Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Clinical features | Tissue handling | Interpretation | Uses by pathologists | Microscopic (histologic) images | Positive staining - normal | Positive staining - disease | Negative staining | Sample pathology report | Practice question #1 | Practice answer #1Cite this page: Tozbikian G. PDL1 22C3. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/stainspdl1.html. Accessed September 15th, 2025.
Definition / general
- Programmed death ligand 1 (PDL1) is an immune checkpoint protein expressed on activated immune cells and tumor cells (Cancer Epidemiol Biomarkers Prev 2014;23:2965, Cancer Immunol Res 2014;2:361)
- Coinhibitory factor that binds receptors programmed cell death 1 (PD1) and B7-1 on the surface of activated T cells to regulate the immune response and limit autoimmunity
- Expression of PDL1 in cancer is an adaptive immune resistance mechanism for avoiding T cell mediated anticancer immune response
Essential features
- PDL1 (22C3) is the FDA approved companion diagnostic to pembrolizumab in certain clinical scenarios for
- Non-small cell lung carcinoma (NSCLC)
- Cervical squamous cell carcinoma and endocervical adenocarcinoma
- Head and neck squamous cell carcinoma (HNSCC)
- Gastric or gastroesophageal junction (GEJ)
- Esophageal squamous cell carcinoma (ESCC)
- Triple negative breast cancer (TNBC)
- Note: indication for urothelial carcinoma was withdrawn on 2/2022
- Scoring of PDL1 (22C3) is tumor site specific
Terminology
- Alternative names: PDCD1 ligand 1, PDCD1L1, cluster of differentiation 274 (CD274), B7 homolog 1 (B7-H1), pharmDX PD-L1 (22C3)
Pathophysiology
- PDL1 is an immune checkpoint protein that regulates the immune response to prevent excessive / chronic autoimmune inflammation
- Normally expressed on activated immune cells (e.g., antigen presenting cells and B cells)
- Expression is induced in response to inflammation and high level cytokine expression (IFNγ)
