Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Molecular / cytogenetics images | Videos | Sample pathology report | Differential diagnosis | Additional references | Practice question #1 | Practice answer #1 | Practice question #2 | Practice answer #2Cite this page: Chapel DB, Bennett J. Aggressive angiomyxoma-vulva. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/vulvaaggressiveangiomyxoma.html. Accessed September 15th, 2025.
Definition / general
- Aggressive angiomyxoma is an infiltrative spindle cell neoplasm arising in the soft tissues of the lower genital tract, perineum and pelvis; approximately 30% recur locally but distant metastasis is exceptionally rare
Essential features
- Unique to the soft tissues of the lower genital tract, pelvis and perineum
- Infiltrative hypocellular myxoid lesion with bland spindle cells and prominent variably sized vessels
- HMGA2 overexpressed by immunohistochemistry in > 90%
- Local recurrence in ~ 30% but distant metastasis and death from disease are exceptionally rare
ICD coding
- ICD-10: D48.1 - neoplasm of uncertain behavior of connective and other soft tissue
Epidemiology
- F > M (~ 5:1) (Hum Pathol 1985;16:621, Int J Gynecol Cancer 2010;20:303, Arch Gynecol Obstet 2020;302:219)
- Age range: 14 - 77 years (median ~ 40 years) (Cancer 1996;78:79, Int J Gynecol Cancer 2005;15:140, Int J Gynecol Cancer 2010;20:303, Arch Gynecol Obstet 2020;302:219)
Sites
- Vulva (Am J Surg Pathol 1983;7:463, Hum Pathol 1985;16:621, Cancer 1996;78:79)
- Perineum
- Deep pelvic (paravaginal, perirectal, ischiorectal) soft tissues
