Table of Contents
Definition / general | Essential features | Terminology | Epidemiology | Pathophysiology | Clinical features | Screening | Donor deferral | Prevention | Laboratory | Radiology images | Case reports | Treatment | Microscopic (histologic) description | Sample assessment & plan | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: George MR. Transfusion related acute lung injury. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/transfusionmedtrali.html. Accessed May 4th, 2024.
Definition / general
- Transfusion related acute lung injury (TRALI) is an adverse outcome of transfusion in which acute respiratory distress occurs within 6 hours of a transfusion
- This is typically an antibody mediated process in which antibodies in the transfused product or less commonly in the recipient attract neutrophils to the pulmonary vasculature
Essential features
- Respiratory distress / acute lung injury developing within 6 hours of cessation of transfusion
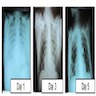
- Radiographic evidence of new bilateral infiltrates (generally without cardiomegaly)
- Hypoxemia
- No previous evidence of acute lung injury (ALI), such as circulatory overload or acute respiratory distress syndrome (ARDS)
Terminology
- Transfusion related acute lung injury (TRALI)
- Acronym TRALI coined in 1985 by Popovsky and Moore (Transfusion 1985;25:573)
Epidemiology
- Difficult to pinpoint exact incidence due to likelihood of underreporting
- Likely decreasing with increased use of leukoreduced products leading to decreased alloimmunization and also reverse TRALI
- 1 in 5,000 transfusions (Transfusion 2003;43:1053, Transfusion 1985;25:573)
- TRALI has been reported following transfusion of all blood products
- Most common with plasma rich blood products (> 60 mL of plasma)
- Plasma from female donors (discussed later under Prevention)
- Directed blood donation from mother to child
Pathophysiology
- A two hit hypothesis has been proposed
- Hit 1: patient's underlying condition
- Hit 2: transfused blood component activates the neutrophils within the pulmonary vasculature, which in turn causes plugging and endothelial damage
- Antibody mediated
- Passive transfer of human leukocyte antigen (HLA) or human neutrophil antigen (HNA) antibodies from donor blood to a transfusion recipient in majority (80%) of cases (Vox Sang 2008;94:277)
- HLA class I molecules: A, B and C antigens carried on nucleated cells (white cells and endothelial cells) and platelets
- HLA class I antibodies can bind to neutrophils and prime them, which can alter their biological processes leading to mechanical plugging of capillary beds and damage to endothelium due to cytokine release
- Antibodies against HLA-A2, a frequent antigen, have been reported (Lancet 1984;1:244, Vox Sang 1986;51:102)
- Antibodies against class II antigens may bind to monocytes → release of cytokines → neutrophil activation (alternate TRALI pathway) (Transfusion 2003;43:177)
- HNA: structures predominantly expressed on neutrophils but are also found on monocytes, lymphocytes and platelets, not expressed on endothelium
- Antibodies against HNA-3a have been identified in numerous TRALI models (Am J Respir Crit Care Med 2001;164:896, Blood 1990;76:1438)
- Exposure to foreign HLA or HNA molecules through transfusion, transplantation or pregnancy can cause alloimmunization against foreign antigens
- Antibodies can cause immediate destruction of cells bearing cognate antigens → cascade of inflammatory events leading to TRALI (Vox Sang 2008;94:324)
- Antibodies are not always found in suspected TRALI cases (JAMA 2002;287:1968, Vox Sang 2007;93:70)
- Mechanism of action of lung damage
- Neutrophils normally tether to endothelium under shear stress
- Neutrophils are normally spherical with a diameter of 6 - 8 μm
- Pulmonary capillaries, which range in diameter from 2 - 15 μm, force neutrophils to flatten out to pass through and make them unable to roll (J Appl Physiol 1995;79:493)
- Priming of neutrophils (caused by HLA and HNA antibodies) disrupts neutrophil shape change and leads to mechanical plugging of pulmonary vessels
- Pulmonary inflammation → emigration of neutrophils from capillary beds into the alveolar spaces (J Appl Physiol 1995;79:493)
- Primed neutrophils release cytokines → damage endothelium → protein rich fluid leak through endothelial gaps → exudate into alveolar spaces → respiratory distress = TRALI (N Engl J Med 2005;353:2788)
- Inverse TRALI (induced by transfusion of neutrophils to patient with preformed antibodies against HLA / HNA)
- HLA or HNA alloimmunized patients receiving blood components that contain neutrophils, e.g. granulocytes
- Leukoreduction should decrease incidence
Clinical features
- Criteria for TRALI
- Respiratory distress / acute lung injury developing within 6 hours of cessation of transfusion
- Radiographic evidence of new bilateral infiltrates (generally without cardiomegaly)
- Hypoxemia defined by ≥ 1 of the following
- PaO2/FiO2 ≤ 300 mg Hg
- O2 saturation < 90% on room air
- Other clinical evidence of hypoxemia
- No previous evidence of ALI, such as circulatory overload or acute respiratory distress syndrome
- Findings that may be helpful but are not required for diagnosis include