- Binds regulatory surface receptors PD1 and B7 on CD8+ T cells
- Inhibits T cell activation and induces T cell exhaustion (Nat Rev Cancer 2012;12:252, Nat Med 2002;8:793)
- Cancers can express PDL1 as an adaptive immune resistance mechanism to attenuate the host antitumor immune response
- Selective blockade of the PD1 / PDL1 axis is used as a therapeutic strategy in the treatment of multiple cancers to prevent T cell inhibition and reactivate T cell mediated tumor cell killing
Clinical features
- PDL1 (22C3) is the companion diagnostic to pembrolizumab, a humanized monoclonal PD1 blocking antibody
- FDA approval for pembrolizumab for non-small cell lung carcinoma is based on the results of KEYNOTE-010 and KEYNOTE-024 clinical trials (Lancet 2016;387:1540, N Engl J Med 2016;375:1823)
- Pembrolizumab is indicated for the treatment of patients with metastatic non-small cell lung carcinoma whose tumors have high PDL1 expression (tumor proportion score ≥ 50%) if
- No EGFR or ALK genomic tumor aberrations and
- No prior systemic chemotherapy treatment for metastatic non-small cell lung carcinoma
- Pembrolizumab is indicated for the treatment of patients with metastatic non-small cell lung carcinoma whose tumors express PDL1 (tumor proportion score ≥ 1%) if there is disease progression on or after platinum containing chemotherapy
- Patients with EGFR or ALK genomic tumor aberrations should have disease progression of FDA approved therapy for these aberrations prior to receiving pembrolizumab
- FDA approval for pembrolizumab for patients with previously treated advanced cervical cancer is based on results of KEYNOTE-158 clinical trial (J Clin Oncol 2019;37:1470)
- FDA approval for pembrolizumab for patients ineligible for cisplatin containing chemotherapy with locally advanced or metastatic urothelial carcinoma is based on the KEYNOTE-052 clinical trial (Lancet Oncol 2017;18:1483)
