Table of Contents
Definition / general | Essential features | Diagrams / tables | WHO 2004 / 2016 PUNLMP | WHO 2004 / 2016 low grade | WHO 2004 / 2016 high grade | WHO 1973 grade 1 | WHO 1973 grade 2 | WHO 1973 grade 3 | Grade heterogeneity | Microscopic (histologic) images | Board review style question #1 | Board review style answer #1Cite this page: van der Kwast T. Grading-bladder. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/bladdergrading.html. Accessed April 25th, 2024.
Definition / general
- Nonmuscle invasive urothelial carcinomas are graded following the 2 tier WHO 2004 / 2016 (endorsed by the American Urologic Association and European Association of Urology) or the 3 tier WHO 1973 grading systems (endorsed by the European Association of Urology)
- WHO 2004 / 2016 classification of urothelial neoplasms includes papillary urothelial neoplasm of unknown malignant potential (PUNLMP)
- Grading of nonmuscle invasive papillary urothelial carcinomas determines the risk of stage progression in recurrent bladder cancer
- Invasive urothelial carcinomas, independent of the degree of invasion, are generally graded as WHO 2004 / 2016 high grade (World J Urol 2019;37:41)
Essential features
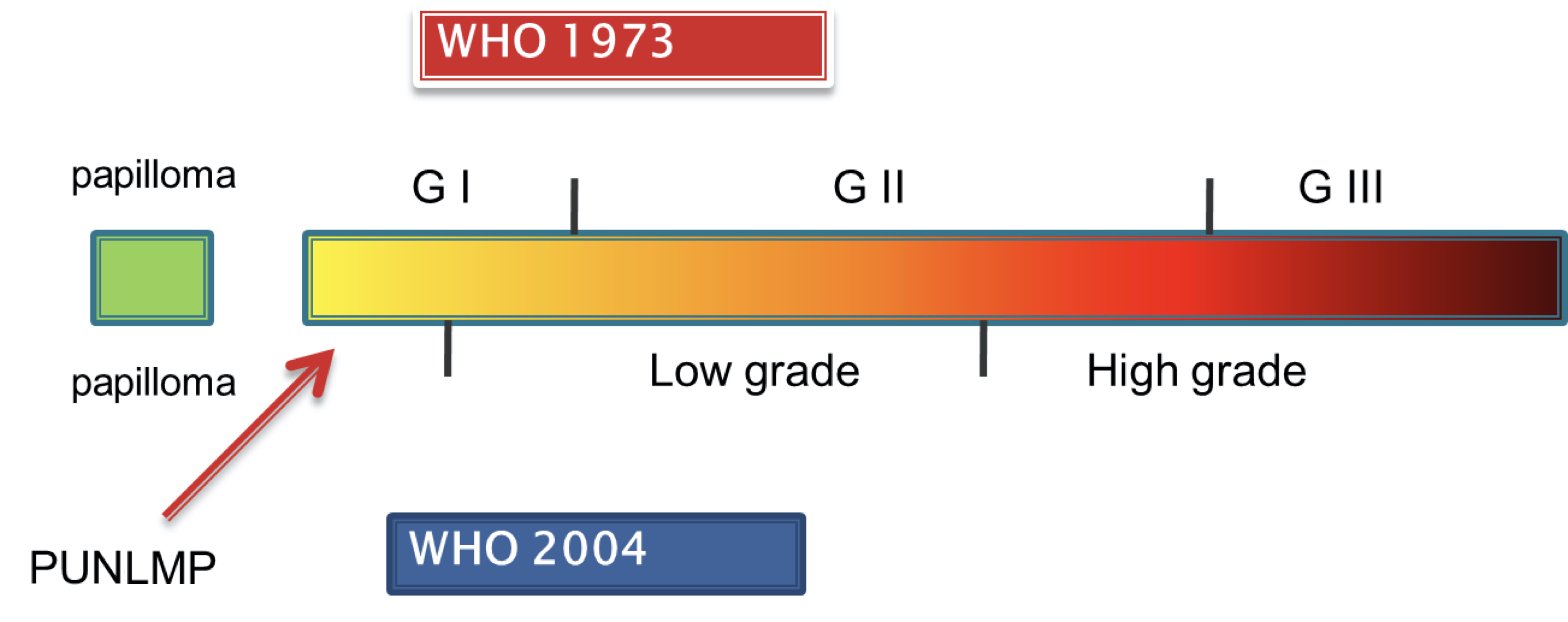
- Grading of (papillary) urothelial carcinomas is based on the level of orderedness of the urothelial lining at intermediate power and nuclear atypia
- Orderedness represents a continuum, varying from very well ordered to chaotic with increasing nuclear atypia (see Diagrams / tables)
- Substantial interobserver variation due to lack of landmarks separating the different grades
- 2 grading systems (WHO 1973 and 2004 / 2016) cannot be translated directly into each other due to overlapping grades (Eur Urol 2010;57:1052)
