Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Positive stains | Electron microscopy description | Videos | Sample pathology report | Differential diagnosis | Additional references | Practice question #1 | Practice answer #1 | Practice question #2 | Practice answer #2Cite this page: Yoshikawa A, Bychkov A. ARDS / DAD. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/lungnontumordiffusealveolardamage.html. Accessed September 16th, 2025.
Definition / general
- In 1994, the American European Consensus Conference (AECC) defined acute respiratory distress syndrome (ARDS) as the acute onset of hypoxemia with bilateral infiltrates on frontal chest radiograph, with no evidence of left atrial hypertension (Am J Respir Crit Care Med 1994;149:818)
- In 2012, the ARDS Task Force revised the new definition (see Diagnosis) (JAMA 2012;307:2526)
- Diffuse alveolar damage (DAD) is manifested by injury to alveolar lining and endothelial cells, pulmonary edema, hyaline membrane formation and later by proliferative changes involving alveolar and bronchiolar lining cells and interstitial cells (Am J Pathol 1976;85:209)
Essential features
- Acute and rapidly progressive hypoxia with bilateral pulmonary edema due to alveolar injury caused by pulmonary or systemic insults
- 40% die within 28 days from onset of ARDS, mainly due to infection / sepsis and multiple organ dysfunction syndrome
- Although DAD is the typical morphology of ARDS, clinical syndrome of ARDS is not synonymous with the pathologic diagnosis of DAD
- DAD pattern is often characterized by hyaline membranes in acute phase but shows a wide variety of findings that makes the diagnosis challenging
Terminology
- Previously known as adult respiratory distress syndrome
- Acute lung injury, an overlapping entity suggested in AECC definition, was removed from Berlin definition; the AECC definition of acute lung injury non-ARDS is compatible with Berlin definition of mild ARDS (JAMA 2012;307:2526)
ICD coding
- ICD-10: J80 - acute respiratory distress syndrome
Epidemiology
- Mean age: 60 years old; from children to elderly
- M:F = 3:2
- Incidence of 10.6 - 78.9 per 100,000 person years (N Engl J Med 2005;353:1685, Intensive Care Med 2009;35:1352)
- Incidence of ARDS in intensive care unit (ICU) (JAMA 2016;315:788):
- 10.4% of all ICU patients
- 67.2% of patients with acute hypoxemic respiratory failure
