Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Case reports | Treatment | Clinical images | Microscopic (histologic) description | Microscopic (histologic) images | Peripheral smear description | Peripheral smear images | Videos | Sample pathology report | Differential diagnosis | Practice question #1 | Practice answer #1 | Practice question #2 | Practice answer #2Cite this page: Bhardwaj S, Teruya-Feldstein J. Megaloblastic anemia. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/bonemarrowmegaloblasticanemia.html. Accessed September 14th, 2025.
Definition / general
- Megaloblastic anemia is a disorder of impaired DNA synthesis (with normal RNA synthesis) that manifests with the presence of megaloblasts in the bone marrow resulting in ineffective and abnormal erythropoiesis and macrocytes / macroovalocytes in the peripheral blood
- Common morphologic manifestation of a multitude of entities causing similar impairment in DNA synthesis
Essential features
- Disorder of impaired DNA synthesis → delayed nuclear maturation → nuclear:cytoplasmic dyssynchrony
- Multitude of etiologies: most commonly vitamin B12 or folate deficiency
- Megaloblasts, macroovalocytes (erythroid lineage) and nuclear hypersegmentation of neutrophils are characteristic morphologic abnormalities
Terminology
- Megaloblast: abnormal erythroid precursors showing nucleo:cytoplasmic dyssynchrony (more immature nucleus for the degree of maturity of the cytoplasm)
- Macrocyte: mature red blood cell with increased mean corpuscular volume (100 - 110 fL)
- Macroovalocyte: mature red blood cell with macrocytosis and a distinct oval shape with reduced or absent central pallor; commonly seen in megaloblastic anemia but not pathognomic
ICD coding
- ICD-10: D53.1 - other megaloblastic anemias, not elsewhere classified
Epidemiology
- Insufficient data regarding incidence and prevalence
- Epidemiology varies depending on the cause of megaloblastic anemia
- Nutritional deficiency of B12 and folate:
- Adults and children in macrobiotic communities and developing countries with rampant malnutrition
- Pregnant women in developing countries with lack of folate supplementation
- Elderly in developed countries
- Pernicious anemia:
- Individuals older than 40 years; incidence of 2% in > 60 years (J Blood Med 2012;3:97)
- More common in individuals of Celtic (e.g. English, Irish, Scottish) or Scandinavian origin
- Patients of any age group on certain chemotherapeutic drugs
- Nutritional deficiency of B12 and folate:
Sites
- Bone marrow
- Peripheral blood
- All rapidly dividing epithelia (gut epithelium, uterine cervical epithelium) can show megaloblastic changes due to impaired DNA synthesis: widespread involvement of epithelia is called megaloblastosis
- Reference: StatPearls: Megaloblastic Anemia [Accessed 11 October 2021]
Pathophysiology
- Normal absorption pathway of cobalamin (B12) (Nat Rev Dis Primers 2017;3:17040):
- Vitamin B12 can be present in diet in free form or bound form
- Free form of B12 / cobalamin binds to protein R binder (TCI or haptocorrin) in the stomach (R binder is produced by salivary glands and gastric mucosal cells)
- This R binder - B12 complex is carried to the duodenum, where R binder is digested and B12 is bound to intrinsic factor (IF) (IF is produced by the parietal cells of the stomach but it binds to B12 in the duodenum)
- B12 - IF complex reaches the terminal ileum, where it is internalized by the cobalamin receptors on the ileal epithelial cells; inside the cytoplasm of ileal epithelial cells, the IF is destroyed
- B12 binds to the protein transcobalamin II (TCII) and is carried bound to TCII to liver (50%) and other organs (50%)
- Normal absorption pathway of folic acid:
- Food folates are present as polyglutamates in diet
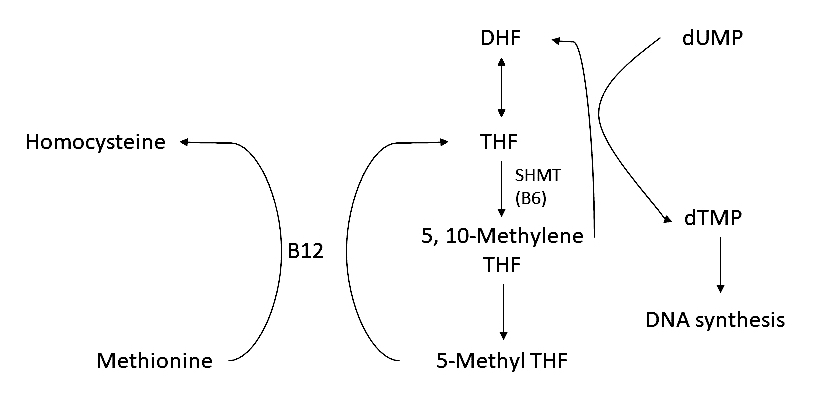
- In the GI tract, polyglutamates are broken down to mono and diglutamates; these are absorbed by the proximal jejunum and converted to dihydrofolate (DHF), tetrahydrofolate (THF) and methyl THF
