Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2 | Board review style question #3 | Board review style answer #3Cite this page: White B, Morris M. Alzheimer disease. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/cnsalzheimer.html. Accessed May 13th, 2024.
Definition / general
- Alzheimer disease is clinically characterized by a progressive dementia and neuropathologically characterized by amyloid plaques and neurofibrillary tangles (NFT)
- Sporadic disease is more common, which typically affects patients over the age of 65 years; however, rare early onset familial forms occur, which affect younger patients
Essential features
- Clinical history of progressive dementia consistent with Alzheimer disease
- Alzheimer disease neuropathologic changes (amyloid plaques, neurofibrillary tangles)
- Can occur in the presence or absence of other neurodegenerative diseases
Terminology
- Senile dementia
- Sporadic Alzheimer disease
- Early onset Alzheimer disease
- Alzheimer disease with amygdala predominant Lewy bodies
- Mixed dementia
ICD coding
- ICD-10
- ICD-11
- 6D80.0 - dementia due to Alzheimer disease with early onset
- 6D80.1 - dementia due to Alzheimer disease with late onset
- 6D80.2 - Alzheimer disease dementia, mixed type, with cerebrovascular disease
- 6D80.3 - Alzheimer disease dementia, mixed type, with other nonvascular etiologies
- 6D80.Z - dementia due to Alzheimer disease, onset unknown or unspecified
Epidemiology
- Alzheimer disease is estimated to affect 6.7 million people in the United States in 2023 (Alzheimers Dement 2022;18:700)
- Alzheimer disease accounts for ~60 - 70% of progressive cognitive impairment in elderly patients (JAMA 2002;287:2335)
- Most significant risk factors include age (over the age of 65 years) and genetics (especially the ɛ4 allele of the apolipoprotein E [APOE] gene) (Lancet Neurol 2021;20:68)
- Protective factors include moderate alcohol consumption, increased cognitive activity and increased physical activity (J Am Geriatr Soc 2004;52:540, Neurology 2007;69:1911, Neurology 2012;78:1323)
- Risk factors include diabetes mellitus, obesity and prior head injuries (Dement Geriatr Cogn Disord 2011;31:424, JAMA Neurol 2022;79:584, J Neurol Neurosurg Psychiatry 2013;84:177)
- F > M; age adjusted comparisons generally do not show significant differences in prevalence between men and women (Alzheimers Dement 2023;19:1598)
Sites
- Most brain regions are affected in advanced cases of Alzheimer disease
- Cortex is affected early by amyloid plaques (Neurology 2002;58:1791, Neurobiol Aging 2018;71:72)
- Mesial temporal lobe structures and specific brainstem nuclei (locus ceruleus, median raphe nuclei) are affected early by neurofibrillary tangles (J Neuropathol Exp Neurol 2011;70:960, Acta Neuropathol 2011;121:171, Neurobiol Aging 2018;71:72)
Pathophysiology
- While the definitive pathophysiology of Alzheimer disease is still under investigation, there are a number of theories that may help explain development of Alzheimer disease
- Beta amyloid may work to drive neurodegeneration