- ARDS is frequently undiagnosed (60.2% recognized by clinician) or late diagnosed (34.0% recognized at the time of criteria fulfillment)
Sites
- Diffusely involves bilateral lobes of the lung without upper or lower lobe predominance
- Distribution can be regional, depending on the degree and cause of the inflammation
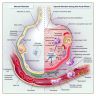
Pathophysiology
- Pathogenesis (N Engl J Med 2000;342:1334, Lancet 2016;388:2416)
- Exudative (acute) phase: 1 - 7 days
- Neutrophil mediated inflammation destroys the alveolar capillary barrier (alveolar epithelium and endothelium), increases its permeability and causes intra-alveolar hemorrhage and edema
- Protein rich edema interacts with alveolar surfactants, resulting in decreased pulmonary compliance
- Hyaline membranes develop on alveolar wall where epithelium is denudated and disrupted
- Proliferative / organizing (subacute) phase: 1 - 3 weeks
- Proliferation of type II pneumocytes and subsequent differentiation into type I pneumocytes
- Proliferation of myofibroblasts
- Drainage of alveolar edema by restored type II pneumocytes
- Fibrotic (chronic) phase: after 3 weeks
- Collagenous fibrosis in alveolar spaces and interstitium
- Refractory rigidity of alveoli due to architectural remodeling
- Exudative (acute) phase: 1 - 7 days
- Pathophysiology of hypoxia
- Collapse of alveoli
- Increased right to left intrapulmonary shunt
- Decreased pulmonary compliance
- Surfactant malfunction
- Fibrosis
- Decreased diffusing capacity due to pulmonary edema
- Increased ventilation perfusion mismatch
- Increased pulmonary vascular resistance
- Mechanism of ARDS to cause multiple organ dysfunction syndrome is not clear
- Ventilator induced lung injury may play a critical role for upregulation of cytokines and eventual development of multiple organ dysfunction syndrome (Curr Opin Crit Care 2011;17:1, Respir Care 2014;59:1422)
Etiology
- Pulmonary and extrapulmonary origin (N Engl J Med 2000;342:1334)
| Direct lung injury | Indirect lung injury |
|---|---|
|
|
|
|
- Infection
- Bacterial: mycoplasma, mycobacteria, legionella, rickettsia
- Viral: influenza virus, herpes virus, cytomegalovirus, hantavirus, SARS-CoV-2 (coronavirus disease 2019, COVID-19) (Histopathology 2020;77:570)
- Fungal: Pneumocystis jirovecii
- Drug (N Engl J Med 2000;342:1334)
- Chemotherapeutic drugs: methotrexate, bleomycin, azathioprine, busulfan