- Folate reaches the plasma as N5 methyl THF; this circulates in the plasma, either loosely bound to albumin or by high affinity binding to folate binding protein
- Inside the cell, N5 methyl THF is utilized for DNA synthesis, with the help of vitamin B12 (see Diagram 1)
- Thus, in the absence of B12 and folate, there is impaired DNA synthesis
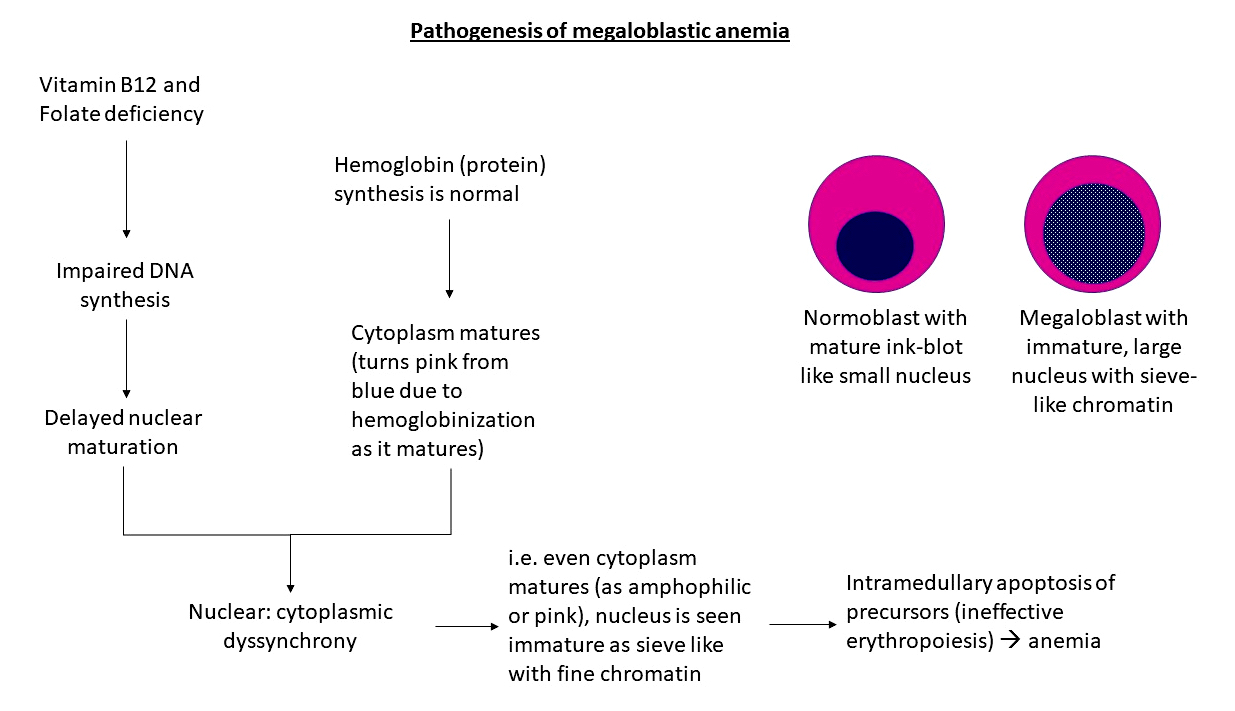
- How impaired DNA synthesis leads to megaloblastic anemia (see Diagram 2):
- As the DNA doesn't synthesize normally, nuclear maturation is delayed, while cytoplasmic maturation continues at normal rate, thus forming a cell with mature cytoplasm with relatively immature, large, open nucleus, known as a megaloblast
- Large number of abnormal precursors undergo intramedullary apoptosis leading to anemia
- Additionally, impaired DNA synthesis leads to ineffective hematopoiesis in all 3 cell lines → pancytopenia
- How impaired DNA synthesis leads to megaloblastic anemia (see Diagram 2):
- Pathophysiology of neurological dysfunction in cobalamin deficiency:
- Cobalamin deficiency leads to demyelination with subsequent axonal disruption and gliosis that can affect all parts of the central nervous system
- Peripheral nerves show axonal degeneration without demyelination
- Involvement of the posterior and lateral columns (including the dorsal, pyramidal and spinocerebellar tracts) leads to the classic myelopathic syndrome of B12 deficiency known as subacute combined degeneration
- Biochemical basis:
- Vitamin B12 is a cofactor for the enzyme methylmalonyl CoA mutase, which converts methylmalonyl CoA to succinyl CoA
- With B12 deficiency, methylmalonic acid (MMA) levels accumulate → elevated levels of MMA and homocysteine lead to myelin damage → neurologic deficits, such as neuropathy and ataxia
- Pathophysiology of pernicious anemia:
- Autoimmune disorder with 2 kinds of antibodies: parietal cell antibody (PCA) and intrinsic factor antibody (IFA) → PCA destroys parietal cells by acting parietal cell proton pump ATPase, IFA destroys IF directly → impaired B12 absorption → B12 deficiency → megaloblastic anemia
- In addition, destruction of parietal cells leads to achlorhydria → atrophic gastritis
Etiology
- Vitamin B12 deficiency
- Dietary deficiency
- Recommended daily intake of 2.4 mcg in ages 14 and older; recent studies recommend a higher level of 4 - 7 mcg (Am J Clin Nutr 2010;91:571)
- Deficiency more common in long term vegans (baseline stores of B12 exceed daily requirement by 1,000 times; thus, it takes years to develop deficiency), breastfed infants of vegan mothers
- Lack of intrinsic factor (IF)
- Chronic gastritis
- Gastric surgery
- Congenital absence of IF
- Pernicious anemia (autoimmune)
- Biologic competition for B12
- Small bowel bacterial overgrowth
- Fish tapeworm disease
- Malabsorption of B12
- Familial: familial selective vitamin B12 malabsorption (Imerslund-Gräsbeck syndrome)
- Drug induced: proton pump inhibitors, H2 receptor antagonists, metformin
- Others: regional enteritis, ileal resection
- Increased requirements
- Pregnancy
- Disseminated cancer
- Dietary deficiency
- Folate deficiency
- Dietary deficiency
- Recommended daily intake of 400 mcg
- Lesser stores than cobalamin, so deficiency develops in 2 - 3 months of dietary restriction
- Uncommon in developed countries due to fortification of foods such as cereals and pasta
- Impaired absorption
- Congenital malabsorption
- Extensive intestinal (jejunal) resection
- Drug interactions: anticonvulsants, trimethoprim, oral contraceptives (J Obstet Gynaecol Can 2015;37:430)
- Increased requirements
- Infancy
- Pregnancy
- Chronic hemolytic anemias
- Myeloproliferative disorders
- Alcoholism (J Nutr 2002;132:2367S)
- Malnutrition
- Direct effects of alcohol on folate metabolism and transport
- Decreased hepatic uptake
- Increased excretion
- Dietary deficiency
- Combined folate and B12 deficiency
- Tropical sprue
- Gluten sensitive enteropathy
- Drug induced suppression of DNA synthesis
- Folate antagonists: methotrexate
