Table of Contents
Definition / general | Essential features | Terminology | Applications | Advantages | Diagrams / tables | QA in gynecologic cytology | QA in nongynecologic and FNA cytology | Cytology laboratory specific quality parameters | Applications of Six Sigma tools in quality management | Cytotechnologist workload | Practice question #1 | Practice answer #1 | Practice question #2 | Practice answer #2Cite this page: Jum’ah H, Ganesan S. Quality assurance for cytopathology. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/managementlabappdigpathqa.html. Accessed September 25th, 2025.
Definition / general
- Cytopathology is an anatomic pathology specialty that is highly regulated; the quality assurance (QA) program in cytopathology is mandated by CLIA '88 and implemented and constantly updated by the College of American Pathologists (CAP) (CMS: Clinical Laboratory Improvement Amendments (CLIA) [Accessed 7 September 2023])
- Such QA measures must be systematically implemented for all test phases (preanalytic, analytic, postanalytic)
- Preanalytical QA measures include review of stain quality, cytologic preparation technical quality and cross contamination checks, among others (CYP.04300 and CYP.04150, respectively)
- Analytical QA measures include prospective 10% QA rescreening (CYP.07478)
- Postanalytical QA measures include retrospective review of all negative gynecologic Paps from the index patient when a new high grade dysplasia is detected (CYP.07517), surgical pathology correlation of nongynecologic cases (CYP.07675) and all gynecologic cases diagnosed as high grade dysplasia (CYP.07543)
- Reference: CAP: Accreditation Checklists [Accessed 7 September 2023]
Essential features
- Cytopathology, especially gynecologic cytology, is considered a prototype screening test; therefore, rigorous regulations revolve around this specialty to reduce (if not eliminate) significant false negative test results, which can have a momentous impact on patient safety and the laboratory's credibility
- There must be a multilayered approach to QA, resulting in a robust quality management plan that conforms to CLIA '88 / CAP laboratory accreditation program
- Therefore, the QA program in cytopathology must integrate prospective, retrospective and correlational QA in gynecologic cytology, retrospective and correlational QA in nongynecologic cytology and fine needle aspiration (FNA) cytology (Cancer Cytopathol 2014;122:3)