- Molecular targeted drugs
- Gold based
- Amiodarone
- Penicillamine
- Nitrofurantoin
- Autoimmune disease
- Polymyositis / dermatomyositis
- Especially in clinically amyopathic dermatomyositis
- Systemic lupus erythematosus
- Scleroderma
- Rheumatoid arthritis
- Polyarteritis nodosa
- Undifferentiated (subclinical) connective tissue disease
- Often positive for serum anti-MDA-5 antibody or anti-ARS antibody (Medicine (Baltimore) 2012;91:220, BMJ Case Rep 2016;2016:bcr2016217624, Tuberc Respir Dis (Seoul) 2016;79:188)
- Polymyositis / dermatomyositis
- Inhalation
- Smoke
- Sulfur dioxide
- Oxygen
- Nitrogen dioxide
Clinical features
- Acute and progressive respiratory failure
- Usually starts 12 - 48 hours after the initial insult
- Shortness of breath; dyspnea on exertion followed by dyspnea at rest
- Hypoxia; PaO2 / FiO2 ≤ 300 mm Hg
- Often followed by sepsis and multiple organ dysfunction syndrome (JAMA 2016;315:788)
- Respiratory dysfunction and physical disability may persist for months after remission of ARDS, with gradual improvement (N Engl J Med 2003;348:683)
Diagnosis
- Diagnosis of ARDS is based on clinical manifestation and its severity is evaluated with ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2 / FiO2)
- Berlin definition (JAMA 2012;307:2526)
- Timing
- Within 1 week of a known clinical insult or new or worsening respiratory symptoms
- Chest imaging
- Assessed by chest Xray or CT
- Bilateral opacities; not fully explained by effusions, lobar / lung collapse or nodules
- Origin of edema
- Respiratory failure not fully explained by cardiac failure or fluid overload
- Need objective assessment (e.g. echocardiography) to exclude hydrostatic edema if no risk factor present
- Oxygenation (with positive end expiratory pressure [PEEP] or continuous positive airway pressure [CPAP] ≥ 5 cm H2O)
- Mild: 200 < PaO2 / FiO2 ≤ 300 mm Hg
- Moderate: 100 < PaO2 / FIO2 ≤ 200 mm Hg
- Severe: PaO2 / FiO2 ≤ 100 mm Hg
- Timing
- Not all ARDS show DAD pattern
- ARDS can present with organizing DAD and acute fibrinous organizing pneumonia as well
- DAD can be seen as a nonspecific manifestation of the agonal phase and shock
- Although rarely performed, lung biopsy can be helpful for critical care and prognosis estimation in patients with ARDS