- Metabolic inhibitors
- Purine antagonists: 6-mercaptopurine, azathioprine
- Pyrimidine antagonists: 5-fluorouracil, cytosine arabinoside
- Alkylating agents: cyclophosphamide, chlorambucil
- Others: nitrous oxide, arsenic
- Inherited disorders of DNA synthesis
- Orotic aciduria
- Lesch-Nyhan syndrome
- Thiamine responsive megaloblastic anemia
- Transcobalamin II deficiency
- Homocystinuria
- Methylmalonic aciduria
Diagrams / tables
Clinical features
- Depends on the underlying cause of megaloblastic anemia; however, general clinical features resulting from anemia are common to all
- Clinical features resulting from anemia (Med Clin North Am 2017;101:297):
- Weakness, shortness of breath, impaired concentration and exercise ability
- Gradual onset of pallor, tachycardia
- Lemon hue of skin due to increased indirect bilirubin levels due to ineffective erythropoiesis
- Clinical features specific to cobalamin deficiency (Nutrients 2013;5:4521):
- Neurological manifestations, such as paraesthesias, balance disorders, lancinating pains (resulting from peripheral neuropathy); visual disturbances due to optic atrophy, dementia in severe cases
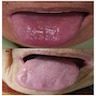
- Beefy red tongue due to atrophy of papillae
- Oral soreness and aphthous stomatitis
- Clinical signs: loss of vibratory sense and proprioception, positive Romberg test, Babinski reflex, hyporeflexia, clonus
- Increased incidence of arterial and venous thrombosis due to raised plasma homocysteine levels
- Clinical features of pernicious anemia:
- Classic triad at presentation: weakness, sore throat, paraesthesiae
- Patients have light blue-gray eyes and premature graying of hair
- Low grade fever, beefy red tongue, tachycardia, blotchy skin pigmentation
- Altered mental status, visual deficits
- Enlarged liver if heart failure is present
- Can be associated with autoimmune diseases, such as type 1 diabetes (3 - 4%), vitiligo (2 - 8%) and autoimmune thyroid disease (3 - 32%) (Nat Commun 2021;12:3761)
Diagnosis
- Complete blood count (anemia), peripheral smear, bone marrow aspirate and biopsy, serum vitamin B12 and folate levels, homocysteine levels (hyperhomocysteinemia), formiminoglutamic acid (FIGLU) levels in urine (increased)
- Gastric biopsy in pernicious anemia
- Reference: StatPearls: Megaloblastic Anemia [Accessed 11 October 2021]
Laboratory
- Complete blood count (CBC): anemia, pancytopenia
- Serum B12 levels (isotope dilution or radioimmunoassay, automated chemiluminescence): reduced (normal = 160 - 950 pg/mL)
- Serum homocysteine levels: raised (normal < 15 mcmol/L)
- Serum methylmalonic acid: raised (normal < 0.40 mcmol/mL)
- Combination of serum B12 levels and homocysteine and methylmalonic acid levels is preferred for establishing diagnosis as the measurement of serum B12 alone can miss 10 - 50% of cases (J Am Geriatr Soc 1992;40:1197, J Am Geriatr Soc 1995;43:1290)
- Assessment of folate levels:
- Serum folate level: reduced (< 4 ng/mL suggests deficiency)
- Red cell folate levels: reduced (< 151 ng/mL suggests deficiency)
- Red cell folate levels were considered the true measure of tissue folate levels; however, it is more costly and time consuming and some recent studies suggest serum folate may be superior to red cell folate (Clin Chem Lab Med 2013;51:555)
Case reports
- 9 week old boy with fever and pancytopenia (J Pak Med Assoc 2020;70:923)
- 7 month old girl with developmental regression (Ital J Pediatr 2020;46:40)
- 39 year old woman with pernicious anemia (Blood 2020;135:1719)
- 69 year old woman with painful tongue (CMAJ 2020;192:E434)
Treatment
- Supplementation of B12 and folate
- Vitamin B12 changes may be ameliorated by folic acid supplementation alone, because the megaloblastic defects are caused by defect in folate utilization; however, folate does not reverse the neurological symptoms and may aggravate them
- When malabsorption is a cause, parenteral supplementation is needed
- Treat the underlying cause (e.g. bacterial overgrowth, etc.)