- FDA approval for pembrolizumab as first line treatment for patients with recurrent or metastatic head and neck squamous cell carcinoma is based on the KEYNOTE-048 clinical trial (Lancet 2019;394:1915)
- FDA approval for pembrolizumab for advanced / metastatic triple negative breast cancer is based on results of KEYNOTE-355 clinical trial (J Clin Oncol 2020;38:1000)
- FDA approval for pembrolizumab for recurrent locally advanced or metastatic esophageal squamous cell carcinoma is based on KEYNOTE-181 clinical trial (J Clin Oncol 2020;38:4138)
- FDA approval for pembrolizumab for first line treatment of patients with locally advanced unresectable or metastatic HER2 positive gastric or gastroesophageal junction (GEJ) adenocarcinoma is based on results of KEYNOTE-811 (Lancet 2023;402:2197)
Tissue handling
- PharmDX PDL1 (22C3) assay is an immunohistochemical assay using monoclonal mouse anti-PDL1 clone 22C3 that targets the extracellular domain of PDL1
- Intended for use in the assessment of PDL1 protein in either tumor cells or tumor infiltrating immune cells in the formalin fixed, paraffin embedded tissues
- Assay includes EnVision FLEX visualization system reagents, performed on an Autostainer Link 48
- Tissue fixed in 10% neutral buffered formalin
- Feasibility studies were performed on tissues fixed for between 12 - 72 hours
- Tissues with fixation times of < 3 hours should not be used for PDL1 assessment
- Not validated for decalcified specimens
- Avoid performing on stored precut unstained slides that are older than 6 months
- Testing must be performed according to standardized analytically validated protocols
- References: Agilent: PD-L1 IHC 22C3 pharmDx Overview [Accessed 2 February 2023], Agilent: PD-L1 IHC 22C3 pharmDx Interpretation Manual - NSCLC [Accessed 2 March 2023]
Interpretation
- Minimum of 100 viable tumor cells must be present for evaluation
- Scoring in necrotic areas should be avoided
- Any degree of staining intensity (1+ to 3+) counts towards scoring
- Scoring is tumor site dependent