- Gluteal region
- Retroperitoneum
- In men, may affect shaft of penis
Pathophysiology
- Histogenesis uncertain
- May arise from site specific stromal cells with capacity for fibroblastic, myofibroblastic and smooth muscle differentiation (Cancer 1996;78:79)
Clinical features
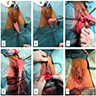
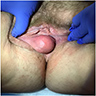
- Typically presents as an ill defined, painless, slowly enlarging mass (Am J Surg Pathol 1983;7:463, Hum Pathol 1985;16:621)
- May cause tenderness, pelvic fullness, dyspareunia or frequent urination (Am J Dermatopathol 1993;15:446, Histopathology 1997;30:3)
- Present for months to years prior to presentation (Am J Dermatopathol 1993;15:446, Histopathology 1997;30:3)
- Can clinically resemble a Bartholin cyst, Gartner duct cyst, lipoma or hernia (Am J Surg Pathol 1983;7:463, Am J Dermatopathol 1993;15:446, Histopathology 1997;30:3)
- Physical exam may substantially underestimate size and extent
- May grow rapidly in pregnancy (J Lab Physicians 2018;10:245, Case Rep Obstet Gynecol 2016;2016:8539704)
Diagnosis
- Radiology may suggest diagnosis and establish extent of local infiltration (Medicine (Baltimore) 2017;96:e6820, World J Clin Cases 2018;6:811)
- Definitive diagnosis on excision specimen
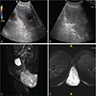
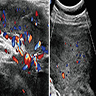
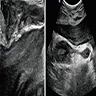
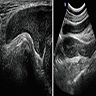
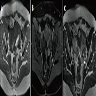
Radiology description
- Ultrasound: shows local infiltration and rich vasculature (Arch Gynecol Obstet 2020;302:219, Medicine (Baltimore) 2017;96:e6820)
