Table of Contents
Definition / general | Essential features | Terminology | Epidemiology | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Case reports | Treatment | Clinical images | Microscopic (histologic) description | Microscopic (histologic) images | Peripheral smear description | Peripheral smear images | Positive stains | Videos | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Raja F, Chitsaz M, Kundrapu S. Iron deficiency anemia. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/hematologyirondefanemia.html. Accessed May 12th, 2024.
Definition / general
- Most common type of anemia worldwide; occurs due to decreased iron levels in the body, which leads to decreased erythropoiesis
- Characterized by hypochromic microcytic red blood cells (RBCs)
Essential features
- Iron deficiency anemia (IDA) is the most frequent presentation of iron deficiency
- Common causes are blood loss, reduced absorption, inadequate dietary intake, pregnancy, intestinal worm colonization and chronic inflammation
- Low ferritin levels; ferritin is an indicator of iron stores and is the most sensitive and specific biomarker
- Hypochromia, microcytosis and anisopoikilocytosis of the RBCs are the characteristic morphologic abnormalities
Terminology
- Iron deficiency anemia (IDA)
- Microcytic, hypochromic anemia with anisopoikilocytosis
- Anemia of chronic disease or anemia of inflammation or acute blood loss anemia or nutritional anemia (depending on the cause)
Epidemiology
- Iron deficiency is defined as ferritin < 15 ng/mL and transferrin saturation < 10% (PLoS One 2020;15:e0232125)
- Iron deficiency is more prevalent than IDA
- F > M; common in women of childbearing age, children and elderly
- Approximately 25% of people worldwide have anemia and 50% of the anemias are due to iron deficiency (StatPearls: Iron Deficiency Anemia [Accessed 16 February 2022])
- Affects > 1.2 billion population worldwide; iron deficiency in the absence of anemia is even more frequent (Blood 2019;133:30)
- Iron deficiency is more common in developing countries than in developed countries like the United States (StatPearls: Iron Deficiency Anemia [Accessed 16 February 2022])
Pathophysiology
- Iron is essential for hemoglobin synthesis during erythropoiesis
- Impaired delivery of iron to erythroid precursors results in decreased erythropoiesis
- Iron deficiency leading to IDA is a chronic process
- Initially normal RBCs are produced
- Later, decreased iron transport to bone marrow results in microcytic hypochromic RBCs
- Daily iron requirement is about 20 - 25 mg
- About 1 - 2 mg is absorbed daily and the remaining comes from recycled iron
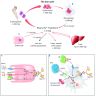
- Iron absorption pathway
- Dietary iron is absorbed into the bloodstream via the small intestine
- Following absorption, iron may be stored in enterocytes or transported to other tissues via transferrin (Medicines (Basel) 2019;6:85)
- Iron homeostasis
- Role of hepcidin
- Peptide hormone produced and secreted by the liver; responsible for regulation of iron levels
- Binds to iron transporter ferroportin, causes its internalization and degradation, thus preventing iron transport and decreasing the circulating levels
- Levels directly correlate with serum ferritin levels, increases in systemic inflammation or infection
- Increased hepcidin causes iron sequestration and reduces supply of erythropoietic iron
- Its production is inhibited with increased erythropoiesis, iron deficiency and tissue hypoxia to facilitate iron absorption and release of iron from body stores (See Diagrams)
- When the iron stores are low, activation of hypoxia inducible factor 2α increases intestinal iron uptake through divalent metal transporter 1 (DMT1) (N Engl J Med 2015;372:1832)
- Iron recycling:
- Majority of the iron required by the body is recycled from senescent RBCs undergoing phagocytosis by the reticuloendothelial macrophages; this iron is either used for hematopoiesis or stored as ferritin and hemosiderin (N Engl J Med 2015;372:1832, Merck Manual: Iron Deficiency Anemia [Accessed 7 March 2022])
- Role of hepcidin
Etiology
- Major causes:
- Blood loss: menstruation, gastrointestinal (GI) bleeding, intestinal worm colonization (developing countries)
- Women of childbearing age: menorrhagia; fibroid
- Adults > 50 years: GI bleeding is the most common cause and should be evaluated for malignancy