- Other approaches such as rapid random rescreening and targeted rescreening (negative Pap test with positive human papillomavirus [HPV] cotesting and including certain high risk cases into the 10% prospective rescreening pool) can be used as additional measures to enhance the QA program (Roum Arch Microbiol Immunol 2013;72:93)
- Creative adaptation of Six Sigma principles such as root cause analysis (RCA), define, measure, analyze, improve, control (DMAIC) and define, measure, analyze, design, verify (DMADV) will offer unique advantage to the laboratory in optimizing workflow, efficiency or accuracy
Terminology
- Quality assurance: the process of ensuring quality
- Quality control: the way the laboratories assure that their results are valid and accurate by evaluating the quality (Clin Lab Med 1983;3:541)
- Quality improvement: improving patient care, system performance, outcomes and cost by systematic change methods and strategies
- CLIA '88: Clinical Laboratory Improvement Amendments of 1988
- Prospective QA: a minimum of 10% rescreening of all slides with negative gynecologic Pap test diagnosis get rescreened before the reports are released
- Retrospective QA: rescreening of all prior negative gynecologic Pap tests from the past 5 years on an index patient when a new high grade dysplasia is detected
- Correlational QA: surgical pathology correlation of all high grade dysplasia in gynecologic cytology, surgical pathology correlation of nongynecologic and FNA cytology cases
- Rapid random rescreening: rescreening of negative Pap tests for a limited duration and at low magnification (Cancer Cytopathol 2011;119:357)
- Targeted rescreening: e.g., rescreening of cases with negative Pap test with positive HPV cotesting
- Cytohistologic correlation (CHC): correlation of cytology diagnoses with review of follow up or concurrent surgical pathology
- Root cause analysis (RCA): a standardized approach to understanding the causes of an adverse event or identifying system flaws for correction
- DMAIC (define, measure, analyze, improve, control): refers to a data driven improvement cycle used for improving, optimizing and stabilizing business processes and designs
- DMADV (define, measure, analyze, design, verify): a Six Sigma framework that focuses primarily on the development of a new service, product or process as opposed to improving a previously existing one
- Six Sigma: a management strategy to improve quality of business processes, which can be applied in the laboratories (Clin Chem Lab Med 2007;45:789)
Applications
- Ongoing QA programs facilitate monitoring the quality metrics and benchmark standards mandated by CLIA '88 and assure quality (CMS: Clinical Laboratory Improvement Amendments (CLIA) [Accessed 7 September 2023])