- CT: variably dense lesion; highlights local extent of mass (Arch Gynecol Obstet 2020;302:219)
- MRI: hyperintense T2 signal and hypointense T1 signal; highlights local infiltration (J Lab Physicians 2018;10:245, Arch Gynecol Obstet 2020;302:219, Medicine (Baltimore) 2017;96:e6820)
Prognostic factors
- Local recurrence in ~30% (Am J Surg Pathol 1983;7:463, Hum Pathol 1985;16:621, Cancer 1996;78:79, Pathol Oncol Res 2017;23:131, Histopathology 1997;30:3)
- Positive surgical margins increase recurrence risk (Hum Pathol 1985;16:621, Pathol Oncol Res 2017;23:131, Arch Gynecol Obstet 2020;302:219)
- Recurrences may occur up to 15 years after diagnosis (Am J Surg Pathol 1983;7:463)
- Multiple recurrences not uncommon (Am J Surg Pathol 1983;7:463, Hum Pathol 1985;16:621, Cancer 1996;78:79)
- Distant metastasis and death from disease extremely rare (Hum Pathol 2003;34:1072, Gynecol Oncol Rep 2018;24:15)
Case reports
- 22 year old woman with vulvar aggressive angiomyxoma (Medicine (Baltimore) 2019;98:e13860)
- 24 year old woman with perineal aggressive angiomyxoma (SAGE Open Med Case Rep 2019;7:2050313X19843391)
- 25 year old pregnant woman with aggressive angiomyxoma (Case Rep Obstet Gynecol 2016;2016:8539704)
- 46 and 61 year old women with pelvic aggressive angiomyxoma (Cureus 2019;11:e4419)
- 47 year old woman with vulvar aggressive angiomyxoma (Gynecol Oncol Rep 2018;24:15)
Treatment
- Optimal primary management is wide local excision with clear margins (Am J Surg Pathol 1983;7:463, Hum Pathol 1985;16:621, Case Rep Obstet Gynecol 2016;2016:8539704)
