Roche companion diagnostic testing library
Copyright: 2020-2024, PathologyOutlines.com, Inc.
Revised: 22 February 2021
For information about Roche Diagnostics, visit diagnostics.roche.com or call 1-800-428-5076.
* This library contains material produced by Roche which has not been peer-reviewed by PathologyOutlines.com.
* Pathologists should contact Dr. Pernick (Nat@PathologyOutlines.com) to suggest additional material to add to this page.
For comprehensive information on the VENTANA PD-L1 (SP142) assay, including training resources and an assay interpretation guide, visit go.roche.com/pdl1SP142.
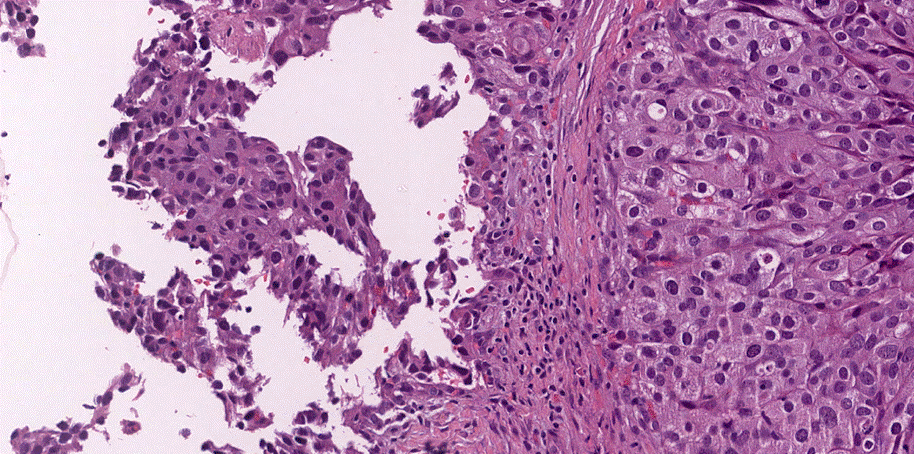
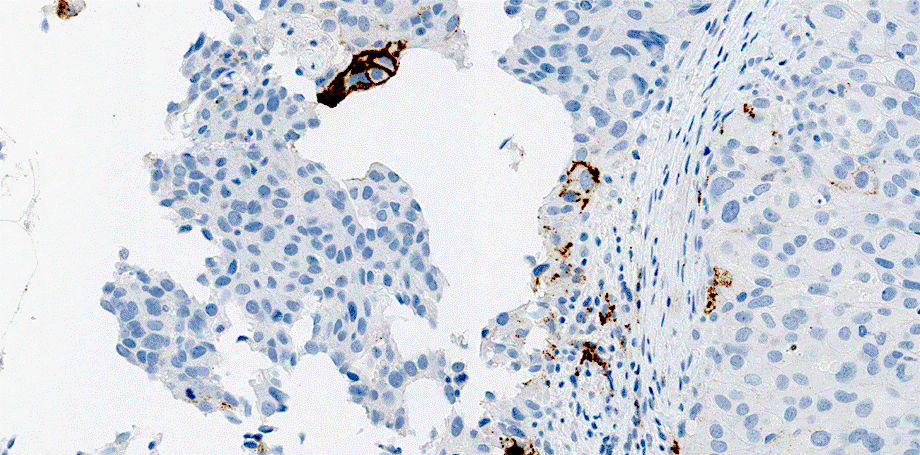
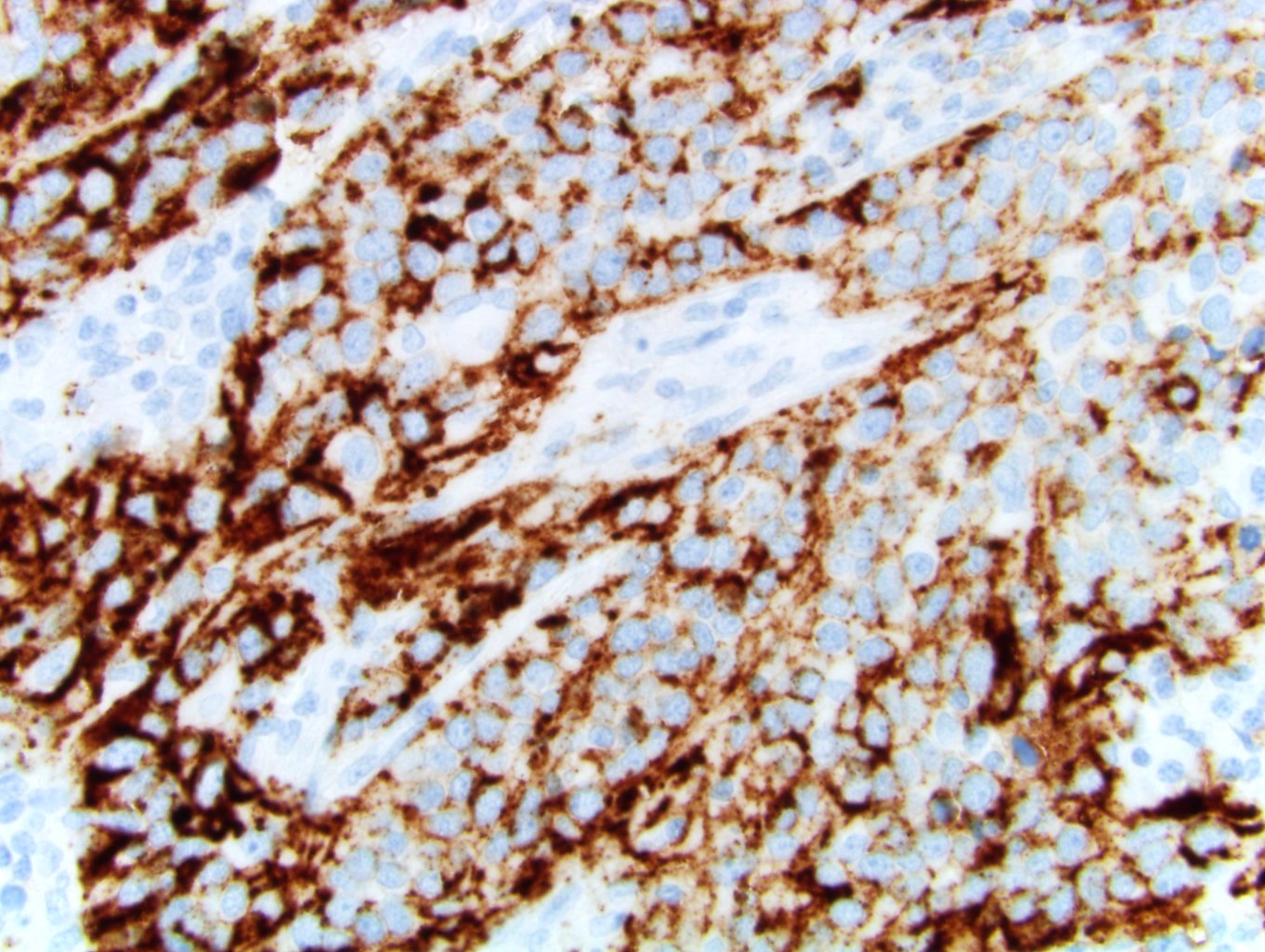
Microscopic (histologic) images:

Urothelial carcinoma
Interpretation guide (PDF)
Videos available on YouTube:
PD-L1 Expression on Immune and Tumor Cells
Dr. Michael Lynch, a pathologist with Roche Diagnostics Medical and Scientific Affairs (MSA), discusses:
- How and why PD-L1 is present on tumor cells, as well as being present on immune cells
- The different tumor types that show PD-L1 expression
- The dramatic response of some patients to anti-PD-L1 or anti-PD-1 therapy, or immunotherapy
- The diagnostic tests that are available for PD-L1 and how they should be used
- The VENTANA PD-L1 (SP142) diagnostic assay and how it stains both tumor cells and immune cells
- The patterns of staining and why they might be important
Tumor Microenvironment and PD-L1 biomarker
Dr. Michael Lynch, a pathologist with Roche Diagnostics Medical and Scientific Affairs (MSA), provides an overview of the function and role PD-L1 plays in tumor biology.
