Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Clinical features | Diagnosis | Prognostic factors | Case reports | Treatment | Clinical images | Microscopic (histologic) images | Virtual slides | Peripheral smear images | Positive stains | Negative stains | Flow cytometry description | Flow cytometry images | Molecular / cytogenetics description | Molecular / cytogenetics images | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1Cite this page: Tang Z, DiNardo CD. Myeloid neoplasms with germline GATA2 mutation. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/bonemarrowneoplasticGATA2.html. Accessed April 26th, 2024.
Definition / general
- Classified as one of the myeloid neoplasms with germline predisposition and other organ dysfunction in the revised 2016 WHO classification (Blood 2016;127:2391)
- Now known to underlie several syndromes: MonoMAC syndrome, dendritic cell, monocyte, B and NK lymphoid (DCML) deficiency and Emberger syndrome
Essential features
- Counts for the cause of 7 - 15% of primary myelodysplastic syndrome cases in children and adolescents and for approximately 37% of myeloid neoplasms with monosomy 7 (-7) or other forms of 7q- / -7 in adolescents and young adults
- Differentiating somatic from germline GATA2 mutations is necessary
- Genetic counseling of family members and timely screening of donors for presence of predisposition germline mutations are necessary prior to hematopoietic stem cell transplant for optimal patient outcomes
- Skin fibroblasts are the gold standard for obtaining germline DNA in patients with myeloid neoplasms
Terminology
- Myeloid neoplasms with germline GATA2 mutation
- Familial myeloid neoplasms
- Familial myelodysplastic syndrome (MDS) / acute myeloid leukemia (AML)
ICD coding
Epidemiology
- Prevalence in general population is unknown
- Approximately 550 myeloid neoplasm cases with germline GATA2 mutations reported in literature (Best Pract Res Clin Haematol 2020;33:101197)
- In children, adolescents and young adults, 7 - 15% of primary myelodysplastic syndrome cases and up to 37% of patients with monosomy 7 (-7) are associated with germline GATA2 mutation (Blood 2016;127:1387, Best Pract Res Clin Haematol 2020;33:101197)
Sites
- Myeloid neoplasms mainly involve the peripheral blood and bone marrow
- Many other organs other than bone marrow (e.g. ear, lung, heart, joints, skin, blood vessels and immune system) can also be involved, especially if a germline GATA2 mutation associated syndrome exists (Blood 2014;123:809)
Pathophysiology
- GATA2 is a member of the GATA2 family of zinc finger transcription factors that regulate transcription of many genes
- Plays an essential role in genesis and function of hematopoietic stem and progenitor cells and thus all subsequent blood cell lineages
- Also involved in autoimmunity, inflammation and developmental processes
- Germline GATA2 mutations may result in loss of function of the mutated allele, which leads to GATA2 haploinsufficiency and then affecting function of multiple cell lineages (Hematol Oncol Clin North Am 2018;32:713)
- 4 types of germline GATA2 mutations are categorized:
- Missense mutations, including all mutations resulting in a mutant protein
- Null mutations, including all nonsense, frameshift mutations and large deletions that result in no protein product
- Regulatory mutations, including all mutations within the enhancer region of intron 5
- Uniallelic mutations, including cases with phenotypical GATA2 deficiency but without a defined gene mutation
- According to Spinner et al, null mutations are closely associated with severe viral infections and earlier onset of those infections, while lymphedema was only observed in patients with null or regulatory mutations (Blood 2014;123:809)
- Large deletions are associated with extramedullary developmental defects