- Reduced absorption: celiac disease, Helicobacter pylori, gastritis, bariatric surgery
- Infants: iron is the most common single nutrient deficiency
- Children consuming cow’s milk are at greater risk of developing iron deficiency
- Higher concentration of calcium in cow’s milk competes with iron for absorption
- Inadequate dietary intake
- Increased demand: pregnancy, lactation and rapid growth phase in children (ASH: Iron Deficiency Anemia [Accessed 16 February 2022])
- Blood loss: menstruation, gastrointestinal (GI) bleeding, intestinal worm colonization (developing countries)
- Medications
- Proton pump inhibitors directly affect iron metabolism by suppressing iron absorption through the inhibition of duodenal ferroportin via hepcidin upregulation (Toxicol Lett 2020;318:86)
- Recombinant erythropoietin used in treatment of chronic kidney disease may increase need and utilization of iron by the hematopoietic tissues (Kidney Int Suppl 1999;69:S107)
- Procedures such as hemodialysis and frequent phlebotomies
- Blood remaining in the dialysis tubing and an increased rate of blood loss during dialysis can cause iron deficiency anemia (Acta Haematol 2019;142:44)
- Rare causes: iron refractory iron deficiency anemia
- Totally or partially resistant (refractory) to treatment with iron
- Occurs due to mutations in TMPRSS6, a hepcidin regulatory gene
- Mutations in other genes such as divalent metal transporter 1 (DMT1), ceruloplasmin (CP), transferrin (TF) (N Engl J Med 2015;372:1832)
Clinical features
- Can frequently be asymptomatic (N Engl J Med 2015;372:1832)
- In addition to anemia, may present with features specific to iron deficiency (Clin Med (Lond) 2021;21:107)
- Anemia symptoms:
- Fatigue
- Altered behavior, irritability
- Exertional dyspnea
- Can lead to decreased cognitive performance and delay mental and motor development in children (N Engl J Med 2015;372:1832)
- Headache
- Sleep disturbance
- Decrease cognitive function and impaired memory
- Palpitations
- Muscle and joint pain
- Features of iron deficiency:
- Glossitis, angular cheilitis
- Craving for ice or clay (pica or pagophagia), especially in pregnant women (American Pregnancy Association: Pica Cravings During Pregnancy [Accessed 16 February 2022])
- Dysphagia with solid foods
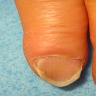
- Koilonychia and brittle nails (see Clinical images) (N Engl J Med 2018;379:e13)
- Restless legs syndrome (Willis-Ekbom disease)
- Severe IDA in pregnancy is associated with an increased risk of preterm labor, low neonatal weight and increased newborn and maternal mortality (N Engl J Med 2015;372:1832)
Diagnosis
- See Laboratory
Laboratory
- Complete blood count
- Low hemoglobin (Hb) and hematocrit (Hct)
- Low mean corpuscular volume (MCV) and mean corpuscular hemoglobin concentration (MCHC)
- Increased red cell distribution width (RDW)
- Decreased reticulocyte index
- Low serum iron and ferritin levels (best initial test)
- Low iron saturation
- Elevated total iron binding capacity (TIBC) or transferrin
- Reticulocyte hemoglobin content (CHr) value is a strong predictor of the early measurement of functional iron deficiency (ASH: Clinical utility of the reticulocyte hemoglobin content in the diagnosis of iron deficiency [Accessed 16 February 2022])
- In adult men, postmenopausal women or younger women with severe anemia, tests to determine the reason for iron deficiency should be performed:
- Fecal occult blood test
- Upper and lower endoscopy
- Test for hematuria or hemoglobinuria
- Gynecologic evaluation in women
- In adult men, postmenopausal women or younger women with severe anemia, tests to determine the reason for iron deficiency should be performed:
Case reports
- 42 year old Hispanic woman with a chief complaint of increasing fatigue and dizziness for 2 weeks (J Med Case Rep 2021;15:472)
- 52 year old man on omeprazole (a proton pump inhibitor) for 25 years developed iron deficiency anemia (Intern Med 2018;57:899)
- 54 year old woman with a 7 year history of hemorrhoid bleeding and developing iron deficiency anemia; although her anemia was treated, her koilonychia persisted (N Engl J Med 2018;379:e13)
Treatment
- Dietary supplementation
- Iron therapy:
- Oral iron for mild to moderate symptoms
- Parenteral iron: unable to absorb oral iron or anemia worsening despite oral iron therapy
