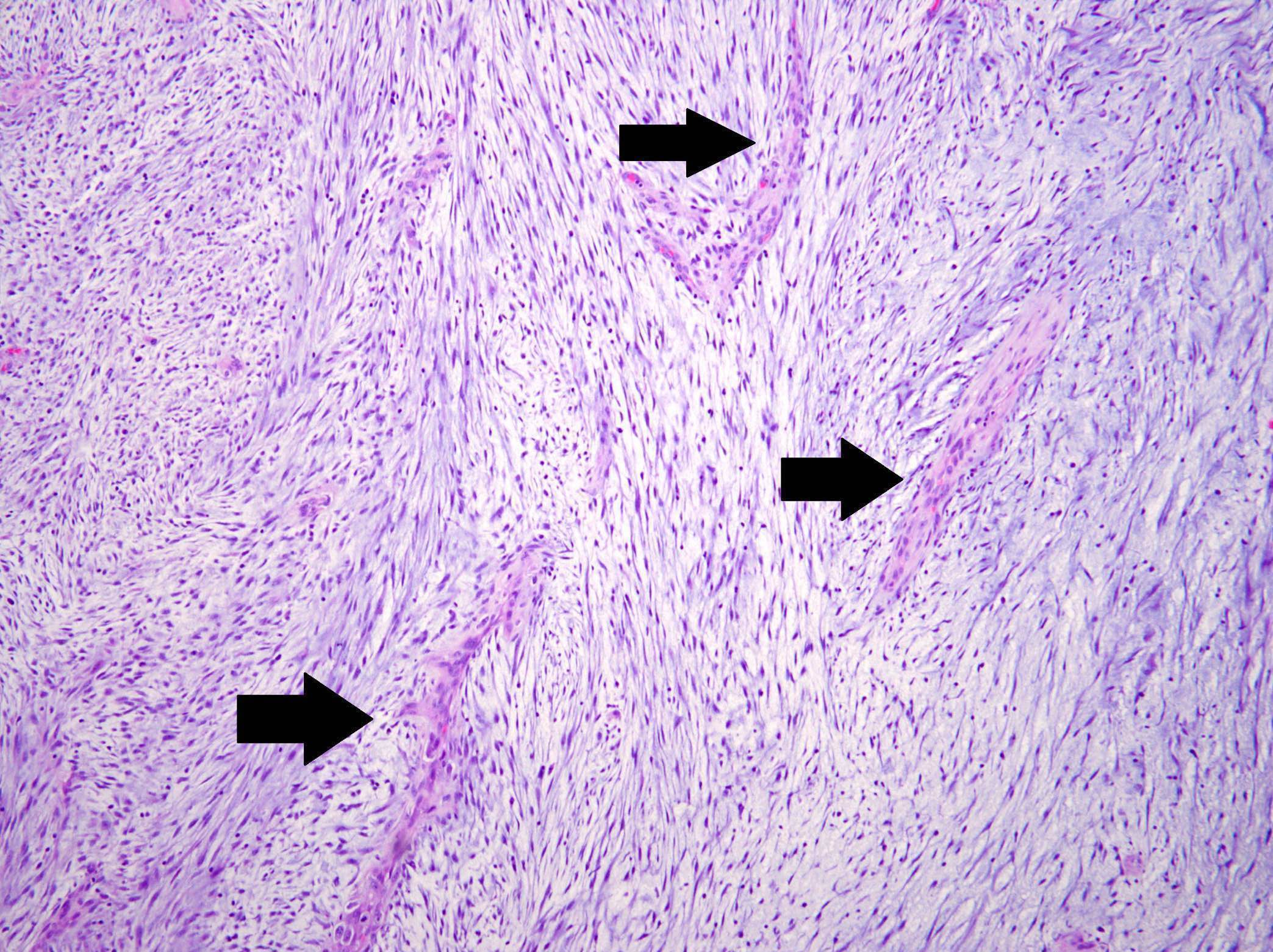
- Borders range from well circumscribed, to focally irregular, to infiltrative
- 3 main growth patterns: myxoid, fascicular / compact, hyalinized (Mod Pathol 2017;30:1489)
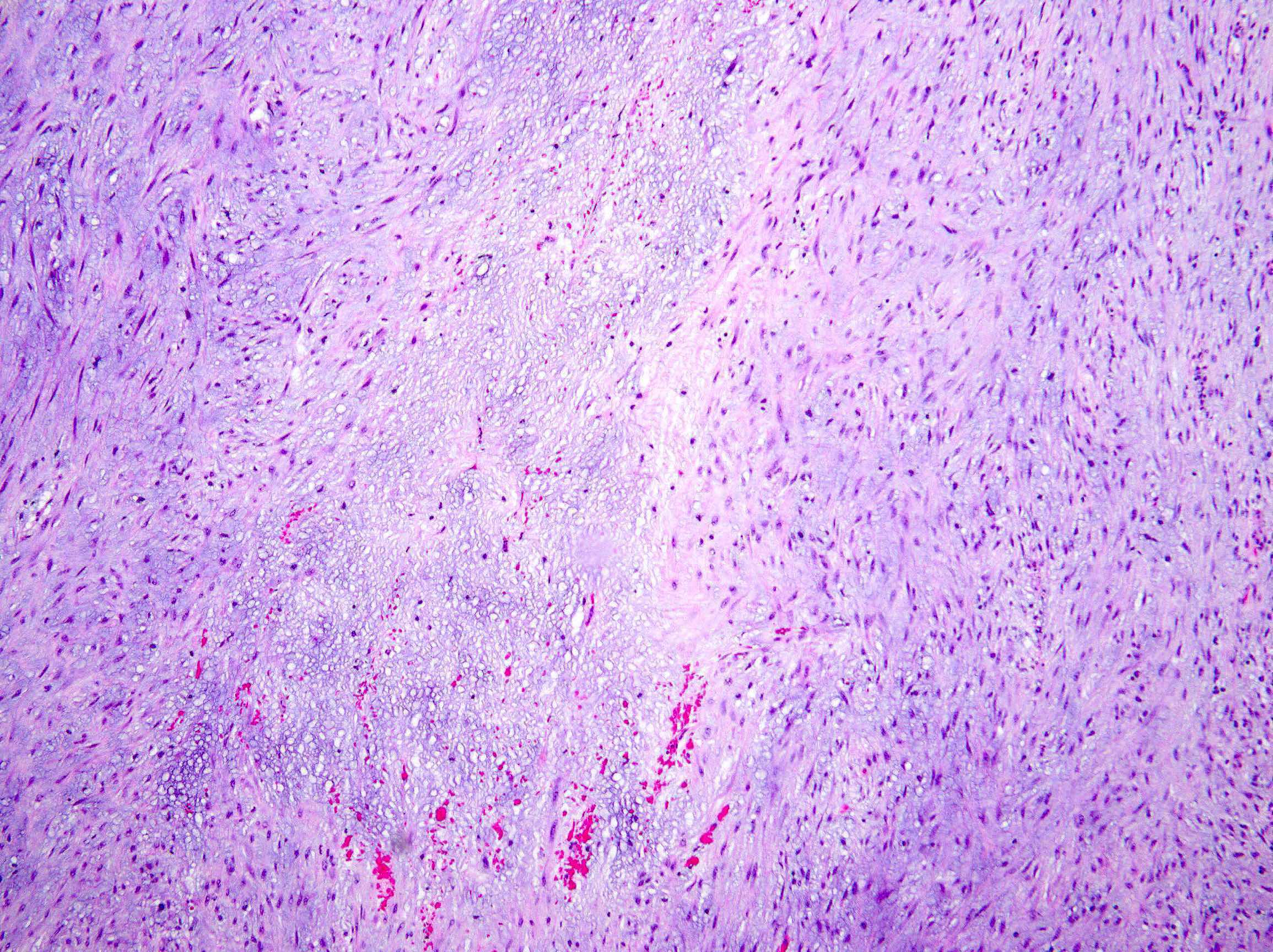
- Myxoid: loosely arranged spindle cells (nodular fasciitis-like) in a myxoid background, hypocellular
- Fascicular / compact: densely arranged spindle cells with fascicular or storiform architecture; may have a smooth muscle appearance or myxoid stroma, hypercellular
- Hyalinized (infrequent): sparsely cellular collagen resembling a scar
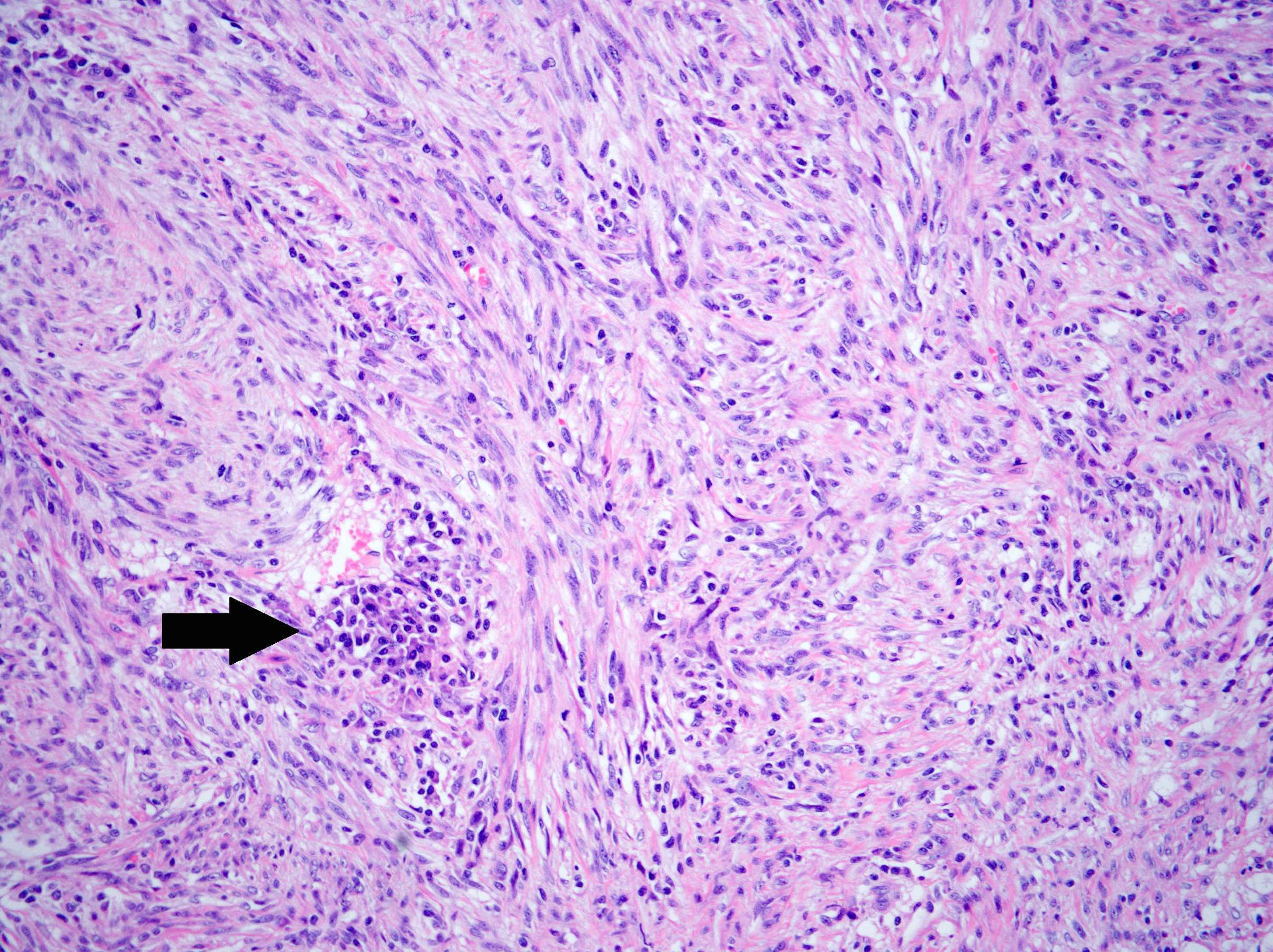
- Variable degree of lymphoplasmacytic inflammation, occasionally lymphocyte predominant
- Lymphoid aggregates, foamy histiocytes, neutrophils, eosinophils and Touton giant cells may be seen
- Thin walled and elongated vessels, occasional thick walled (leiomyoma-like) or staghorn vessels (Mod Pathol 2017;30:1489, Am J Surg Pathol 2019;43:64)
- Spindle cells have open vesicular nuclei with variable cytologic atypia (rarely severe)
- Ganglion-like cells (abundant eosinophilic cytoplasm, eccentric nuclei, prominent nucleoli) may be seen and typically comprise a minority of the tumor
- Spindle cells may show a decidualized appearance during pregnancy (Mod Pathol 2017;30:1489, Am J Surg Pathol 2020;44:970, Hum Pathol 2020;106:62)