- For tumors not amenable to resection, GnRH agonist or radiation therapy may be used at clinical discretion (BMJ Case Rep 2019;12:e227475)
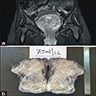
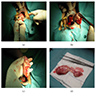
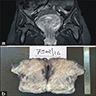
Clinical images
Gross description
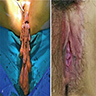
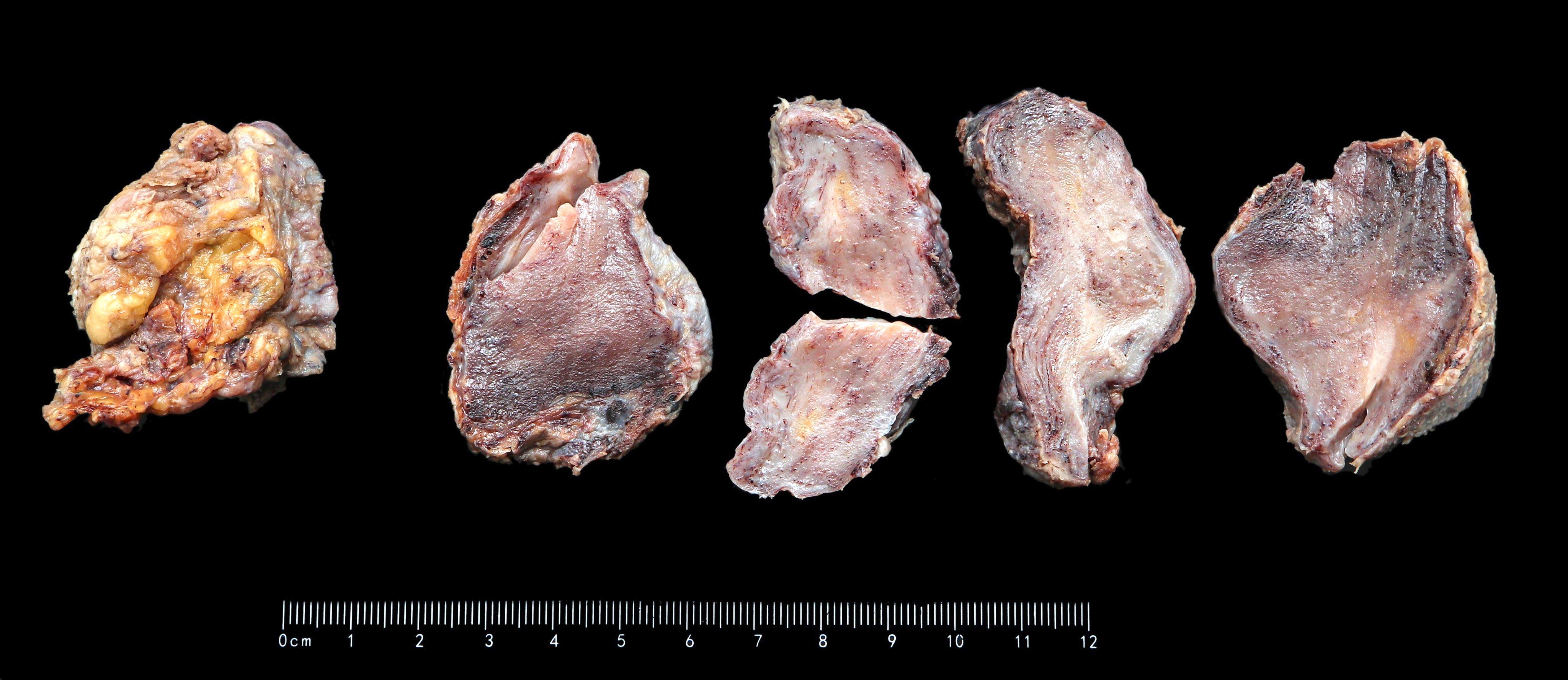
- Large lesions, range 3 - 60 cm (most > 10 cm) (Am J Surg Pathol 1983;7:463, Cancer 1996;78:79, Int J Gynecol Cancer 2005;15:140)
- Poorly defined with gross infiltration of surrounding soft tissue (Histopathology 1997;30:3)
- Lobulated, blue-gray to tan-gray to pink, gelatinous to myxoid to edematous cut surface (Am J Surg Pathol 1983;7:463)
- Punctate hemorrhage in some cases (Hum Pathol 1985;16:621)
- Necrosis absent (Hum Pathol 1985;16:621, Histopathology 1997;30:3)
Gross images
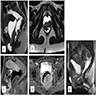
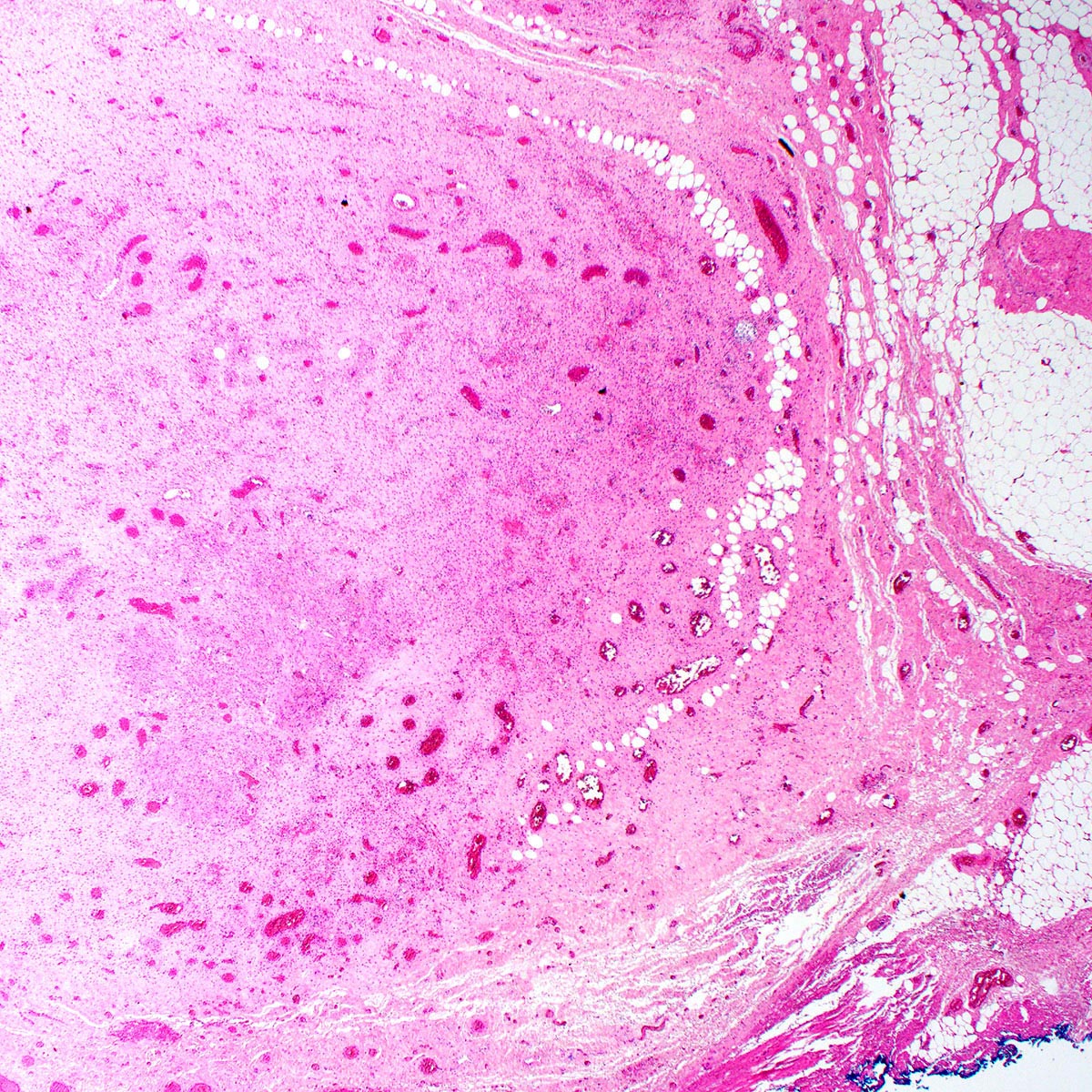
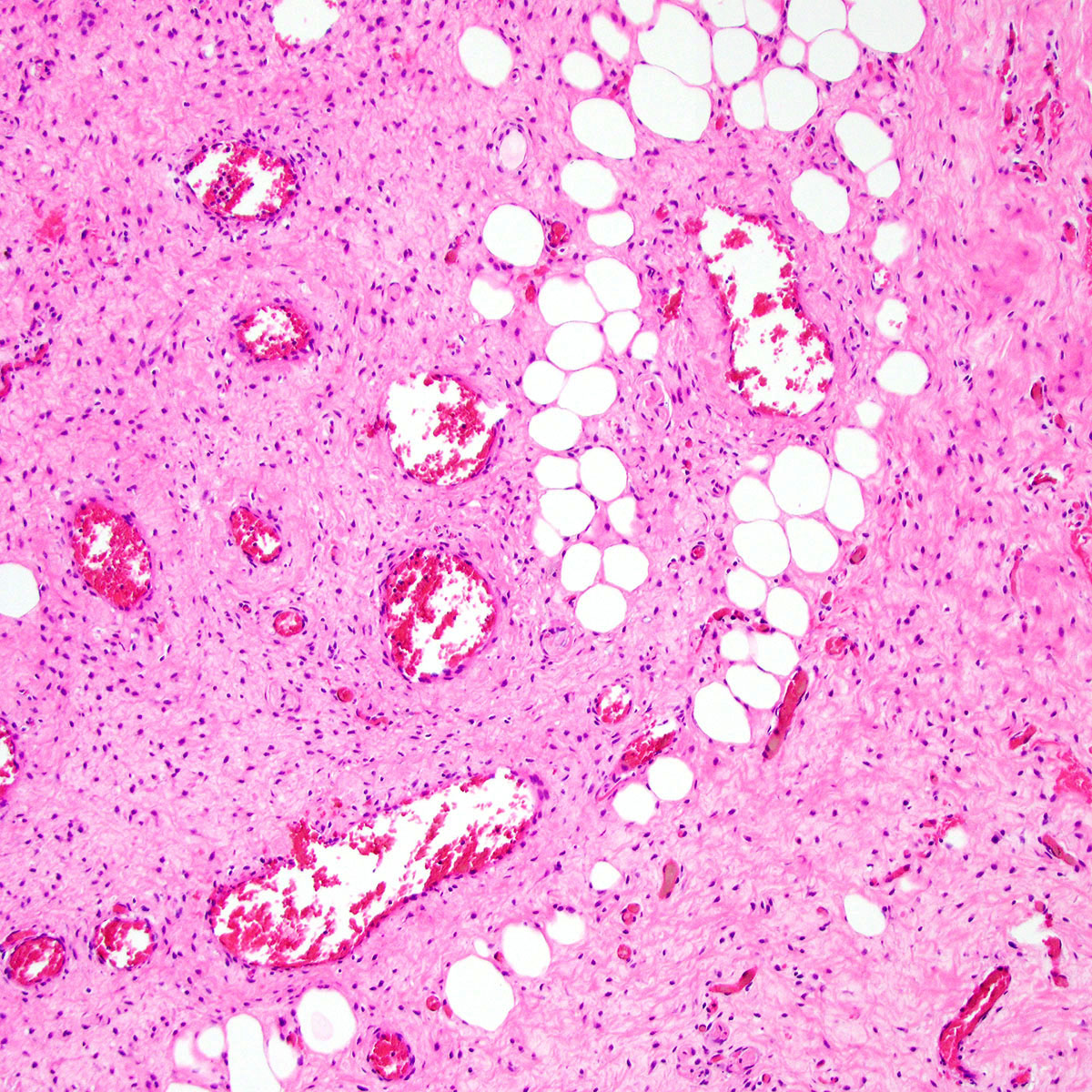
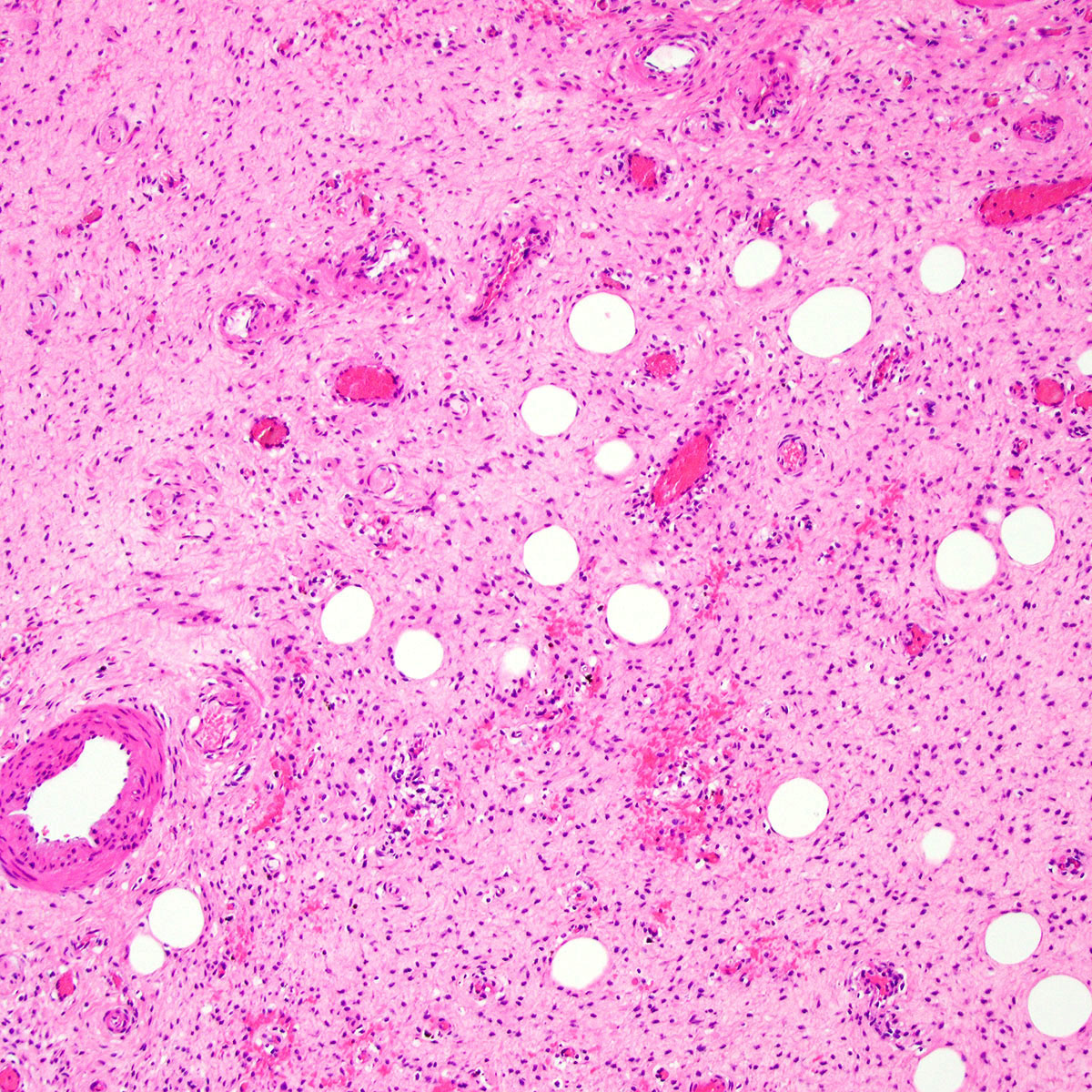
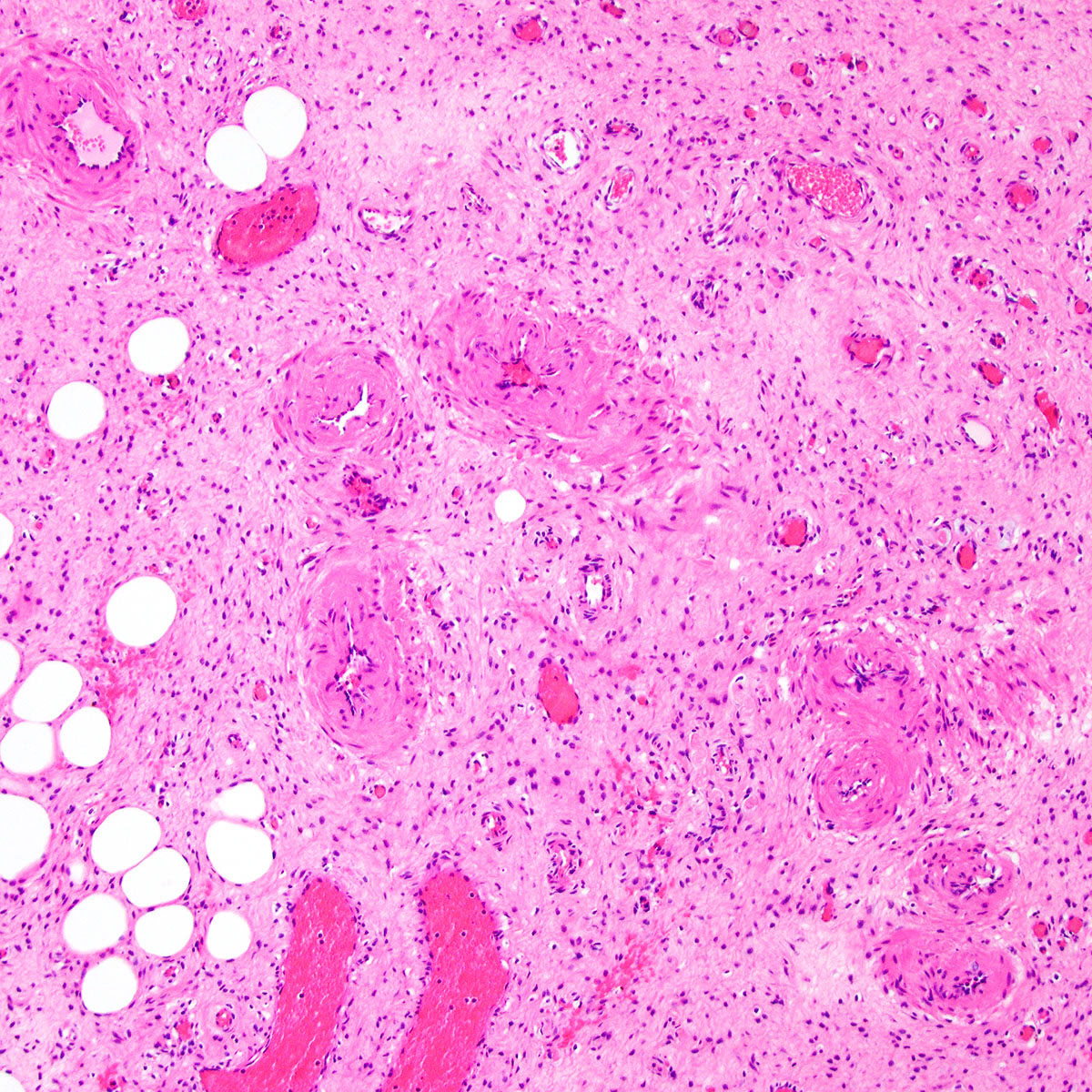
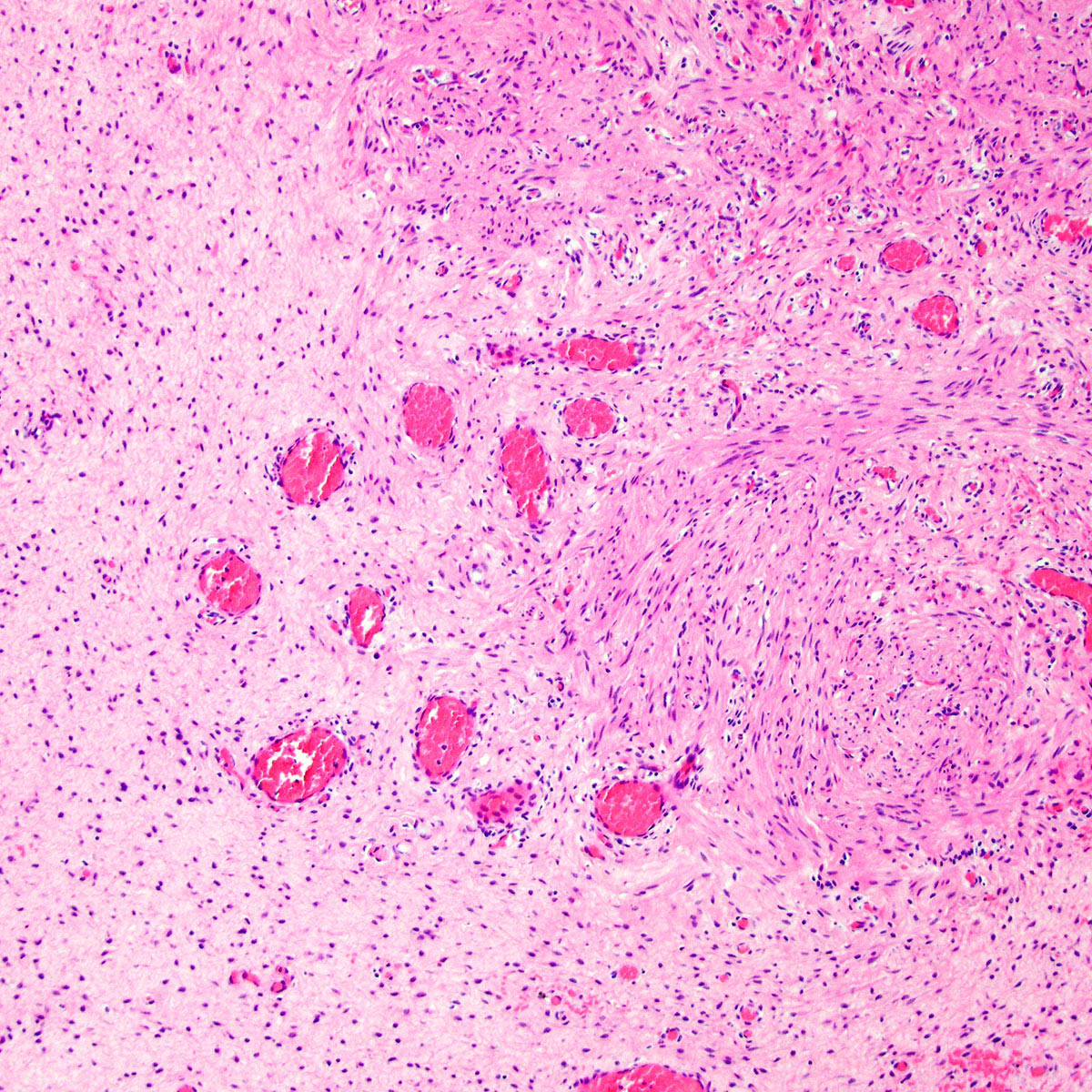
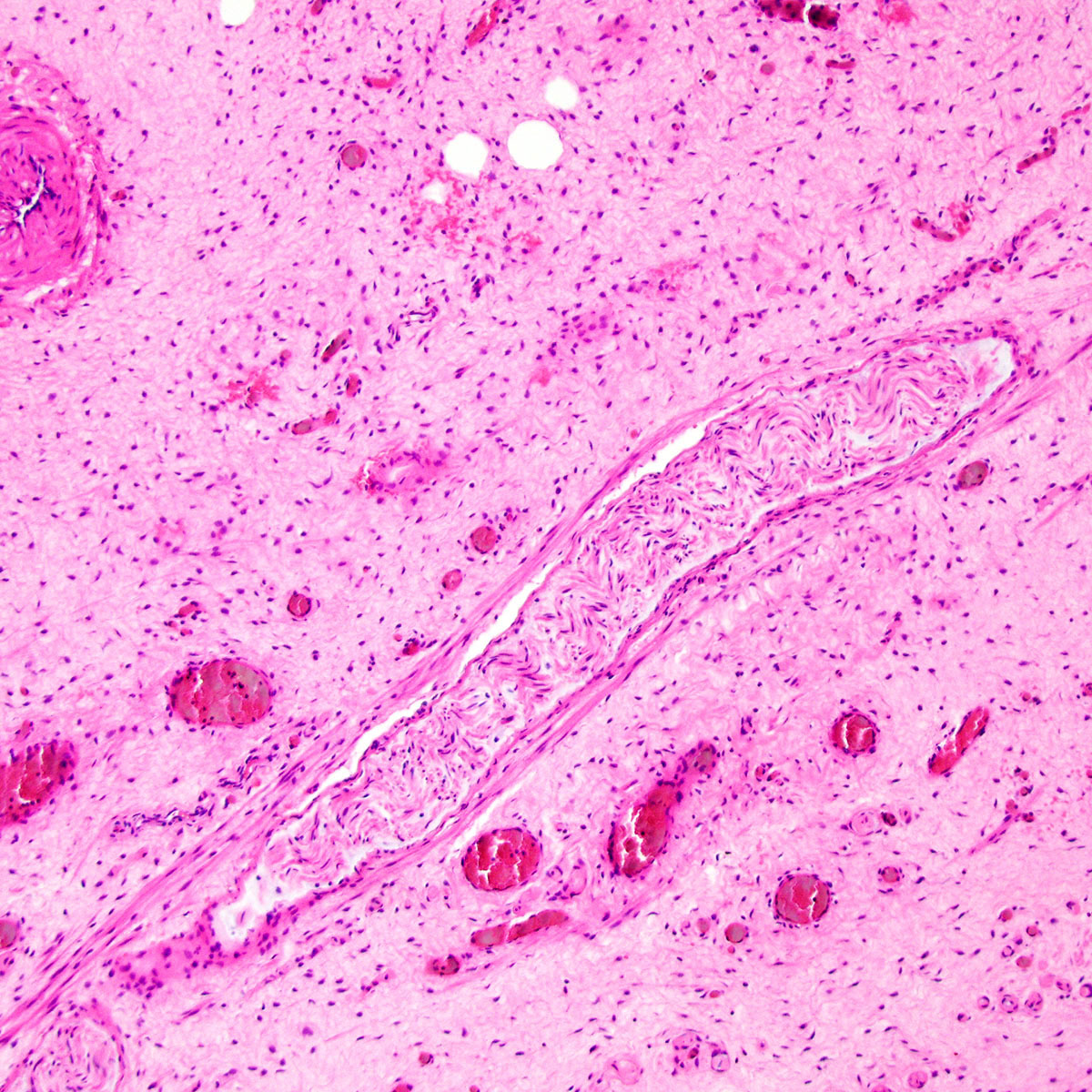
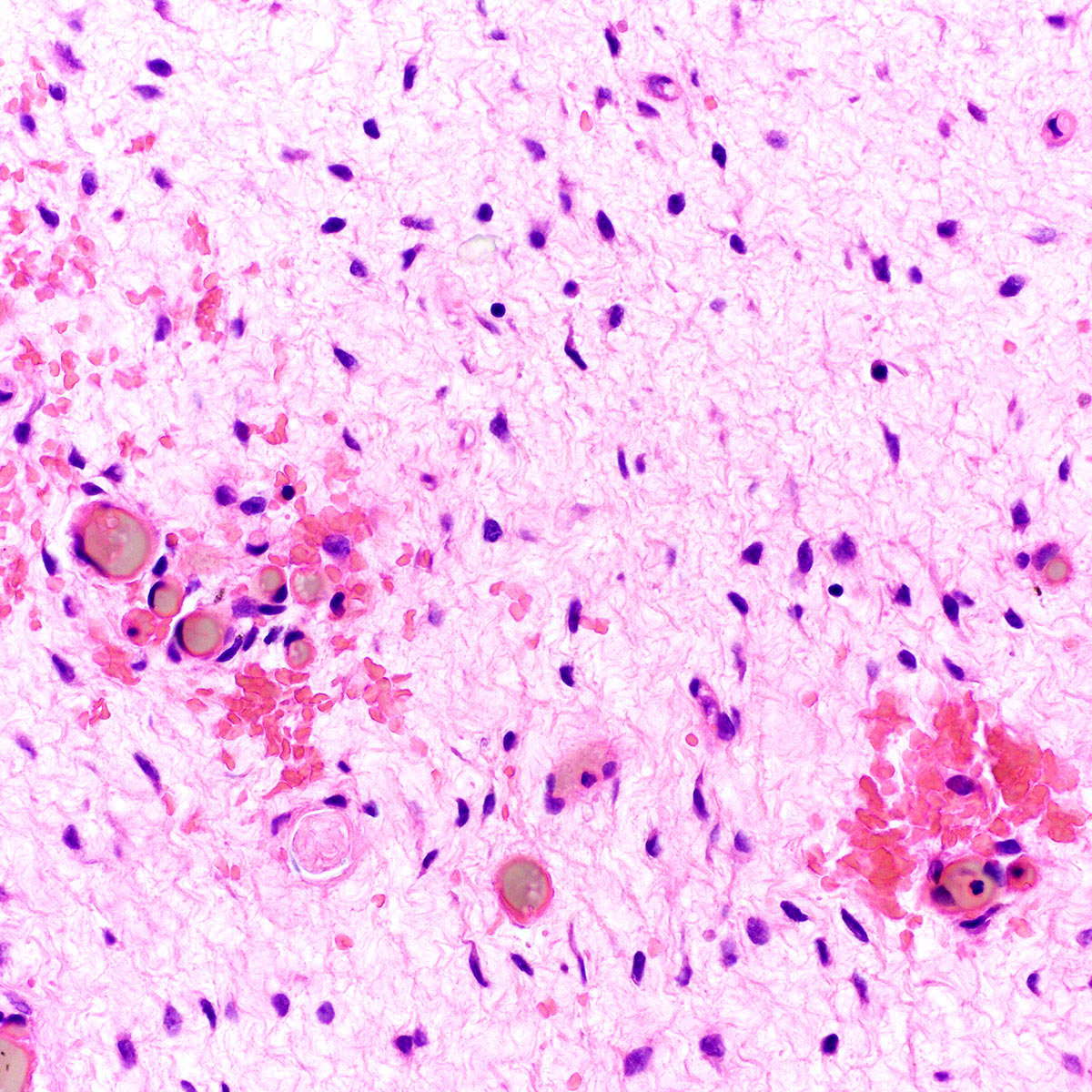
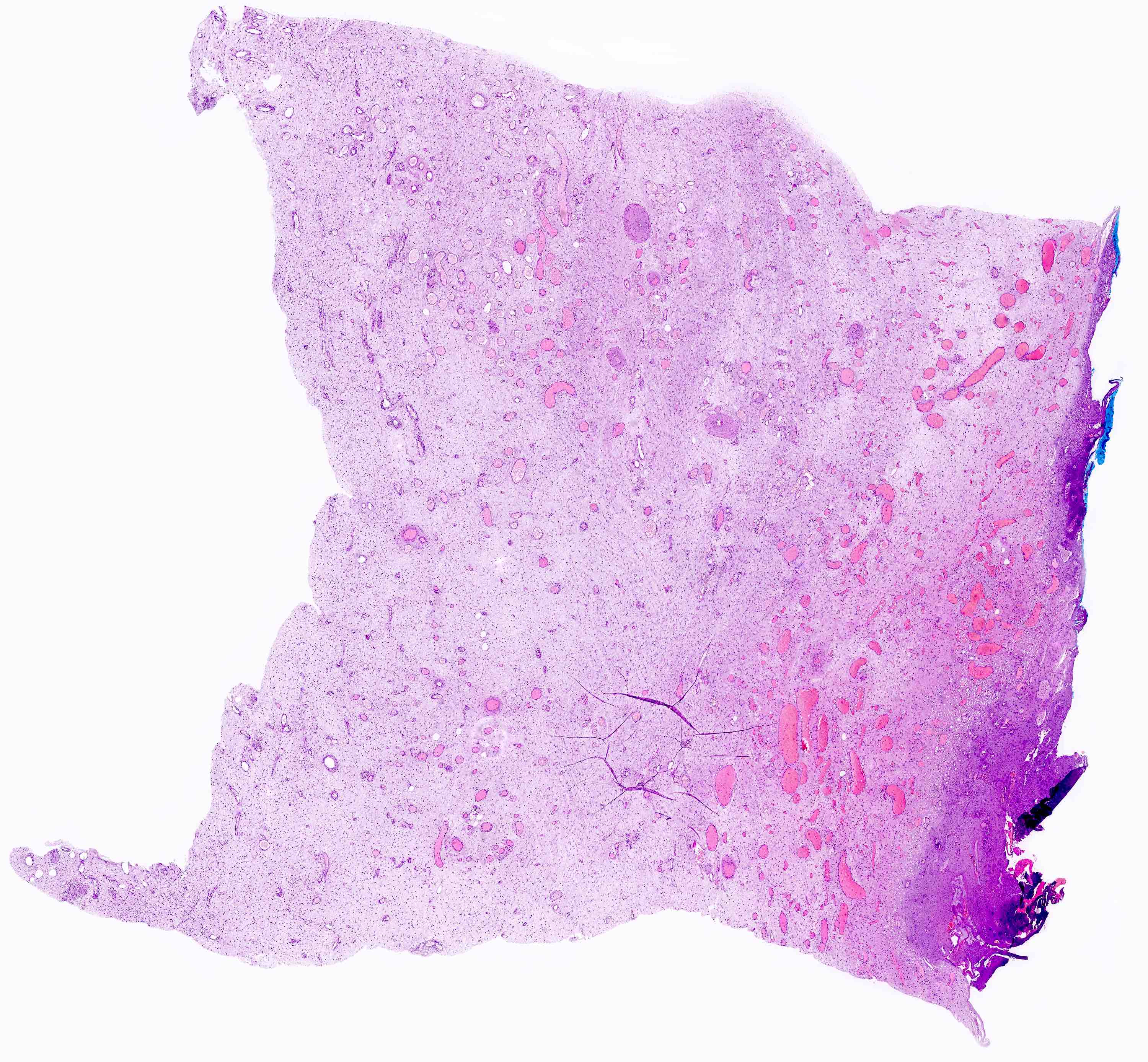
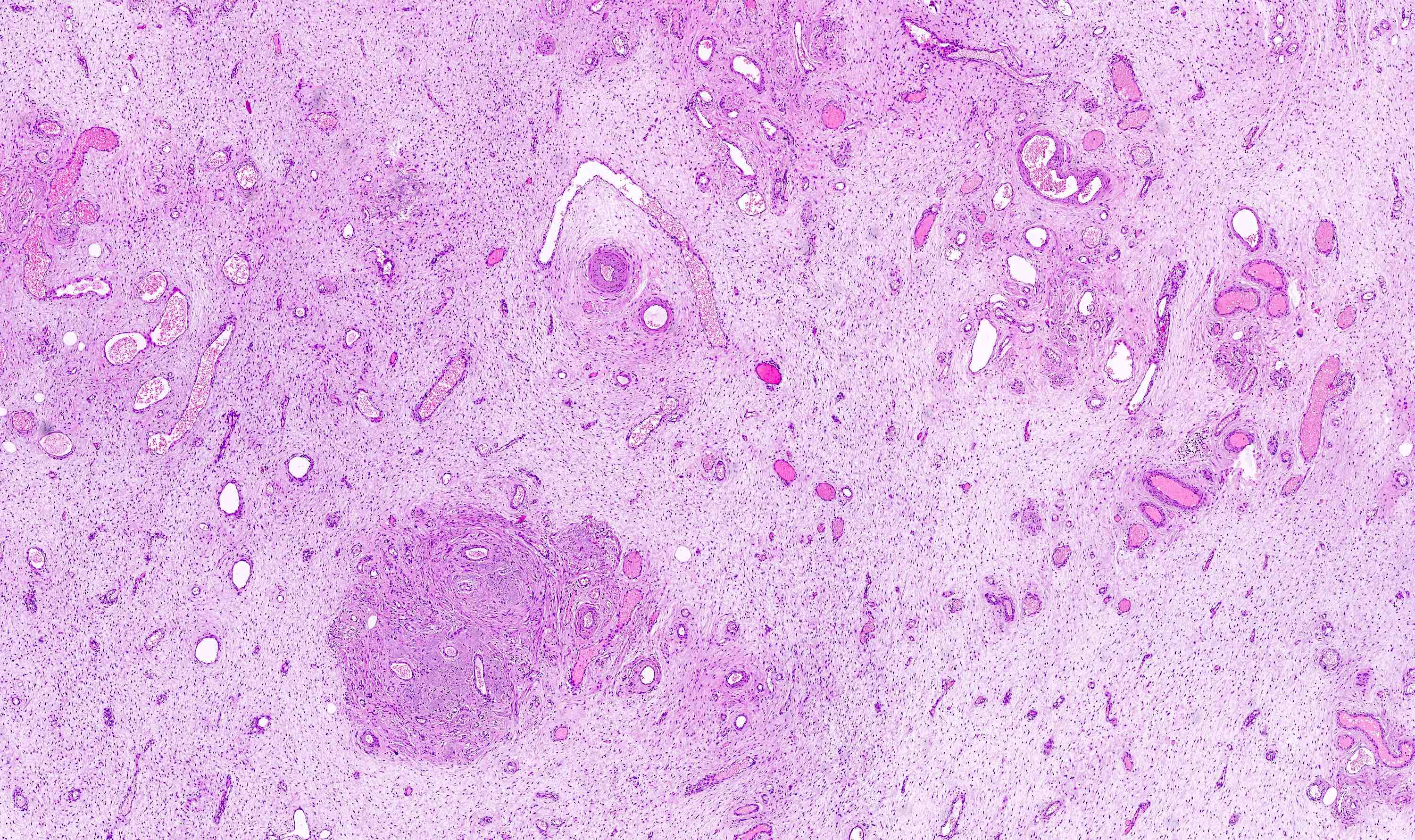
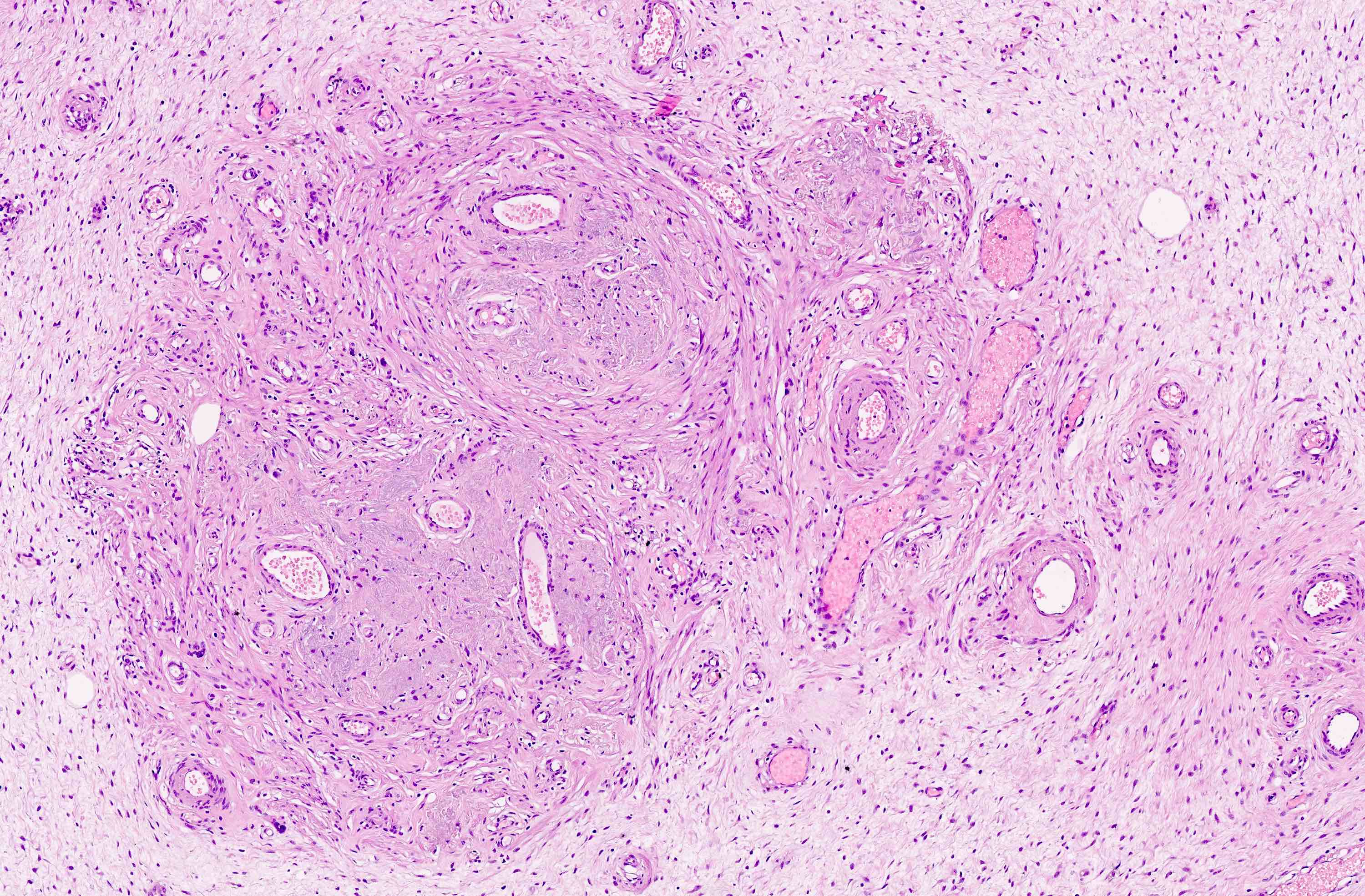
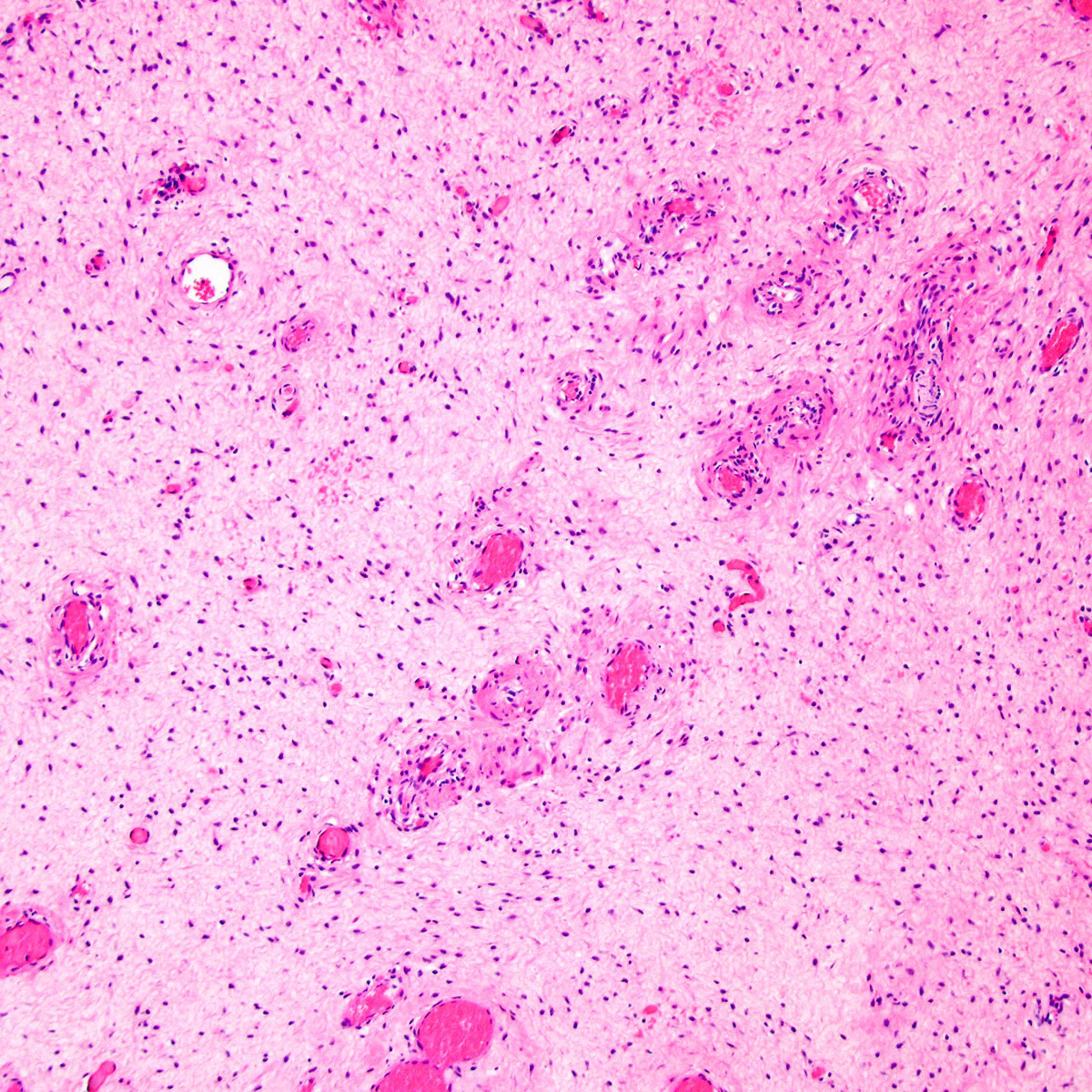
Microscopic (histologic) description
- Unencapsulated and locally infiltrative (Am J Surg Pathol 1983;7:463, Hum Pathol 1985;16:621, Am J Dermatopathol 1993;15:446, Histopathology 1997;30:3)