- Familiarity with the robust QA and quality management program of the cytology laboratory enhances knowledge of the pathologist to be an efficient medical director and prudent CAP inspector for laboratory accreditation
Advantages
- RCA is a valuable QA tool that identifies the steps that have led to a sentinel event, capture early trends and identify prevention strategies for process improvement by reducing variability in the process (Diagn Cytopathol 2021;49:633)
- This can be initiated by setting internal triggers based upon CAP benchmark standards or the voice of the customer
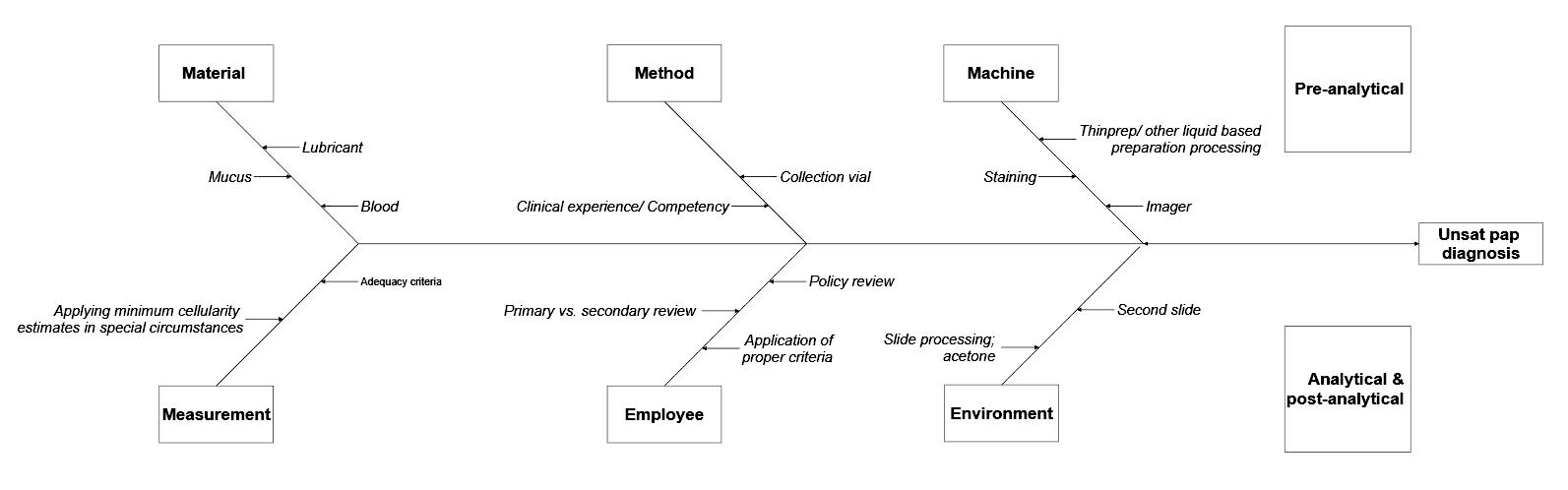
- Real life example
- One of the clinicians reached out to a cytopathology lab for the high number of unsatisfactory Pap smear diagnoses
- At the same time, that lab also noticed that the overall unsatisfactory rate doubled in that month
- That was a trigger to start RCA
- Fishbone diagram (see Diagram 1) shows the preanalytical, analytical and postanalytical factors to consider when investigating for the previous example
- Plan of action should be developed based on the findings
- Familiarity with Six Sigma principle (DMAIC) and creative adaptation offer innovative approaches to improve an existing process (DMAIC) in optimizing workflow, efficiency or accuracy
- Familiarity with Six Sigma principle (DMADV) aids in developing a new process and integration of new technology such as telecytology, automation and digital transformation
Diagrams / tables
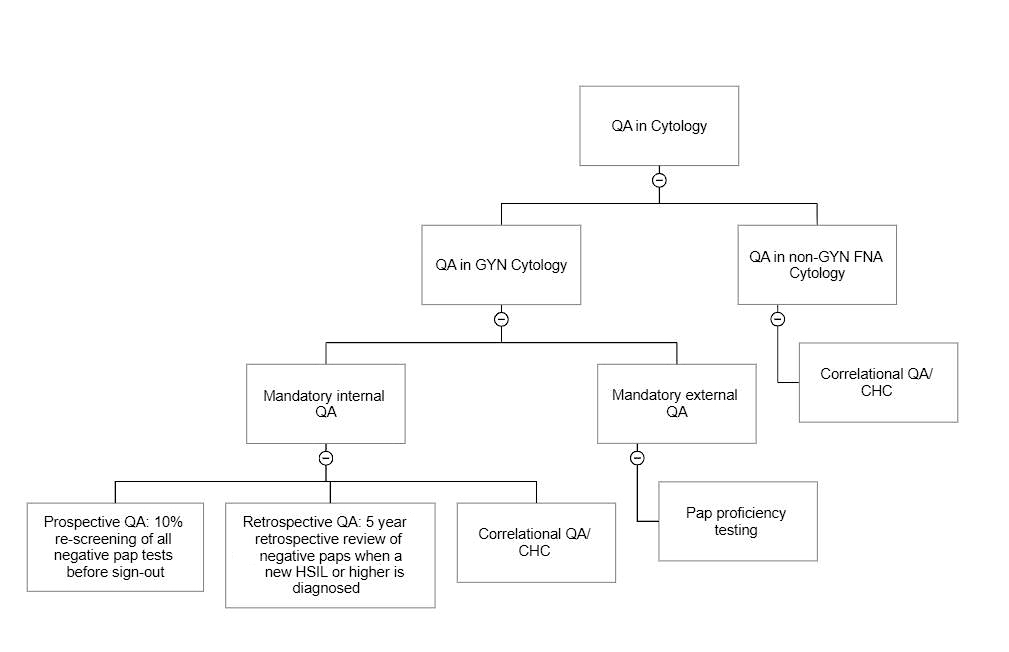
QA in gynecologic cytology
Internal quality assurance
Atypical squamous cells (ASC)/squamous intraepithelial lesions (SIL) ratio
External quality assurance
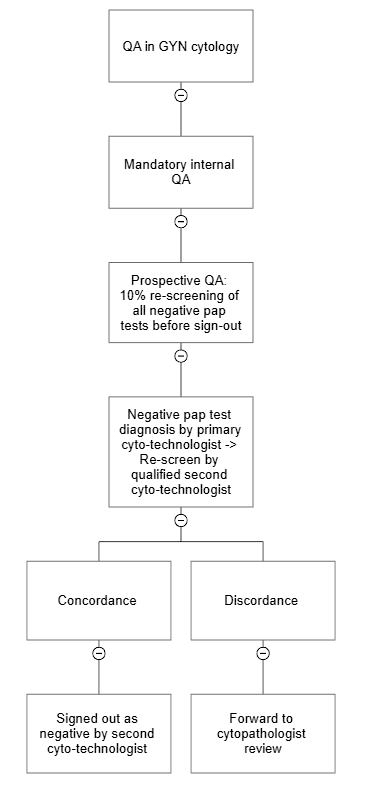
- Prospective QA (10% rescreening)
- CAP checklist, requirement CYP.07478: at least 10% of each cytotechnologist's gynecologic cases that have been interpreted to be negative are rescreened
- A minimum 10% rescreening of all slides with negative gynecologic Pap test diagnosis get rescreened by a qualified cytotechnologist before the cases are signed out (prospective rescreen) (CAP: Accreditation Checklists [Accessed 7 September 2023])
- If an epithelial cell abnormality (ECA) is detected, then the case is reflexed for pathologist's review
- This 10% rescreen error rate is an effective tool to monitor the quality on an ongoing basis