- Frothy pink secretions in ventilator tubing
- No response to diuretics, as opposed to transfusion associated circulatory overload, which does respond
- Short lived, sudden drop in white blood cell count, particularly with neutropenia, resulting from neutrophil sequestration in small alveolar blood vessels
- While presence of antibodies against HLA or HNA are helpful for diagnosis, they are not absolutely required
- References: Transfusion 1992;32:589, Transfusion 1985;25:573, CDC: National Healthcare Safety Network Biovigilance Component Hemovigilance Module Surveillance Protocol [Accessed 16 January 2020]
Screening
- Women who have been pregnant have a higher likelihood of developing HLA and HNA antibodies due to alloimmunization from the fetus
- Most significant in plasma rich products such as fresh frozen plasma and apheresis platelets
- Initial efforts to limit products from female donors → use of male only plasma in U.K. and U.S. → reduced rate of TRALI dramatically
- Screening donors for anti-HLA
- References: Transfus Med 2008;18:348, Vox Sang 2008;95:313, Hematology Am Soc Hematol Educ Program 2018;2018:585
Donor deferral
- Donor lookback and deferral is critical to protect future patients
- Very important to identify cocomponents
- Hospital should notify blood center following a potential case of TRALI
- Investigate pregnancy, transfusion or transplantation history of the donor
- Determine if donor has anti-HLA or HNA antibodies; if yes, then patient is also tested to see whether an antigen - antibody interaction could be implicated
- Donors with anti-HLA or HNA antibodies matched to recipient antigens in a possible case of TRALI are deferred indefinitely (AABB: TRALI Risk Mitigation for Plasma and Whole Blood for Allogeneic Transfusion [Accessed 8 January 2020])
Prevention
- Defer donors confirmed to be implicated in TRALI
- Use only male plasma to avoid risk of anti-HLA antibodies formed in women during pregnancy
- Screen female donors of apheresis platelets for HLA antibodies, indefinitely defer those with antibodies
- References: Hematology Am Soc Hematol Educ Program 2018;2018:585, Vox Sang 2017;112:694, AABB: TRALI Risk Mitigation for Plasma and Whole Blood for Allogeneic Transfusion [Accessed 8 January 2020], Transfusion 2010;50:1312, AABB: Standards for Blood Banks and Transfusion Services, 31st Edition, 2018, Fung: Technical Manual, 19th Edition, 2017
Laboratory
- Short lived, sudden drop in white blood cell count, particularly with neutropenia, resulting from neutrophil sequestration in small alveolar blood vessels
- No elevation in brain natriuretic peptide (BNP) as in transfusion associated circulatory overload
- Pleural fluid is exudate rather than transudate (as in transfusion associated circulatory overload) with a protein content of > 2.9 g/dL and fluid protein to serum protein ratio of > 0.5, and cholesterol content of > 45 mg/dL
- References: Hematology Am Soc Hematol Educ Program 2018;2018:585, Fung: Technical Manual, 19th Edition, 2017, Transfusion 2004;44:1689, Transfusion 2005;45:1056, Transfusion 2008;48:2167, Vox Sang 2008;94:277, Curr Opin Hematol 2011;18:452, Transfusion 2004;44:1683, Vox Sang 2007;93:70
Case reports
- 4 month old girl and 78 year old woman, transfusions between mothers and daughters (Am J Clin Pathol 2004;121:590)
- 2 cases in children (Transfus Med 2006;16:343)
- 28 and 31 year old men with thalassemia (Mediterr J Hematol Infect Dis 2017;9:e2017060)
- 67 year old man with transfusion for iron deficiency anemia (CMAJ 2007;177:149), recurrence 2 years later (Transfus Med 2007;17:192)
- 77 year old woman with common variable immune deficiency (Transfusion 2014;54:3088)
- Patient with hematologic malignancy and neutropenia associated pneumonitis (Transfusion 2003;43:1683)
Treatment
- Stop the transfusion
- Report the transfusion to transfusion medicine service to start the investigation
- Supportive management only
- Mechanical ventilation may be needed in severe cases
- Most patients improve within 48 - 96 hours
- Mortality is 5 - 10%
- Indefinite deferral of blood donor to prevent future cases
- Deaths from TRALI: during fiscal years 2012 - 2016, the combined TRALI and possible TRALI cases caused the highest number of reported fatalities, 64 out of 186 total fatalities (34%) (FDA: Transfusion Related Acute Lung Injury (TRALI) [Accessed 8 January 2020])
Microscopic (histologic) description
- Acute respiratory distress syndrome with extensive intra-alveolar edema and presence of neutrophils
- Hyaline membranes and distortion of pulmonary architecture
- Capillary leukostasis
- Electron microscopy shows degranulation of neutrophils in contact with injured endothelium (Transfusion 1986;26:278, Chest 2001;119:219)
Sample assessment & plan
- Assessment: John Doe is a 75 year old man who developed respiratory distress 2 hours after a packed red blood cell (pRBC) transfusion. He had normal ejection fraction, no elevation in brain natriuretic peptide (BNP) and treatment with diuretics did not resolve his respiratory distress. The blood supplier was notified and performed testing of donor sample. Testing of the donor sample identified anti-HLA DR11, DR13 and DR52. Patient had matching cognate antigens - HLA type DR11, DR13 and DR52.