- WHO 1973 but not WHO 2004 / 2016 grading of pT1 bladder cancer is prognostic for stage progression (BJU Int 2011;107:404)
- In urothelial carcinomas with grade heterogeneity, the highest grade is reported
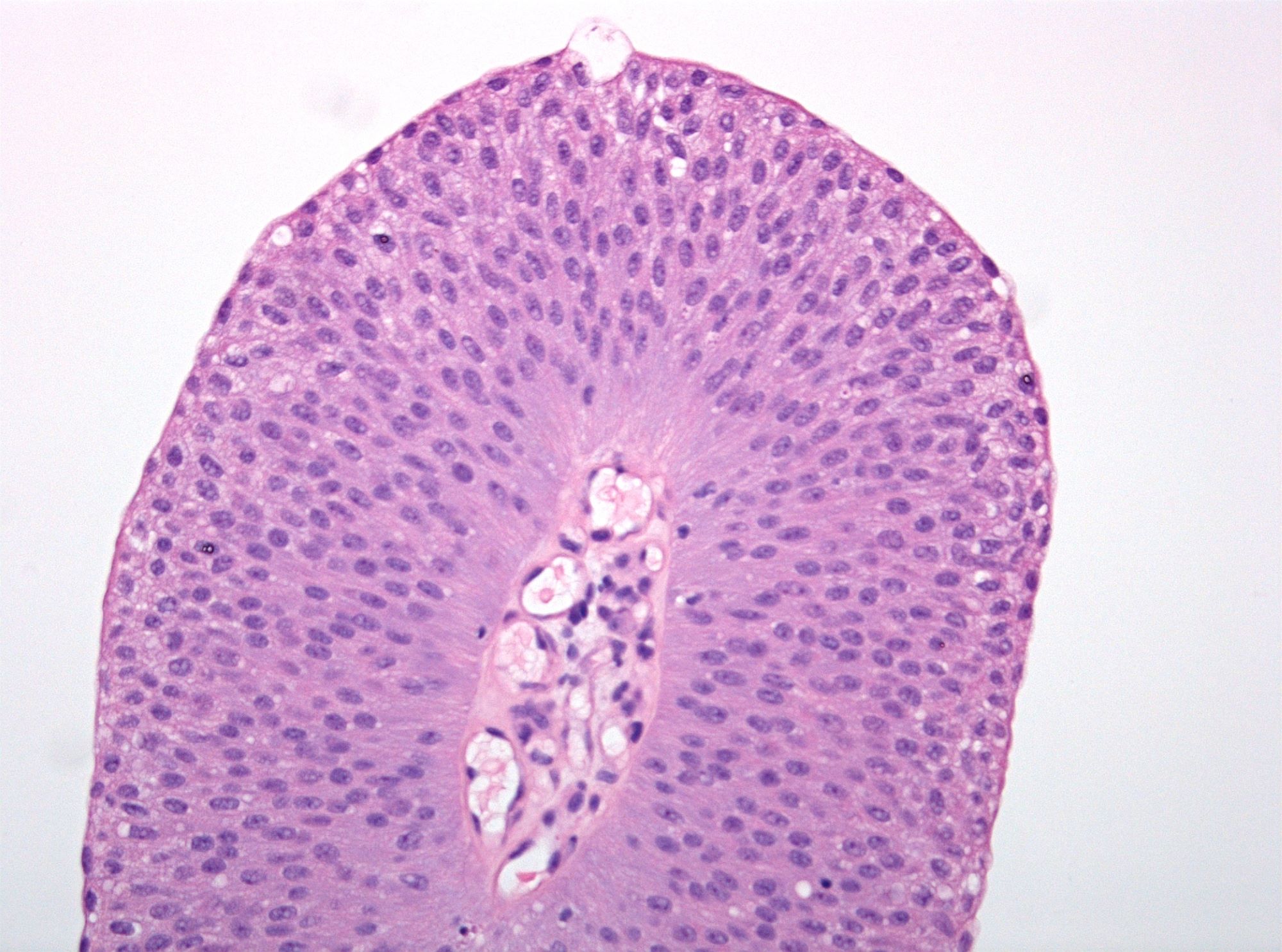
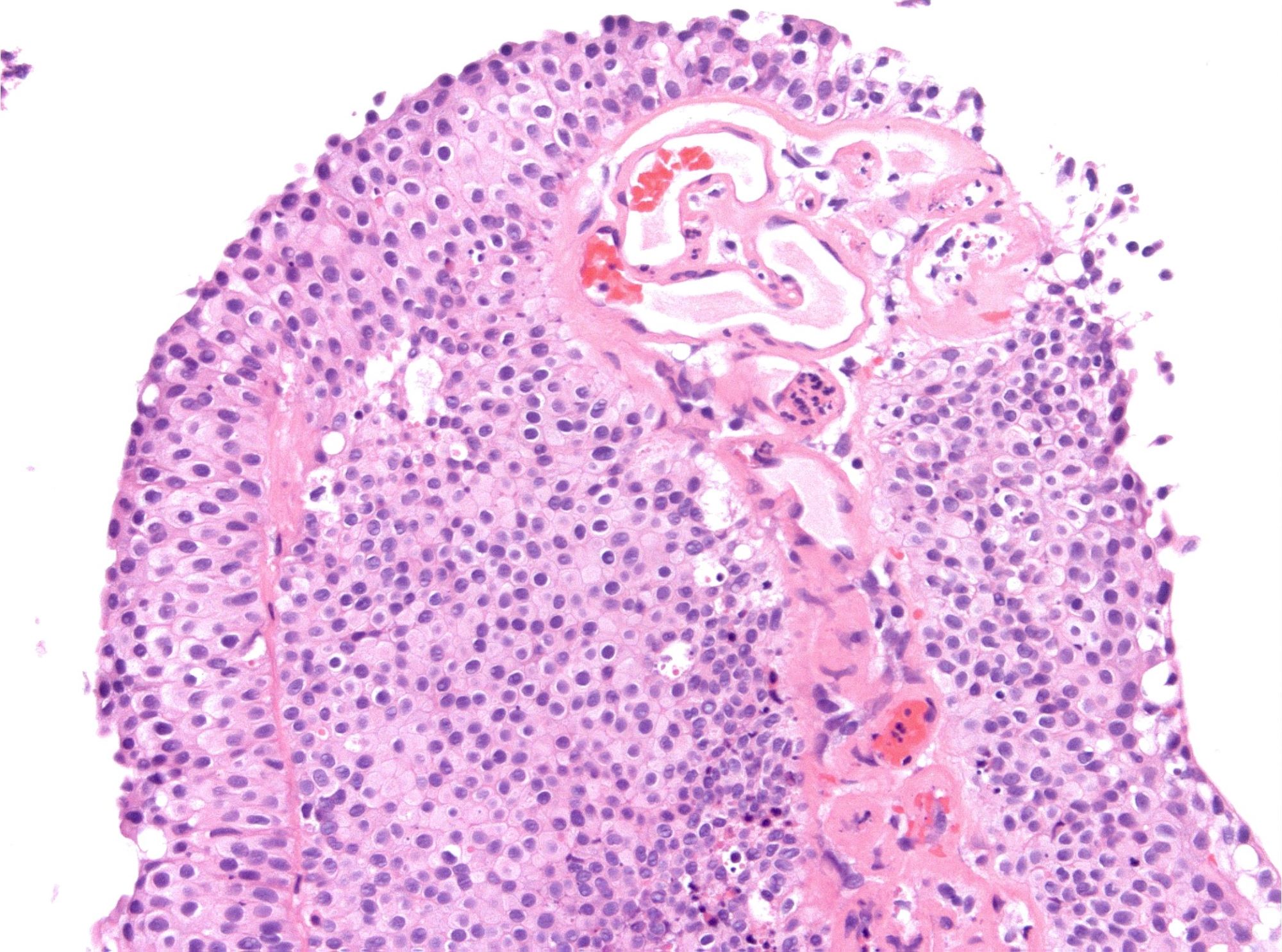
WHO 2004 / 2016 PUNLMP
- Clinical description
- Frequency: < 5% of noninvasive papillary neoplasms with controversy regarding use of this designation; some experts advise elimination of this category (Urol Oncol 2020;38:440, Histopathology 2020;77:525)
- Manifestation by micro or gross hematuria
- Urine cytology negative
- Cystoscopy shows exophytic sea anemone-like tumor
- Microscopic description
- Increased thickness of papillary structures with slender fibrovascular cores
- Ordered layering (streaming) of uniform nuclei with preserved polarity
- Inconspicuous nucleoli