- Mitotic index is usually low and tumor cell necrosis is uncommon
Microscopic (histologic) images
Positive stains
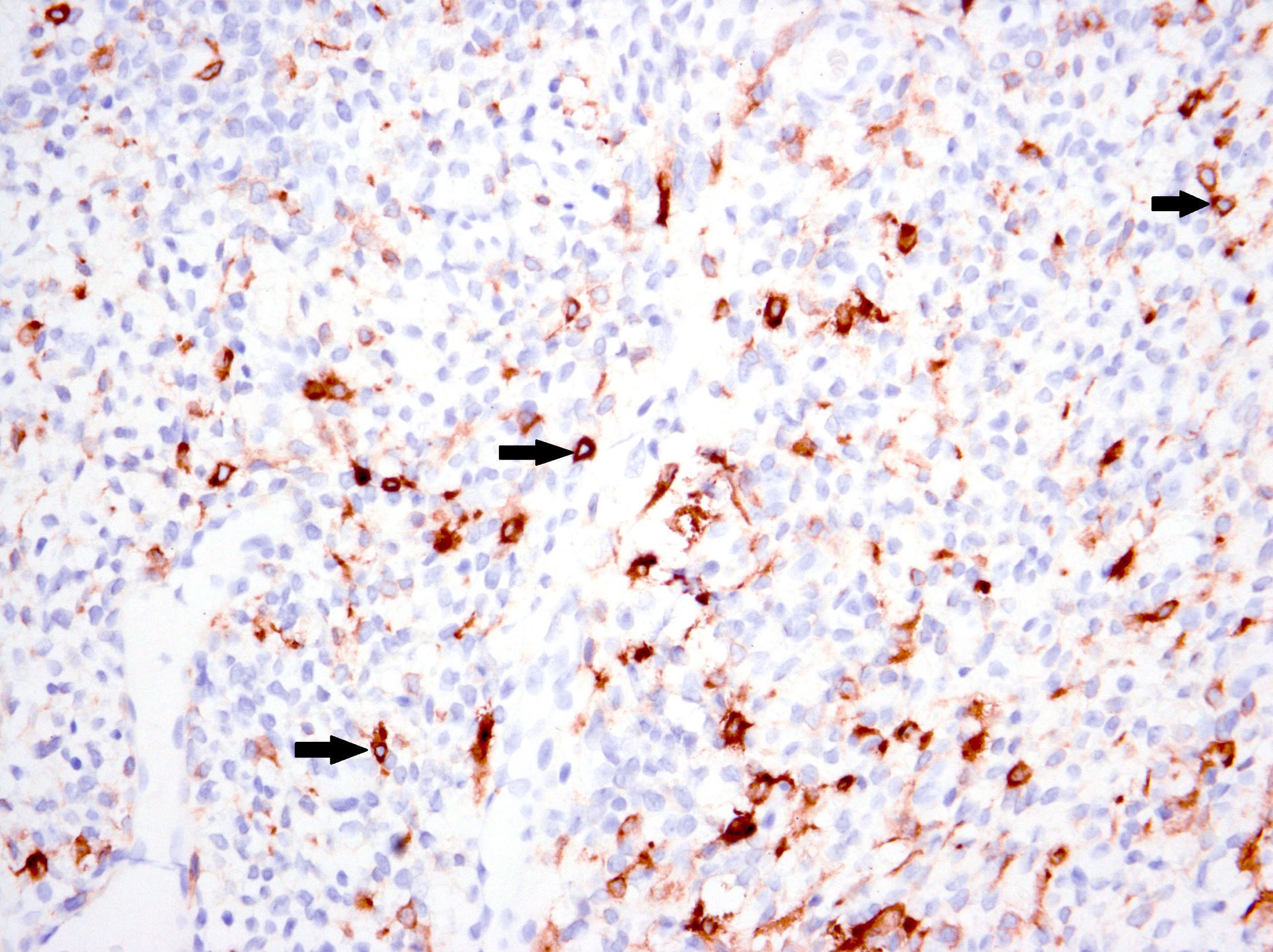
- ALK (granular cytoplasmic staining with or without perinuclear accentuation):
- Variable staining intensity and extent
- D5F3 clone most sensitive (Int J Gynecol Pathol 2021;40:28)
- Negative in tumors with non-ALK fusions
- Usually positive for smooth muscle (desmin, caldesmon, smooth muscle actin, transgelin) and stromal (CD10, IFITM1) markers (Am J Surg Pathol 2020;44:1441, Surg Pathol Clin 2022;15:315)
- Variable staining intensity and extent
- p16 usually patchy but aberrant in 35% (strong and diffuse or completely absent) (Am J Surg Pathol 2020;44:1441)
- Pregnancy associated tumors: PR positive, ER variable (Am J Surg Pathol 2020;44:970)
Negative stains
- Keratin
- p53 wild type
- BCOR (may be focal / weak) (Am J Surg Pathol 2020;44:1441)
Electron microscopy description
- Spindle cells show features of myofibroblasts and are focally surrounded by basal lamina-like material (Int J Gynecol Pathol 1987;6:275)
- Nondilated rough endoplasmic reticulum
- Thin filaments with peripheral dense bodies
- Pinocytic vesicles, occasional Golgi bodies, lipid droplets