- Somatic ASXL1 mutations are commonly observed in patients with germline GATA2 mutations and are considered as an important second hit for myeloid transformation observed in these patients (Haematologica 2014;99:276)
- Inheritance: spontaneously arising but transmitted mainly as autosomal dominant (approximately 80%) (Blood 2014;123:809, Best Pract Res Clin Haematol 2020;33:101197)
- Penetrance was estimated at 90% by the age of 60 years; cases with sporadic germline GATA2 mutations have been reported as well (Br J Haematol 2013;161:701)
Clinical features
- Onset of myeloid neoplasm in patients with germline GATA mutation(s) is between ages of 5 months and 78 years (median 20 years) (Blood 2014;123:809, Best Pract Res Clin Haematol 2020;33:101197)
- Spectrum of the diseases include pediatric MDS or familial MDS / AML, chronic myelomonocytic leukemia (CMML) and myeloproliferative neoplasms (MPNs); especially, germline GATA2 mutations count for approximately 7% of all primary MDS cases in children but are absent in children with secondary MDS
- Initial presentations in these patients are heterogeneous, ranging from asymptomatic, mild or moderate cytopenia (e.g. refractory cytopenia of childhood, RCC) to mild to severe immunodeficiency (e.g. lymphedema, pulmonary diseases, vascular problems, virus or bacteria infections, skin lesions or congenital deafness) (Best Pract Res Clin Haematol 2020;33:101197)
- Can present or be diagnosed as a standalone disease / syndrome prior to myeloid neoplasm as well as coexist with myeloid neoplasm
Diagnosis
- The following conditions can be indications to start a workup for potential germline GATA2 mutations that are associated with existing or preexisting myeloid neoplasm:
- Idiopathic cytopenia (especially neutropenia, CD4 lymphocytopenia, depletion of monocytes, B cells, NK cells and dendritic cells), idiopathic bone marrow failure, susceptibility to refractory virus or other pathogen infection (e.g. human papillomavirus [HPV], herpes virus, nontuberculous mycobacteria [NTM] and Histoplasma capsulatum, etc.), pulmonary alveolar proteinosis (PAP) and ventilatory defects, skin lesions (e.g. flat warts), idiopathic lymphedema, solid tumor, especially those being associated with HPV, EBV infections and so on
- Diagnostic criteria for each type of germline GATA2 mutation associated myeloid neoplasm (e.g. MDS, MPN or AML) are the same as that for cases without germline GATA2 mutation; the prevalence of MDL, AML and CMML in a study of 57 patients with GATA2 deficiency were 84%, 14% and 8% respectively and all the AML cases were evolved from MDS (Blood 2016;127:1387)
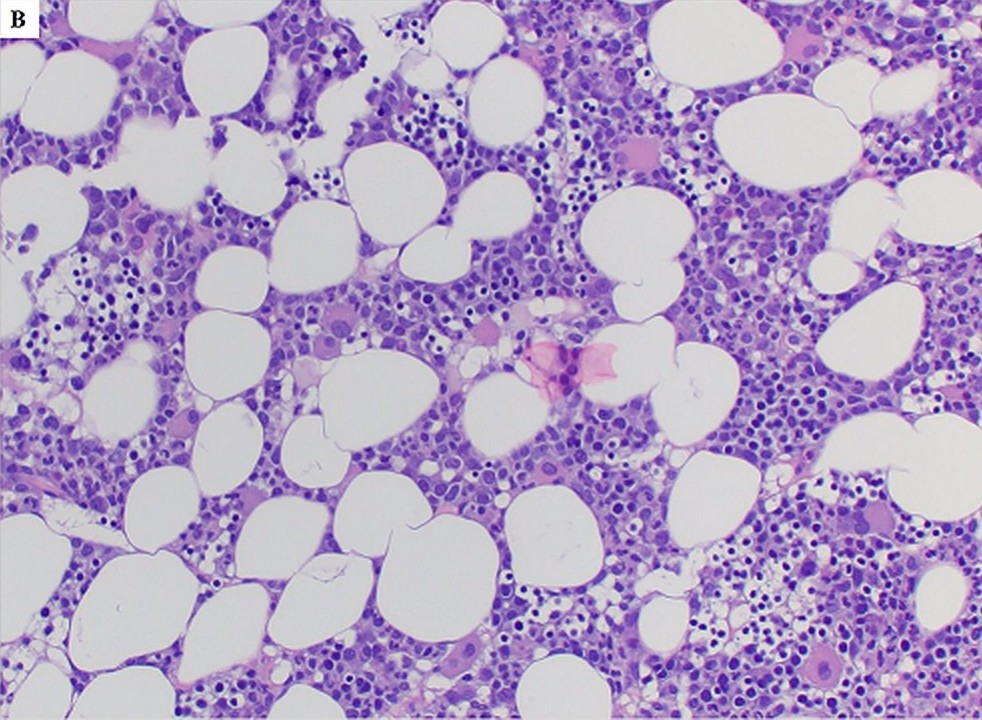
- Different from de novo MDS cases with a bone marrow feature of hypercellularity, germline GATA2 mutation associated MDS patients mostly present with hypocellular bone marrow for their ages, also frequently with increased fibrosis and dysplastic magakryocytes (Blood 2016;127:1387)
Prognostic factors
- In general, the status of germline GATA2 mutation does not influence overall survival and outcome of hematopoietic stem cell transplantation (HSCT) (Blood 2014;123:809, Blood 2016;127:2391, Semin Hematol 2017;54:81)
- However, children and adolescence with germline GATA2 mutation(s) and monosomy 7 had a shorter overall survival than their controls with wild type GATA2 (Blood 2016;127:1387, Semin Hematol 2017;54:81)
- Acquired somatic mutations, such as monosomy 7, SETBP1 and ASXL1 mutations that are more frequently detected in adolescents and adults usually indicate a disease progression (Blood 2016;127:2391, Blood 2016;127:1387)
Case reports
- 16 year old boy with acute myeloid leukemia and severe bilateral warts on hands and fingers since childhood (Leuk Lymphoma 2020;61:3010)
- 22 year old woman with acute monoblastic leukemia diagnosed at age 13 (Br J Haematol 2020;188:768)