- Response to treatment (Klin Lab Diagn 2004;(5):42):
- Reversal of megaloblastic to normoblastic changes: 12 - 48 hours
- Increase in reticulocyte count: within 3 days (starts on second day, peaks at sixth day)
- Normalization of MMA and plasma homocysteine levels: first 5 days
- Rise in white blood cell and platelet counts (if they had been low): 1 week
- Sustained normalization of serum cobalamin: following 2 weeks of therapy
- Neutrophil hypersegmentation persists for 2 weeks or more
- Neurological improvement: first few weeks
- Correction of peripheral blood macrocytosis: first month of treatment
- Normalization of blood counts: before 8 weeks
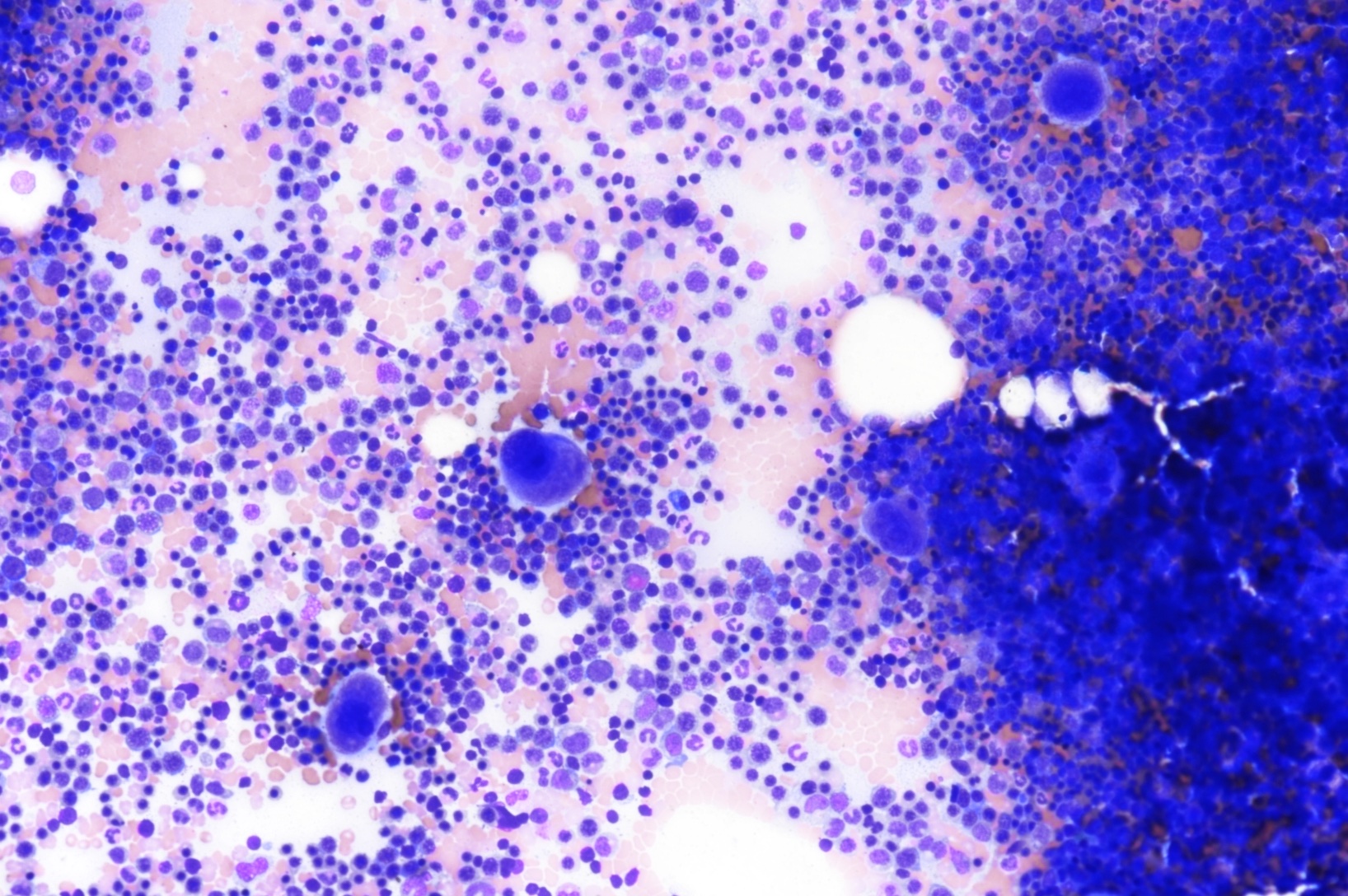
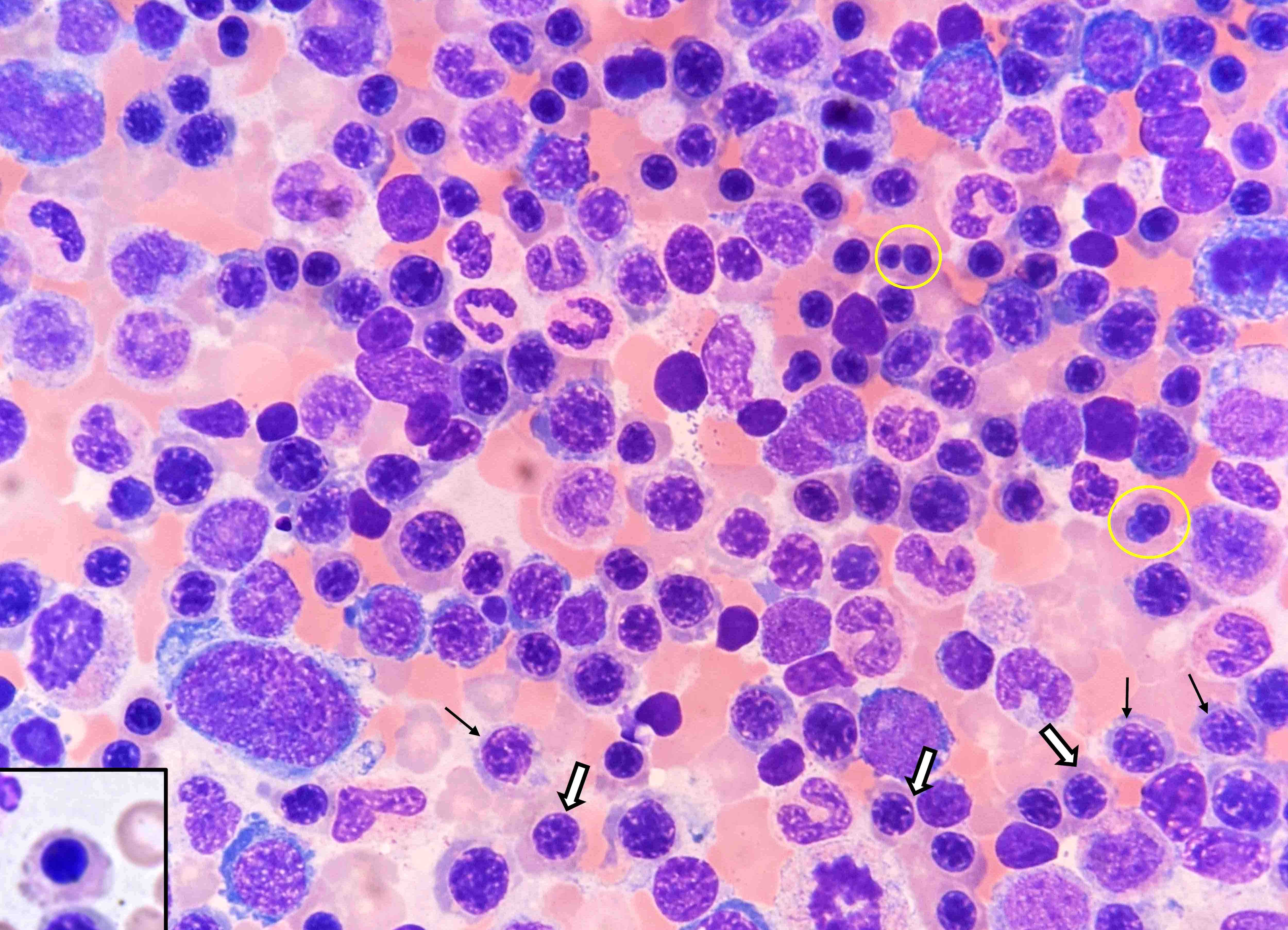
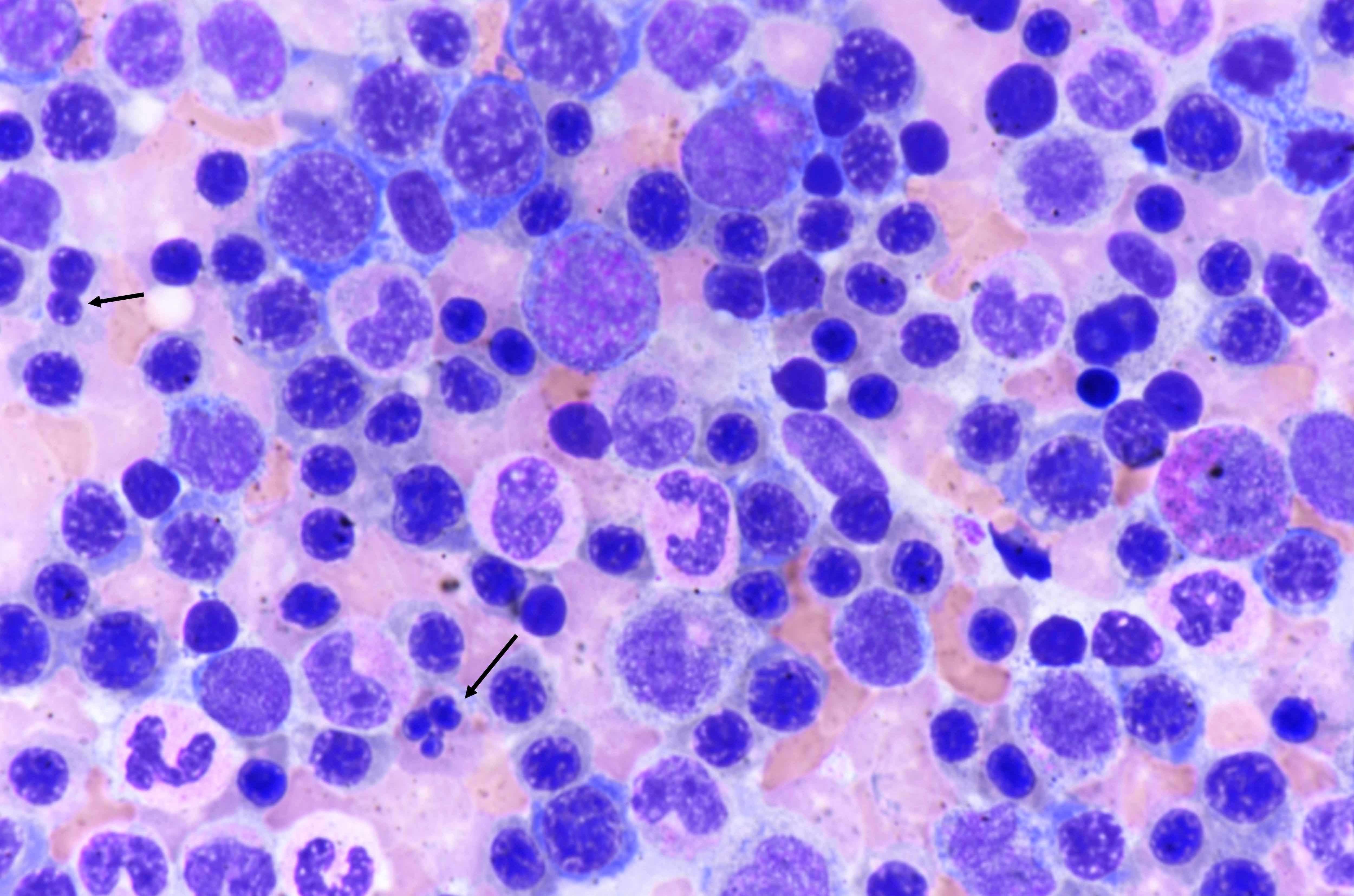
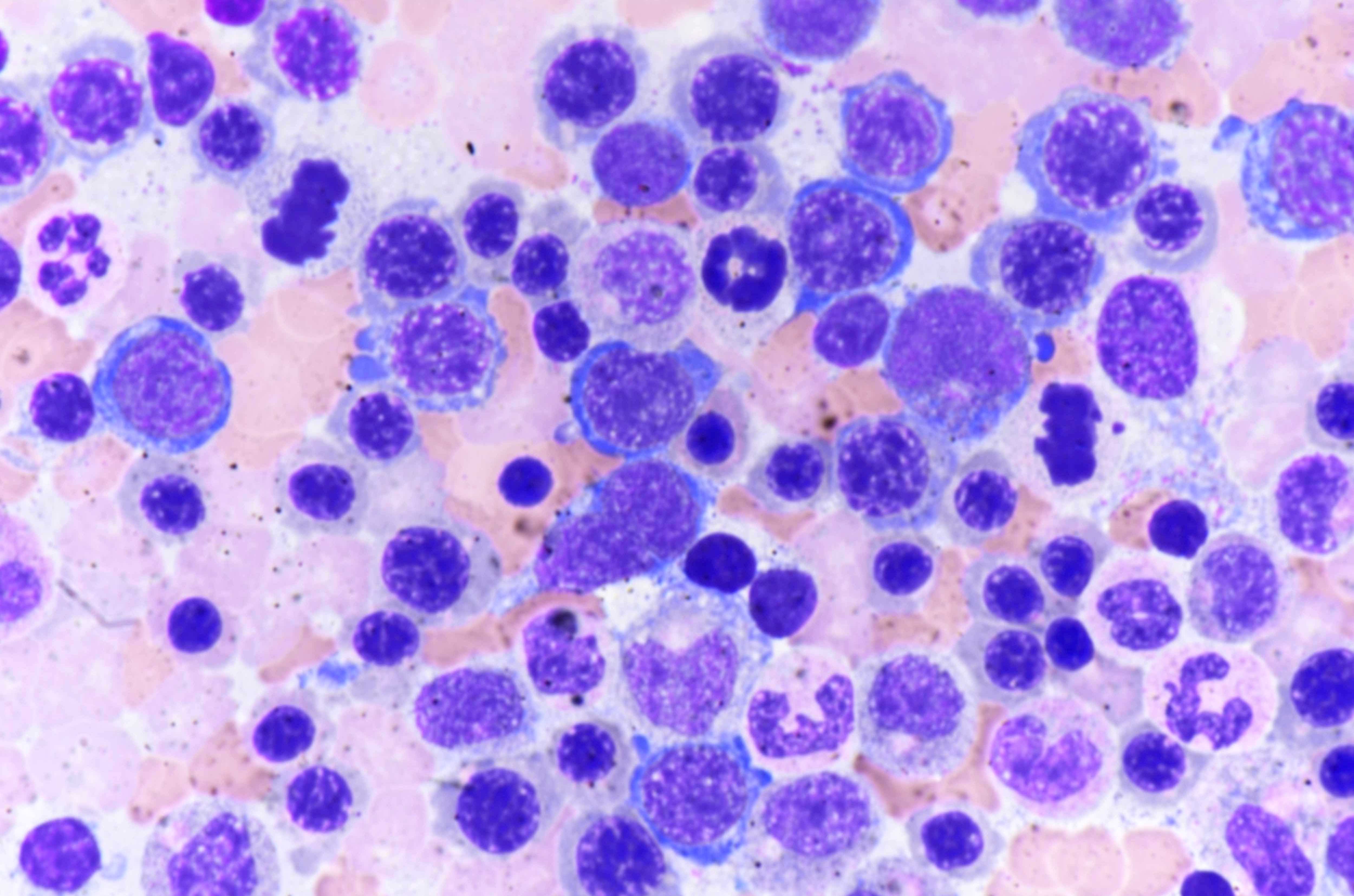
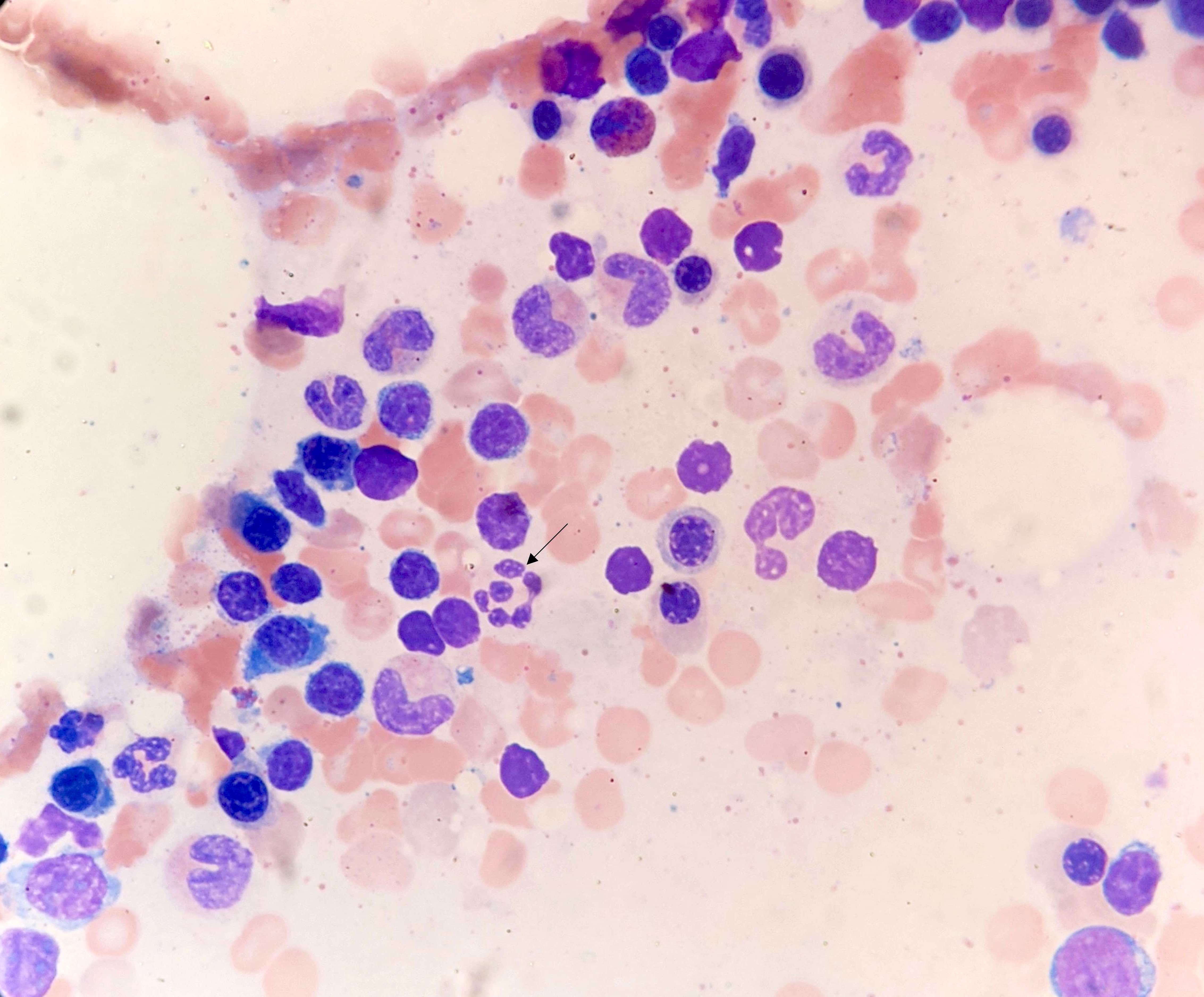
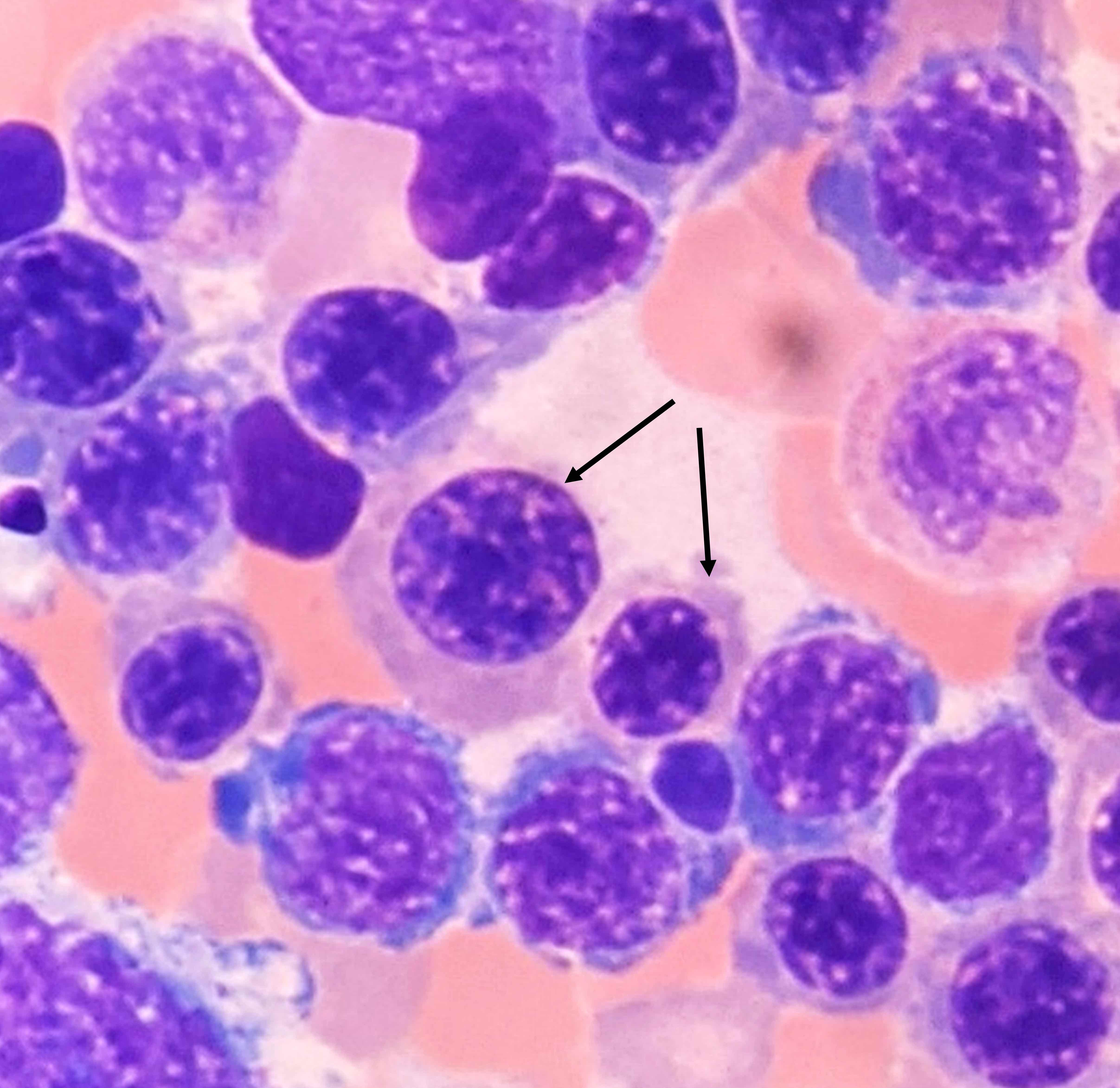
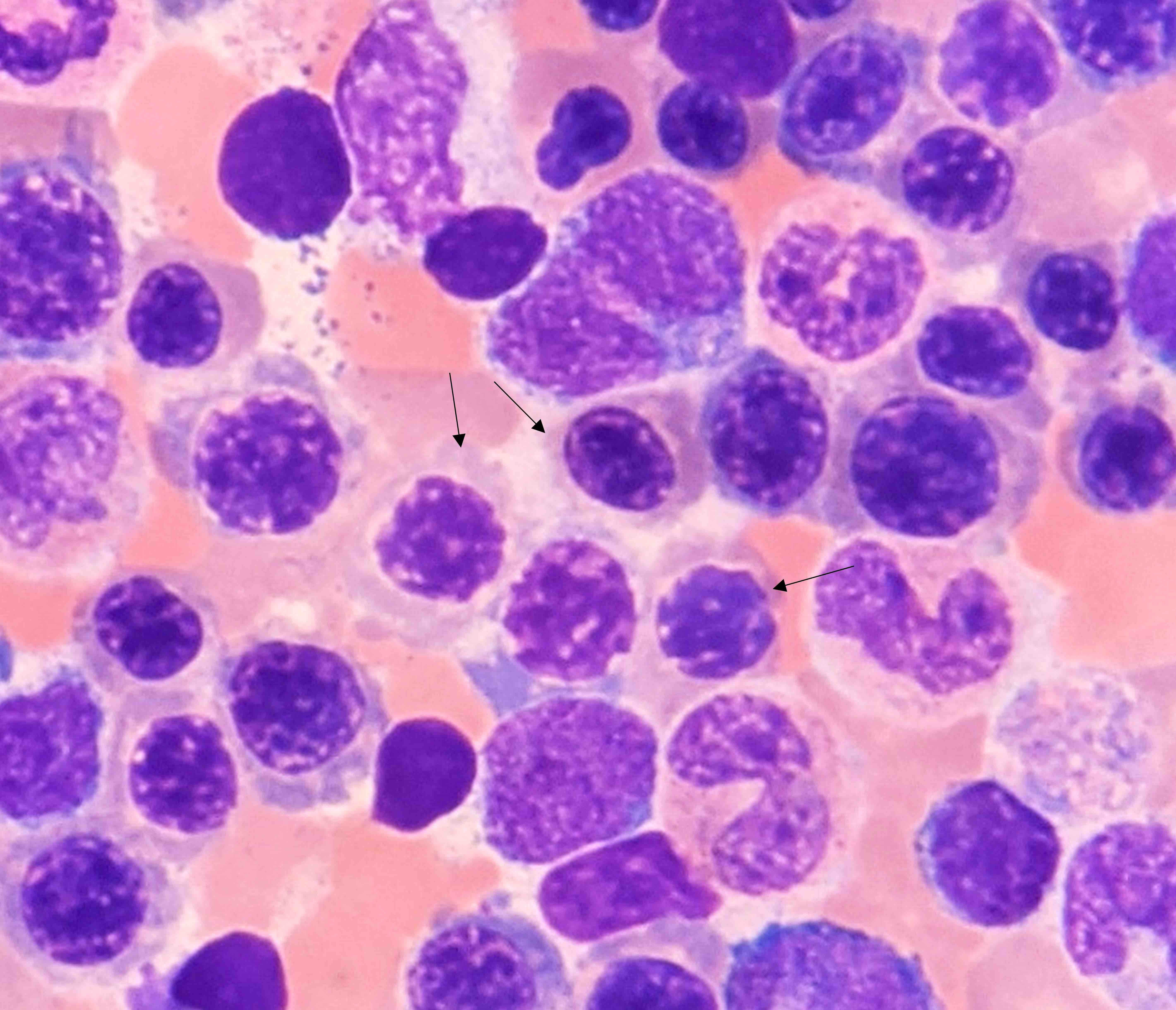