The presentation focuses on:
- The tumor microenvironment
- The cancer immunity cycle
- The checkpoint immunity system
- The role of PD-L1 in the checkpoint immunity system
- Patterns of staining that can be seen with the H&E stain and the VENTANA PD-L1 (SP142) IHC assay
PD-L1 testing and SP142 assay as a diagnostic tool in triple negative breast cancer (TNBC)
Dr. Michael Lynch, a pathologist with Roche Diagnostics Medical and Scientific Affairs (MSA), discusses the VENTANA PD-L1 (SP142) assay, the first FDA-approved assay for metastatic triple negative breast cancer (TNBC). Testing for PD-L1 identifies patients who could benefit from TECENTRIQ + nab-paclitaxel in the first line treatment for this disease.
The presentation focuses on:
- How the assay is used in TNBC
- System used for staining
- Scoring algorithm
- Tumor area definition
- Specimen requirement
- Case review microscopy session
Case Study: Assessing PD-L1 in triple negative breast cancer (TNBC) - Moderate immune cell (IC) staining without tumor cell (TC) staining
Dr. Michael Lynch, a pathologist with Roche Diagnostics Medical and Scientific Affairs (MSA), reviews three cases that show the most common tissue staining pattern a pathologist will see in the triple negative breast cancer indication with the VENTANA PD-L1 (SP142) assay.
Starting with an H&E slide, Dr. Lynch goes through a four-step assessment:
1. Do I have 50 viable tumor cells with associated stroma?
2. Identify the tumor area
3. Assess the pattern of immune infiltrate
4. Identify the area of exclusion
In evaluating the IHC slide, Dr. Lynch also goes through a four-step assessment:
1. Is there staining in the tumor area?
2. Assess if staining is on TC or IC
3. Aggregates or single-cell spread staining
4. Apply scoring algorithm for percentage of tumor area (cut-off at greater than or equal to 1 percent) with IC staining
Case Study: Assessing PD-L1 in triple negative breast cancer (TNBC) - Scoring cases with common staining patterns
Dr. Michael Lynch, a pathologist with Roche Diagnostics Medical and Scientific Affairs (MSA), reviews three cases that show the most common pattern of tissue staining a pathologist will see in the triple negative breast cancer indication with the VENTANA PD-L1 (SP142) assay.
Starting with an H&E slide, Dr. Lynch goes through a four-step assessment:
1. Do I have 50 viable tumor cells with associated stroma?
2. Identify the tumor area
3. Assess the pattern of immune infiltrate
4. Identify the area of exclusion
In evaluating the IHC slide, Dr. Lynch also goes through a four-step assessment:
1. Is there staining in the tumor area?
2. Assess if staining is on TC or IC
3. Aggregates or single-cell spread staining
4. Apply scoring algorithm for percentage of tumor area (cut-off at greater than or equal to 1 percent) with IC staining
Case Study: Assessing PD-L1 in triple negative breast cancer - Select cases with staining near the scoring algorithm cut-off
Dr. Michael Lynch, a pathologist with Roche Diagnostics Medical and Scientific Affairs (MSA), reviews three cases that show the borderline tissue staining of IC around 1% of tumor area that a pathologist will see in the triple negative breast cancer indication with the VENTANA PD-L1 (SP142) assay.
Starting with an H&E slide, Dr. Lynch goes through a four-step assessment:
1. Do I have 50 viable tumor cells with associated stroma?
2. Identify the tumor area that includes the tumor cells, associated intratumoral, and contiguous peritumoral stroma
3. Assess the pattern of immune infiltrate
4. Identify the area of exclusion
In evaluating the IHC slide, Dr. Lynch also goes through a four-step assessment:
1. Is there staining in the tumor area?
2. Assess if staining is on TC or IC
3. Aggregates or single-cell spread staining
4. Apply scoring algorithm for percentage of tumor area (cut-off at greater than or equal to 1 percent) with IC staining
Related PathologyOutlines.com resources: PDL1 SP142