- Staining can be present in both immune cell (IC) and tumor cell (TC) components
- Tumor cells
- Only membrane staining should be evaluated
- Partial or complete membrane staining is included
- Cytoplasmic staining is not included
- Immune cells
- Membrane and cytoplasmic staining are often indistinguishable due to high N:C ratio
- Therefore, both membrane and cytoplasmic staining of inflammatory cells are included
- Only staining in lymphocytes and macrophages is counted
- Staining in neutrophils, eosinophils and plasma cells is not counted
- Recommended control tissue: benign human tonsil
Non-small cell lung carcinoma (NSCLC)
- Evaluated using a tumor proportion score (TPS)
- TPS is the percentage of viable tumor cells showing partial or complete membrane staining (≥ 1+) relative to all viable tumor cells present in the sample
- Specimen should be considered to have PDL1 expression if TPS ≥ 1% and high PDL1 expression if TPS ≥ 50%
TPS = Number of PDL1 positive tumor cells × 100 Total number of PDL1 positive + PDL1 negative tumor cells
- Divided into 3 levels
- TPS < 1%: no PDL1 expression
- TPS 1 - 49%: PDL1 expression
- TPS ≥ 50%: high PDL1 expression
Cervical carcinoma
- Expression is determined by using combined positive score (CPS)
- CPS is the number of PDL1 staining cells (tumor cells, lymphocytes, macrophages) divided by the total number of viable tumor cells, multiplied by 100
- Specimen should be considered to have PDL1 expression if CPS is ≥ 1
- Cells in the numerator include all viable invasive tumor cells and mononuclear immune cells directly associated with the response to the tumor (lymphocytes and macrophages within tumor nests and adjacent supporting stroma); adjacent mononuclear immune cells are defined as being within the same 20x field as the tumor
CPS = Number of PDL1 staining cells (tumor cells, lymphocytes, macrophages) × 100 Total number of viable tumor cells
- CPS value can be > 100 but the maximum value reported is 100
- Divided into 2 groups
- CPS < 1: no PDL1 expression