Microscopic (histologic) description
- Bone marrow is markedly hypercellular with erythroid hyperplasia and reversal of M:E ratio (Greer: Wintrobe's Clinical Hematology, 13th Edition, 2014)
- Hallmark is nuclear:cytoplasmic dyssynchrony / dissociation = megaloblastosis
- Erythroid lineage abnormalities: most apparent
- Megaloblasts: have large, immature nucleus with open, sieve-like chromatin
- Early megaloblasts > intermediate megaloblasts > late megaloblasts
- Some megaloblasts show Howell-Jolly bodies and chromatin stippling
- Frequent normal and abnormal mitoses
- Other features of dyserythropoeisis may also be seen: nuclear budding, irregular nuclear membrane, nuclear fragments, etc.
- Myeloid lineage abnormalities:
- Giant metamyelocytes
- Giant band forms
- Neutrophil hypersegmentation: often precedes anemia
- Misshapen neutrophils → ineffective myelopoiesis → neutropenia
- Megakaryocyte abnormalities: least common
- Nuclei with open chromatin and nuclear lobular hypersegmentation
- Fragmentation → ineffective thrombocytopoiesis
Microscopic (histologic) images
Peripheral smear description
- Pancytopenia
- Macrocytosis and macroovalocytosis: macroovalocytes are unique to megaloblastic anemia; large, oval, egg shaped cells with diminished / absent central pallor
- Anisopoikilocytosis
- Some red cells show Howell-Jolly bodies, Cabot rings (acidophilic, figure of 8 shape, arginine rich), basophilic stippling
- Reference: Clin Med Res 2006;4:236
Peripheral smear images
Videos
Megaloblastoid erythroids, basophils, mast cells in a bone marrow smear
Sample pathology report
- Bone marrow, right posterior iliac crest, aspirate smears, trephine biopsy:
- Hypercellular marrow (80%) showing erythroid hyperplasia, with reversal of M:E ratio (1:5); erythroid precursors show megaloblastic changes and mild to moderate dyserythropoeisis (see comment)