- Hypocellular (though focally increased cellularity is common) (Histopathology 1997;30:3)
- Tumor cells:
- Spindle to stellate with delicate cytoplasmic processes
- Bland chromatin with small nucleoli
- Mitoses rare or absent
- Tumor stroma:
- Myxoid stroma with scattered delicate collagen fibers
- Stromal mucin positive for Alcian blue
- Stroma peripherally entraps fat, nerves and muscle
- Extravasated red blood cells common (Histopathology 1997;30:3)
- Necrosis absent
- Vasculature:
- Conspicuous haphazard dilated capillaries
- Scattered large, thick walled (medial hypertrophy) or hyalinized vessels
- Vessels are nonanastomosing but may cluster together
- Stromal smooth muscle bundles cluster around tumor vessels (Cancer 1996;78:79, Histopathology 1997;30:3)
- Recurrent lesions may show increased cellularity, increased vasculature and dense stromal collagen
- Rare cases may show minor foci with morphologic features of angiomyofibroblastoma (Histopathology 1997;30:3)
Microscopic (histologic) images
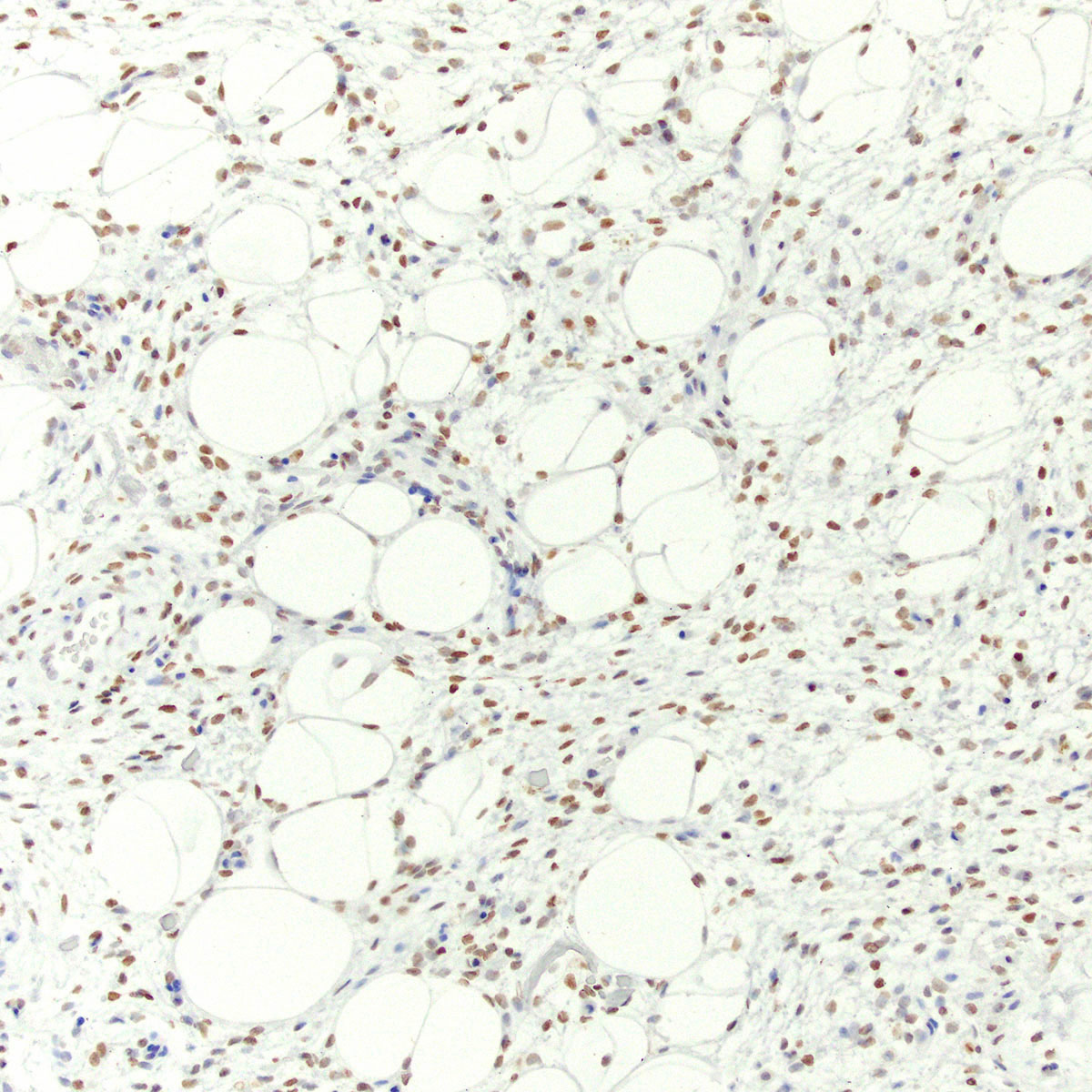
Positive stains
- Myogenic markers: desmin, SMA, calponin, MSA
- May be focal
- Highlight perivascular myoid bundles (Cancer 1996;78:79, Histopathology 1997;30:3)
- Hormone receptors: ER, PR (Cancer 1996;78:79, Int J Gynecol Pathol 2007;26:494, Ultrastruct Pathol 2003;27:227)
- HMGA2 (90%) (Genes Chromosomes Cancer 2001;32:172, Mod Pathol 2010;23:1657)
- CD34 (~ 50%) (Cancer 1996;78:79, Ultrastruct Pathol 2003;27:227)
- CDK4 often positive but without MDM2 co-expression (Virchows Arch 2005;446:157)
Negative stains
Electron microscopy description