- CPS ≥ 1: PDL1 expression
Gastric or gastroesophageal junction (GEJ) adenocarcinoma
- Expression is determined by using CPS
- CPS is the number of PDL1 staining cells (tumor cells, lymphocytes, macrophages) divided by the total number of viable tumor cells, multiplied by 100
- Specimen should be considered to have PDL1 expression if CPS is ≥ 1
- Cells in the numerator include all viable invasive tumor cells and mononuclear immune cells directly associated with the response to the tumor (lymphocytes and macrophages within tumor nests and adjacent supporting stroma); adjacent mononuclear immune cells are defined as being within the same 20x field as the tumor
CPS = Number of PDL1 staining cells (tumor cells, lymphocytes, macrophages) × 100 Total number of viable tumor cells
- CPS value can be > 100 but the maximum value reported is 100
- Divided into 2 groups
- CPS < 1: no PDL1 expression
- CPS ≥ 1: PDL1 expression
Head and neck squamous cell carcinoma (HNSCC)
- Expression is determined by using CPS
- CPS is the number of PDL1 staining cells (tumor cells, lymphocytes, macrophages) divided by the total number of viable tumor cells, multiplied by 100
- PDL1 expression (CPS ≥ 1) is used to inform patient eligibility for first line therapy with pembrolizumab
- Cells in the numerator include all viable invasive tumor cells and mononuclear immune cells directly associated with the response to the tumor (lymphocytes and macrophages within tumor nests and adjacent supporting stroma); adjacent mononuclear immune cells are defined as being within the same 20x field as the tumor
- Divided into 3 groups
- CPS < 1: no PDL1 expression
- CPS ≥ 1: PDL1 expression
- CPS ≥ 20: PDL1 expression*
- *PDL1 expression level CPS ≥ 20 may be of interest to treating physicians but does not determine eligibility for first line therapy
Esophageal squamous cell carcinoma (ESCC)
- Expression is determined by using CPS