- Open lung biopsy provided a specific diagnosis for 56 - 84% of ARDS patients and an alternative treatment for 73% of cases (Ann Thorac Surg 2014;98:1254, Crit Care 2015;19:228, Intensive Care Med 2015;41:222)
- ARDS with DAD is related to worse prognosis (see Prognostic factors)
- Carefully consider biopsy if there is a high prediction for beneficial result and the risk of empirical therapy is not suitable or unsuccessful (Chest 2015;148:1073)
Laboratory
- Arterial blood gas test
- Hypoxemia
- Ratio of pulse oximetric oxygen saturation to FIO2 (SpO2 / FiO2) may be helpful for instant follow up (Chest 2007;132:410)
- SpO2 / FiO2 of 235 ≈ PaO2 / FiO2 of 200 (sensitivity: 85%, specificity: 85%)
- SpO2 / FiO2 of 315 ≈ PaO2 / FiO2 of 300 (sensitivity: 91%, specificity: 56%)
- Other blood tests
- Increased C reactive protein
- Increased procalcitonin in septic ARDS
- Increased ferritin (Am J Respir Crit Care Med 1999;159:1506)
- Increased KL-6
- Brain natriuretic peptide (BNP) test may helpful to distinguish ARDS and cardiogenic pulmonary edema (Chest 2007;131:964)
- BNP ≤ 200 pg/mL is suggestive for ARDS (sensitivity: 40%, specificity: 91%)
- BNP ≥ 1,200 pg/mL is suggestive for cardiogenic pulmonary edema (sensitivity: 52%, specificity: 92%)
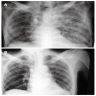
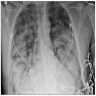
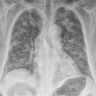
Radiology description
- General
- Heterogeneous bilateral shadows due to pulmonary edema
- Rule out atelectasis, pleural effusion and mass
- Takes 12 - 24 hours from onset to be apparent
- Chest Xray
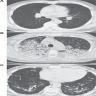
- Exudative phase: ground glass opacity and consolidation with air bronchogram
- Proliferative / organizing and fibrotic phases: reticular shadow and volume reduction
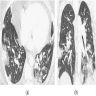
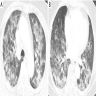
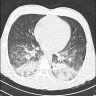
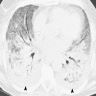
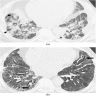
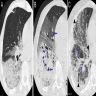
- Chest CT (Radiology 1999;211:859)
- Exudative phase
- Patchy to diffuse ground glass opacity, with or without interlobular septal thickening
- Dorsal consolidation due to infiltrate
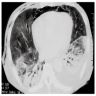
- Proliferative / organizing phase
- Ground glass opacity with bronchiolectasis and bronchiectasis
- Volume reduction
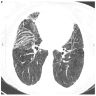
- Fibrotic phase
- Septal thickening and reticular shadow in ground glass opacity