- Variable fibroblastic and myofibroblastic differentiation (Am J Surg Pathol 1983;7:463, Hum Pathol 1985;16:621, Am J Dermatopathol 1993;15:446, Ultrastruct Pathol 2003;27:227)
Molecular / cytogenetics description
- Balanced HMGA2 rearrangement (chr 12) in approximately 33% (Genes Chromosomes Cancer 2007;46:981)
- HMGA2 rearrangements and overexpression are confined to stromal spindle cells (Genes Chromosomes Cancer 2001;32:172)
- HMGA2 breakpoints and fusion partners highly variable (Genes Chromosomes Cancer 2001;32:172, Virchows Arch 2006;448:838, Int J Gynecol Pathol 2007;26:494, Cancer Genet Cytogenet 2008;181:119)
- Mechanism of HMGA2 overexpression in cases without HMGA2 rearrangement is unclear
Videos
Histopathology
Sample pathology report
- Vulva, mass, wide local excision:
- Aggressive angiomyxoma with involvement of the deep margin (see comment)
- Comment: Microscopic examination reveals a poorly circumscribed hypocellular lesion with myxoid stroma and abundant vasculature, infiltrating fibroadipose tissue. By immunohistochemistry, lesional stromal cells are positive for desmin, ER, PR and HMGA2. The morphologic and immunophenotypic findings are most consistent with aggressive angiomyxoma. Although distant metastasis is exceptionally rare, approximately 30% of such lesions recur locally. Clinical follow up is advised.
Differential diagnosis
- Angiomyofibroblastoma:
- Well circumscribed, noninfiltrative
- Hypo and hypercellular foci
- Epithelioid to plasmacytoid tumor cells
- Tumor cells cluster around small to medium sized, nonhyalinized, thin walled vessels