- Initial accounts of the disease from the early 1900s demonstrated senile plaques based upon silver based histochemical stains (Clin Anat 1995;8:429)
- Beta amyloid was later isolated from these plaques in the 1980s (Clin Anat 1995;8:429)
- Following this discovery, the progression of Alzheimer disease was thought to be based on the generation of beta amyloid plaques (Clin Anat 1995;8:429)
- This was supported by the presence of autosomal dominant mutations in the amyloid pathway that lead to clinical Alzheimer disease, including APP and presenilin 1 and 2 gene (PSEN1 / PSEN2) mutations as well as trisomy 21
- These mutations lead to an increase in the prevalence of soluble and insoluble beta amyloid isomers (Clin Anat 1995;8:429)
- Additionally, the biologic therapies directed at beta amyloid have met some success in delaying the progression of dementia
- Despite this evidence in support of the amyloid pathway, there is reproducible evidence showing that beta amyloid accumulation and deposition does not correlate well with neuronal loss and cognitive decline (Arch Neurol 2008;65:1509)
- Study of beta amyloid neurotoxicity in vivo shows that it does not reproduce the in vitro toxicity and that beta amyloid likely acts synergistically with tau to drive neuronal loss (JAMA Neurol 2014;71:505)
- Initial accounts of the disease from the early 1900s demonstrated senile plaques based upon silver based histochemical stains (Clin Anat 1995;8:429)
- Abnormal microtubule associated tau (MAP tau or tau) may drive functional cognitive loss by neuronal dysfunction
- While beta amyloid aggregates are found years or decades before the onset of clinical dementia, tau neurofibrillary tangles are strongly correlated with neurodegeneration and cognitive impairment
- Cognitive impairment often coincides with the spreading of tau neurofibrillary tangles out of the medial temporal lobes and into the surrounding isocortex (Nat Med 2018;24:29)
- There is a stereotypical spread of tau that is believed to be secondary to cell to cell spread through tau transmission across synapses
- This idea is supported by animal models and spatial tau PET signal spreading along distributions reminiscent of functional brain networks (Neuron 2012;73:685, Brain 2018;141:568)
- Neuroinflammation is also theorized to contribute to the pathophysiology of Alzheimer disease
- Role of inflammation's contribution to Alzheimer disease has come into focus more recently with the recognition that beta amyloid activates microglial cells causing a release of proinflammatory cytokines
- These cytokines induce production of amyloid precursor protein (APP) and thus the production of beta amyloid
- This is further supported by genome wide analysis which suggests that several genes that increase risk of Alzheimer disease are associated with the clearance of misfolded proteins and immune responses
- Clinical data show external factors including systemic inflammation and obesity are likely to interfere with these processes and further promote disease progression (Lancet Neurol 2015;14:388)
Etiology
- True etiology of Alzheimer disease is still under investigation; however, there are epidemiological risk factors that contribute to the development of the disease