- Comment: The above findings of bone marrow hypercellularity, erythroid hyperplasia and megaloblastic changes are consistent with megaloblastic anemia in the right clinical context. Advised correlation with serum vitamin B12 and folate levels.
- Peripheral smear: Peripheral smear examination shows pancytopenia. Macrocytes and macroovalocytes are seen along with rare cells with basophilic stippling. Nucleated red cells are seen with immature nucleus (4/100 WBCs). Neutrophils show hypersegmentation.
- Bone marrow aspirate: Adequate for evaluation. Bone marrow aspirate smear is hypercellular with erythroid hyperplasia, reversal of M:E ratio (1:5). Erythroid precursors show megaloblastic changes, nuclear budding and nuclear irregularity. Myeloid lineage cells are reduced in number. Giant metamyelocytes are seen. Neutrophils show hypersegmentation. Megakaryocytes are reduced in number.
- Bone marrow biopsy: Adequate for evaluation. Bone marrow biopsy is hypercellular for age (80%), showing marked erythroid hyperplasia on CD61 staining.
Differential diagnosis
- Myelodysplastic syndrome (MDS):
- Marked dysplasia
- Dysplastic features unique to MDS not seen in megaloblastic anemia include nuclear hyposegmentation and hypogranularity, hypolobation of megakaryocytes, micromonolobated megakaryocytes, hypogranular platelets, increased blasts
- Nonmegaloblastic macrocytic anemias:
Practice question #1
Practice answer #1
Practice question #2
Where is the vitamin B12 - intrinsic factor complex absorbed?
- Duodenum
- Ileum
- Jejunum
- Stomach
Practice answer #2
B. Ileum. B12 - IF complex is absorbed in the terminal ileum where it is internalized by the cobalamin receptors on the ileal epithelial cells.
Comment Here
Reference: Megaloblastic anemia
Comment Here
Reference: Megaloblastic anemia