- Peripheral cystic and honeycomb-like lesions due to fibrosis
- Exudative phase
Radiology images
Prognostic factors
- Septic shock and multiple organ dysfunction syndrome account for about 50% of deaths in patients with ARDS, while respiratory failure is responsible for 10 - 20% of deaths (Am J Respir Crit Care Med 2013;187:761, Chest 2005;128:525, Chest 1989;96:885, Clin Chest Med 2006;27:725)
- First 7 - 10 days are the most relevant for the prognosis of ARDS (JAMA 2016;315:788)
- Of all ARDS patients, 93.1% developed ARDS in the first 48 hours
- Mortality rate at day 28 and at hospital discharge was 29.6% and 34.9% for mild ARDS, 35.2% and 40.3% for moderate and 40.9% and 46.1% for severe, respectively
- Mortality is higher for ARDS with DAD (71.9%) than ARDS without DAD (45.5%) (Crit Care 2015;19:228, PLoS One 2017;12:e0180018, Chest 2016;149:1155)
- Prognosis of ARDS patients improved over the last decades (Chest 2008;133:1120)
Case reports
- 31 year old man with ARDS / DAD and eosinophilic pneumonia due to amphetamine smoking (Chin J Physiol 2014;57:295)
- 41 year old woman died of ARDS / DAD due to influenza A virus infection (Jpn J Infect Dis 2010;63:72)
- 43 year old man died of ARDS due to clinically amyopathic dermatomyositis (Case Rep Crit Care 2016;2016:7379829)
- 45 year old woman died of ARDS / DAD due to rabies (Kaohsiung J Med Sci 2006;22:94)
- 49 year old man died of ARDS / DAD due to EBV positive diffuse large B cell lymphoma (Int J Clin Exp Pathol 2014;7:1742)
- 72 year old woman died of ARDS / DAD due to scrub typhus (J Korean Med Sci 2000;15:343)
- 72 year old man died of ARDS / DAD due to influenza B virus infection (Respirol Case Rep 2015;3:61)
- 75 year old man died of ARDS / DAD due to gemcitabine chemotoxicity (Intern Med 2003;42:1022)
- 77 year old woman died of ARDS / DAD due to acute interstitial pneumonia (Case Rep Med 2011;2011:628743)
- 93 year old woman with segmented DAD due to COVID-19 (Pathol Int 2020;70:820)
Treatment
- Removal of the original insult
- Oxygen therapy for respiratory failure (Lancet 2016;388:2416)
- Mechanical ventilation
- PEEP or CPAP ≥ 5 cm H2O
- With or without neuromuscular blockade, prone position and extracorporeal membrane oxygenation
- Mechanical ventilation
- Other supportive care
- Fluid management
- Pharmacotherapy
- Glucocorticoid
- Anticoagulant