- Laboratory can utilize the 10% rescreen error rate towards cytotechnologist diagnostic accuracy calculation, especially when 2 step discrepancy is detected; the laboratory can also utilize as one of the triggers for targeted rapid screening, in the rare instance when a low grade dysplasia is detected upon 10% prospective rescreen (CAP: Accreditation Checklists [Accessed 7 September 2023])
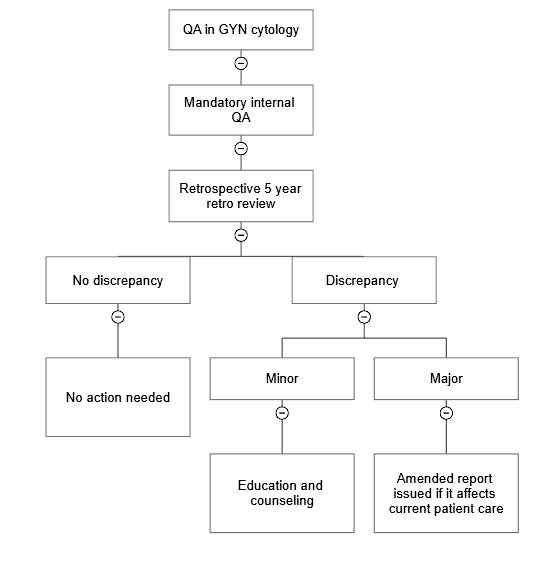
- Retrospective QA (5 year retrospective review of cases diagnosed as high grade squamous intraepithelial lesion [HSIL] or higher)
- CAP checklist, requirement CYP.07517: all available (either onsite or in storage) previously negative slides received within the past 5 years are reviewed whenever a new HSIL (moderate or severe dysplasia, carcinoma in situ, cervical intraepithelial neoplasia [CIN] II or III) or malignant cervical / vaginal cytology is reported
- Any previously negative Pap smears from the patient need to be reviewed by the cytology supervisor or a qualified cytotechnologist
- CAP checklist, requirement CYP.07530: amended report needs to be issued if a discrepancy that could affect the current patient care is identified (CAP: Accreditation Checklists [Accessed 7 September 2023])
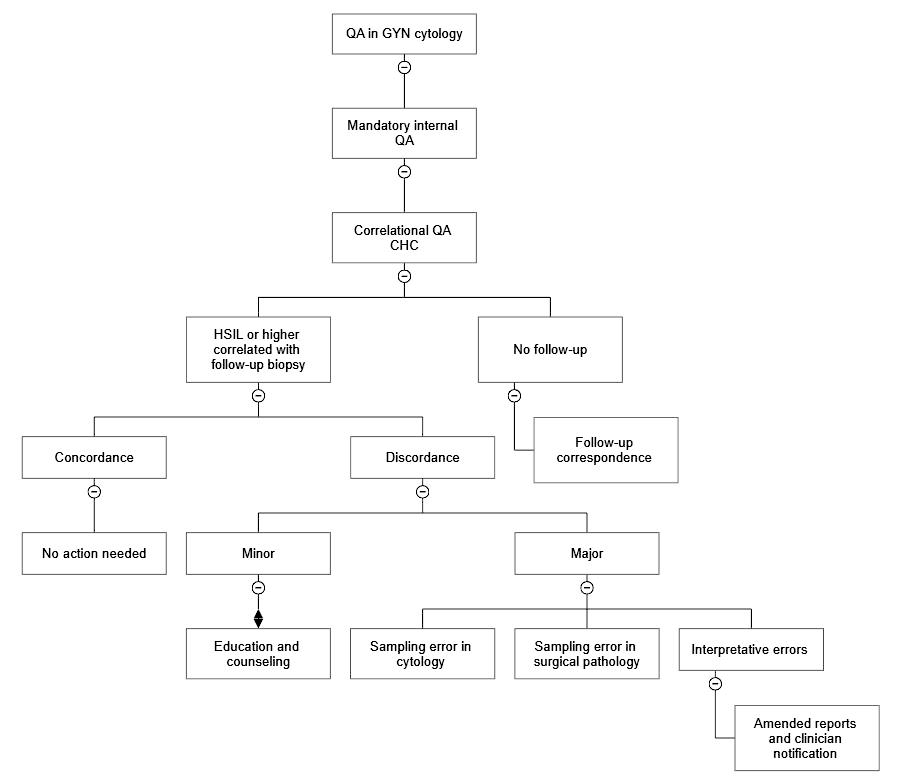
- Correlational QA (cytohistological correlation [CHC])
- CAP checklist, requirement CYP.07543: when the histologic diagnosis is available, correlation to the cytologic findings must be recorded and these records must be readily accessible; the number of cases that have histologic correlation must be recorded
- Review of follow up surgical pathology on all cases diagnosed as high grade dysplasia or higher by cytology
- Any discrepancy between cytology and surgical pathology is determined as cytology sampling, surgical pathology sampling, acceptable minor deviation in interpretation in both cytology and surgical pathology and major interpretation errors in both cytology and surgical pathology
- The major cause of discordant pairs in CHC remains sampling error (Arch Pathol Lab Med 2013;137:199)
- Reason for discordance gets documented in QA report and communicated with the clinician as appropriate
- CAP checklist, requirement CYP.07556: while looking for follow up surgical pathology on all cases diagnosed as high grade cytology, if no follow up surgical pathology is available, there should be records of attempts to obtain follow up histological information for correlative review (e.g., follow up correspondence, etc.) (CAP: Accreditation Checklists [Accessed 7 September 2023])
Atypical squamous cells (ASC)/squamous intraepithelial lesions (SIL) ratio