- Plan: The patient presentation and laboratory workup are consistent with a diagnosis of transfusion related acute lung injury (TRALI).
Differential diagnosis
- Transfusion associated circulatory overload:
- Fluid overload → increased pulmonary capillary pressure → cardiogenic edema = congestive heart failure
- Patient often has predisposition to congestive heart failure
- Hypertension rather than the hypotension typically seen in TRALI
- Elevated brain natriuretic peptide (BNP), e.g. post to pretransfusion ratio of BNP ≥ 1.5 (indicates stretched ventricular muscle due to volume overload)
- Transudative rather than exudative alveolar edema
- All blood components have been implicated, volume issue, no particular product is more commonly associated
- Transfusion associated dyspnea:
- Diagnosis of exclusion that does not meet criteria for TRALI or transfusion associated circulatory overload
- No specific underlying pathophysiology has been identified
Additional references
Board review style question #1
One key feature seen in transfusion related acute lung injury but not in transfusion associated circulatory overload is
- Decreased oxygen saturation
- Hypotension
- More likely to have underlying cardiac dysfunction
- New pulmonary infiltrates
- Response to diuretics
Board review style answer #1
B. Hypotension is often seen in transfusion related acute lung injury, as opposed to hypertension seen in transfusion associated circulatory overload, as a result of expansion of intravascular fluid volume, making choice B the correct response. The answer choices of new pulmonary infiltrates and decreased oxygen saturation are seen in both. Response to diuretics and pre-existing, underlying cardiac dysfunction are more commonly seen in transfusion associated circulatory overload.
Comment Here
Reference: Transfusion related acute lung injury (TRALI)
Comment Here
Reference: Transfusion related acute lung injury (TRALI)
Board review style question #2
A 55 year old woman with acute myeloid leukemia, status postallogeneic bone marrow transplant with severe neutropenia and unresponsive fungal infection is receiving granulocytes. She develops severe respiratory distress shortly after the transfusion is completed, necessitating mechanical ventilation. Her fluid balance is neutral and she has no pre-existing cardiac dysfunction. What laboratory testing would help confirm a diagnosis of inverse transfusion related acute lung injury?
- Anti-HLA / HNA antibodies in the donor that match antigens in the recipient
- Anti-HLA / HNA antibodies in the recipient that match antigens in the donor
- Elevated brain natriuretic peptide (BNP)
- Pleural fluid with protein content < 2.5 g/dL and fluid protein to serum protein ratio of < 0.5 and cholesterol content of < 45 mg/dL
- Short lived, sudden increase in neutrophils in complete blood count
Board review style answer #2
B. Anti-HLA / HNA antibodies in the recipient that match antigens in the donor. Inverse transfusion related acute lung injury refers to the recipient having antibodies against antigens in the donor, so choice B. Elevated brain natriuretic peptide and transudative pulmonary fluid are seen in transfusion associated circulatory overload. Choice A describes traditional transfusion related acute lung injury occurring when antibodies in the donor match cognate antigens in the recipient. Choice E is incorrect because transfusion related acute lung injury may feature a sudden, short lived decrease rather than increase in neutrophils in a complete blood count.
Comment Here
Reference: Transfusion related acute lung injury (TRALI)
Comment Here
Reference: Transfusion related acute lung injury (TRALI)