- HMGA2 negative (rarely positive) (Int J Gynecol Pathol 2021;40:185)
- Lacks HMGA2 rearrangements
- Superficial angiomyxoma:
- Myxoid neurofibroma:
- Characteristic buckled nuclei
- Vasculature less conspicuous
- Contain intrinsic nerve fibers or Meissner (neuroid) bodies
- S100 positive
- Subset occur in context of neurofibromatosis
- Myxoid smooth muscle tumor:
- Cigar shaped nuclei with eosinophilic cytoplasm, at least focally
- Less abundant, large, thick walled vessels
- SMA, desmin and caldesmon positive
- HMGA2 IHC may be positive in a subset (Int J Gynecol Pathol 2021;40:185)
- Myxoid liposarcoma:
- Rarely primary in gynecologic tract (Int J Gynecol Pathol 2015;34:390)
- Lipoblasts present; may be more conspicuous peripherally
- Subset shows cellular, less differentiated round cell component
- Characteristic plexiform (crow's feet) vasculature
- ER and PR negative
- DDIT3 - FUS (t(12;16)) fusion
- Atypical lipomatous tumor / well differentiated liposarcoma:
- Rarely primary in gynecologic tract (Int J Gynecol Pathol 1998;17:17, Int J Gynecol Pathol 2015;34:390)
- Atypical mature fat with scattered hyperchromatic cells
- Vasculature less conspicuous
- CDK4 and MDM2 co-expressed
- ER and PR negative
- Underlying MDM2, CDK4, HMGA2 (chr 12) amplification
- Low grade myxofibrosarcoma:
- Low grade fibromyxoid sarcoma:
Additional references
- Vulvar soft tissue tumor review (Semin Diagn Pathol 2021;38:85), HMGA2 rearrangement in aggressive angiomyxoma (Int J Gynecol Pathol 2006;25:403), fallopian tube prolapse mimicking aggressive angiomyxoma (Int J Gynecol Pathol 2005;24:292), aggressive angiomyxoma mimicking myxoid liposarcoma (Am J Dermatopathol 2000;22:368)