- ASC/SIL ratio is number / percentage of atypical squamous cells of uncertain significance (ASCUS) and atypical squamous cells - cannot exclude high grade dysplasia (ASC-H) diagnosed cases divided by the number / percentage of low (LSIL) and high (HSIL) grade squamous intraepithelial lesions and malignant cases (Am J Clin Pathol 2007;128:653)
- For example, if the percentage of ASCUS and ASC-H is 5% and the percentage of LSIL, HSIL and malignant cases is 3%, the ASC/SIL ratio will be 1.6
- Investigation needs to be initiated if the ASC/SIL ratio for gynecologic cases falls outside of the 5th or 95th percentile
- CAP checklist requirement CYP.07650: if the laboratory's annual ASC/SIL ratio for gynecologic cases falls outside of the 5th or 95th percentiles, the laboratory determines and records the reason(s) (CAP: Accreditation Checklists [Accessed 7 September 2023])
- See tables from cytopathology checklist CAP accreditation program (CAP: Accreditation Checklists [Accessed 7 September 2023])
External quality assurance
- Gynecologic Pap proficiency testing (PT) participation
- Laboratory and all individuals who examine gynecologic preparations participate in the CAP Gynecologic Cytology PT Program (PAP PT) or another proficiency testing program in gynecologic cytopathology approved by the Centers for Medicare and Medicaid Services (CMS) (CMS: Cytology Proficiency Testing [Accessed 7 September 2023])
- Annual proficiency testing is mandated for all cytotechnologists and pathologists who sign out Pap tests
- A score of < 90% is failure; an initial test failure requires reexamination (CAP TODAY: Cytopathology in Focus - Pap Proficiency Testing - What's Permitted, What's Not [Accessed 7 September 2023], CAP: Accreditation Checklists [Accessed 7 September 2023])
QA in nongynecologic and FNA cytology
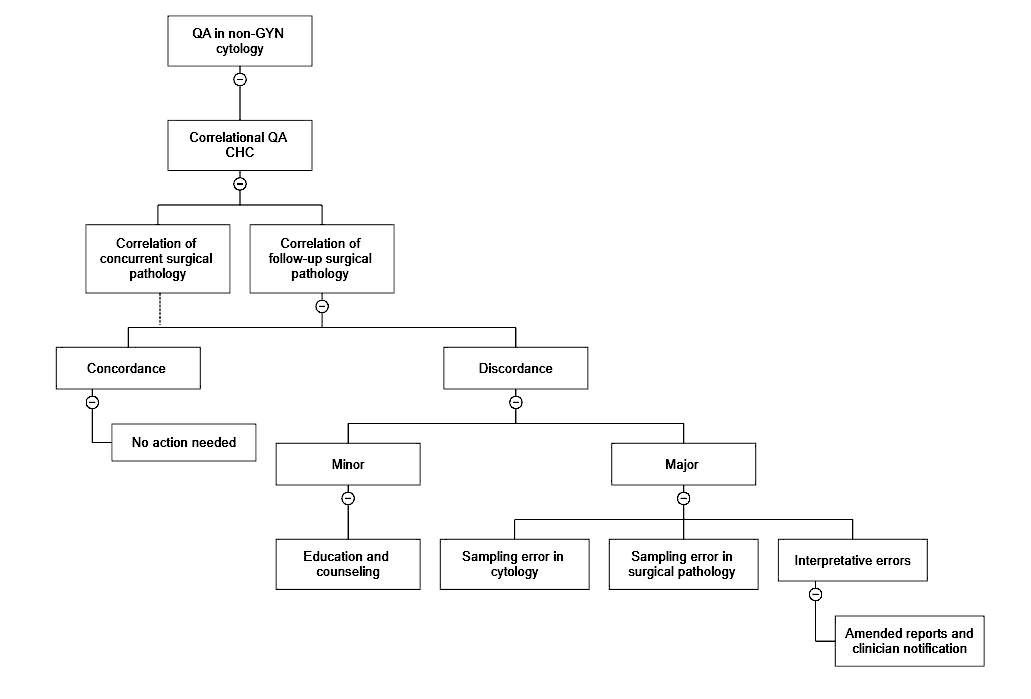
- Correlational QA in FNA and nongynecologic cytology (cytohistological correlation [CHC])
- CAP checklist, requirement CYP.07675: correlation of all or a subset of nongynecologic cytology specimens should be performed
- Methods of correlation should be recorded in the laboratory procedure manual and selected reports can be reviewed to confirm practice
- Possible mechanisms for correlation of histology include correlation of current specimens, focused review of specific specimen / organ types or follow up of suspicious / positive specimens, possible clinical correlation (CAP: Accreditation Checklists [Accessed 7 September 2023])