- In addition, there are genetically linked forms of autosomal dominant Alzheimer disease that will cause dementia including mutations in APP and PSEN1 / PSEN2
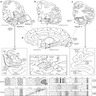
Diagrams / tables
Clinical features
- Patients typically present with a history of progressive short term memory disturbances for ~1 - 3 years, which can be clinically characterized as mild cognitive impairment (Alzheimers Dement 2011;7:263)
- This is followed by progressive cognitive or behavioral impairment (Alzheimers Dement 2011;7:263)
- It is common to have concurrent agitation or depression
Diagnosis
- Diagnosis of Alzheimer disease requires both a clinical diagnosis as well as neuropathological changes
- Clinical dementia is diagnosed as possible or probable Alzheimer disease dementia when there are cognitive or behavioral symptoms that (Alzheimers Dement 2011;7:263)
- Interfere with the ability to function at work or usual activities
- Represent a decline from previous levels of functioning
- Are not explained by delirium or major psychiatric disorder
- Involve a minimum of 2 cognitive domains listed below
- Inability to acquire and remember new information
- Impaired reasoning and handling of complex tasks, poor judgment
- Impaired visuospatial abilities
- Impaired language functions
- Changes in personality, behavior or comportment
- Alzheimer disease neuropathologic changes are graded using higher levels of pathology associated with dementia
- Alzheimer disease neuropathologic changes are currently reported as an ABC score, which includes 3 separate diagnostic schemes (Alzheimers Dement 2012;8:1)
- A score represents the spread of amyloid deposition (Thal phase) (Neurology 2002;58:1791)
- B score represents the spread of neurofibrillary tangles (Braak stage) (Acta Neuropathol 2006;112:389)
- C score represents a semiquantitative scoring of neuritic plaques (CERAD score) (Neurology 1991;41:479)
Laboratory
- Elevated homocysteine levels are associated with the development of Alzheimer disease
- Assays investigating beta amyloid 42/40 ratios in the cerebral spinal fluid (CSF) have been approved by the Food and Drug Administration (FDA) in patients over the age of 55 years who present with cognitive impairment for the evaluation of Alzheimer disease
- Similar assays for the beta amyloid 42/40 ratio using blood plasma have cleared Clinical Laboratory Improvement Amendments (CLIA) regulations but have not yet been approved by the FDA
- Assays for phosphorylated tau are being investigated for diagnostic purposes (Ann Neurol 2009;65:403)
- Proteomic assays of the CSF have been reported and provide enhanced diagnostic and prognostic information in excess of the beta amyloid and tau assays (Sci Transl Med 2023;15:eadg4122)
- Alzheimer disease biomarkers typically help establish the presence of Alzheimer disease neuropathologic changes but do not exclude the presence of other pathologies contributing to dementia (i.e., mixed dementia)
Radiology description
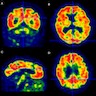