Molecular / cytogenetics description
- ALK fusions:
- Classic rearrangement with split 3' and 5' signals (~75%)
- Abnormal patterns:
- Isolated 5' signal (Am J Surg Pathol 2016;40:285, Mod Pathol 2017;30:1489)
- Isolated 3' signal (Am J Surg Pathol 2019;43:64, Int J Gynecol Pathol 2020;39:152)
- Numerous isolated 3' signals (Am J Surg Pathol 2019;43:64)
- False negative FISH can result from complex genetic rearrangements (intrachromosomal inversion) (Am J Surg Pathol 2017;41:773):
- Fusion partners identified include TIMP3, THBS1, IGFBP5, DES, FN1, DCTN1, SEC31, TPM3, PPP1CB, TNS1, SYN3 (Surg Pathol Clin 2022;15:315)
- TIMP3 and THBS1 common in pregnancy associated tumors (Am J Surg Pathol 2020;44:970)
- Fusions involve ALK exons 12, 17, 18, 19 or 20
- ROS1, RET and ETV6::NTRK3 fusions uncommon (Hum Pathol 2020;100:45, Genes Chromosomes Cancer 2021;60:822, Hum Pathol 2020;97:29, J Int Med Res 2018;46:3498)
- CDKN2A deletions have been reported in a subset of malignant tumors (Am J Surg Pathol 2020;44:1441, Am J Surg Pathol 2022;46:105)
Sample pathology report
- Uterus, hysterectomy:
- Inflammatory myofibroblastic tumor (see comment)