- No variation in nuclear size, contour or shape
- No nuclear hyperchromasia
- Minimal mitotic activity confined to basal layers
- Presence of umbrella cell layer
- References: Eble: Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs, 1st Edition, 2004, Pathol Int 2010;60:1
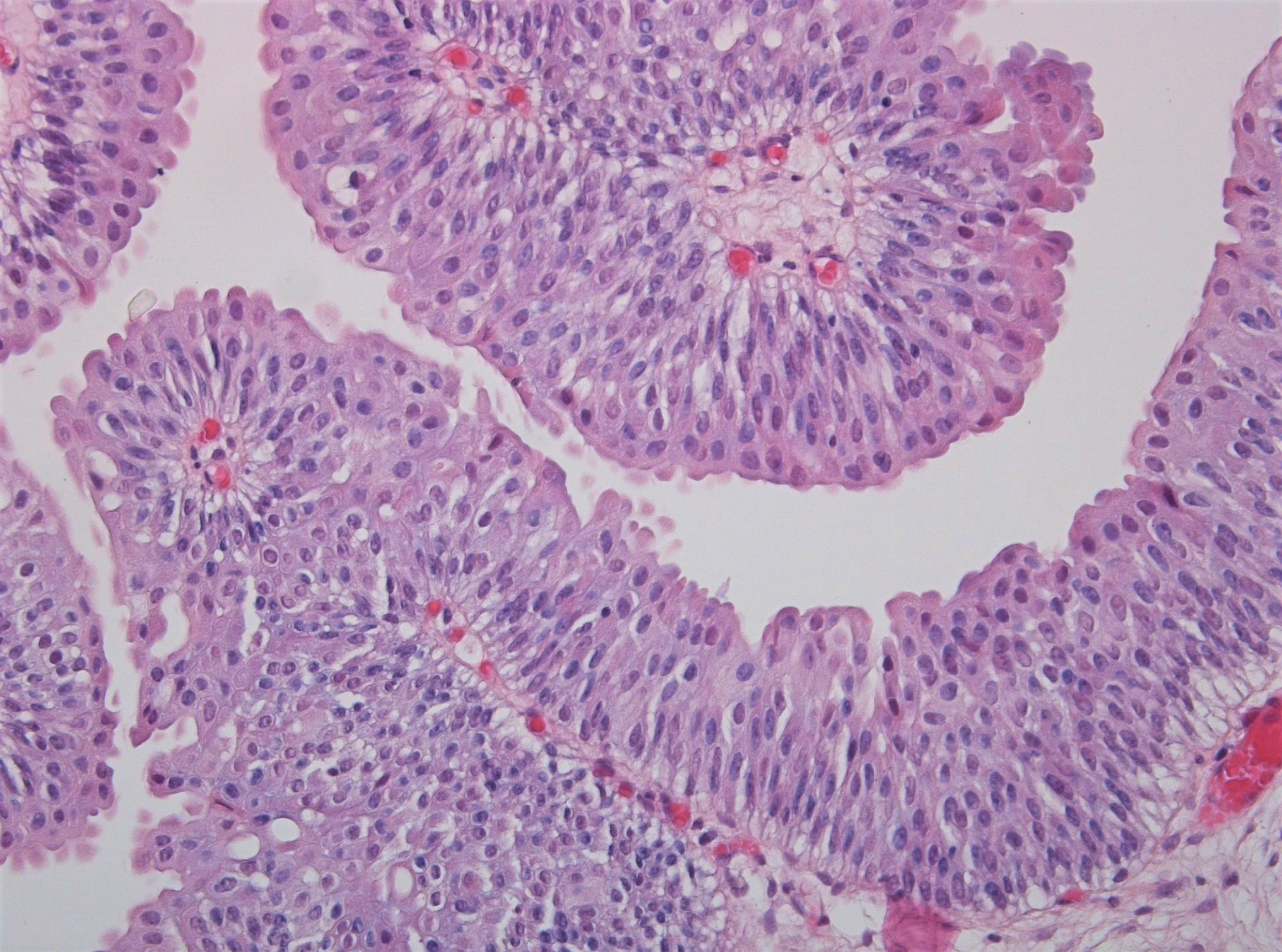
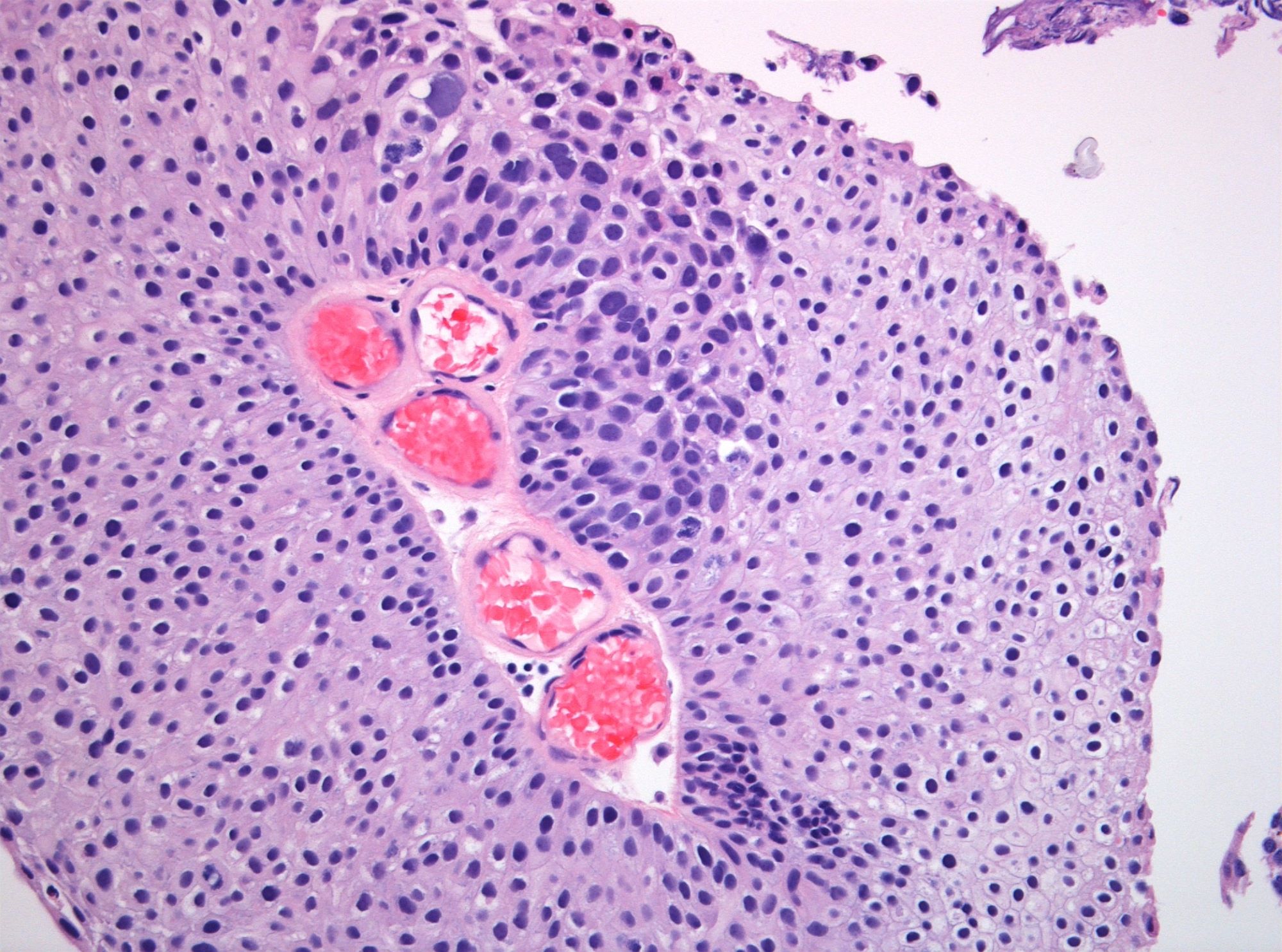
WHO 2004 / 2016 low grade
- Clinical description
- Frequency: 60 - 70% in Ta, 10 - 20% in T1 (Eur Urol Oncol 2021;4:182)
- Manifestation by micro or gross hematuria
- Urine cytology almost always negative
- Cystoscopy shows exophytic sessile or polypoid lesion
- Microscopic description
- Increased thickness of papillary structures with slender fibrovascular cores
- Ordered layering of somewhat enlarged nuclei with variation in polarity
- Mild variation in nuclear size, contour or shape
- Limited mitotic activity may extend to suprabasal layers