- Current radiological workup takes advantage of multiple modalities to assess structural and functional metrics used in Alzheimer disease workups (Int J Mol Sci 2022;23:6079)
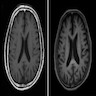
- Structural magnetic resonance imaging (MRI) demonstrates cerebral atrophy and ventricular enlargement; hippocampal volumes can be helpful in both structural and functional assessments
- Fluorodeoxyglucose positron emission tomography (FDG PET) imaging can highlight accumulations of amyloid and tau as well as glucose metabolism; these tests are FDA approved for diagnostic purposes
- Functional MRI can be used for the detection of hyper and hypoactivation while investigating task related regions
- Electroencephalography can be used to assess altered electrical function
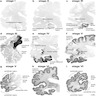
Radiology images
Prognostic factors
- Shorter survival in clinically diagnosed Alzheimer disease associated with older age, male sex, increasing comorbidities and medications, lower cognitive function at diagnosis or suspected mixed dementia (Neurology 2020;94:e538)
Case reports
- 51 year old woman with dementia, progressive limb apraxia and choreiform movements (BMJ Case Rep 2015;2015:bcr2014207011)
- 61 year old woman presented with progressive gait difficulties and cognitive impairment over 5 years (Dement Neurocogn Disord 2018;17:32)
- 78 year old man with lower extremity weakness, gait disturbance and cognitive impairment (Ann Geriatr Med Res 2019;23:38)
- Woman in her 70s presented with dementia with both a PSEN1 mutation and a APOE3 Christchurch mutation (Nat Med 2019;25:1680)
Treatment
- NMDA receptor antagonists (i.e., memantine) are used to slow the cognitive decline by blocking neuronal excitotoxicity (CNS Drug Rev 2003;9:275)
- Acetylcholinesterase inhibitors (i.e., rivastigmine) can help symptomatically improve neuropsychiatric symptoms such as apathy through slowing the degradation of neurotransmitters
- Monoclonal antibodies directed against beta amyloid isoforms have gained FDA approval and work to slow the progression of cognitive impairment
- These include aducanumab and lecanemab (N Engl J Med 2023;388:9)
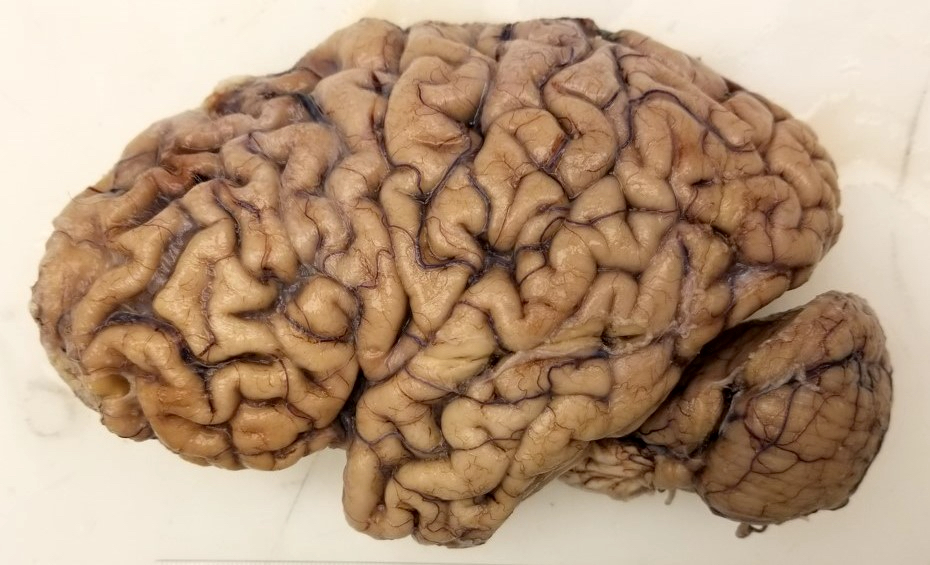
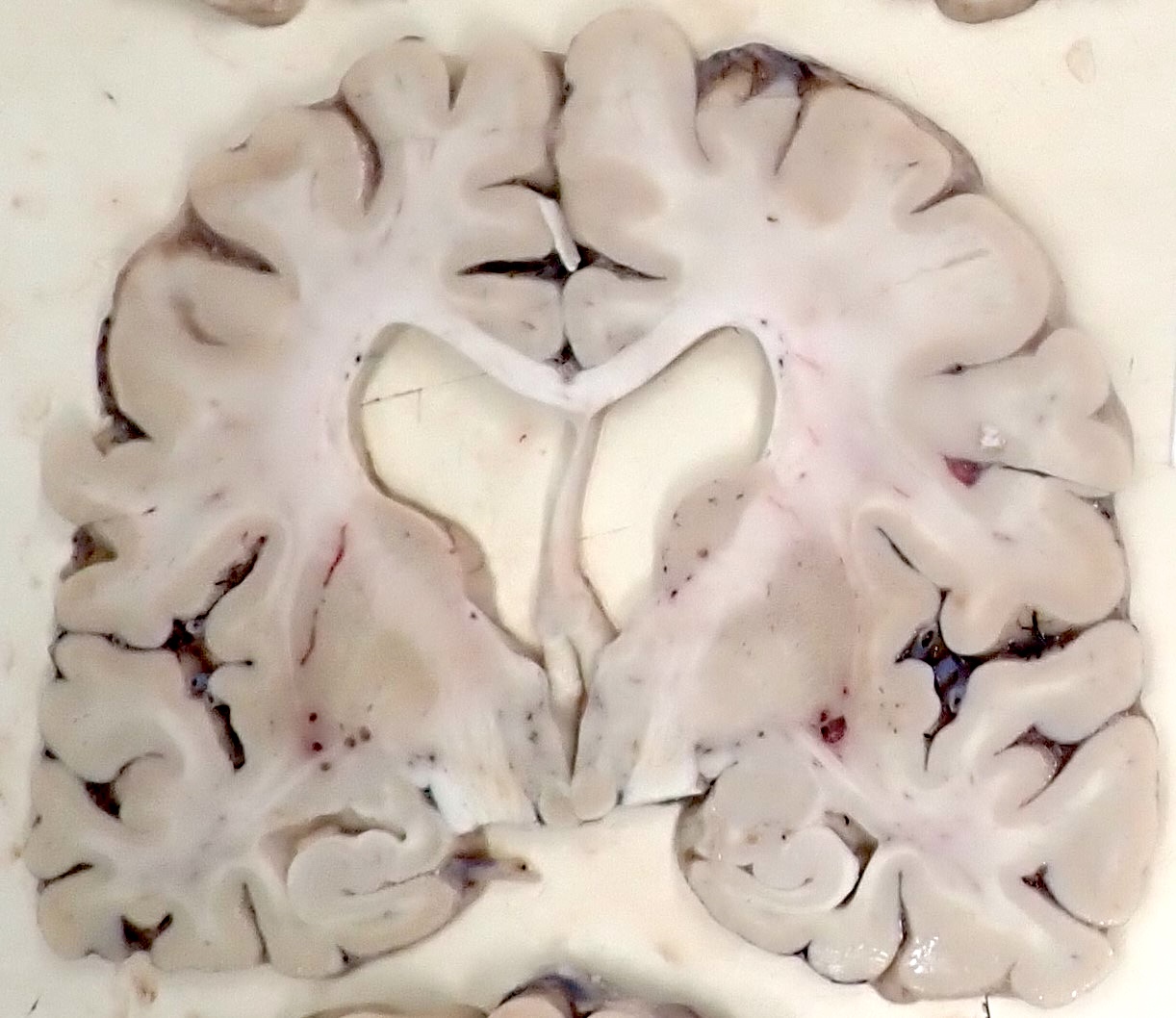
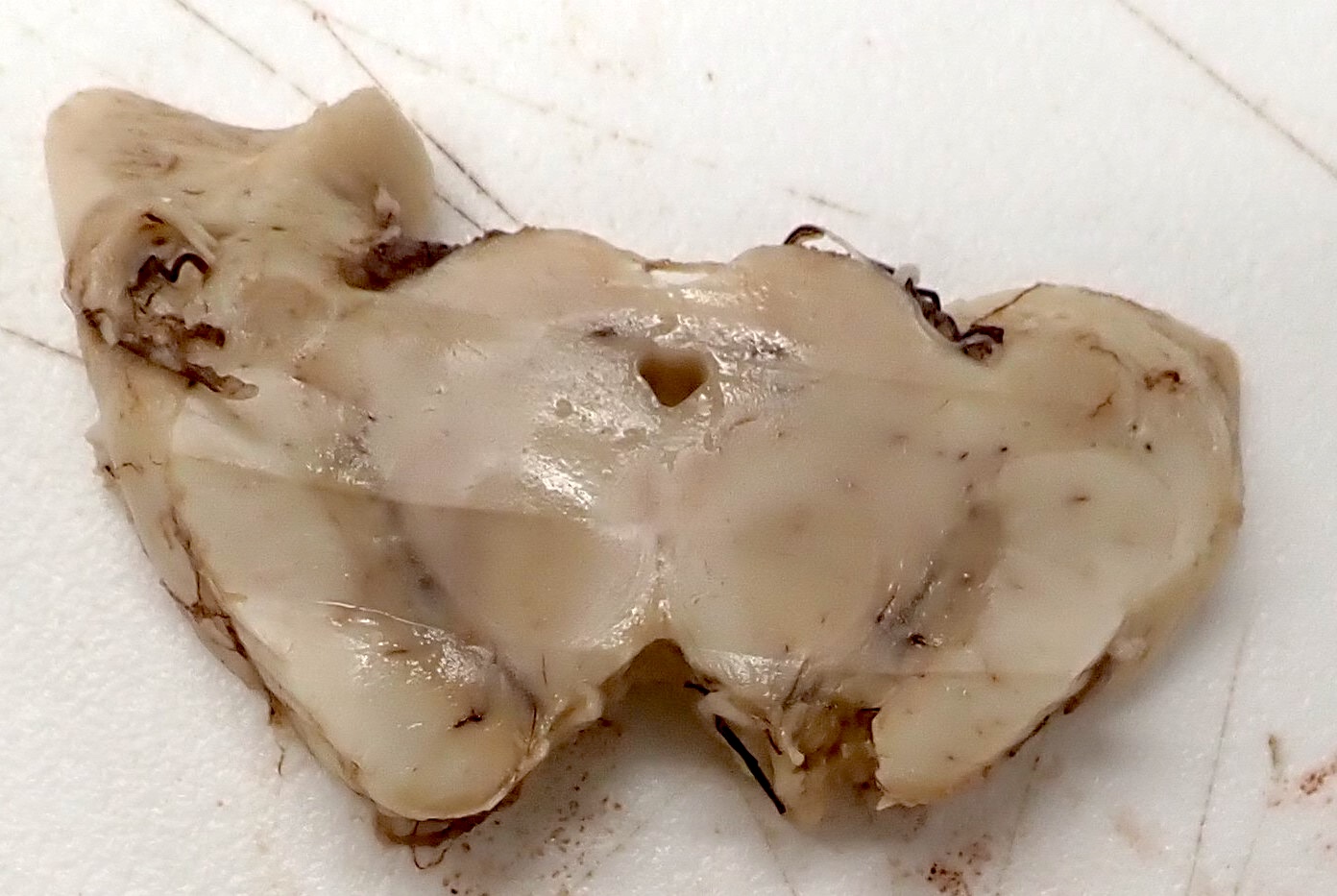
Gross description
- Cortical atrophy can be externally observed with narrowing of the gyri and widening of the sulci
- This can be associated with a reduced brain weight
- Atrophy selectively affects the frontal, temporal and parietal lobes with relative sparing of the occipital lobe
- There can be significant atrophy of the amygdala and hippocampus
- Hydrocephalus ex vacuo, shown by symmetric dilation of the lateral ventricles and third ventricle, is common
- Pallor of the locus ceruleus is common
- Pallor of the substantia nigra may occur in ~25 - 33% of cases and may be associated with extrapyramidal signs (Neurology 2005;64:1397, Arch Pathol Lab Med 1993;117:132)
Gross images
Microscopic (histologic) description
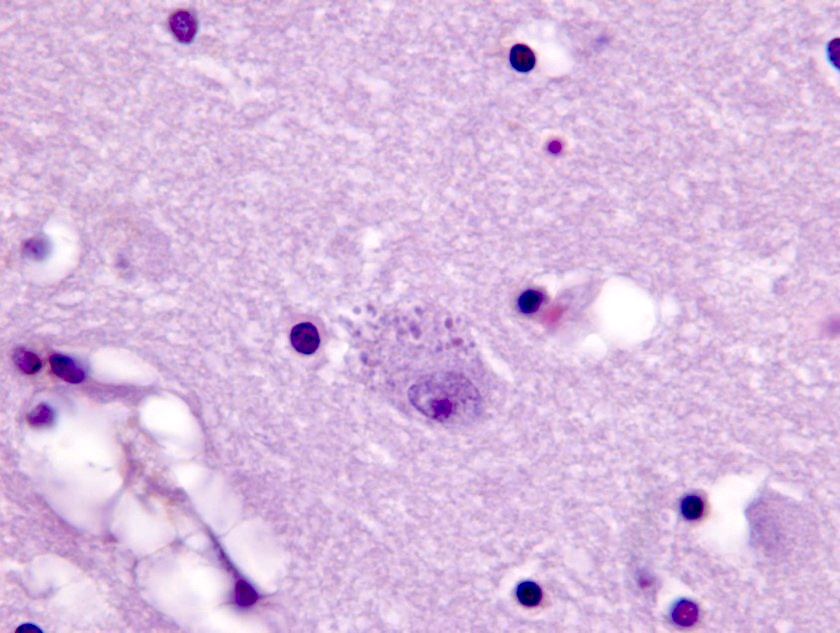
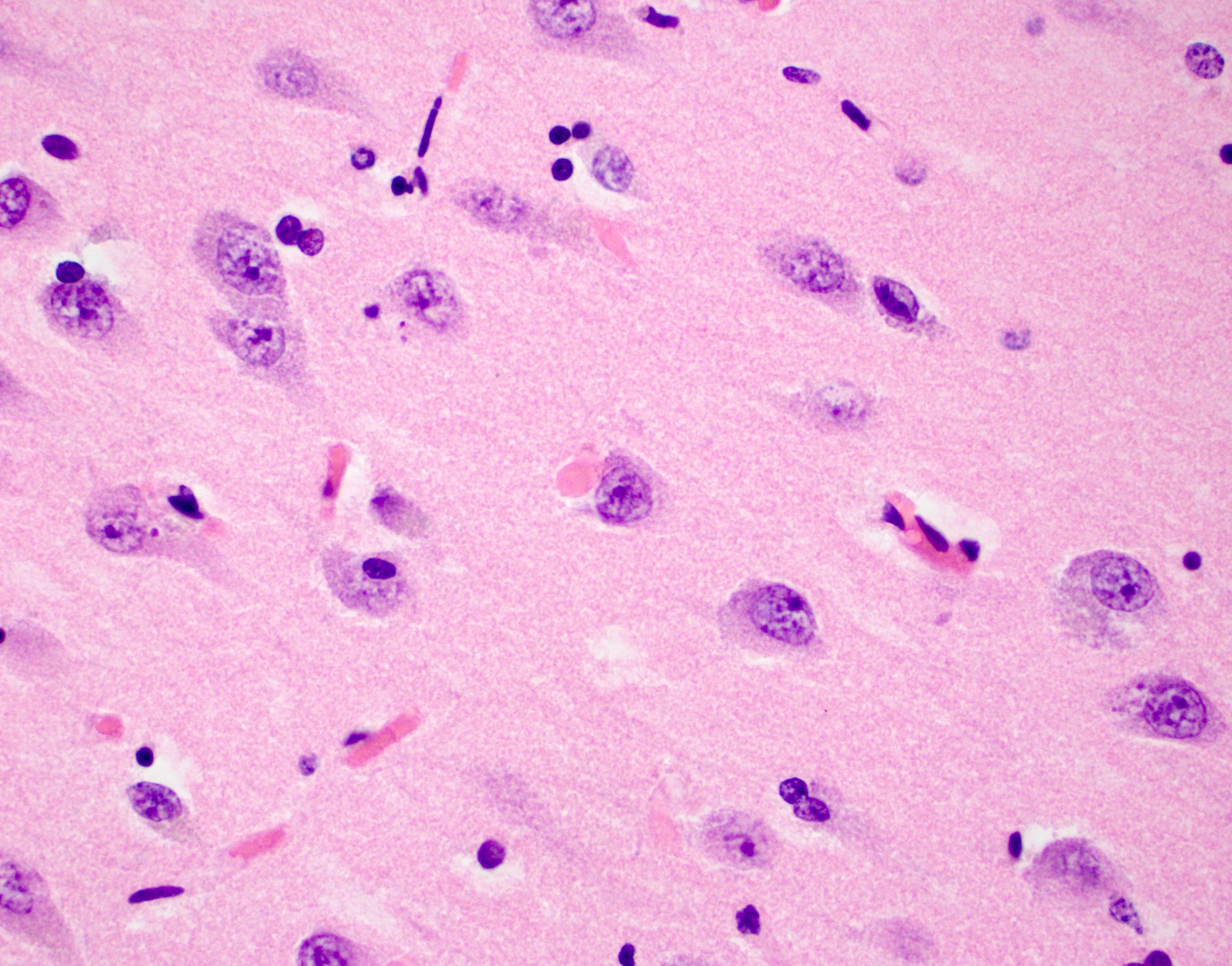
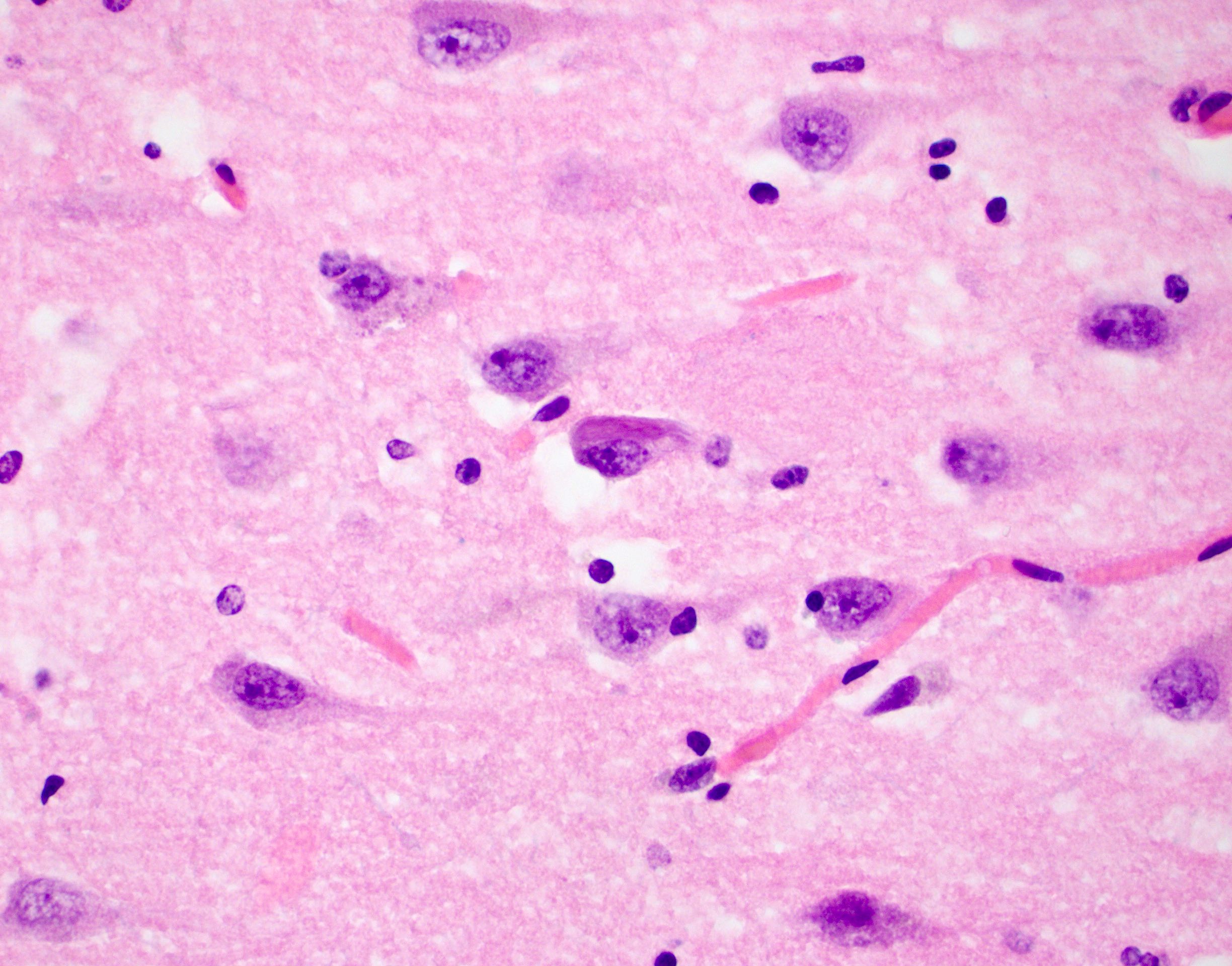