- CPS is the number of PDL1 staining cells (tumor cells, lymphocytes, macrophages) divided by the total number of viable tumor cells, multiplied by 100
- Specimen should be considered to have PDL1 expression if CPS is ≥ 10
- Cells in the numerator include all viable invasive tumor cells and mononuclear immune cells directly associated with the response to the tumor (lymphocytes and macrophages within tumor nests and adjacent supporting stroma); adjacent mononuclear immune cells are defined as being within the same 20x field as the tumor
- Divided into 2 groups
- CPS < 10: no PDL1 expression
- CPS ≥ 10: PDL1 expression
Triple negative breast cancer (TNBC)
- Expression is determined by using CPS
- CPS is the number of PDL1 staining cells (tumor cells, lymphocytes, macrophages) divided by the total number of viable tumor cells, multiplied by 100
- Specimen should be considered to have PDL1 expression if CPS is ≥ 10
- Divided into 2 groups
- CPS < 10
- CPS ≥ 10
Urothelial carcinoma
- Following the FDA's Oncologic Drugs Advisory Committee (ODAC) meeting (2021), the approved pembrolizumab indication was updated for the treatment of patients with locally advanced or metastatic urothelial carcinoma who are not eligible for any platinum containing chemotherapy; due to this update, PDL1 testing to determine eligibility of urothelial carcinoma patients for treatment with pembrolizumab is no longer indicated
- References: Agilent: PD-L1 IHC 22C3 pharmDx Overview [Accessed 2 February 2023], Agilent: PD-L1 IHC 22C3 pharmDx Interpretation Manual - NSCLC [Accessed 2 March 2023], Dako: PD-L1 IHC 22C3 pharmDx Interpretation Manual - Esophageal Squamous Cell Carcinoma (ESCC) [Accessed 16 January 2023]
Uses by pathologists
- Current companion diagnostic indications for PDL1 (22C3) include non-small cell lung carcinoma (NSCLC), cervical carcinoma (including cervical squamous cell carcinoma and endocervical adenocarcinoma), urothelial carcinoma, head and neck squamous cell carcinoma (HNSCC), advanced / metastatic triple negative breast cancer (TNBC), esophageal squamous cell carcinoma (ESCC), HER2 positive gastric or gastroesophageal junction (GEJ) adenocarcinoma