- Since no treatment drastically improves the respiratory failure of ARDS, respiratory and systemic supportive care is needed until the patient survives from ARDS
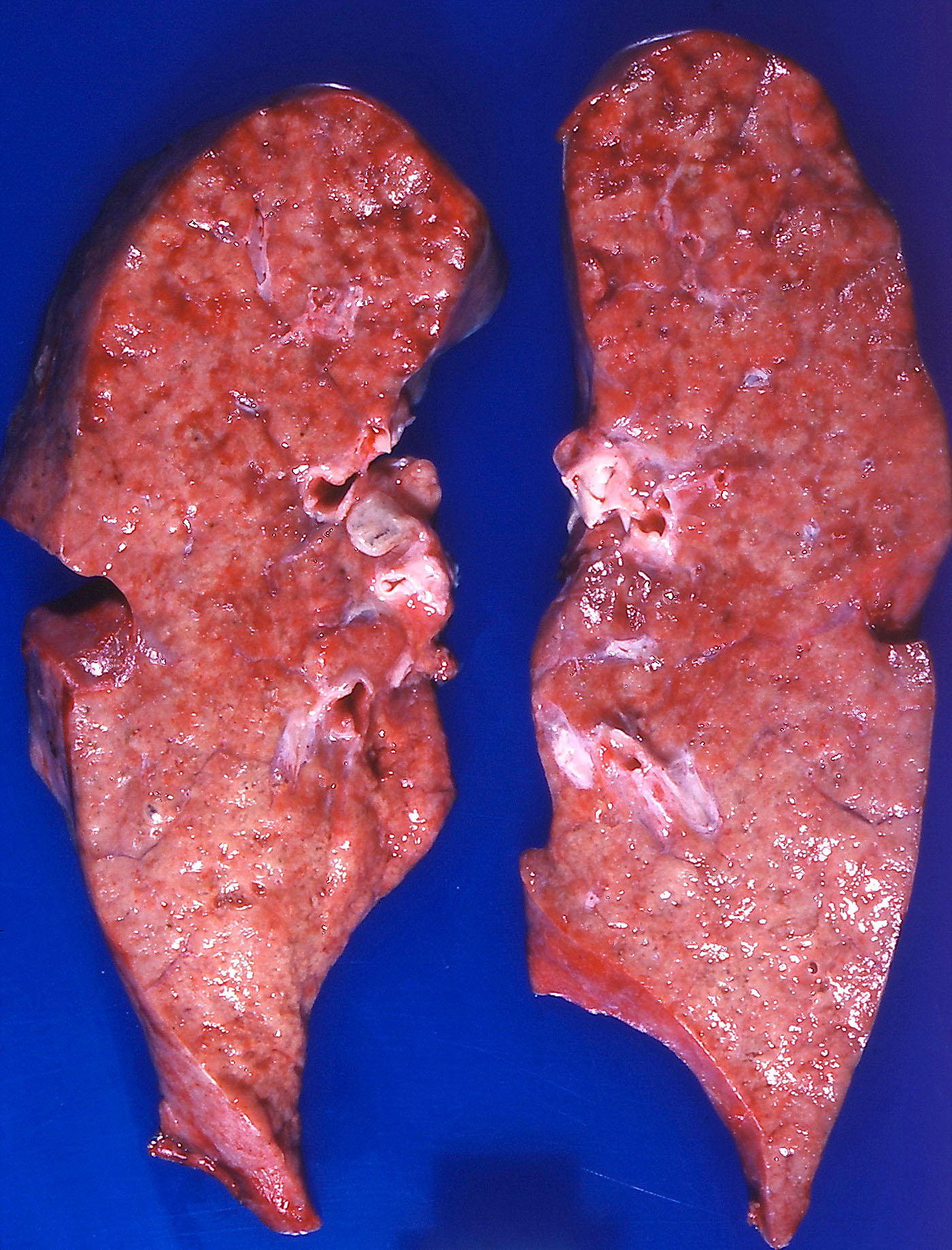
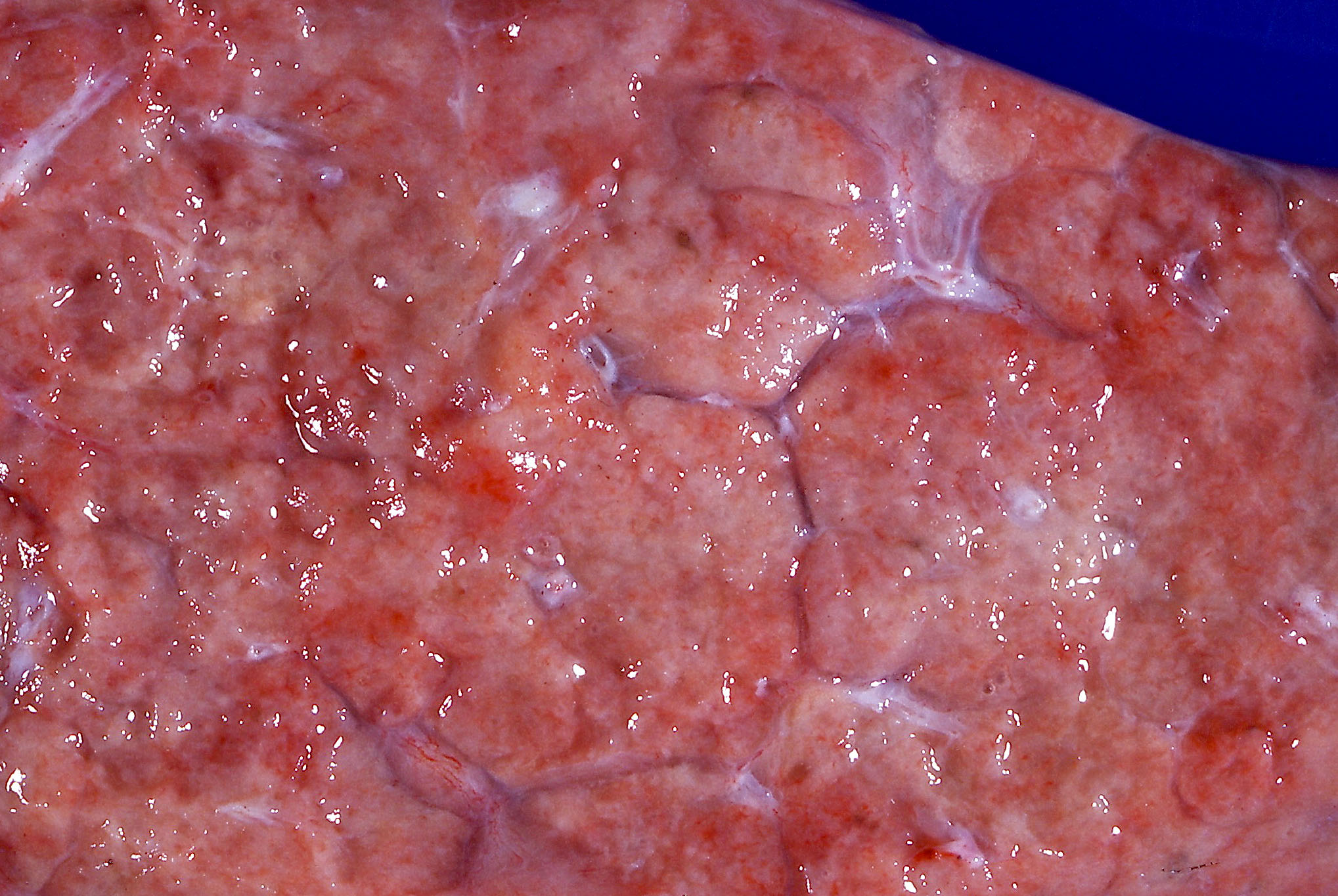
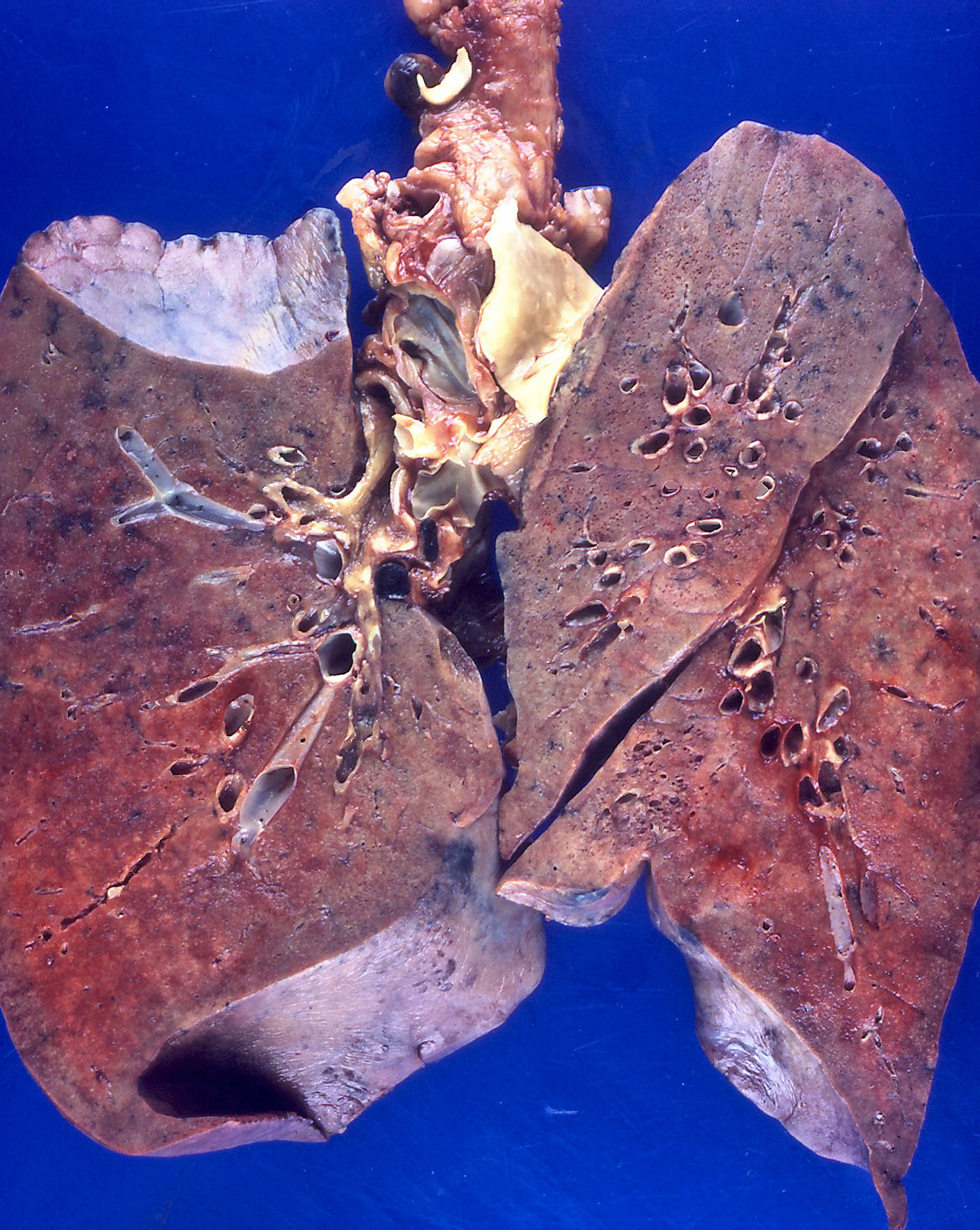
Gross description
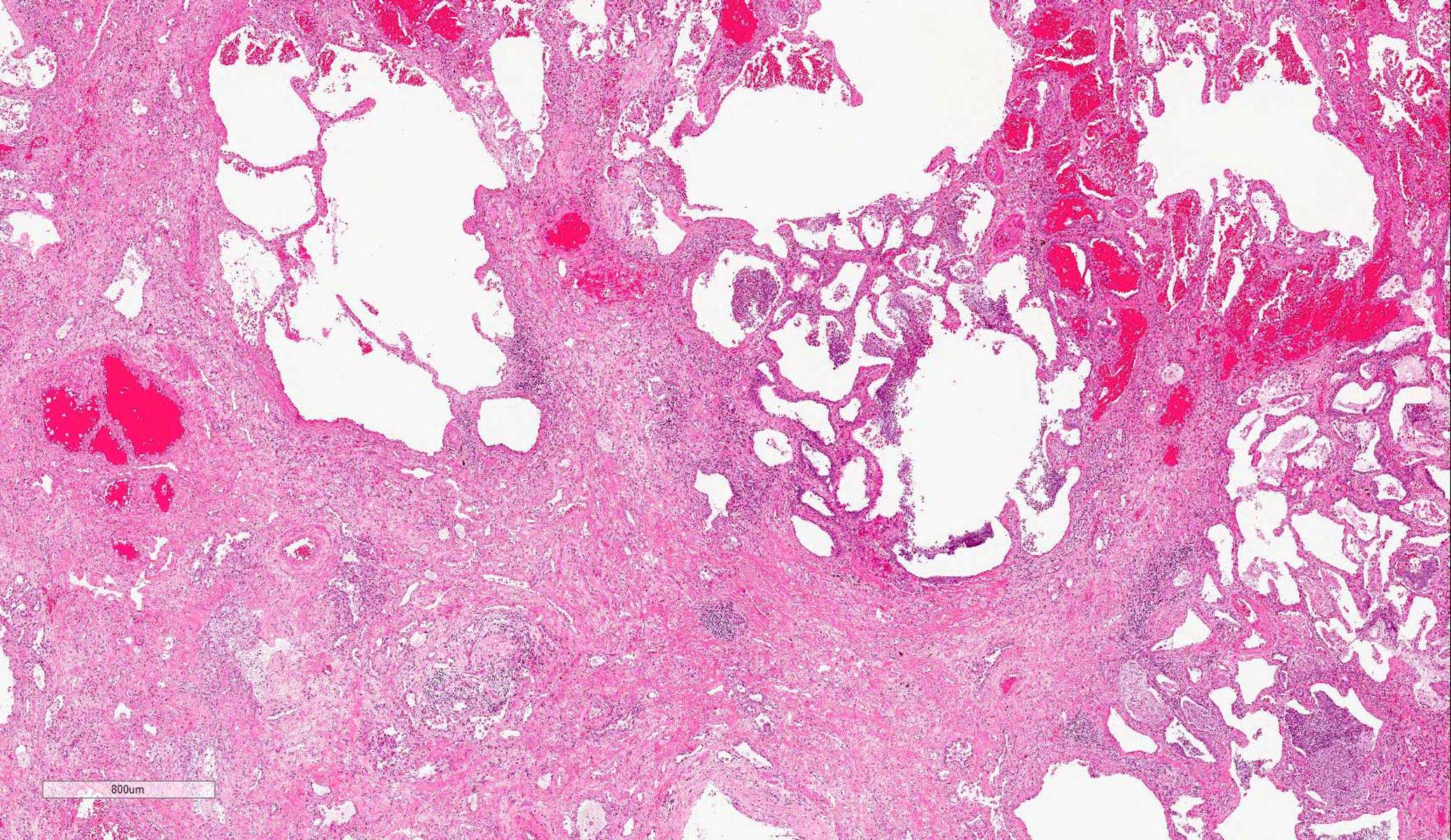
- Brown to gray consolidative lesion with indistinct borders, diffusely involving bilateral lobes
- Elastic hard and greasy due to transudate within alveolar spaces and interstitial edema
- Heavy and shrunken due to fibrosis and collapse of the tissue
- Dots of hemorrhage on pleural surface
Gross images
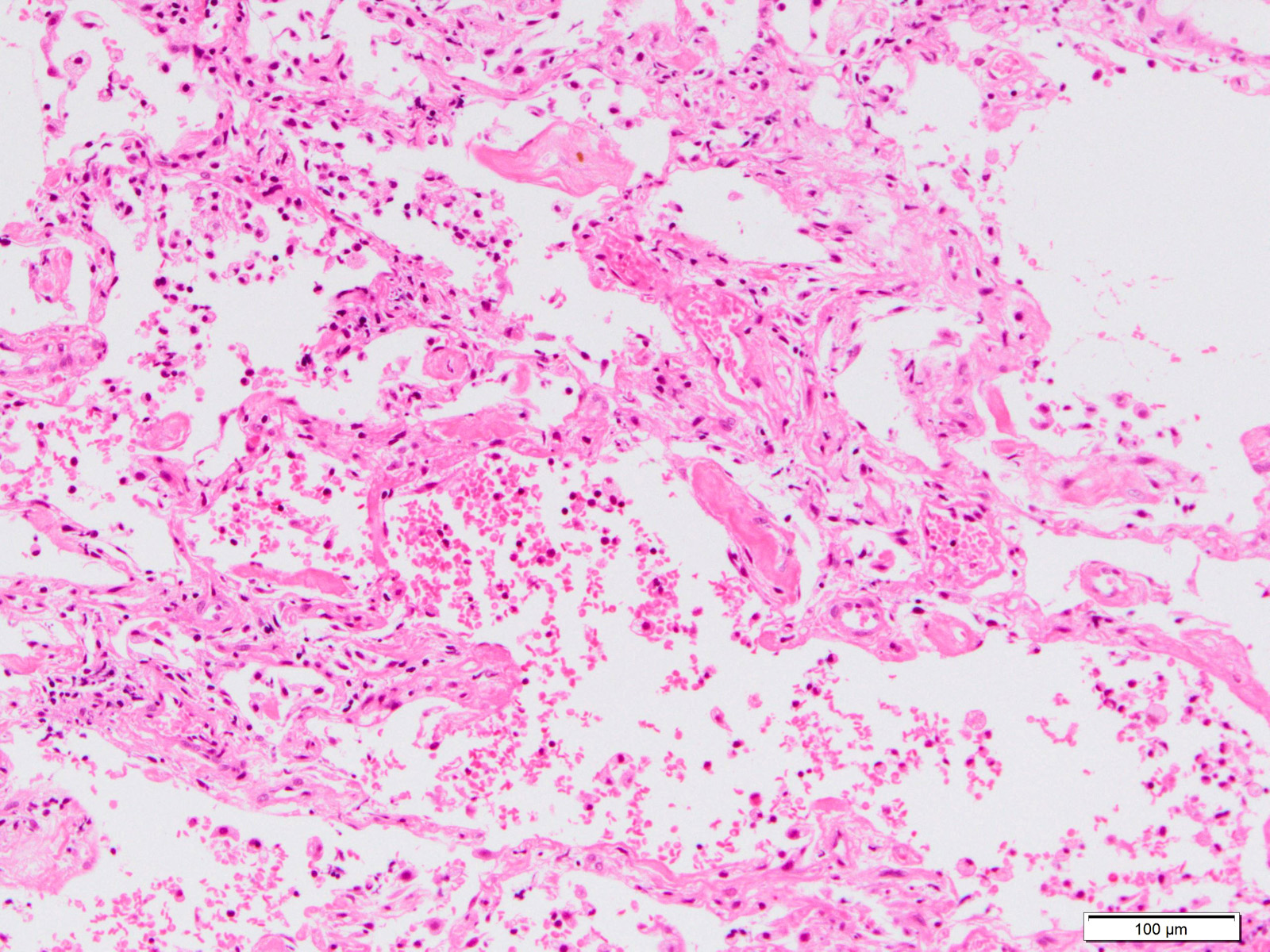
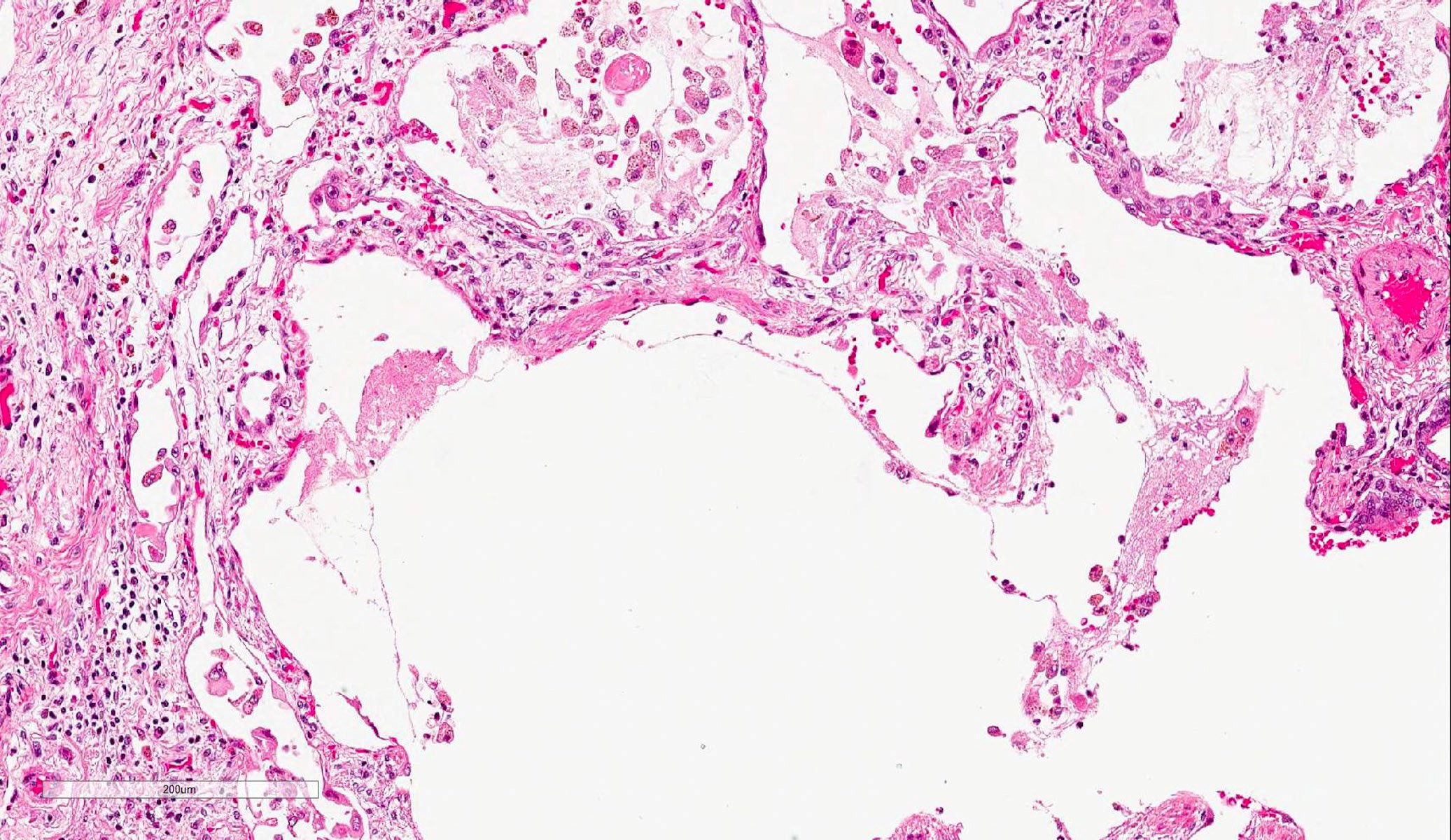
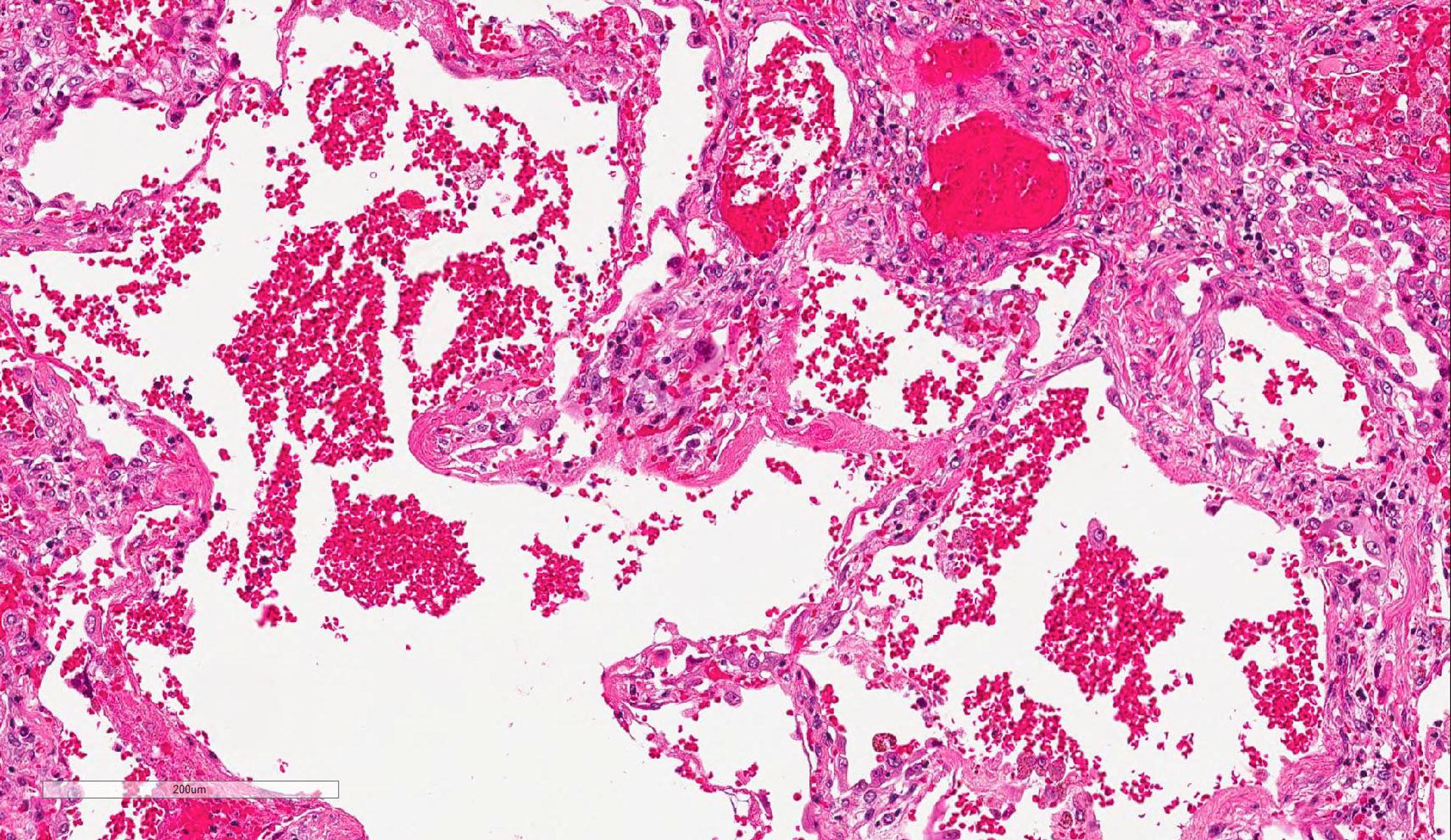
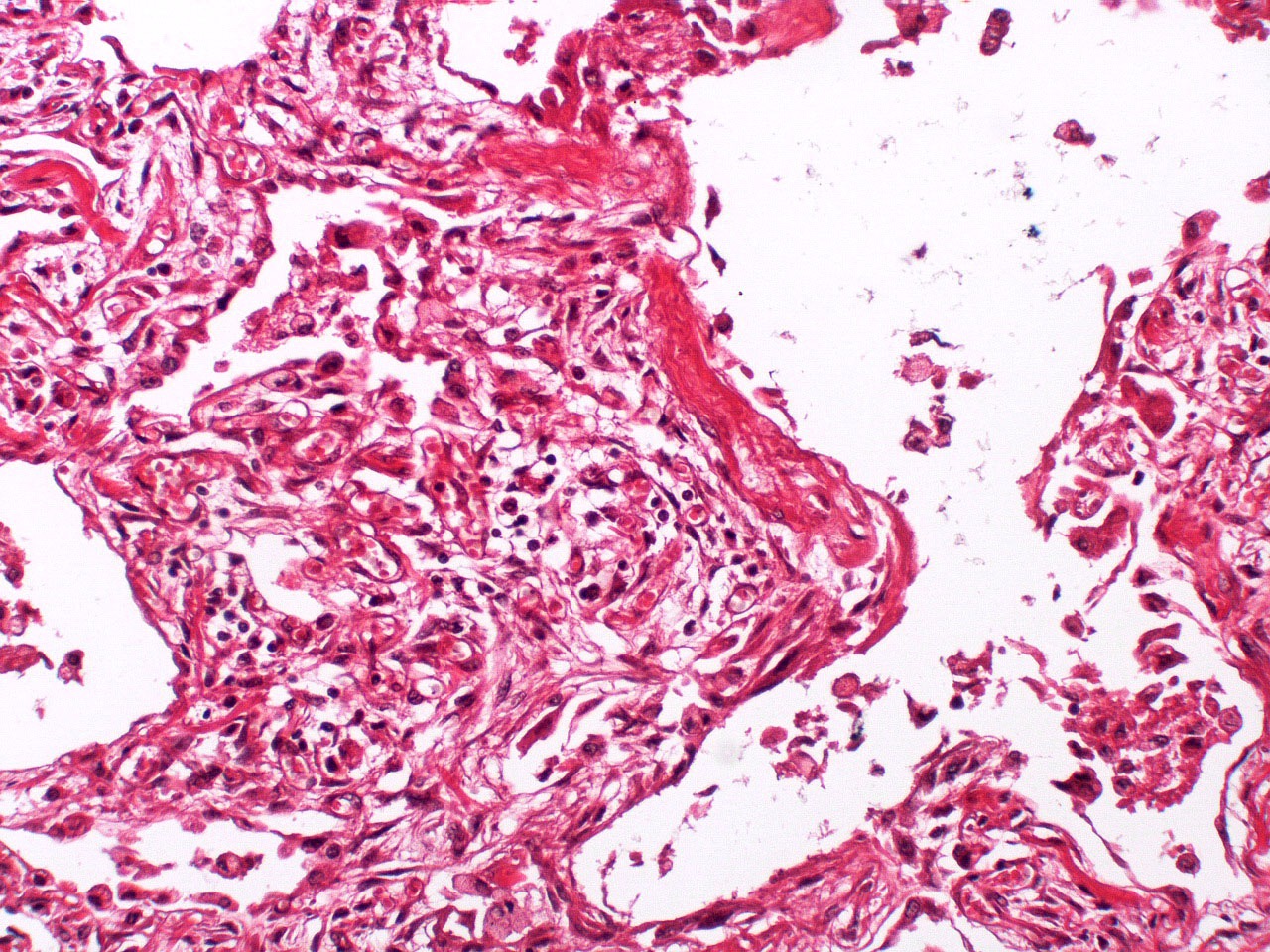
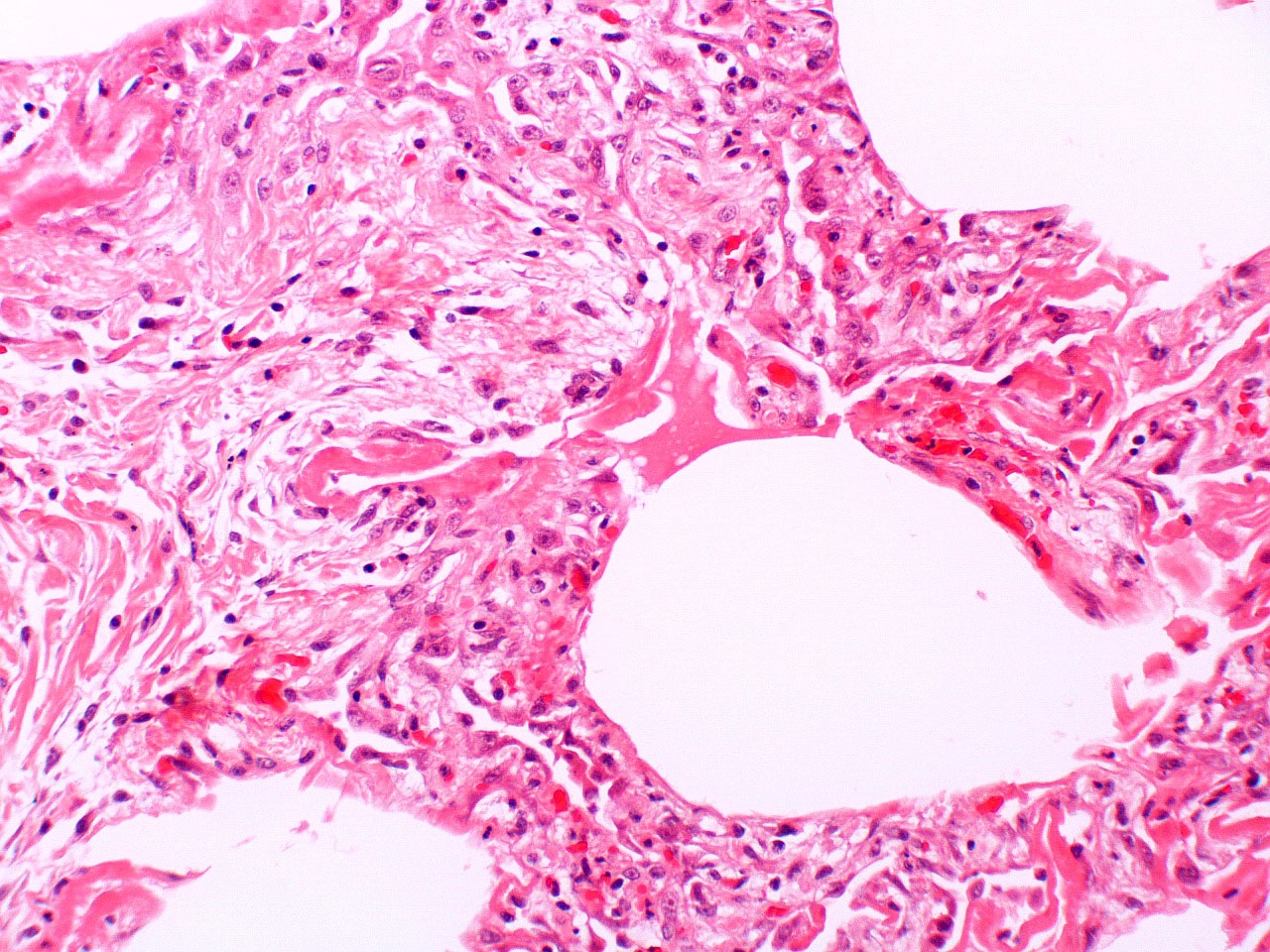
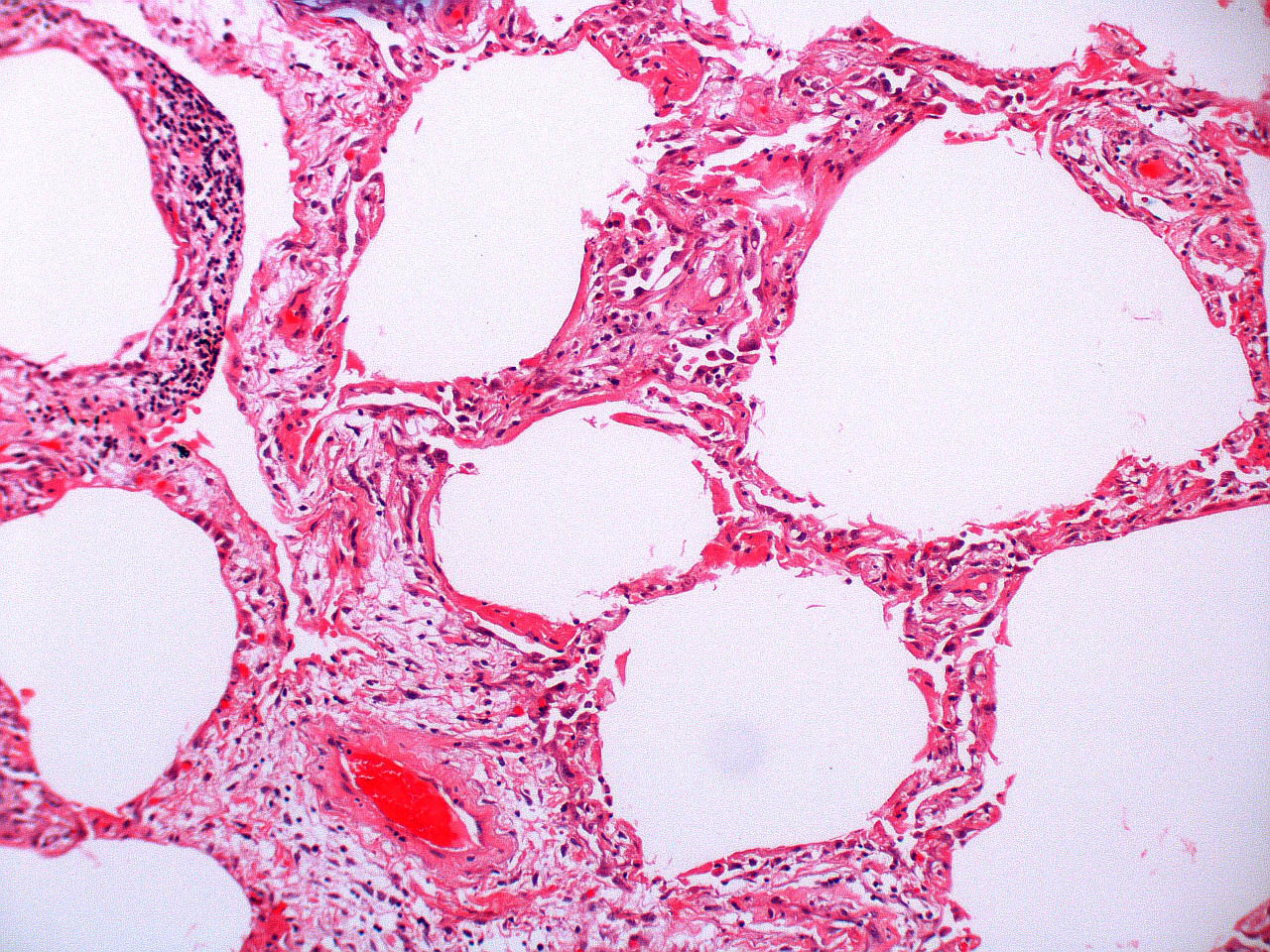
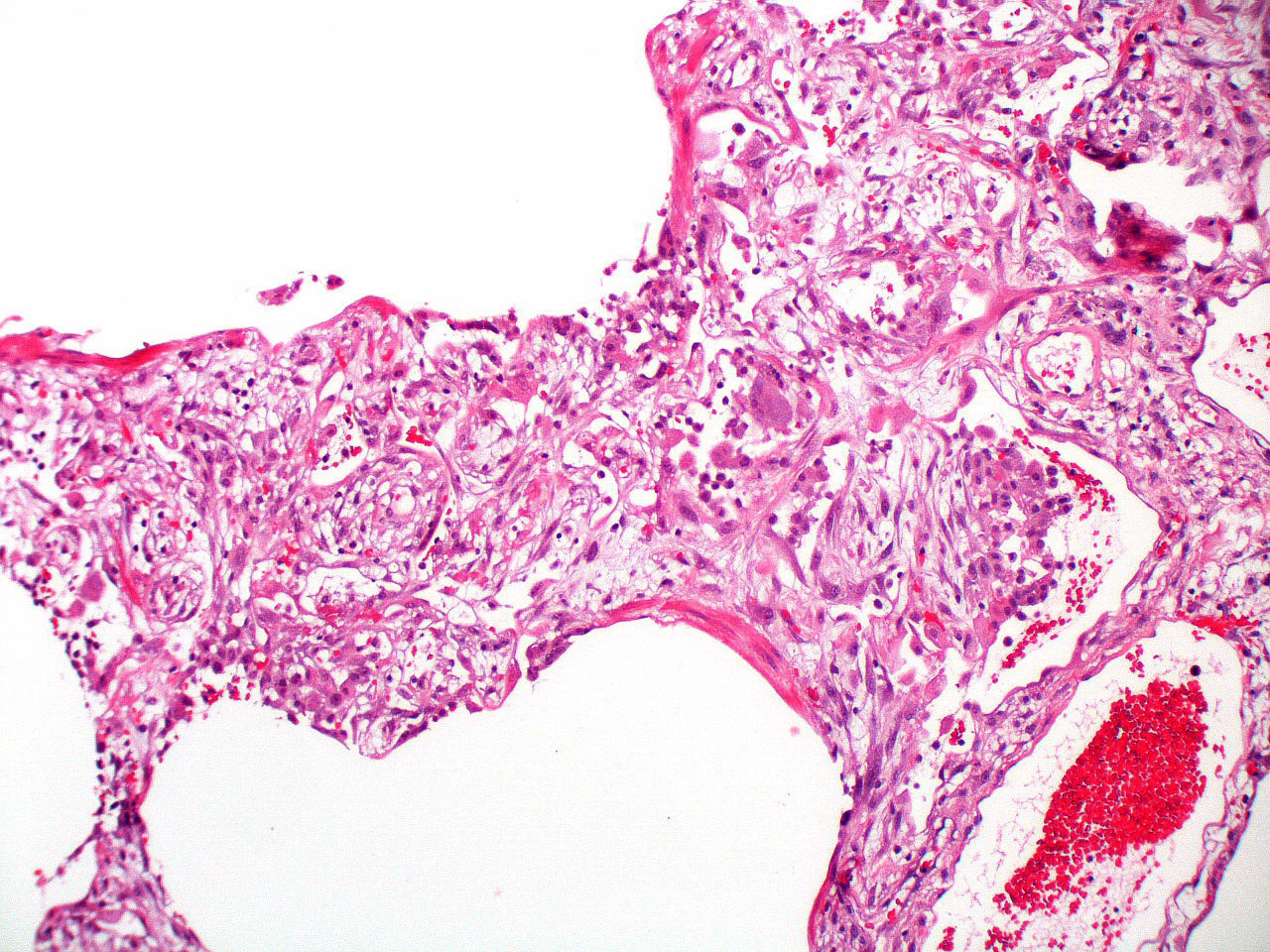
Microscopic (histologic) description
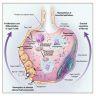
- Histopathology of DAD progresses from exudative (acute) phase through proliferative / organizing (subacute) phase to chronic fibrotic phase roughly corresponding to the period of ARDS (Am J Pathol 1976;85:209, Arch Pathol Lab Med 2010;134:719, Clin Chest Med 2000;21:435, N Engl J Med 2000;342:1334)
- Phase of the disease is almost synchronous throughout the lung
- Features of different phases may be combined in the transitional period or with repeated bouts of lung injury
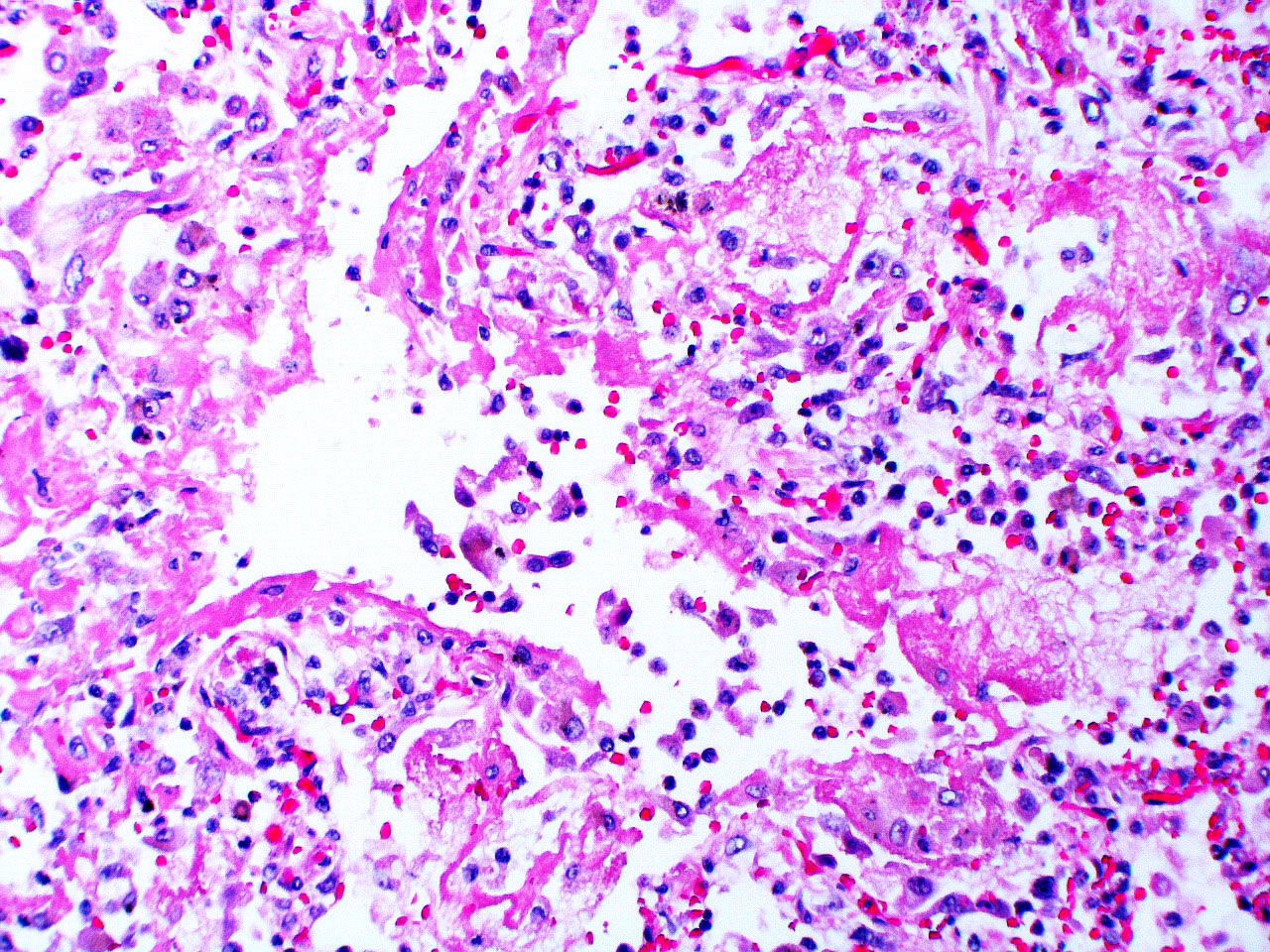
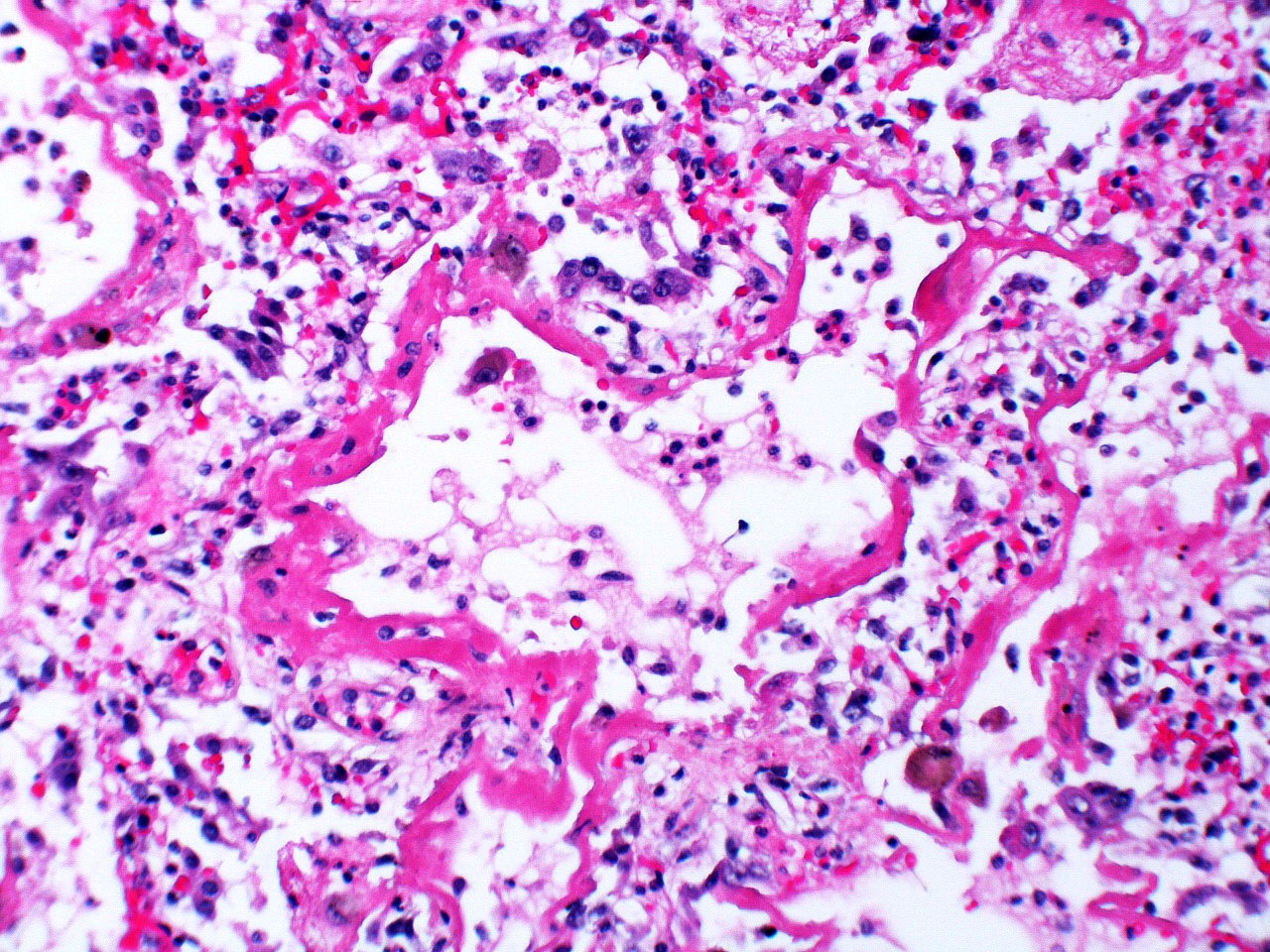
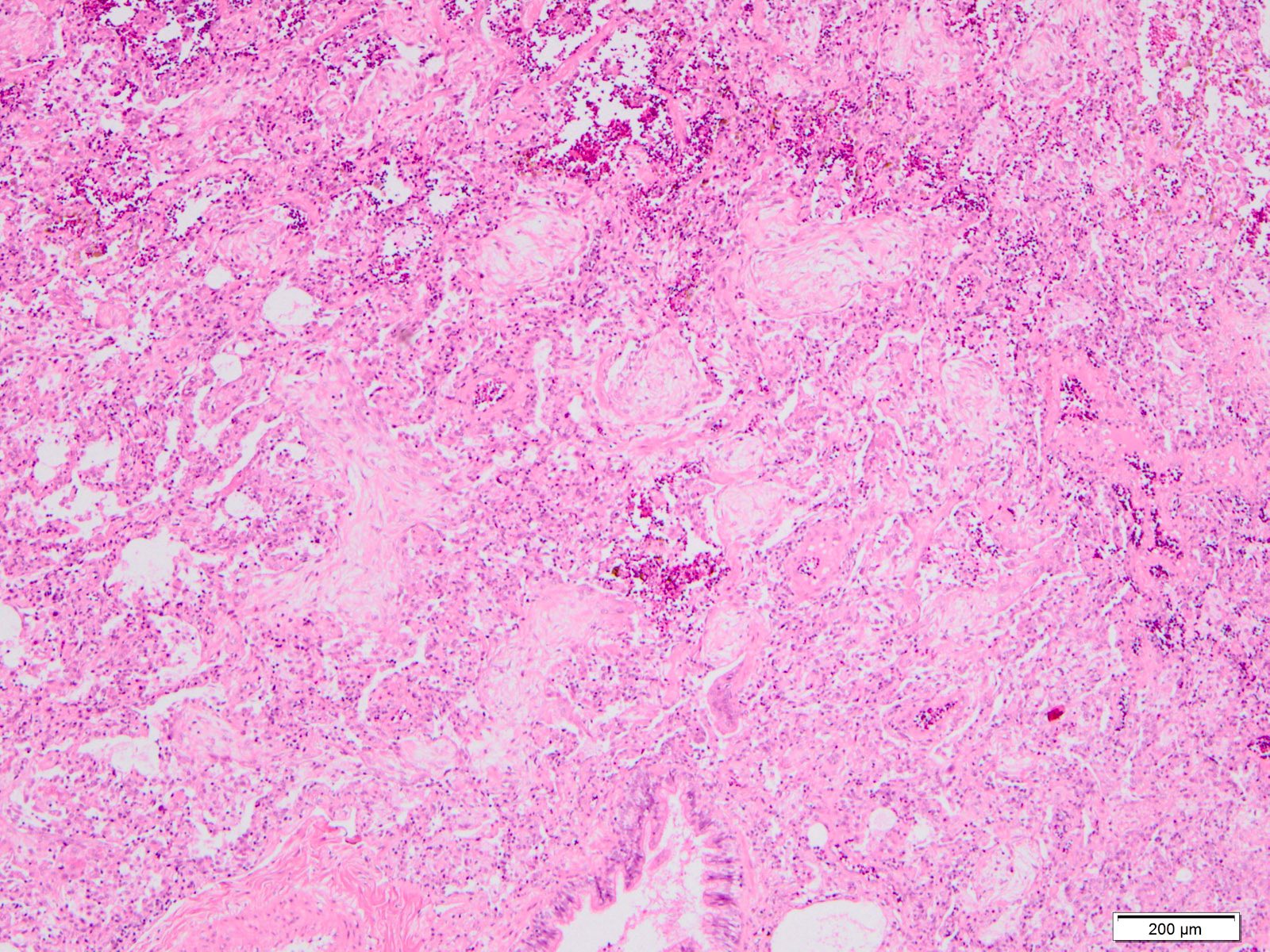
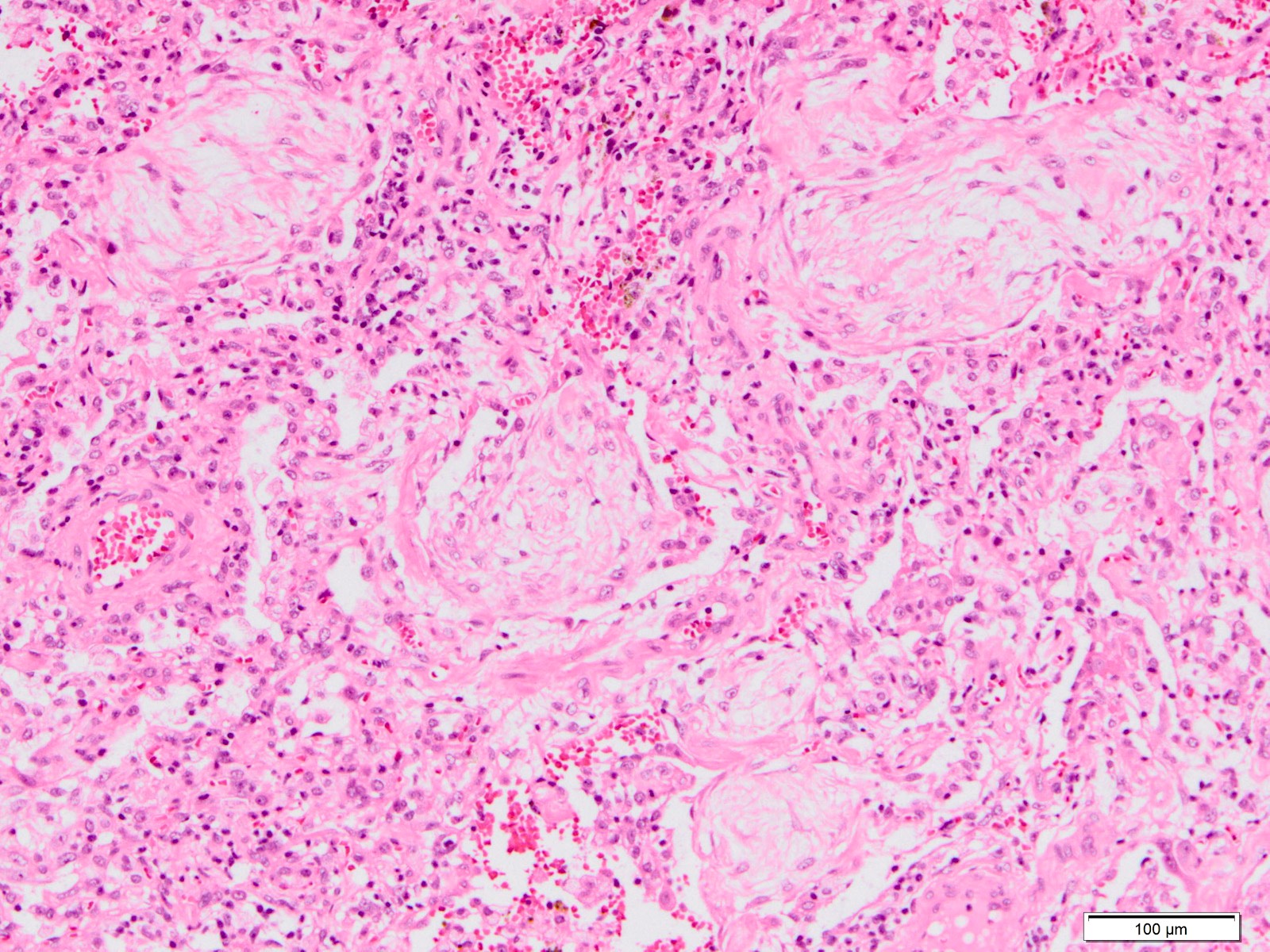
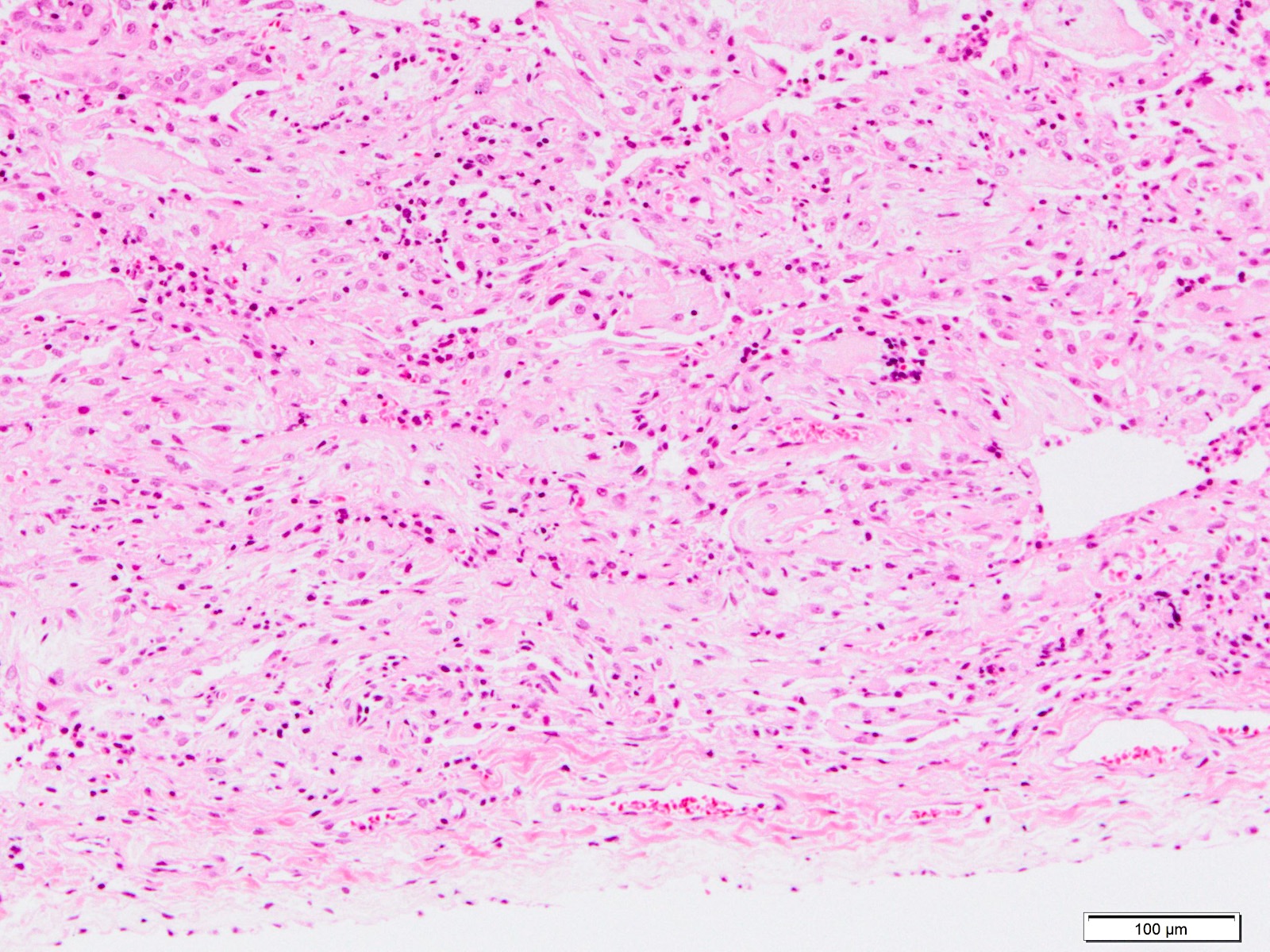
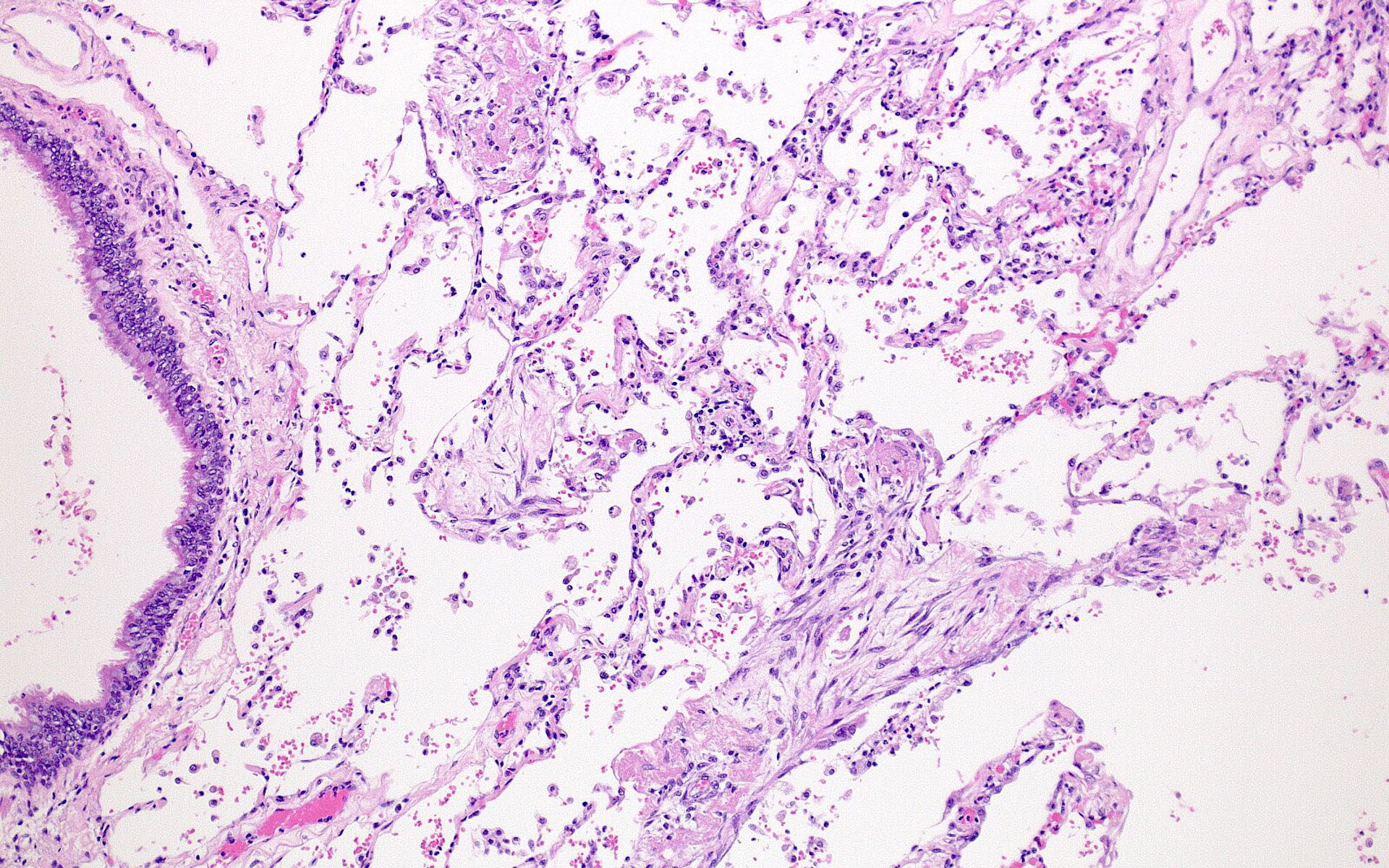
- Exudative (acute) phase
- Alveolar change
- Hyaline membranes on alveolar duct or sacs
- Interstitial and intra-alveolar edema
- Collapsed alveoli
- Epithelial change
- Denudation and necrosis of type I pneumocytes
- Vascular change
- Necrosis of endothelial cells
- Neutrophil aggregation
- Microthromboemboli
- Hemorrhage
- Alveolar change
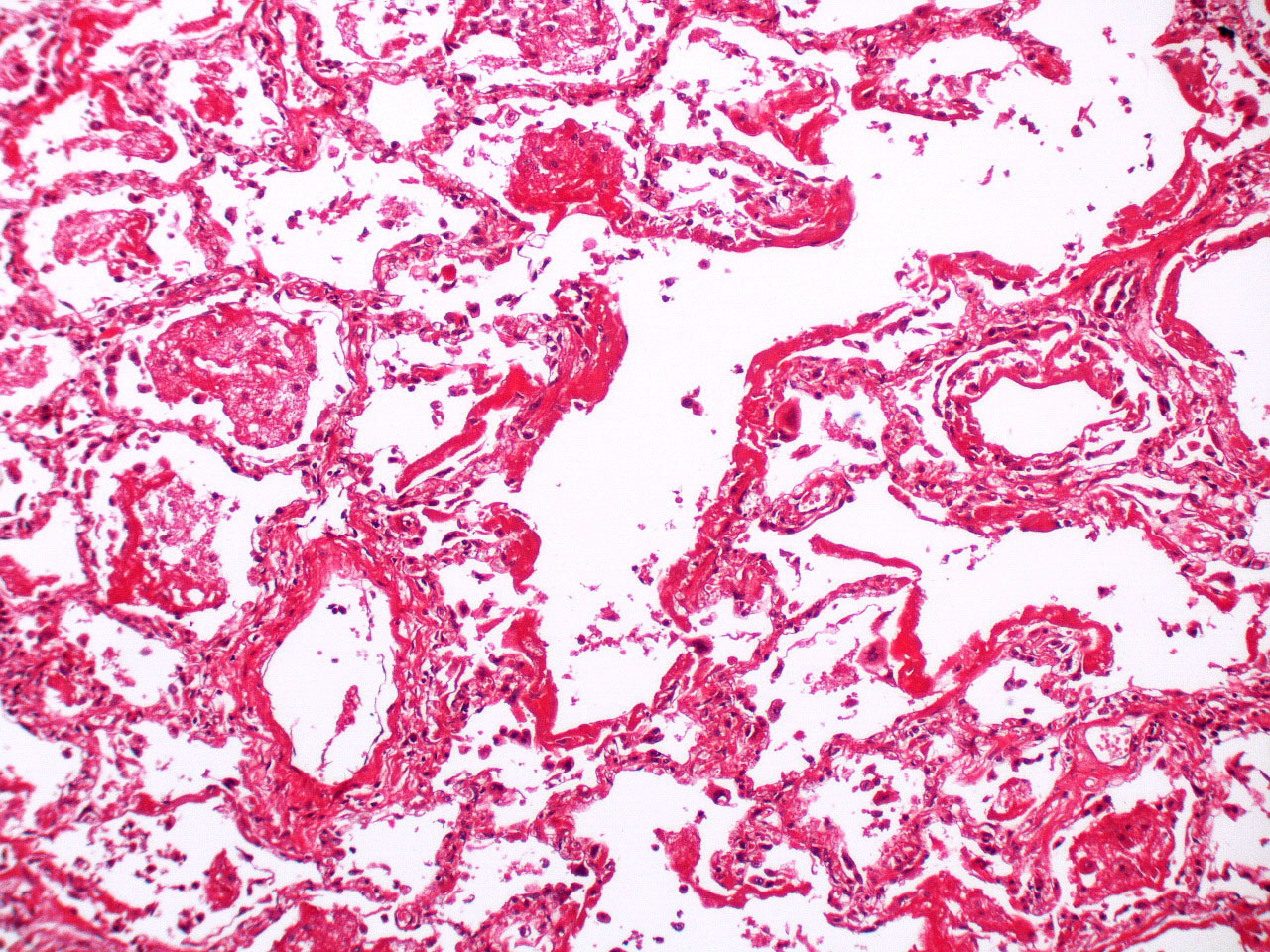
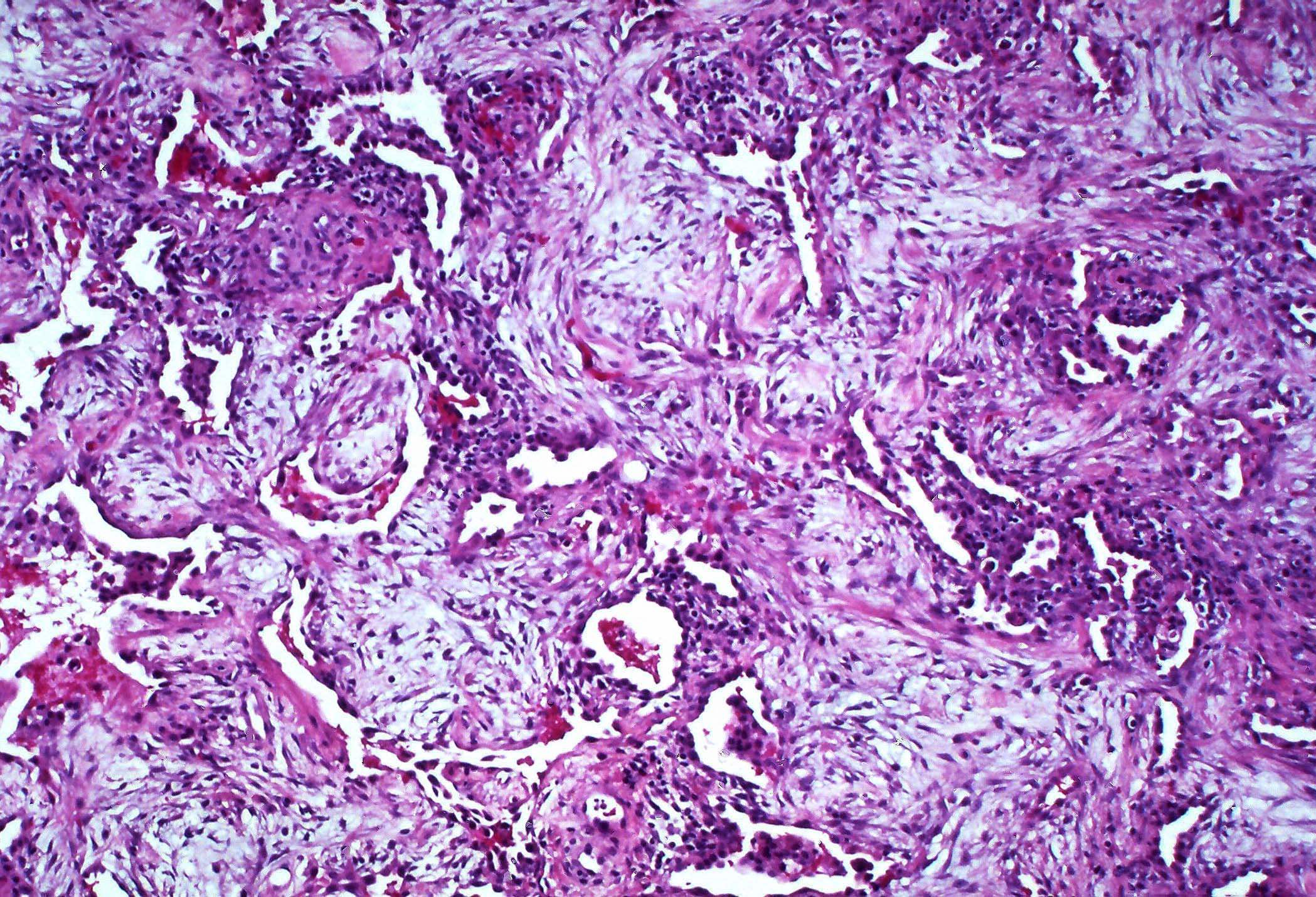
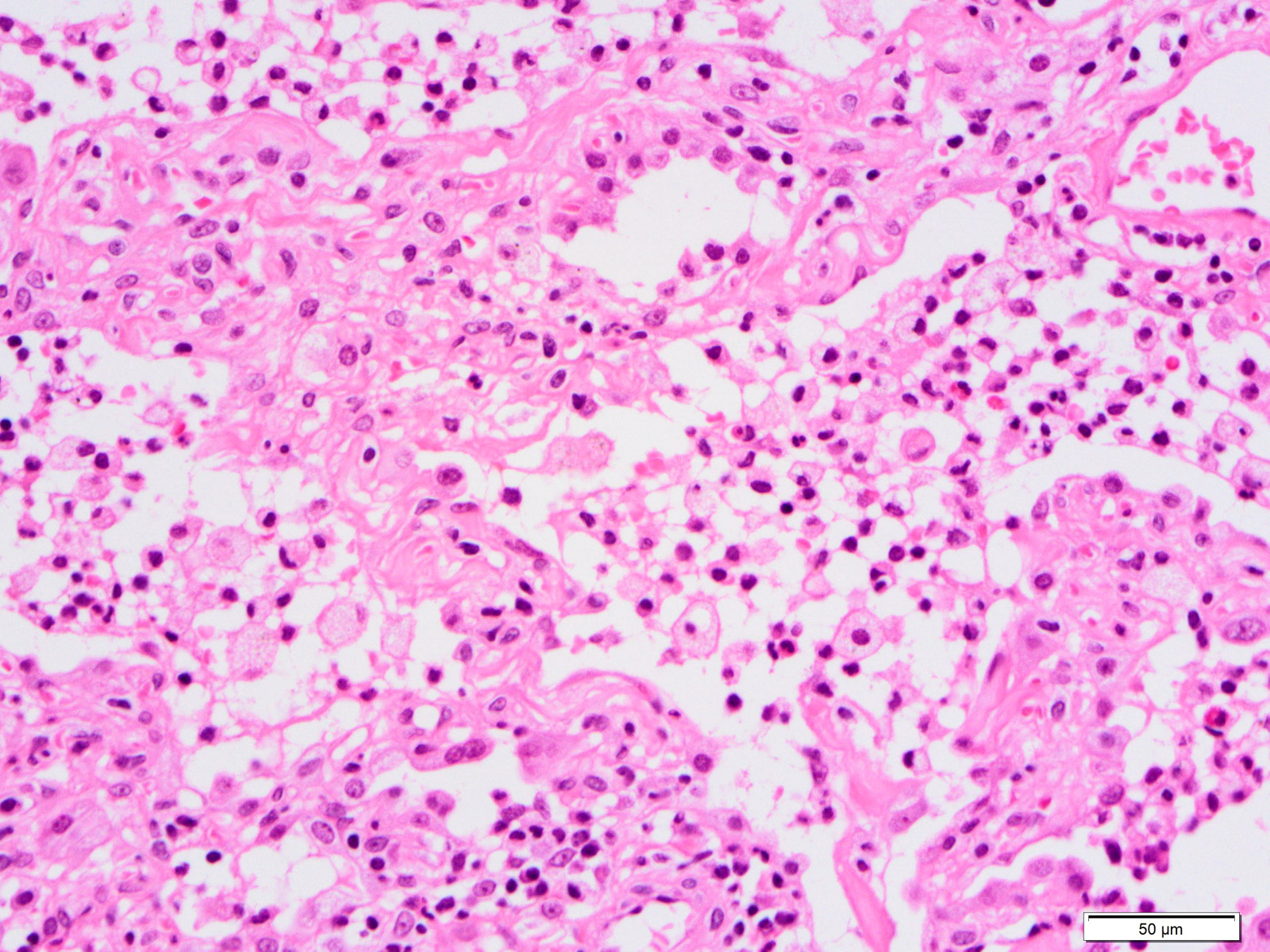
- Proliferative / organizing (subacute) phase
- Alveolar change
- Remnants of hyaline membrane with or without organization
- Interstitial and intra-alveolar proliferation of myofibroblasts