- RBC transfusions in patients with acute bleeding or in severe symptoms of anemia or coronary insufficiency (ASH: Iron Deficiency Anemia [Accessed 3 January 2022])
Microscopic (histologic) description
- Peripheral blood smear:
- Microcytic and hypochromic RBCs with marked anisopoikilocytosis in chronic cases of iron deficiency anemia
- Platelets may be increased (reactive thrombocytosis) (Am J Hematol 2014;89:524)
- Target cells are absent, unlike in thalassemia
- In more acute cases, dimorphic population of RBCs with increased RDW is the earliest evidence of iron deficient erythropoiesis
- Normocytic cells produced before bleeding and microcytic cells produced after bleeding
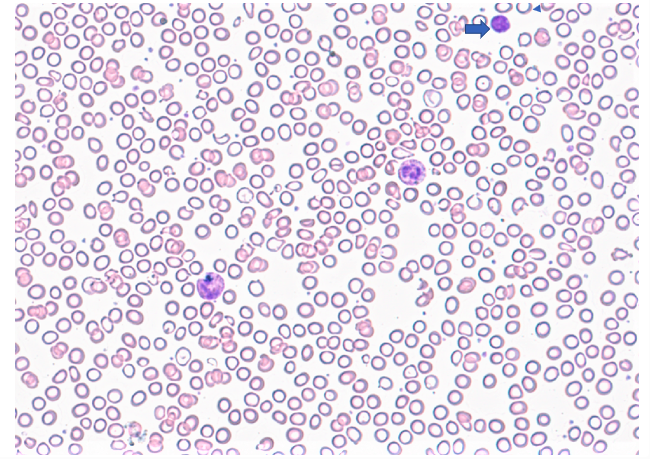
Peripheral smear description
- Blood smear in iron deficiency anemia shows many hypochromic (pale, relatively colorless) and small red blood cells and may also show poikilocytosis (variation in shape) and anisocytosis (variation in size)
- With more severe iron deficiency anemia, the peripheral blood smear may show hypochromic, pencil shaped cells and, occasionally, small numbers of nucleated RBCs
- Platelet count may be slightly above the high limit of normal in IDA (mild thrombocytosis) but severe cases can present with thrombocytopenia (low platelet count)
- Reference: Wikipedia: Iron deficiency anemia [Accessed 17 Januuary 2022]
Positive stains
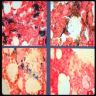
- Prussian blue stain of the bone marrow is considered the gold standard for evaluating marrow iron stores (see Cytology images)
Videos
Iron deficiency anemia
Differential diagnosis
- Anemia of chronic disease / anemia of inflammation:
- Normal to increased ferritin levels
- Normal MCV and serum transferrin receptors
- C reactive protein (CRP) increases unlike in iron deficiency anemia, where it is normal
- Thalassemia:
- Serum iron levels normal to high
- Serum ferritin levels normal to slightly decreased
- Serum TIBC is normal unlike in iron deficiency anemia, where it is high
- Hb electrophoresis is diagnostic
- Lead poisoning:
- Also causes hypochromic, microcytic anemia with characteristics basophilic stippling
- Serum iron levels are variable; however, serum ferritin and TIBC are at normal levels
- Copper deficiency:
- Deficiency can cause sideroblastic anemia (iron containing red blood cells); morphology varies from microcytic to macrocytic
- Increased serum iron concentration (decrease utilization) and ferritin levels
- Normal to mildly decreased TIBC
Additional references
Board review style question #1
A 27 year old Asian woman, gravida 1, para 0, comes to the office at 26 weeks gestation for prenatal care. The patient denies abdominal pain, vaginal bleeding and leakage of fluid. Fetal movement is normal. For the past few weeks, she has become increasingly fatigued and has been having exertional dyspnea. She is taking proper vitamins as prescribed. On examination, she has general pallor and scooping of nails. Her pregnancy has been uncomplicated. Hemoglobin (Hb) electrophoresis does not reveal abnormal Hb and her initial complete blood count (CBC) results are shown below:
RBCs: 4.2 (4.3 - 5.6 x 1012/L)
Hb: 8.0 (11.6 - 15 grams/dL)
MCV: 60 (80 - 100 fL)
Platelets: 452 x 103/uL (150 - 450 x 103/uL)
WBC count: 5.2 k/ul (4.5 - 11.5/uL)
What is most likely diagnosis and treatment for the patient?
- Anemia due to hemoglobinopathy and requires immediate blood transfusion
- Anemia due to increased nutrient requirement during pregnancy and requires iron supplementation
- Anemia due to physiologic changes of pregnancy and requires no further treatment
- Anemia due to the lack of prenatal vitamins; folic acid supplementation is indicated
Board review style answer #1
B. Iron deficiency anemia due to increased nutrient requirement during pregnancy and requires iron supplementation.
Comment Here
Reference: Iron deficiency anemia
Comment Here
Reference: Iron deficiency anemia
Board review style question #2
Which of the following is the single best test for diagnosis of iron deficiency anemia?
- Decreased serum ferritin
- Decreased serum iron
- Decreased soluble serum transferrin receptor
- Decreased total iron binding capacity
Board review style answer #2
A. Decreased serum ferritin. Decreased levels of serum ferritin is the best test for iron deficiency anemia.
Comment Here
Reference: Iron deficiency anemia
Comment Here
Reference: Iron deficiency anemia