- Any discrepancy between cytology and surgical pathology is determined as cytology sampling, surgical pathology sampling, acceptable minor deviation in interpretation in both cytology and surgical pathology and major interpretation errors in both cytology and surgical pathology; the same documented in QA report and communicated with the clinician as appropriate
- Laboratory determines and records the reasons
Cytology laboratory specific quality parameters
- Monitoring of unsatisfactory diagnoses rate on a monthly basis to capture early trends, shifts and drifts, set triggers for when to start RCA
- Monitoring indeterminate diagnosis rate (e.g., atypical FNA diagnosis on thyroids, pancreas, etc.) (Cancer Cytopathol 2017;125:502)
- Monitoring of HPV negative ASCUS cases and set triggers for when to start RCA
- Automated image assisted screening failure rate to assess efficiency of image assisted screening and subsequent full manual review as an indirect measure for laboratory's productivity
Applications of Six Sigma tools in quality management
- DMAIC (Rev Environ Health 2019;34:427)
- This approach (define, measure, analyze, improve, control) can be creatively adapted to improve existing processes in cytology laboratory and can be applied to process improvement (PI) projects
- Key phase in DMAIC methodology is the control phase, which needs to be strictly followed to ascertain consistency and keeping the PI project on track
- Control phase is a vital step once the PI project goes in to effect; in the healthcare and laboratory setting, the following steps will keep the QI plan on track
- Periodic monitoring of the plan
- Review of data for any shift or drift
- Regular in service to all involved parties
- Orienting new staff appropriately
- DMADV (Int J Health Care Qual Assur 2012;25:254)
- This approach (define, measure, analyze, design, verify) is especially useful when implementing new strategies and initiatives because of its basis in data, early identification of success and thorough analysis
- Define: define requirements for the project
- Measure: collect data and requirement for the new process that are critical to quality (CTQ)
- Analyze: identify bottlenecks or areas where problems are likely to happen
- Design: work towards finished product
- Verify: tie loose ends and transition the process to go live
- DMADV can be creatively adapted in the integration of new technology such as telecytology, automation and digital transformation
- RCA (StatPearls: Root Cause Analysis and Medical Error Prevention [Accessed 7 September 2023])
- It is one of the main components of the continuous improvement process