- Lymphocytic infiltration
- Epithelial change
- Proliferation / hyperplasia of type II pneumocytes
- Vascular change
- Endothelial injury and thromboemboli in arterioles
- Alveolar change
- Fibrosis phase
- Alveolar change
- Collagenous fibrosis
- Microscopic honeycomb-like change
- Traction bronchiolectasis
- Epithelial change
- Squamous metaplasia / hyperplasia
- Vascular change
- Remodeling of arteries
- Intimal fibrosis
- Thickening of media
- Alveolar change
- Others
- May have superimposed pneumonia
- Fibrin deposition
- DAD with prominent organizing pneumonia is also called organizing DAD
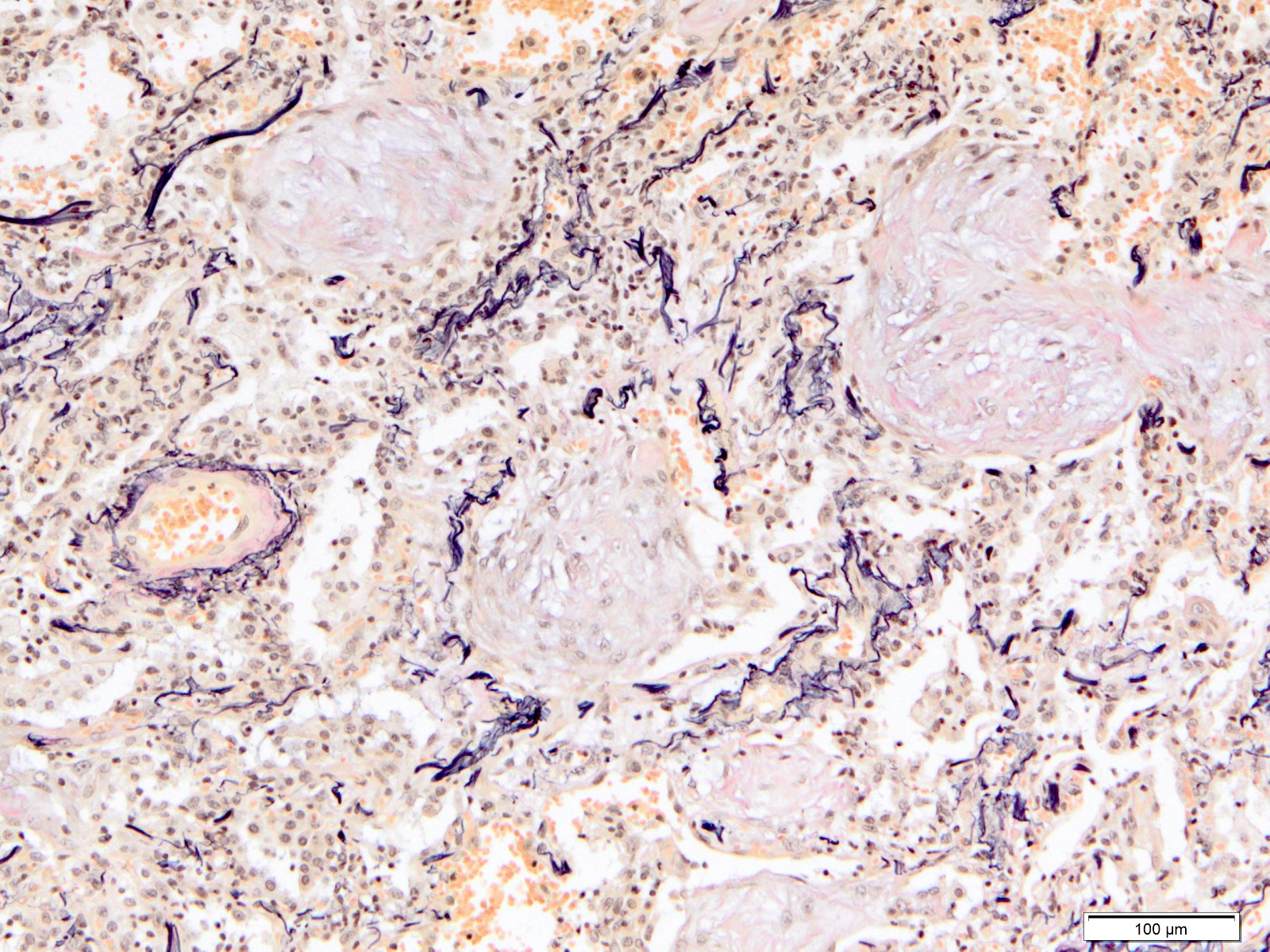
Microscopic (histologic) images
Contributed by Akira Yoshikawa, M.D. and Yale Rosen, M.D.
Cytology description
- Bronchoalveolar lavage (BAL) fluid
- Increased neutrophils in ARDS
- Can be helpful for the diagnosis of underlying disease
Positive stains
- Fiber staining (e.g. elastica van Gieson) is helpful to evaluate fibrosis and to evaluate the destruction of the alveolar architecture
- Giemsa, Grocott and Ziehl-Neelsen stains are helpful to identify pathogens
- Immunohistochemistry (not of practical utility):
- Cytokeratin highlights collapsed alveoli and lung architecture
Electron microscopy description
- Changes in epithelium, endothelium and interstitium (N Engl J Med 2000;342:1334)
- Exudative phase
- Vacuolization in damaged endothelial cells
- Replacement of epithelial cells by hyaline membrane on the basement membrane
- Proliferative / organizing and fibrotic phases
- Reepithelialization of type II pneumocytes with microvilli and lamellar bodies with surfactant
- Flattening of cytoplasm and loss of lamellar bodies and microvilli of type II pneumocyte, indicating transformation to type I pneumocyte
- Collagen deposition
- Exudative phase
Videos
Histology of DAD
Sample pathology report
- Lung, autopsy:
- Diffuse alveolar damage (see comment)
- Comment: Histologic sections reveal diffuse alveolar damage in subacute and chronic phases involving bilateral lobes; the former is represented by fibrin deposition, organizing pneumonia and focal hyaline membranes and the latter by interstitial fibrosis, fibroblastic foci and the destruction of alveolar architecture. Acute bacterial pneumonia is also superimposed.
Differential diagnosis
- Major entities:
- Acute exacerbation of usual interstitial pneumonia / idiopathic pulmonary fibrosis (UIP / IPF):