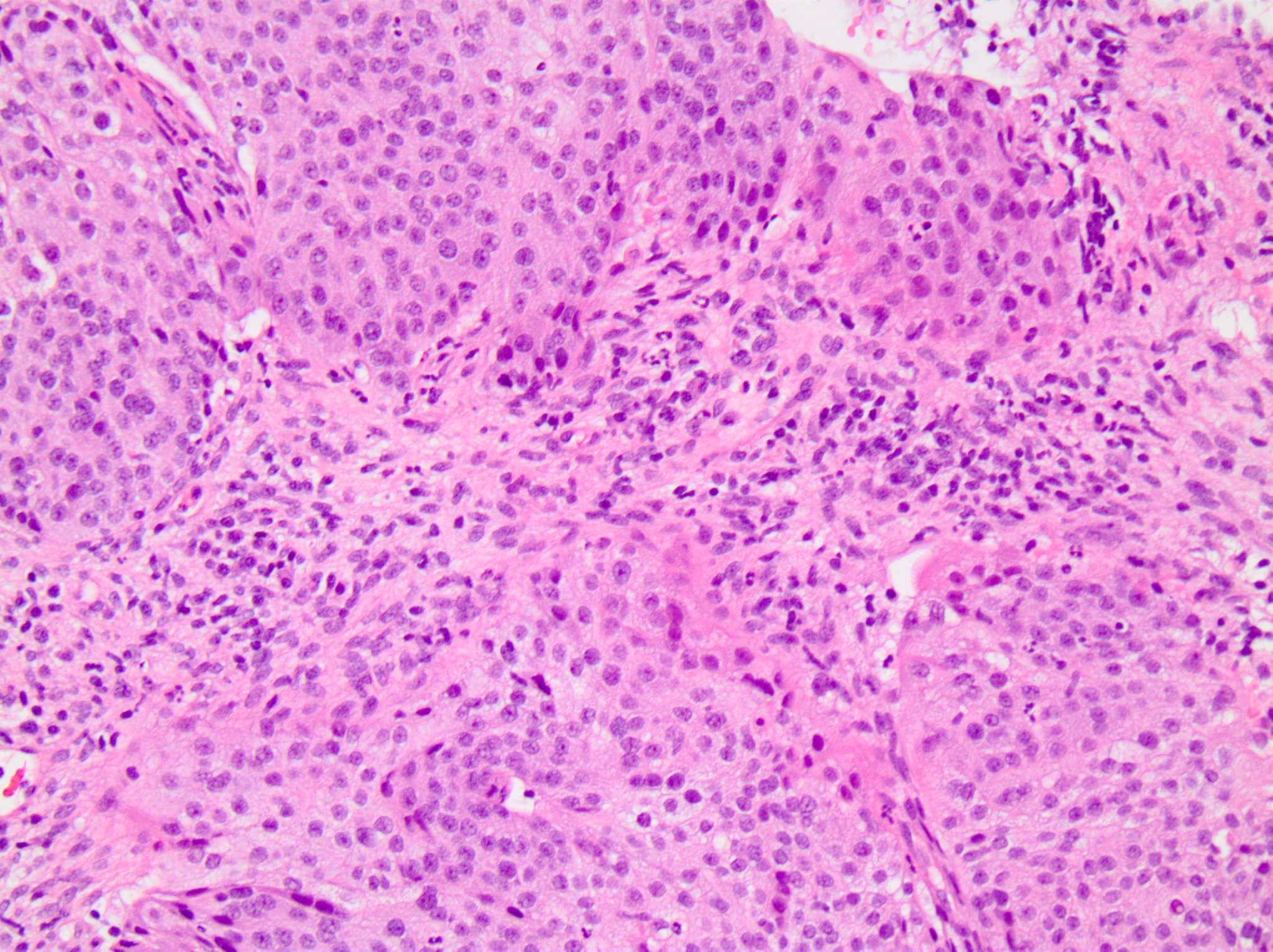
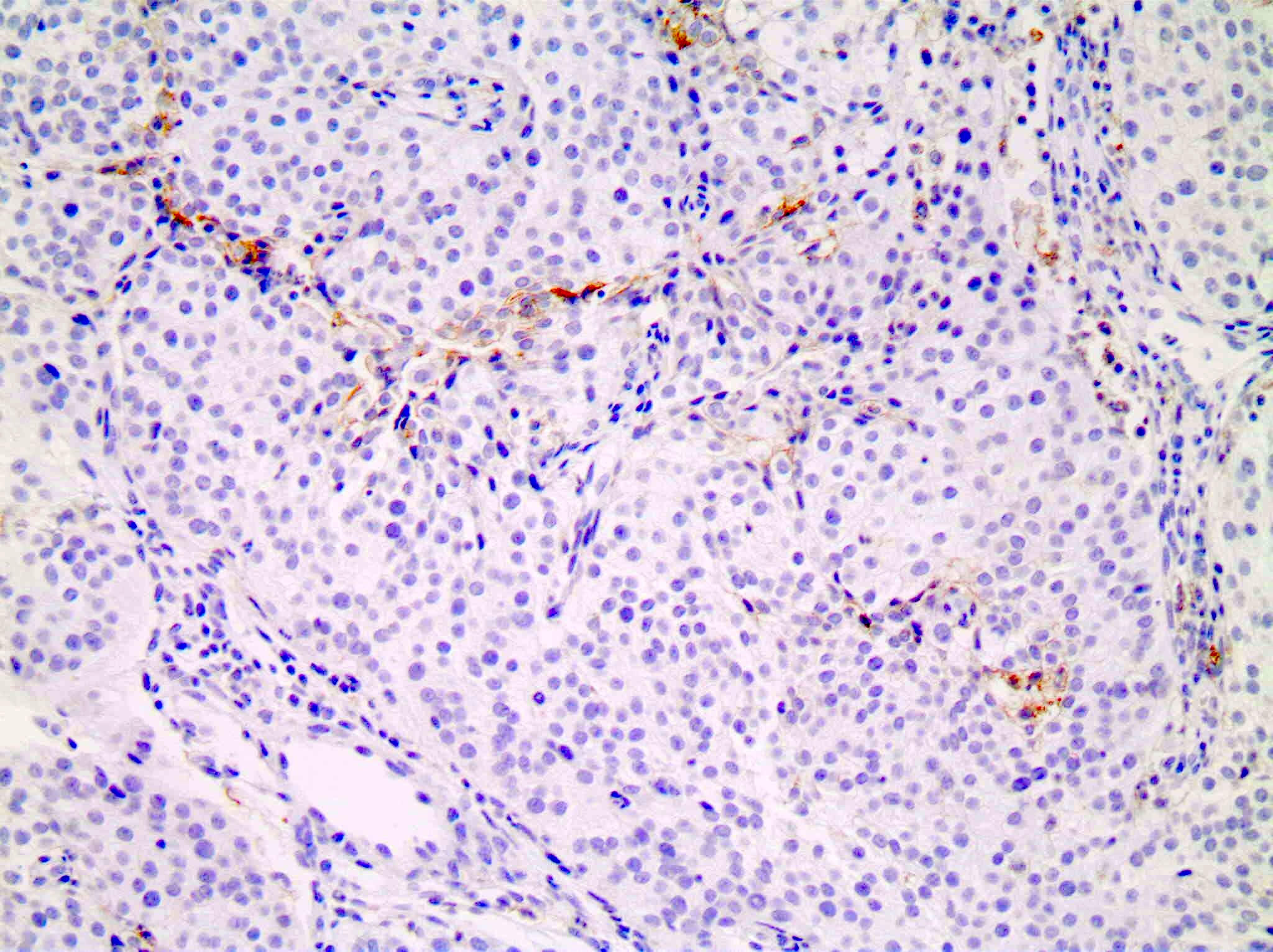
Microscopic (histologic) images
Positive staining - normal
- Immune cells (nontumoral)
- Tonsil (control tissue) in follicular macrophages (weak moderate) and reticulated crypt epithelial cells (strong)
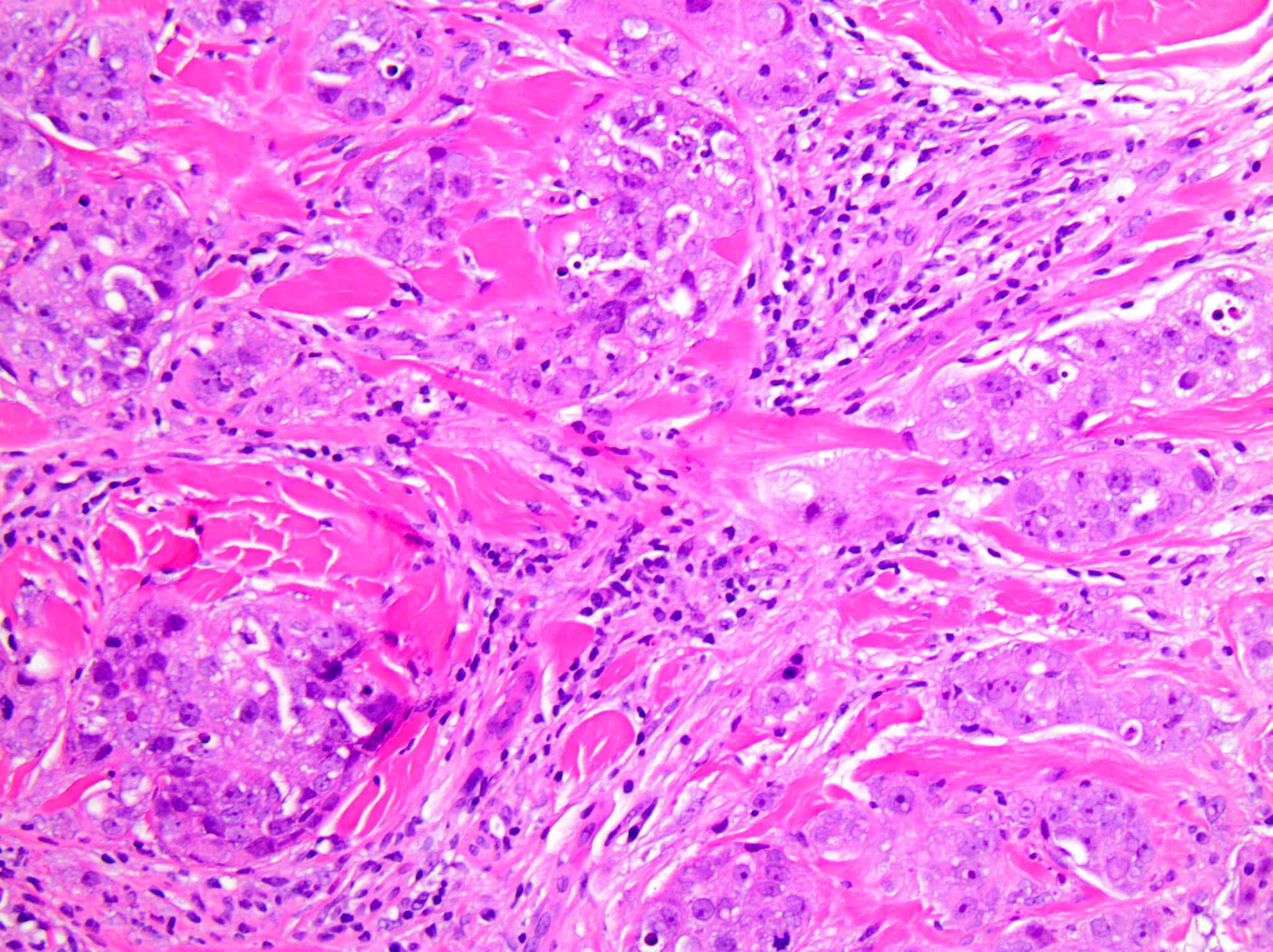
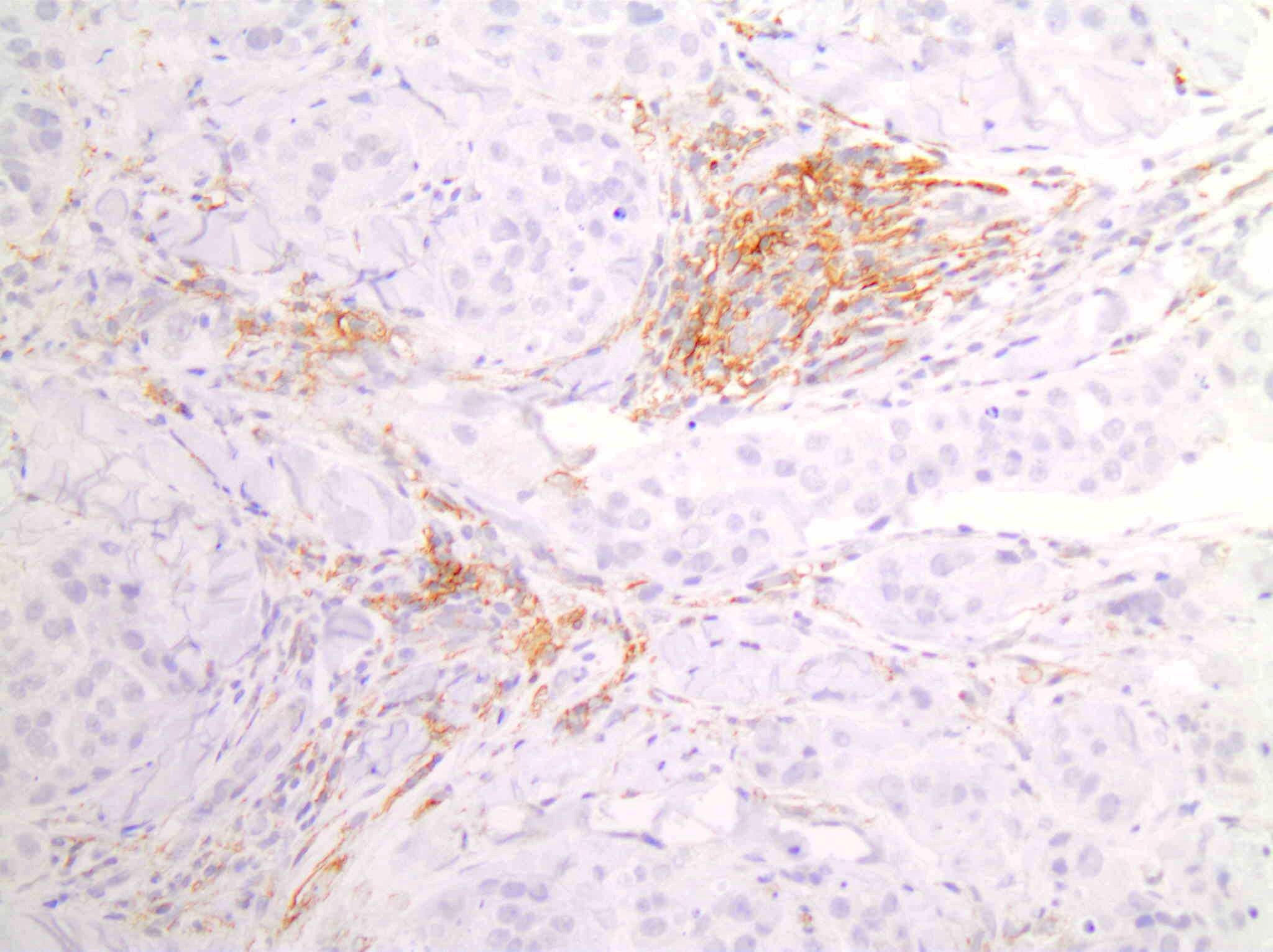
Positive staining - disease
- Non-small cell lung carcinoma: 22.8 - 30.2% with tumor proportion score ≥ 50% and 34.2 - 39.1% with tumor proportion score 1 - 49% (Lancet 2016;387:1540, N Engl J Med 2016;375:1823)
- Cervical cancer: 85% with combined positive score ≥ 1 (J Clin Oncol 2019;37:1470)
- Head and neck squamous cell carcinoma: 85% with combined positive score ≥ 1 and 43% with combined positive score ≥ 20 (Lancet 2019;394:1915)
- Urothelial carcinoma: 30.5% with combined positive score ≥ 10 (Lancet Oncol 2017;18:1483)
- Triple negative breast cancer: 28% with combined positive score ≥ 10 (J Clin Oncol 2020;38:1000)
- Esophageal squamous cell carcinoma: 35% with combined positive score ≥ 10 (J Clin Oncol 2020;38:4138)
- Gastric or gastroesophageal junction adenocarcinoma: 85% with combined positive score ≥ 1 (Lancet 2023;402:2197)
Negative staining
- Tonsil (control tissue) in superficial squamous epithelium, fibroblasts and endothelium
Sample pathology report
- Liver, core needle biopsy:
- Metastatic breast carcinoma involving liver (see comment)
- PDL1 (Ventana 22C3): Positive (CPS ≥ 10)
- Comment: PDL1 IHC (clone 22C3, pharmDx) is an FDA approved companion diagnostic for selecting patients with triple negative breast cancer for pembrolizumab performed on a Dako Autostainer Link 48. Pembrolizumab (Keytruda) is indicated for the treatment of patients with unresectable locally advanced or metastatic triple negative, PDL1 positive breast cancer.