- Comment: The tumor consists of spindled cells in a myxoid stroma admixed with lymphoplasmacytic inflammation. The cells show minimal cytologic atypia, without appreciable mitoses or tumor cell necrosis. The tumor is positive for ALK (granular cytoplasmic staining), desmin (patchy) and CD10 (patchy) but negative for BCOR and caldesmon. The morphology and immunohistochemical profile is consistent with an inflammatory myofibroblastic tumor. If clinically indicated, FISH or RNA fusion analysis can be performed.
Differential diagnosis
- Myxoid smooth muscle tumors (leiomyoma, smooth muscle tumor of uncertain malignant potential / STUMP, leiomyosarcoma) (Am J Surg Pathol 2016;40:285, Histopathology 2017;70:1138, Mod Pathol 2019;32:1688):
- Elongated nuclei with tapered ends (cigar shape)
- Typically lack a lymphoplasmacytic infiltrate
- ALK negative
- Subset with PLAG1 fusions
- Myxoid / fibromyxoid low grade endometrial stromal sarcoma (Int J Gynecol Pathol 1999;18:310):
- Tongue-like invasion
- Prominent small arterioles
- Areas of conventional endometrial stromal sarcoma at least focally present
- No lymphoplasmacytic infiltrate
- ALK negative
- BCOR / BCORL1 altered high grade endometrial stromal sarcoma (Mod Pathol 2017;30:1251, Mod Pathol 2018;31:674, Am J Surg Pathol 2018;42:335, Mod Pathol 2021;34:2200):
- Epithelioid inflammatory myofibroblastic sarcoma (Int J Gynecol Pathol 2018;37:468, Am J Surg Pathol 2022;46:105):