- Acute exacerbation of IPF and DAD are clinically and morphologically similar
- IPF shows chronic and dense collagen fibrosis with smooth muscle hyperplasia, while fibrosis in DAD is not so dense and usually lacks smooth muscle hyperplasia
- Acute interstitial pneumonia:
- So called Hamman-Rich syndrome or idiopathic DAD
- Lack of clinical criteria of ARDS
- Cardiogenic pulmonary edema:
- BNP ≥ 1,200 pg/mL
- Congestive heart failure on echocardiograph
- Right heart catheterization is now not recommended due to negative influence to prognosis
- Drug induced lung injury:
- History of causative drug
- Remission of symptoms after withdrawal of the responsible drug
- Acute exacerbation of usual interstitial pneumonia / idiopathic pulmonary fibrosis (UIP / IPF):
- Minor entities:
- Organizing pneumonia:
- Migratory sign on imaging
- Good response to corticosteroid
- Eosinophilic pneumonitis:
- Current smoker or history of particle inhalation
- Eosinophilia (> 25%) in bronchoalveolar lavage
- Good response to corticosteroid
- Hypersensitivity pneumonitis:
- History of antigen exposure
- Remission of symptoms after removal of the causative antigen
- Lymphocytosis (> 30%) in bronchoalveolar lavage
- Diffuse centrilobular nodular shadow with ground glass opacity, usually in upper lobes
- Miliary tuberculosis:
- Diffuse nodular shadow (≤ 3 mm) involving whole lobes
- Mycobacterium tuberculosis in bronchoalveolar lavage or biopsy specimen
- Lymphangitic carcinomatosis:
- Known malignancy
- Tumor cells in bronchoalveolar lavage or biopsy specimen
- Organizing pneumonia:
Additional references
Practice question #1
Practice answer #1
E. Proliferative / organizing (subacute). Masson body, polypoid proliferation of spindle shaped fibroblasts, is noted in the alveoli.
Comment Here
Reference: ARDS / DAD
Comment Here
Reference: ARDS / DAD
Practice question #2
Which of the following findings is most suggestive of acute respiratory distress syndrome (ARDS) / diffuse alveolar damage (DAD)?
- Bacterial pneumonia
- Diffuse collagenous fibrosis
- Hyaline membranes
- Organizing pneumonia
- Proliferation of atypical pneumocytes
Practice answer #2
C. Hyaline membranes. Hyaline membranes morphologically represent the damage of pneumocytes and endothelium in DAD.
Comment Here
Reference: ARDS / DAD
Comment Here
Reference: ARDS / DAD