- Tumor cells and mononuclear inflammatory cells (within tumor nests or adjacent supporting stroma) with partial or complete linear membrane staining if at least 1+ intensity are scored as positive. PDL1 expression in triple negative breast cancer is determined by using combined positive score (CPS), which is the number of PDL1 staining cells (tumor cells, lymphocytes, macrophages) divided by the total number of viable tumor cells, multiplied by 100. The combined positive score is estimated by manual quantification. Scoring is as follows: CPS < 10 negative, CPS ≥ 10 positive. The sample is adequate if ≥ 100 tumor cells are present. Certain tissue processing factors, such as decalcification, formalin fixation time outside an acceptable range (< 3 hours), prolonged time to fixation and use of tissue from blocks that are 6 months or older, can affect PDL1 staining / expression and results should be interpreted with caution in such instances. This assay is not validated for decalcified specimens.
- All controls show appropriate reactivity. All immunohistochemistry, in situ hybridization and histochemical tests were developed by and are performed at the ___ Laboratory. All tests reported here, except those addressing HER2, ALK and PDL1 expression as predictive markers, have not been cleared by or approved by the U.S. FDA. The laboratory is regulated under CLIA as qualified to perform high complexity testing. The tests are used for clinical purposes. They should not be regarded as investigational or for research.
- Reference: J Clin Oncol 2020;38:1000
- Metastatic breast carcinoma involving liver (see comment)
Practice question #1
A PDL1 (22C3) immunostain is shown above. Which of the following statements regarding PDL1 (22C3) is correct?
- PDL1 (22C3) is the FDA approved companion diagnostic assay for nivolumab
- In advanced / metastatic triple negative breast cancer, PDL1 (22C3) is determined by using combined positive score (CPS), with a positive score cutoff of CPS ≥ 1
- In non-small cell lung carcinoma, PDL1 (22C3) is determined by using combined positive score, with a positive score cutoff of CPS ≥ 10
- In esophageal squamous cell carcinoma, PDL1 (22C3) is determined by using tumor proportion score (TPS), with a positive score cutoff of TPS ≥ 1%
- In non-small cell lung carcinoma, scoring of PDL1 (22C3) is only evaluated in the tumor cell component
Practice answer #1
E. In non-small cell lung carcinoma, scoring of PDL1 (22C3) is only evaluated in the tumor cell component. Answer A is incorrect because PDL1 (22C3) is the FDA approved companion diagnostic assay for pembrolizumab. Answers B and D are incorrect because in triple negative breast cancer and esophageal squamous cell carcinoma, 22C3 is scored using combined positive score, with a cutoff of CPS ≥ 10. Answer C is incorrect because in non-small cell lung carcinoma, 22C3 is scored using a tumor proportion score. Tumor proportion score is scored by evaluating the PDL1 expression in the tumor cell component only.
Comment Here
Reference: PDL1 22C3
Comment Here
Reference: PDL1 22C3