- Alzheimer disease is pathologically characterized by extracellular amyloid plaques and intracellular neurofibrillary tangles
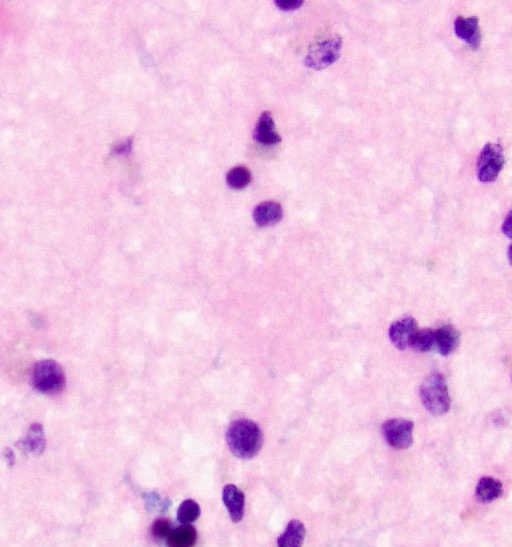
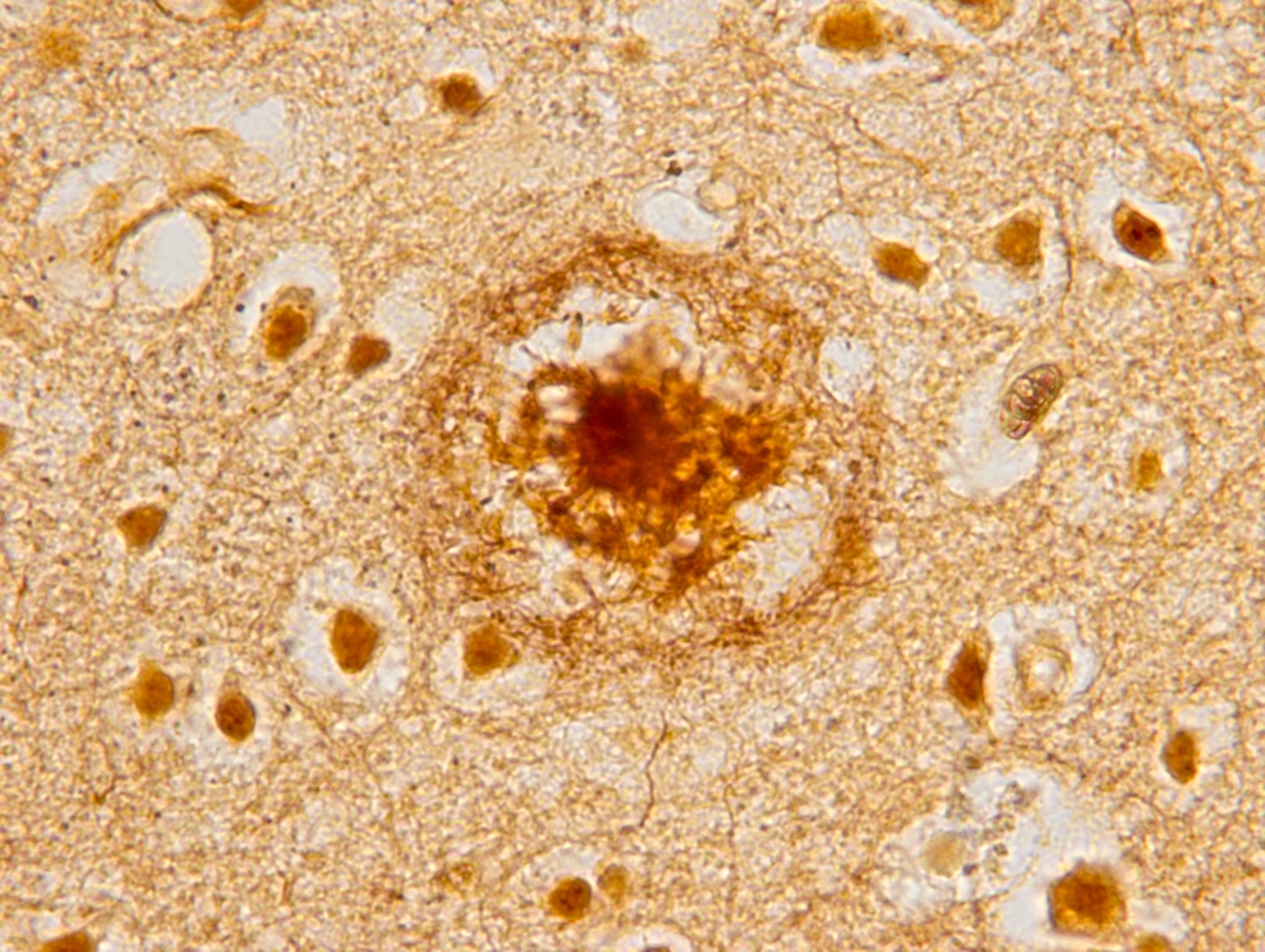
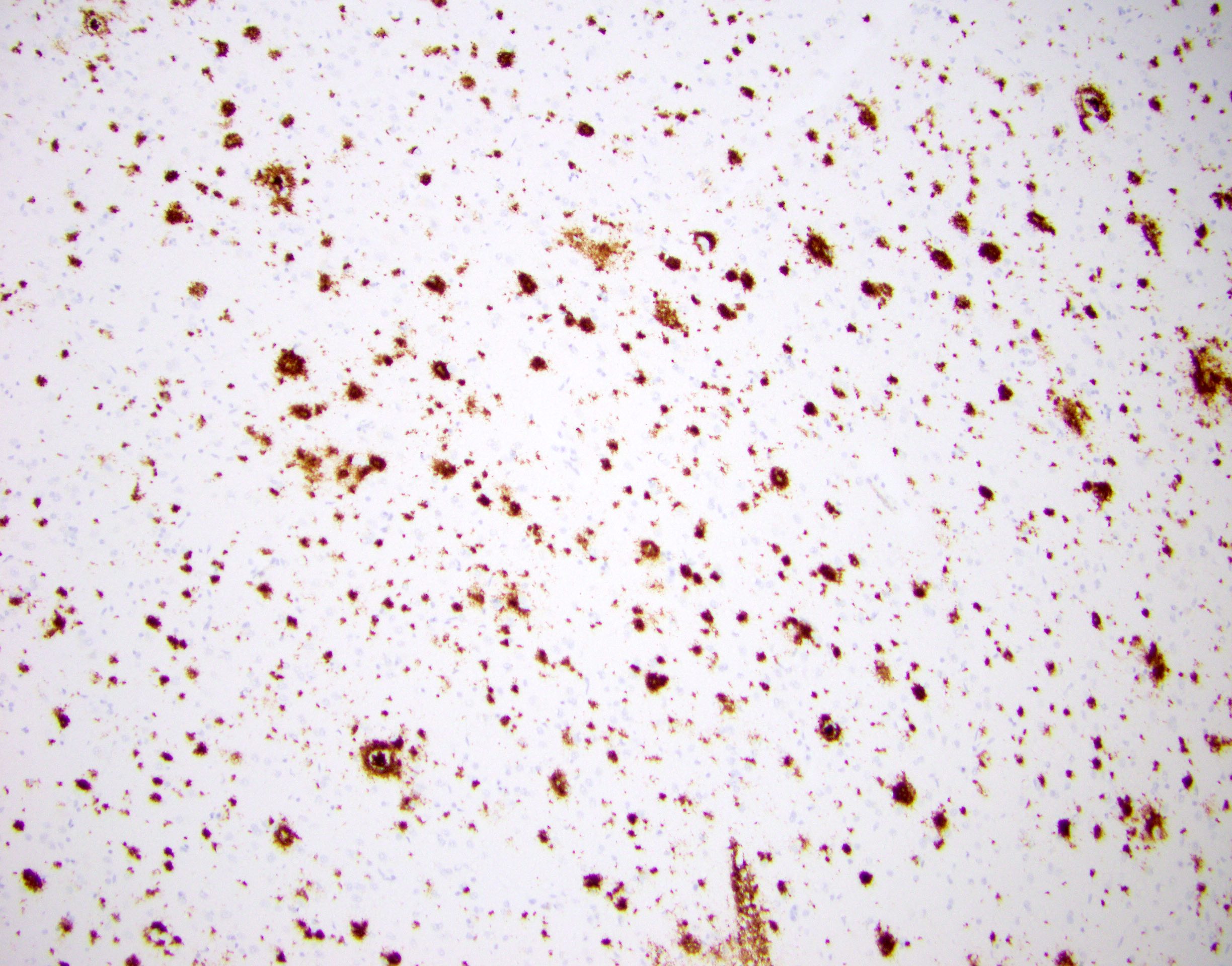
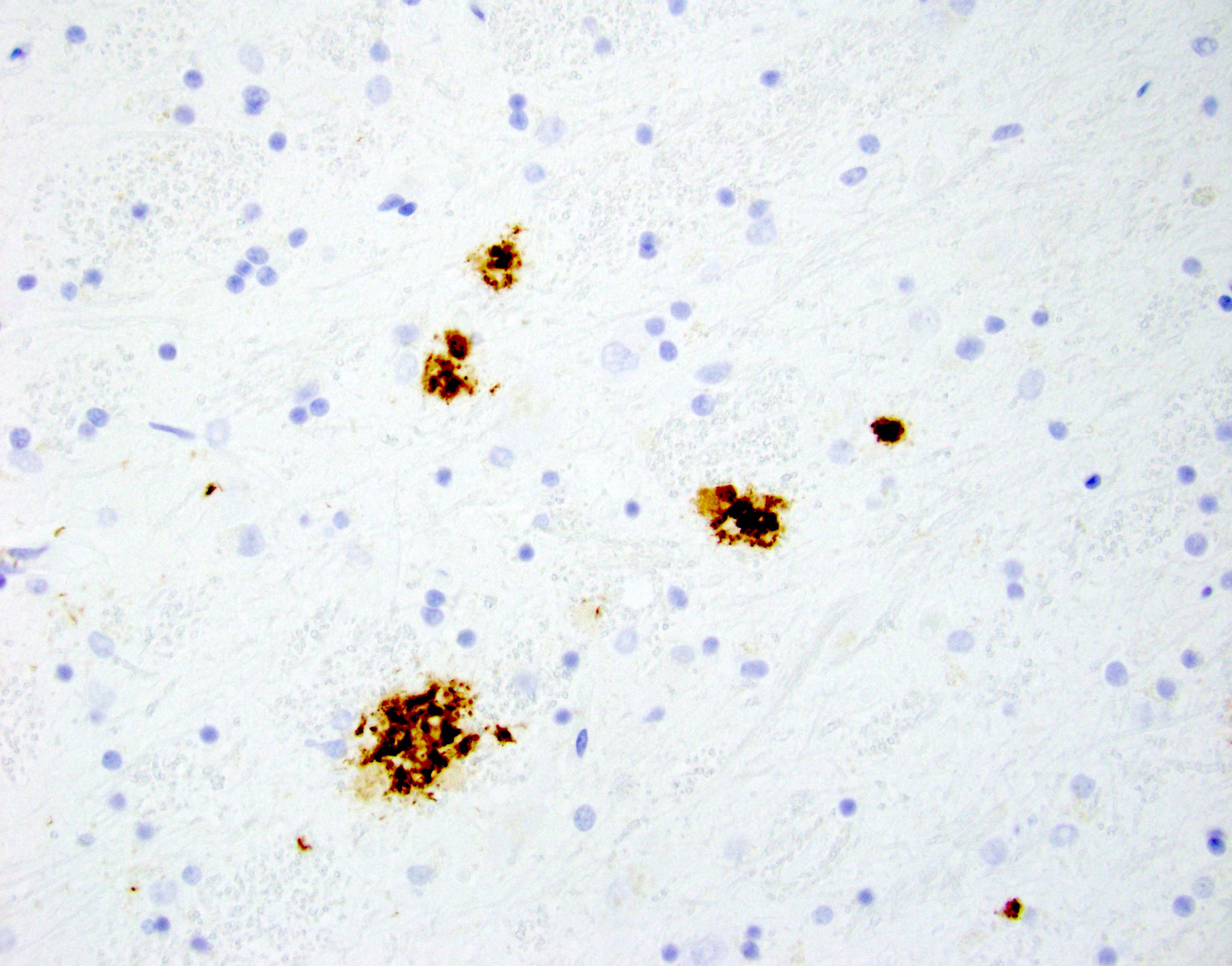
- Amyloid plaques are visible on H&E staining as a circular disorganization of the neuropil, which may be accompanied by activated microglia and a dense amorphous eosinophilic core
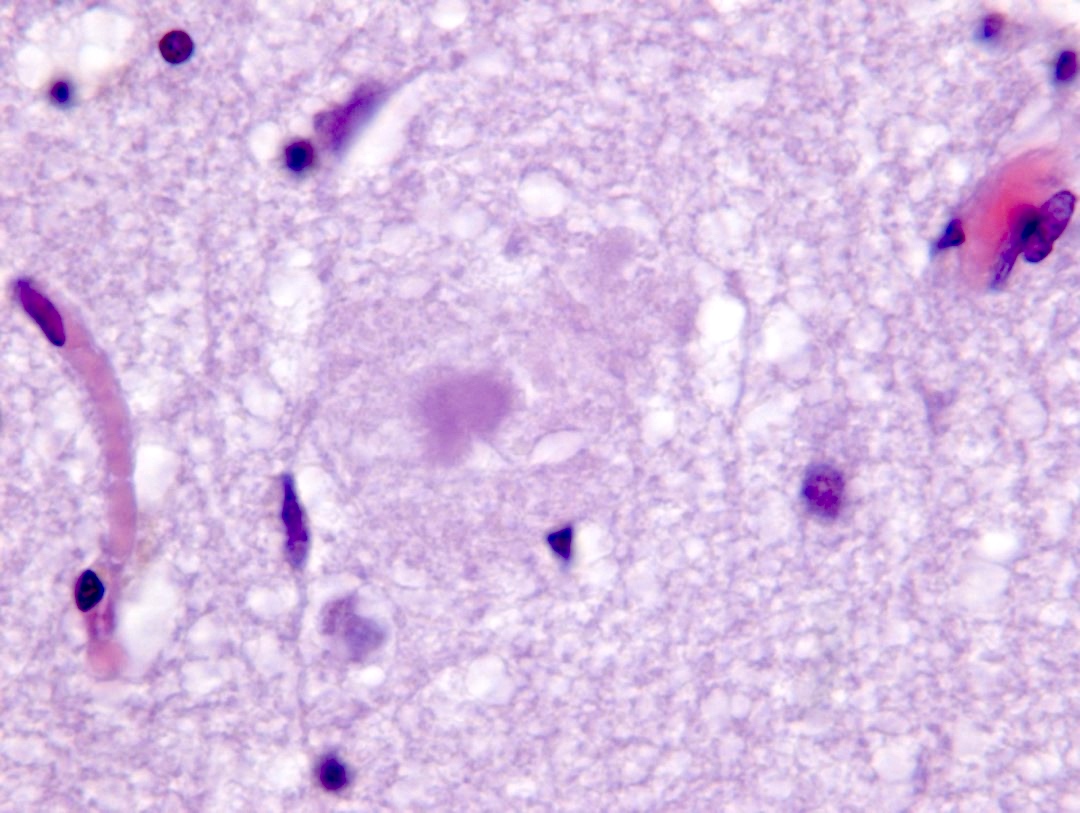
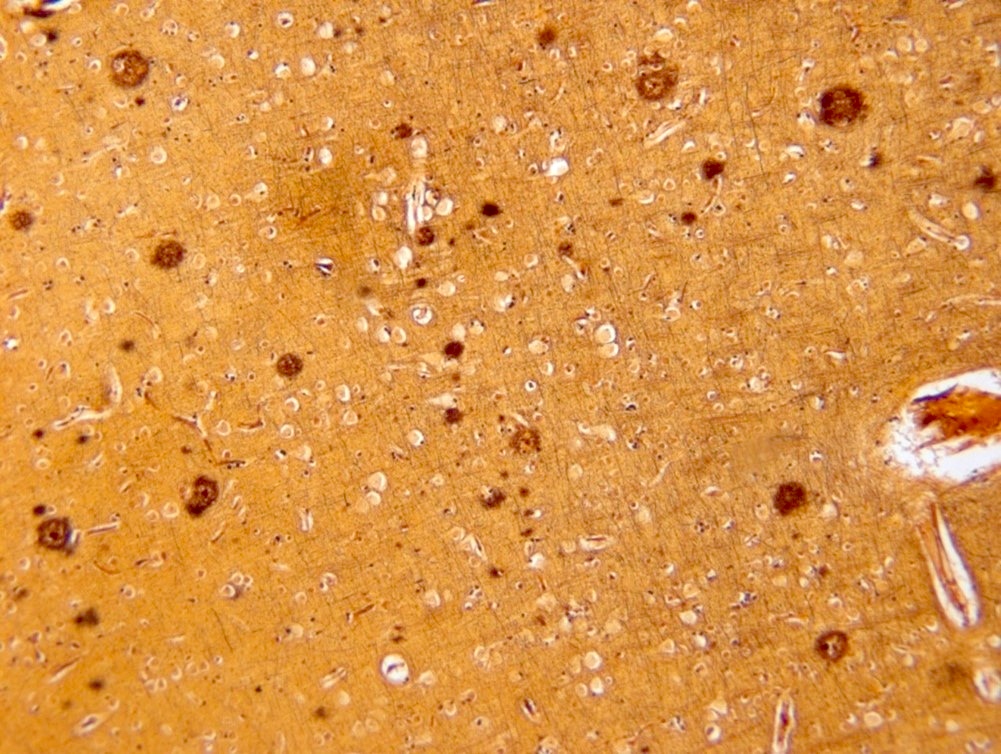
- Amyloid plaques are dark on silver staining and are separated into 2 types: diffuse and neuritic
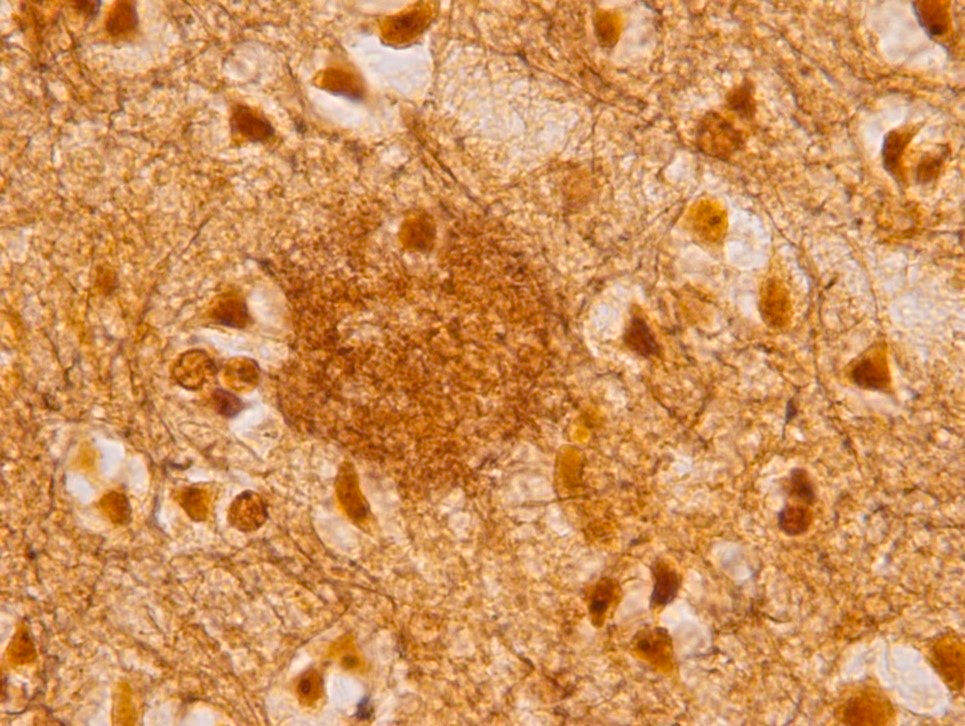
- Neuritic plaques (NP) show abnormal dystrophic neurites associated with the plaque on silver or phosphorylated tau immunostaining; these plaques count toward the C and A scores
- Diffuse plaques lack central cores or dystrophic neurites and can be detected on silver or amyloid staining; these count toward the A score
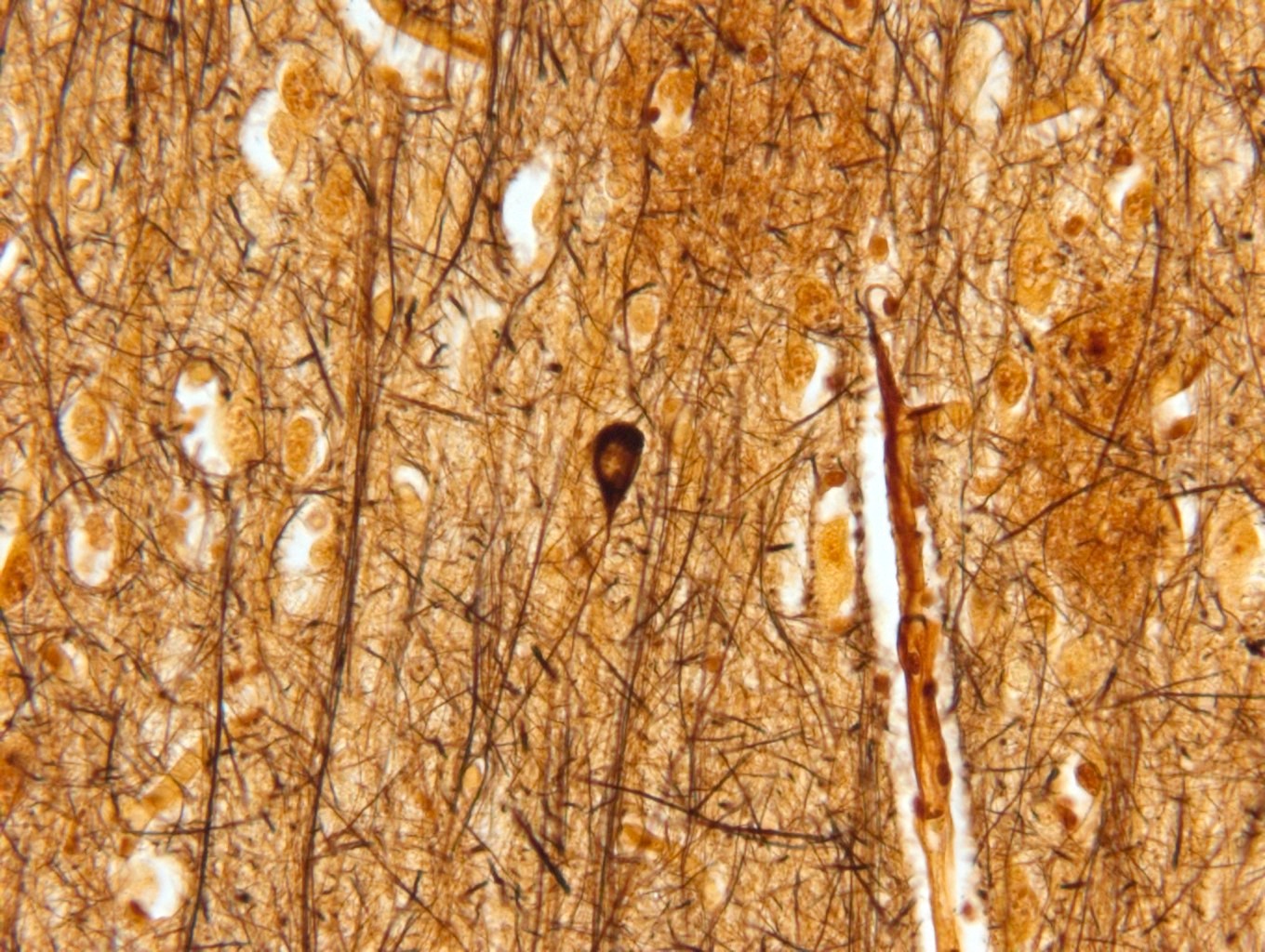
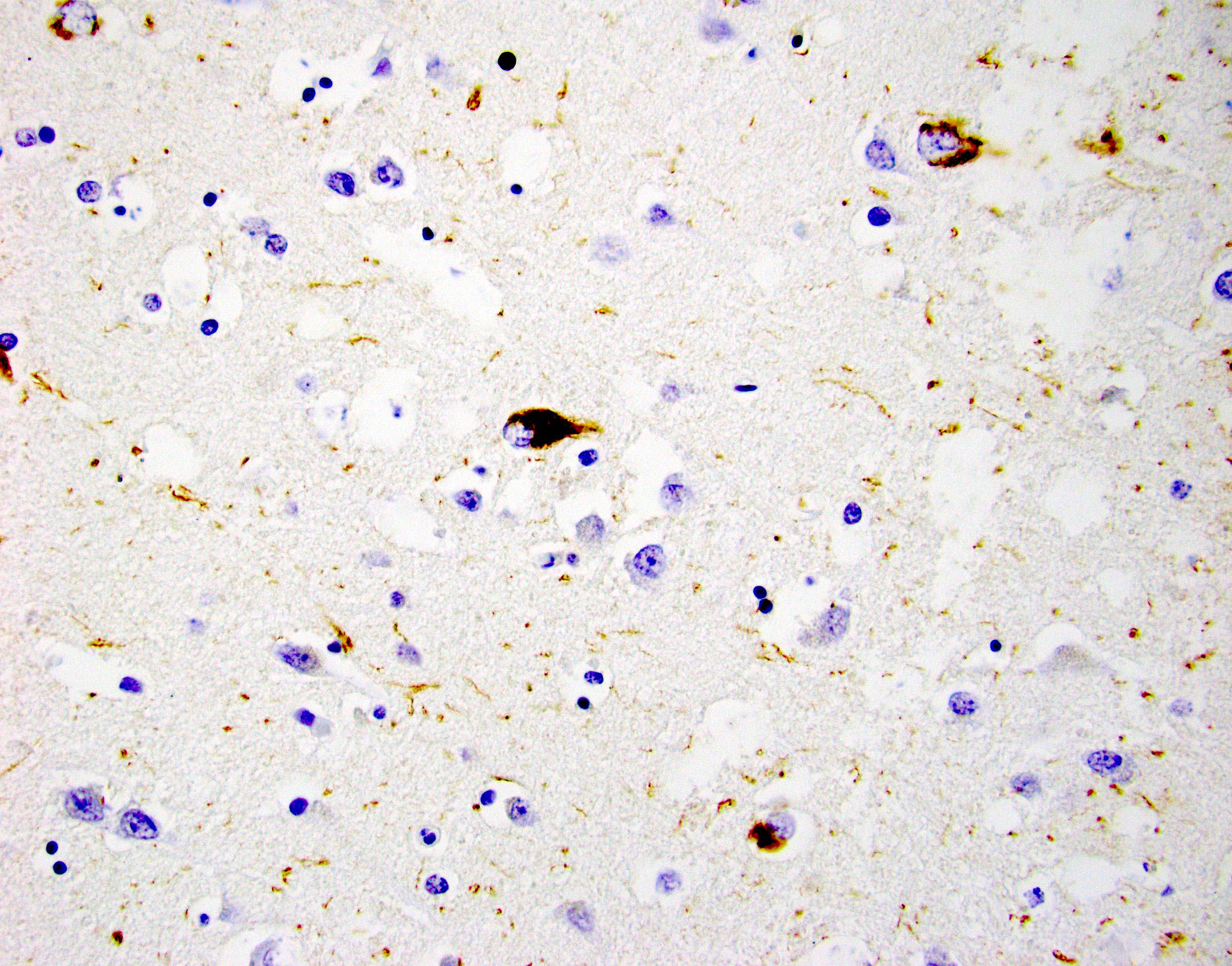
- Neurofibrillary tangles are visible on H&E staining as pale fibrillar basophilic aggregates within neuronal cytoplasm
- Neurofibrillary tangles are dark fibrillar aggregates in neurons on silver staining
- Phosphorylated tau immunostaining can highlight both neurofibrillary tangles and more granular pretangle aggregates
- Both aggregates are better visualized for staging purposes on silver staining or immunostaining; H&E is not recommended for staging
- Alzheimer disease neuropathological change is currently assessed by the ABC scoring system using a 10x objective when density assessment is required (Alzheimers Dement 2012;8:1)
- A score is derived from the Thal phase, which assesses regional involvement by beta amyloid deposits (Neurology 2002;58:1791)
- Thal phase 1 - 2 is designated A1 and includes beta amyloid in the neocortex (Thal 1) and extends into the entorhinal region and CA1 of the hippocampus (Thal 2)
- Thal phase 3 is designated A2 and includes extension of beta amyloid into the thalamus and deep gray matter structures
- Thal phase 4 - 5 is designated A3 and includes extension of beta amyloid into brainstem areas such as the substantia nigra (Thal 4) and molecular layer of the cerebellum (Thal 5)
- B score is derived from the Braak stage, which assesses regional involvement by neurofibrillary tangles (Acta Neuropathol 2006;112:389)
- Braak stage 1 - 2 is designated B1, neurofibrillary tangles start in the transentorhinal cortex (Braak 1) and extend into the entorhinal cortex and hippocampus (Braak 2)
- Braak stage 3 - 4 is designated B2, neurofibrillary tangles extend to involve the neocortex starting in the fusiform and lingual gyri (Braak 3) and extending in a fan-like distribution into other association areas (Braak 4)
- Braak stage 5 - 6 is designated B3, neurofibrillary tangles extend into the peristriate (Braak 5) and then the primary visual cortex (Braak 6) in the occipital lobe
- C score is derived from the CERAD score and is a semiquantitative assessment of neuritic plaques in the frontal, parietal and temporal lobes (the occipital lobe is excluded from this scheme) (Neurology 1991;41:479)