- 24 year old man with pancytopenia and recurrent extensive warts on bilateral hands and recurrent panniculitis (JCO Precis Oncol 2019;3:PO.18.00301)
- 27 year old man with myelodysplastic syndrome diagnosed at age 9 and a history of recurrent viral and bacterial infections and congenital deafness (Br J Haematol 2020;188:768)
- 36 year old woman with prolonged pancytopenia, chronic active Epstein-Barr virus infection and later onset of acute myeloid leukemia (JCO Precis Oncol 2019;3:PO.18.00301)
Treatment
- Intensive chemotherapy is not suitable for patients with germline GATA2 mutation associated myeloid neoplasm due to the underlying immunodeficiency and stem cell defects; therefore, timely HSCT is necessary for patients with disease progression or high risk karyotypes (Blood 2014;123:809, JCO Precis Oncol 2019;3:PO.18.00301, Best Pract Res Clin Haematol 2020;33:101197, Blood 2016;127:2391)
- However, genetic screening of related donors to avoid reintroduction of germline mutation with predisposition is necessary
Virtual slides
Positive stains
- Variable, depending on the type and stages of myeloid neoplasm but not specifically associated with the underlying germline GATA2 mutation
Negative stains
- Variable, depending on the type and stages of myeloid neoplasm but not specifically associated with the underlying germline GATA2 mutation
Flow cytometry description
- Variable, depending on the type and stages of myeloid neoplasm but not specifically associated with the underlying germline GATA2 mutation; for example, in childhood myelodysplastic syndrome with germline GATA2 mutation, characteristic immunophenotyping changes include but are not limited to granulocytopenia, monocytopenia, decreased B cells and NK cells, presence of CD56+ plasma cells, etc. (Haematologica 2011;96:1221, Hematol Oncol Clin North Am 2018;32:713)
Molecular / cytogenetics description
- Molecular findings:
- Mutation of various types (missense, nonsense, indels, etc.), involving exon(s) or intron(s) of GATA2 gene; differentiating somatic from germline GATA2 mutations is necessary (see below)
- Cytogenetic findings:
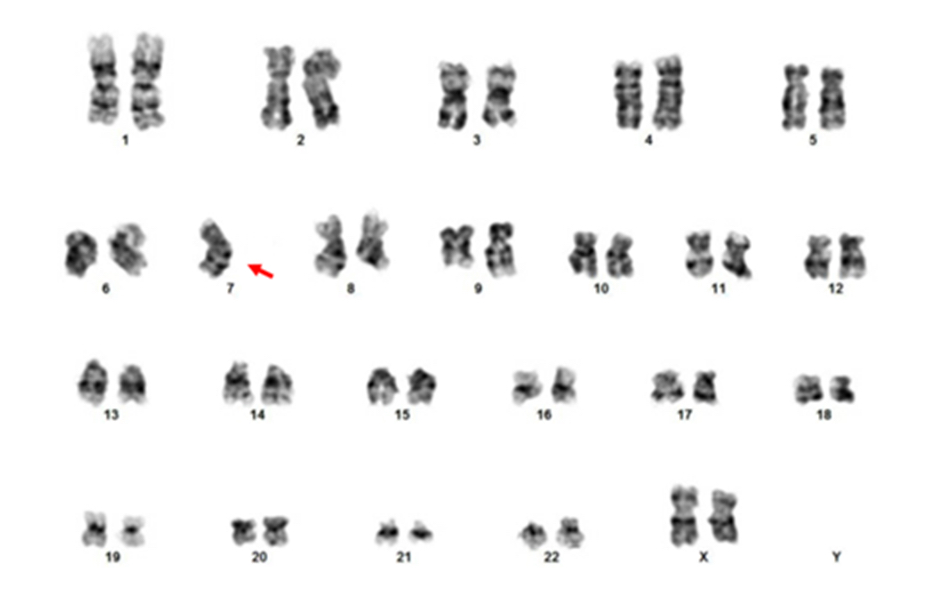
- In the study by Wlodarski et al, the frequent chromosomal abnormalities were monosomy 7 (68%), trisomy 8 (9%), der(1;7)(q10;p10) resulting loss of 1p and 7q (7%); the rest of the cases had a normal karyotype (Blood 2016;127:2391)
- Other chromosomal abnormalities, such as trisomy 21 and t(2;12)(p21;p13), have also reported (JCO Precis Oncol 2019;3:PO.18.00301, Best Pract Res Clin Haematol 2020;33:101197)
- Next generation sequencing (NGS): in 10 - 15% cases, large deletions or mutations in intronic region of GATA2 gene may be involved and they can be missed with standard gene panel based NGS; therefore, NGS based whole exome (WES) or even whole genome (WGS) sequencing can be applicable to identify rare mutation(s) or those located within an intron (Leuk Lymphoma 2020;61:3010, JCO Precis Oncol 2019;3:PO.18.00301)
- Approximately 20% of all cases are de novo mutations without a family history (IUBMB Life 2020;72:142)
- Sanger sequencing, especially for known mutation(s)
- Conventional cytogenetic analysis or FISH, with focus on monosomy 7 or 7q abnormality, trisomy 8 and trisomy 21
- Note: somatic GATA2 mutations reportedly coexist with other germline mutations (e.g. ASXL1, CEBPA) or other acquired drive mutations in myeloid neoplasm cases; differentiating somatic from germline GATA2 mutations is necessary
- Additional reference: Blood 2014;123:809
Molecular / cytogenetics images
Sample pathology report
- Bone marrow biopsy, right posterior iliac crest:
- Gross measurement: 1.2 cm in aggregate. Quality: limited, subcortical. Cellularity: near empty marrow with only 2 small foci of cellular areas with erythroid predominant. Megakaryocytes: present. Infiltrate: no increase in immature cells.