- Epithelioid cell dominant with only minor spindle cell component
- Typically neutrophil rich but lymphocytes, plasma cells and eosinophils may also be present
- Ring-like (nuclear membrane) staining for ALK
- RANBP2::ALK or RRBP1::ALK fusion
- Only 2 have been reported in the gynecologic tract (ovary and uterine corpus)
- Solitary fibrous tumor (Histopathology 2018;72:749, Am J Surg Pathol 2022;46:363):
- Postoperative spindle cell nodule:
- Prior history of surgery or instrumentation
- Most common in the vagina
- Prominent vasculature
- Brisk mitoses
- Cytokeratin positive
Additional references
Practice question #1
A 44 year old woman presents with an infiltrative spindle cell neoplasm in the myometrium that has prominent myxoid stroma and a dense lymphoplasmacytic infiltrate. It is positive for desmin, smooth muscle actin and ALK by immunohistochemistry and shows ALK rearrangement by FISH. What is the most likely diagnosis?
- BCOR rearranged endometrial stromal sarcoma
- Inflammatory myofibroblastic tumor
- Myxoid endometrial stromal sarcoma
- Myxoid leiomyosarcoma
- Postoperative spindle cell nodule
Practice answer #1
Practice question #2
Which gene is rearranged in the majority of uterine inflammatory myofibroblastic tumors?
- ALK
- BCOR
- RANBP2
- RRBP1
- STAT6
Practice answer #2