- Presence of umbrella cell layer
- References: Eble: Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs, 1st Edition, 2004, Pathol Int 2010;60:1
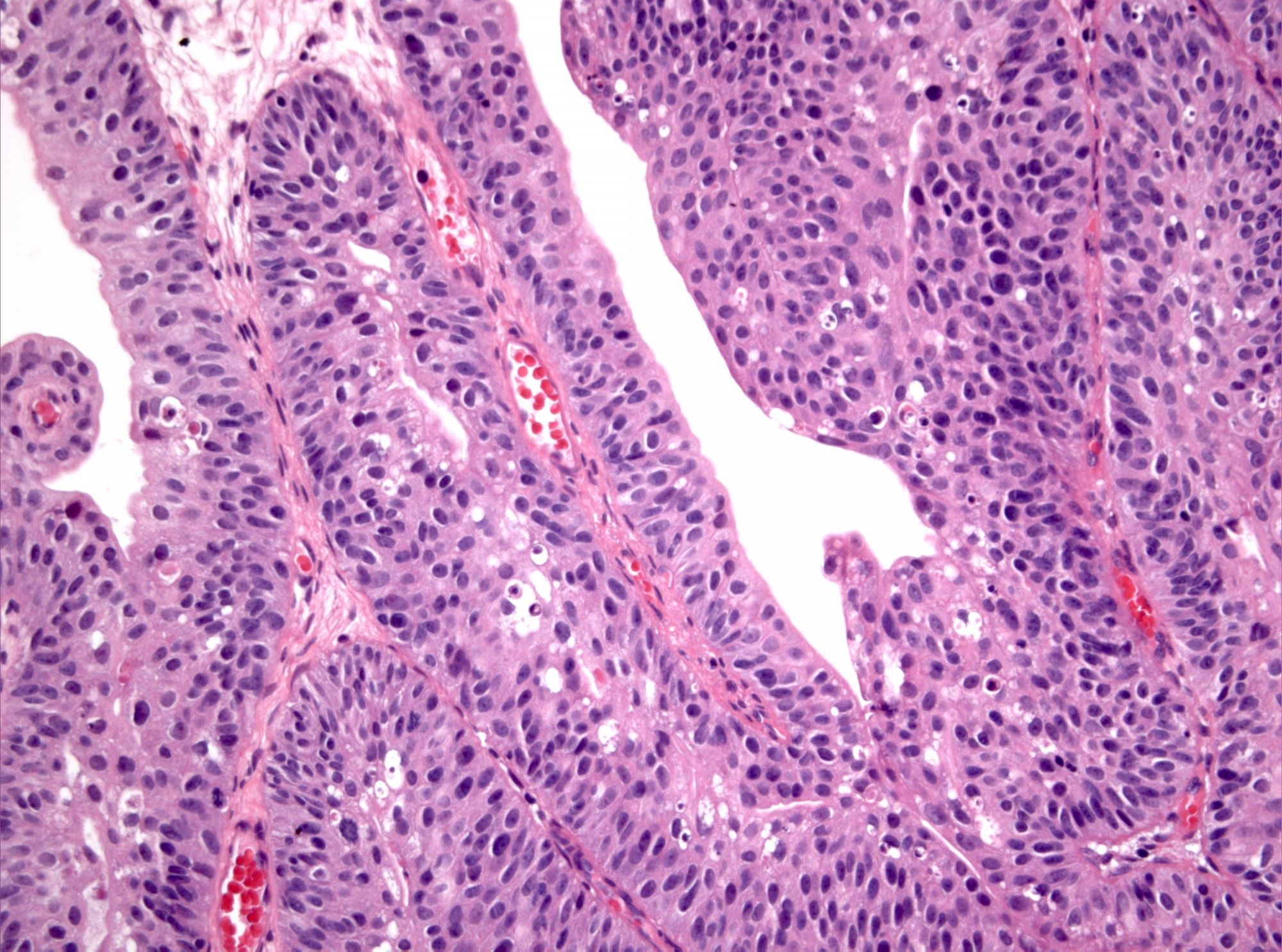
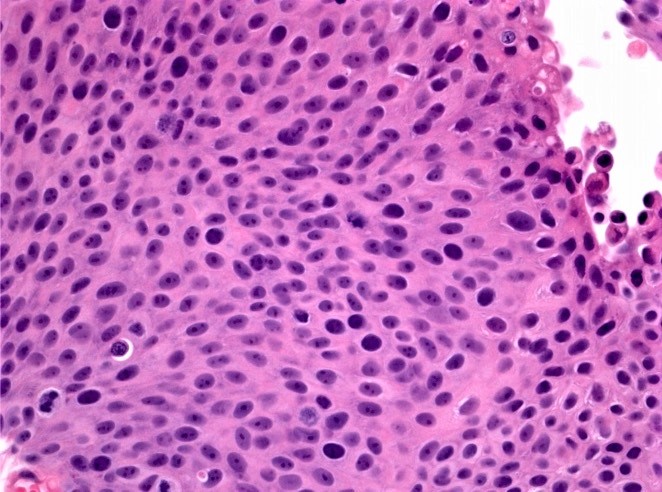
WHO 2004 / 2016 high grade
- Clinical description
- Frequency: 30% in Ta, 80 - 90% in T1 (Eur Urol Oncol 2021;4:182)
- Manifestation by gross or microscopic hematuria
- Urine cytology often positive
- Cystoscopy shows exophytic sessile, solid or polypoid lesion
- Microscopic description
- Papillary structures of variable thickness with fibrovascular cores
- Disordered layering with loss of polarity
- Variably enlarged nuclei and nuclear crowding
- Variation in nuclear size, contour or shape
- Nuclear hyperchromasia may be present
- Mitotic activity may extend to upper layers
- Umbrella cell layer generally indiscernible