- CERAD sparse neuritic plaques are designated C1
- CERAD moderate neuritic plaques are designated C2
- CERAD frequent neuritic plaques are designated C3
- This grading scheme only recognizes neuritic plaques; diffuse plaques are not included in the criteria
- A score is derived from the Thal phase, which assesses regional involvement by beta amyloid deposits (Neurology 2002;58:1791)
- Low level of Alzheimer disease neuropathologic changes is unlikely to account for a clinical history of dementia but can contribute to dementia in combination with other neurodegenerative or vascular pathologies
- Cerebral amyloid angiopathy is a common finding associated with Alzheimer disease pathology
- Characterized by amorphous eosinophilic beta amyloid accumulation around cerebral vessels in a double barrel pattern
- More common in individuals with APOE ɛ4 allele (Alzheimers Dement 2012;8:1)
- Lewy bodies, aggregates of alpha synuclein in neurons, restricted to the olfactory bulb and amygdala can occur with Alzheimer disease neuropathologic changes (J Neuropathol Exp Neurol 2006;65:685, Acta Neuropathol 2008;116:17)
- Vacuolization of the superficial layers of cortex is commonly associated with atrophy in Alzheimer disease but is not specific
- Hirano bodies and granulovacuolar degeneration can be seen at higher levels in the hippocampus associated with Alzheimer disease neuropathologic changes but are nonspecific
- Other age related vascular and neurodegenerative pathologies commonly co-occur with Alzheimer disease but are reported as separate diagnoses
Microscopic (histologic) images
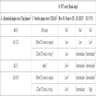
Positive stains
- Beta amyloid immunostaining highlights beta amyloid deposition in both diffuse plaques and neuritic plaques
- Thioflavin S can be used to highlight amyloid plaques and neurofibrillary tangles
- Phosphorylated tau immunostaining highlights pretangle aggregates, neurofibrillary tangles and dystrophic neurites in neuritic plaques
- Silver staining methods can highlight amyloid plaques and neurofibrillary tangles, as well as other pathologic processes
- References: Alzheimers Dement 2012;8:1, Neurology 1991;41:479, Neurology 2002;58:1791, Acta Neuropathol 1991;82:239
Negative stains
- Presence of additional neurodegenerative disease pathology leads to the diagnosis of dementia with mixed pathologies
- Alpha synuclein immunostaining should be negative for aggregates outside of the amygdala and olfactory bulb
- TDP-43 immunostaining should not show additional aggregates unless other neurodegenerative pathologies are present
- References: Alzheimers Dement 2012;8:1, Neurology 1991;41:479, Neurology 2002;58:1791, Acta Neuropathol 1991;82:239
Molecular / cytogenetics description
- Major genetic risk allele for sporadic Alzheimer disease is associated with the ɛ4 allele of the APOE gene, while the ɛ2 allele is protective (Ann Neurol 1996;39:395)
- Other genetic risk alleles confer less risk compared with APOE