- Bone marrow clot:
- Quality: adequate, 10 - 20% cellularity, morphology similar to biopsy.
- Bone marrow smear / touch preparations:
- Quality: adequate. Granulocytes: decreased with maturation and dysplasia. Erythrocytes: relatively increased with maturation and occasional dyserythropoiesis. Megakaryocytes: rare. Lymphocytes: not increased. Plasma cells: not increased. Blasts: not increased.
- Stains of bone marrow smears, clots and biopsy:
- Iron: slightly increased, no ringed sideroblasts. Reticulin / trichrome: inadequate for reticulin and trichrome stain evaluation. MPO: positive in scattered cells, decreased. CD71 / glycophorin A: highlight increased erythroids. CD61: highlights few scattered megakaryocytes.
- Bone marrow diagnosis:
- Hypocellular marrow with erythroid predominance and granulocytic dysplasia, 2% blasts, morphologically and immunophenotypically consistent with myelodysplastic syndrome (MDS). An underlying congenital condition should be considered (see comment).
- Comment: 23 year old man with a history of recurrent skin infections and warts, CBC with mild pancytopenia and marrow biopsy showed hypocellularity. Concurrent flow cytometry analysis demonstrated aberrant myeloblasts, lack of hematogones, virtual absence of monocytes, minimal NK cells and only 1.1% of B cells. In addition, a tiny atypical T cells population is also detected, CD2+, CD3+, CD5 dim, CD4-, CD8 partial, CD56+, CD57 bright. The overall picture is highly suspicious for a congenital GATA2 mutation. Correlation with pending NGS result will be helpful for confirmation. Correlation with pending ancillary study results is recommended for complete evaluation.
Differential diagnosis
- Germline versus somatic GATA2 mutation
- Syndromic versus nonsyndromic germline GATA2 mutation
- 1 case with somatic GATA2 mutation but mimicking clinical presentation of GATA2 deficiency of germline GATA2 mutations has been reported (Blood Adv 2018;2:904)
Table 1. Differentials of Germline and Somatic GATA2 mutations
| ~ 150 | ~ 50 | |
| Whole GATA2 gene, ZF2 especially | ZF1 | |
| Multiple (see pathophysiology) | Mostly point mutations | |
| GATA2 deficiency of loss of function of affected copy | Gain of function | |
| Syndromic and nonsyndromic with or without myeloid neoplasm; myeloid neoplasm as standalone, especially MDS and AML in children and young adults | Mainly MDS, AML, CML blast phase in all age groups | |
| Other germline mutation, somatic ASXL1, etc. | Biallelic CEBPA mutations; other germline mutations (e.g. RUNX1) | |
| Both disease affected and nonaffected tissues; especially skin biopsy | Disease affected tissue only (e.g. bone marrow and skin biopsy will be mostly negative) |
Board review style question #1
What is the gold standard to obtain reliable germline DNA in a patient with a hematologic malignancy?
- Bone marrow biopsy and cell culture
- Buccal swabs and saliva
- Nails or hair
- Peripheral blood and cell culture
- Skin biopsy and cell culture
Board review style answer #1
E. Skin biopsy and cell culture. Genetic testing of cultured skin fibroblasts is the gold standard for obtaining germline DNA in patients with hematologic malignancies. DNA from nails or hair can be also used if skin biopsy and cell culture are not feasible. Peripheral blood and bone marrow can be affected by myeloid neoplasm or other hematological malignancies; they should be used cautiously as a source of germline DNA. Buccal swabs and saliva are often contaminated with blood cells and they should also be cautiously used as resource of germline DNA (2016 WHO classification).
Comment Here
Reference: Myeloid neoplasms with germline GATA2 mutation
Comment Here
Reference: Myeloid neoplasms with germline GATA2 mutation