- References: Eble: Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs, 1st Edition, 2004, Pathol Int 2010;60:1
WHO 1973 grade 1
- Clinical description
- Frequency: 35% in pTa, < 5% in pT1
- Manifestation by micro or gross hematuria
- Urine cytology almost always negative
- Cystoscopy shows exophytic sessile or polypoid lesion
- Microscopic description
- Increased thickness of papillary structures with slender fibrovascular cores
- Ordered layering with streaming of uniform nuclei
- No or minimal nuclear enlargement
- No or mild variation in nuclear size, contour or shape
- Nuclear grooves
- No nuclear hyperchromasia
- Limited mitotic activity may extend to suprabasal cell layers
- Presence of umbrella cell layer
- References: WHO: Histological Typing of Urinary Bladder Tumours [Accessed 14 June 2021], Eur Urol Focus 2021 Mar 23 [Epub ahead of print]
WHO 1973 grade 2
- Clinical description
- Frequency: 55% in pTa, 40% in pT1
- Manifestation by micro or gross hematuria
- Urine cytology often positive
- Cystoscopy shows exophytic sessile or polypoid lesion
- Microscopic description
- Not WHO 1973 grade 1 or 3
- Reference: WHO: Histological Typing of Urinary Bladder Tumours [Accessed 14 June 2021]
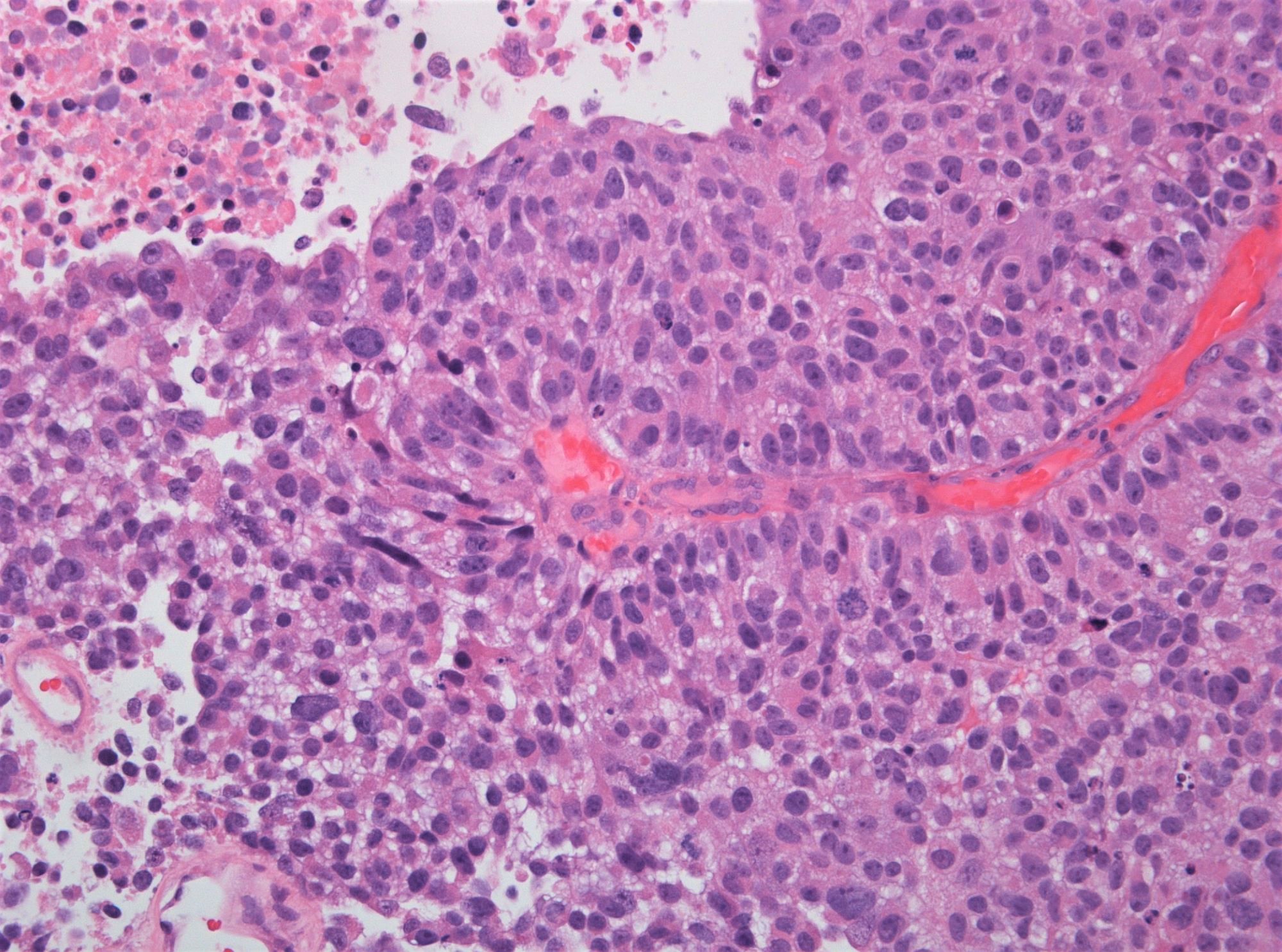
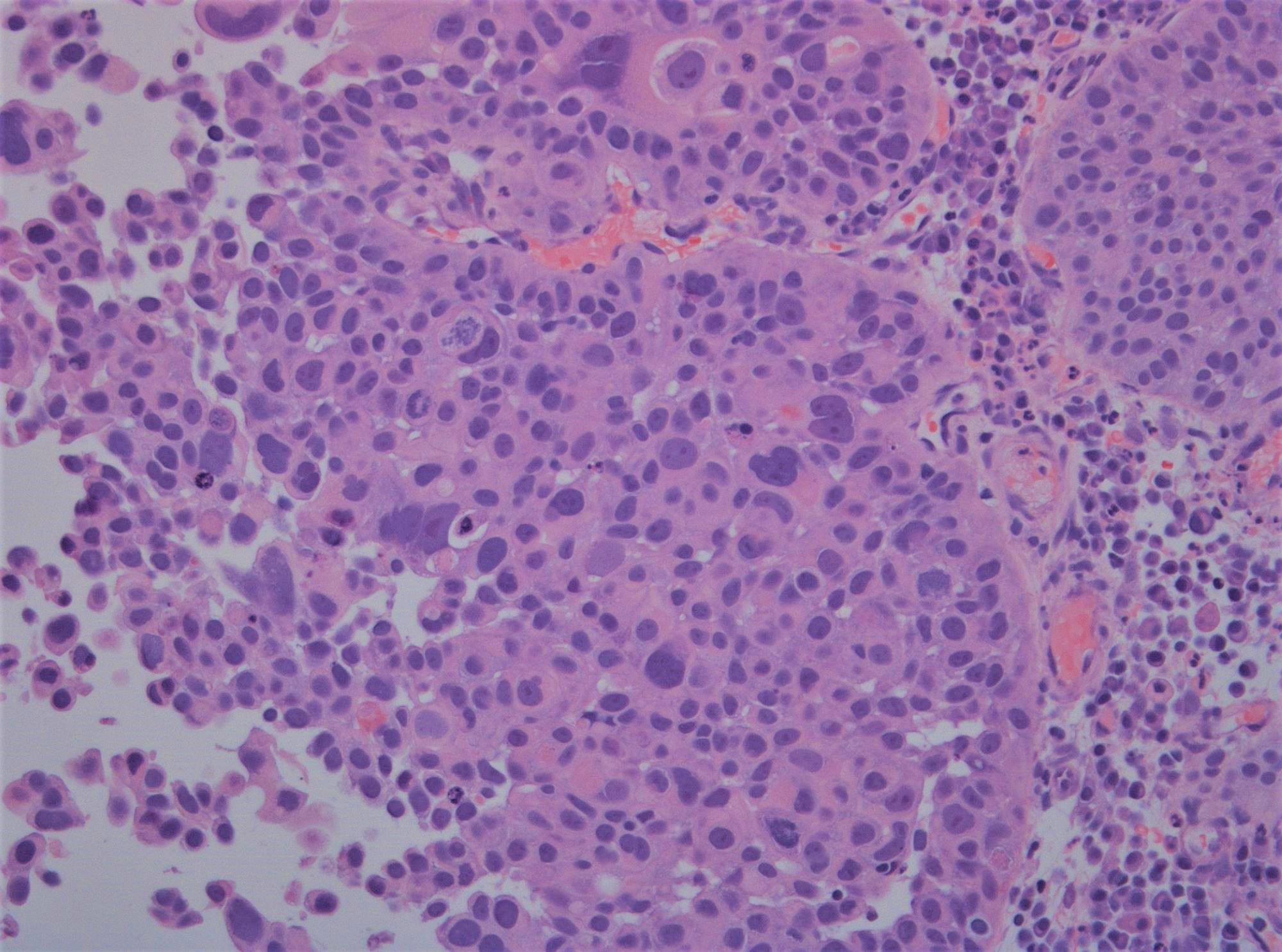
WHO 1973 grade 3
- Clinical description
- Frequency: 12% in pTa, 55% in pT1 (World J Urol 2019;37:41)
- Manifestation by gross or microscopic hematuria
- Positive urine cytology
- Cystoscopy shows exophytic sessile, solid or polypoid lesion
- Microscopic description
- Papillary structures of variable thickness with fibrovascular cores
- Disordered layering with variability in polarity and nuclear crowding
- Substantially increased nuclear size
- Strong variation in nuclear size, contour or shape
- Marked nuclear hyperchromasia
- Prominent mitotic activity extending into upper layers
- Umbrella cell layer absent
- Reference: WHO: Histological Typing of Urinary Bladder Tumours [Accessed 14 June 2021], Eur Urol Focus 2021 Mar 23 [Epub ahead of print]
Grade heterogeneity
- Clinical description
- Frequency: up to 30% (Cancer 2000;88:1663)
- Manifestation by microscopic or gross hematuria
- Occasionally positive urine cytology
- Cystoscopy shows exophytic sessile, solid or polypoid lesion
- Microscopic description
- Distinct areas of low and high grade urothelial carcinoma
- Clear demarcation of separate areas
- Reporting
- By convention, the highest grade is reported if comprising > 5% of the carcinoma
- If < 5%, a comment on its presence is made
Microscopic (histologic) images
Board review style question #1
Board review style answer #1