Practice question #1
A 35 year old woman presented to her gynecologist with a complaint of pelvic discomfort and a slowly growing, ill defined vulvar mass. Radiology showed a vascular lesion extending from the vulva into deep pelvic soft tissues. An excision was performed. A representative photomicrograph of the lesion is shown. By immunohistochemistry, the lesional stromal cells were positive for desmin, ER and PR. Which of the following statements about this lesion is true?
- Approximately 50% of patients develop distant metastases
- FISH reveals a characteristic DDIT3 rearrangement
- HMGA2 is positive (overexpressed) by immunohistochemistry
- Immunohistochemistry for S100 is characteristically positive
- Less than 10% of patients experience local recurrence
Practice answer #1
C. HMGA2 is positive (overexpressed) by immunohistochemistry. This is an aggressive angiomyxoma.
Comment Here
Reference: Aggressive angiomyxoma-vulva
Comment Here
Reference: Aggressive angiomyxoma-vulva
Practice question #2
Which of the following most accurately describes the expected immunophenotype of aggressive angiomyxoma?
- Desmin, ER, PR, HMGA2 positive; S100 negative
- S100, EMA, NF positive; ER, PR negative
- SMA, CD34 positive; ER, PR negative
- SMA, desmin, caldesmon positive; S100 negative
- SMA, ER, PR positive; Rb negative (lost)
Practice answer #2
A. Desmin, ER, PR, HMGA2 positive; S100 negative
Comment Here
Reference: Aggressive angiomyxoma-vulva
Comment Here
Reference: Aggressive angiomyxoma-vulva