- Early onset Alzheimer disease is most commonly associated with mutations in the presenilin 1 and 2 genes (PSEN1, PSEN2), mutations in the amyloid precursor protein gene (APP) or with trisomy 21 (APP is located on chromosome 21)
Sample pathology report
- Adult brain (1,300 grams), autopsy:
- Alzheimer disease neuropathological changes, high level (A3, B3, C3) (see comment)
- External examination: The fresh brain weight is 1,300 grams. A portion of dorsal cerebral dura is available for examination and shows no significant epidural or subdural hemorrhage. The superior sagittal sinus is patent. The leptomeninges are thin and translucent without significant subarachnoid hemorrhage. There is moderate generalized cortical atrophy. The circle of Willis has a normal anatomic configuration and no atherosclerosis. No aneurysms are identified. The cranial nerves occupy appropriate locations without anomalies or lesions. There is no evidence of cingulate gyrus, uncal or cerebellar tonsillar herniation. The brainstem and cerebellum have unremarkable external appearances.
- Coronal sections: Serial coronal sections of the cerebral hemispheres reveal the cortical and subcortical gray and white matter structures to be of normal configuration without hemorrhages, infarcts or mass lesions. There is mild atrophy of the hippocampus. The basal ganglia, thalamus, amygdalae and mamillary bodies bilaterally are of normal size, color and consistency. The ventricular system shows moderate hydrocephalus of the lateral and third ventricles. Serial sections of the brainstem and cerebellum reveal no lesions and show pallor of the locus ceruleus. The substantia nigra is well pigmented.
- Microscopic description: H&E stained sections show superficial spongiosis of the gray matter with scattered amyloid plaques. Neurofibrillary tangles are present in the entorhinal cortex, hippocampus and locus ceruleus. Hirano silver stains show frequent neuritic plaques in the frontal, parietal and temporal cortices, which is considered C3 using the updated National Institute on Aging and Alzheimer's Association (NIA AA) criteria (Alzheimers Dement 2012;8:1). Neurofibrillary tangles are frequent in the entorhinal cortex, hippocampus and temporal cortex, while moderate neurofibrillary tangles are present in the frontal cortex, parietal cortex and nonstriated occipital cortex. Sparse neurofibrillary tangles are present in the striate cortex, corresponding to B3 using the updated NIA AA criteria (Alzheimers Dement 2012;8:1). Amyloid beta immunohistochemistry is positive in multiple areas, including the thalamus, midbrain and cerebellum, which corresponds to A3 in the NIA AA system (Alzheimers Dement 2012;8:1). According to the 2012 NIA AA criteria, this level of amyloid, NFT and NP pathology (A3, B3, C3) is considered a high level.
- Comment: The high level of Alzheimer disease neuropathologic changes support the clinical impression of Alzheimer disease.
Differential diagnosis
- Dementing diseases span a broad spectrum; many are classified by the predominant protein that is thought to drive the disease
- Non-Alzheimer tauopathies (progressive supranuclear palsy [PSP], corticobasal degeneration [CBD], Pick disease, etc.)
- Clinical history does not match Alzheimer disease
- More pronounced frontal and temporal lobe atrophy or atrophy of specific subcortical brain regions (subthalamic nucleus, substantia nigra, areas involved with progressive supranuclear palsy [PSP])
- Abundant tau inclusions
- Amyloid is usually absent
- Alpha synucleinopathies (Parkinson disease, Lewy body dementia, multiple system atrophy, etc.):
- Clinical history does not match Alzheimer disease
- Cortical atrophy is not as pronounced
- Alpha synuclein positive aggregates
- More frequently associated with a pale substantia nigra and locus ceruleus
- Atrophy of the cerebellum or putamen (multiple system atrophy)
- TDP-43 proteinopathies (limbic predominant age related TDP-43 encephalopathy [LATE], frontotemporal lobar degeneration [FTLD], etc.):
- LATE can be seen in the setting of Alzheimer disease
- Clinical findings of FTLD do not usually overlap with Alzheimer disease
- TDP-43 protein aggregates are present
- Vascular dementia:
- Marked by infarcts
- Can commonly occur with Alzheimer disease
- May present clinically with a step-like neurocognitive decline
- Non-Alzheimer tauopathies (progressive supranuclear palsy [PSP], corticobasal degeneration [CBD], Pick disease, etc.)
Additional references
Board review style question #1
Board review style answer #1
B. Beta amyloid. The diagnostic criteria for the assessment of Alzheimer disease hinges on both beta amyloid and tau pathologies. Answer A is incorrect because alpha synuclein is commonly seen with Lewy body dementia and Parkinson disease. Answer C is incorrect because GFAP is typical of reactive astrocytosis, which can be seen with vascular dementia. Answer D is incorrect because TDP-43 is present in both frontotemporal lobar degenerations and limbic predominant age related TDP-43 encephalopathy (LATE disease).
Comment Here
Reference: Alzheimer disease
Comment Here
Reference: Alzheimer disease
Board review style question #2
Which genetic marker is most associated with sporadic Alzheimer disease?
- APOE ɛ4
- APP mutations
- Presenilin mutations
- Trisomy 21
Board review style answer #2
A. APOE ɛ4. APOE ɛ4 is commonly associated with sporadic Alzheimer disease with the risk for the disorder increasing with the number of alleles present. Answers B and C are incorrect because APP mutations and presenilin mutations are associated with autosomal dominant Alzheimer disease. Answer D is incorrect because trisomy 21 is associated with early onset Alzheimer disease.
Comment Here
Reference: Alzheimer disease
Comment Here
Reference: Alzheimer disease
Board review style question #3
Which is the most common form of dementia?
- Alzheimer disease
- Lewy body dementia
- Pick disease
- Progressive supranuclear palsy
Board review style answer #3
A. Alzheimer disease. Alzheimer disease is the most common form of dementia and is responsible for 60 - 70% of cases. Answer B is incorrect because Lewy body dementia is attributed to ~20% of cases. Answers C and D are incorrect because the remaining 10% is comprised of a diverse set of dementing diseases.
Comment Here
Reference: Alzheimer disease
Comment Here
Reference: Alzheimer disease