- It can be requested in response to an identified problem and may include 1 or more of the following: events that led to a significant or sentinel event, failure to achieve performance goals, variation in a process contributing to one of the above or identify prevention strategies for process improvement
- Facilitates identifying early trends, reducing variability in the process and promoting interdepartmental dialogue (Diagn Cytopathol 2021;49:633)
- See Advantages section for an example and Diagrams / tables for a fishbone diagram
Cytotechnologist workload
- Factoring in image assisted screening as opposed to full manual screening to conform to workload limits for cytotechnologists mandated by CLIA '88
- CAP checklist, requirement CYP.08450: each individual screening cytology slides by manual microscopic technique examines no more than 100 gynecologic slides per 24 hours
- When automated image assisted Pap screening methods (e.g., ThinPrep imaging system) are used by the laboratory, then the following will be applicable
- Cytotechnologist review of imaged slide (only looking at the field of view [FOV] marked by the imager) is counted as 0.5 slide
- Cytotechnologist review of imaged slide + full manual review (FMR) (upon detection of abnormality) is counted as 1.5 slides
- Manual cytotechnologist review of slide without prior imaging (full manual review) is counted as 1 slide
- Turnaround time standards must be established prudently factoring in laboratory's epithelial cell abnormality (ECA) rate, manual screening of Pap tests or use of automated image assisted Pap screening methods, while abiding by stringent quality standards mandated by CLIA '88 (ASC: ASC's Workload Recommendations for Automated Pap Test Screening [Accessed 7 September 2023])
Practice question #1
Mandatory internal quality assurance (QA) mechanisms in the cytology laboratory include which one of the following procedures?
- Correlational QA and review of follow up surgical pathology on all cases diagnosed as low grade dysplasia (LSIL) by cytology
- Gynecologic Pap proficiency testing (PT)
- Retrospective review of previously negative Pap slides within the past 5 years on an index patient whenever a new high grade squamous intraepithelial lesion (HSIL) or malignant cervical / vaginal cytology is reported
- Thorough rescreening of all (100%) negative Pap cases by a qualified cytotechnologist prior to sign out
Practice answer #1
C. Retrospective review of previously negative Pap slides received within the past 5 years whenever a new HSIL or malignant cervical / vaginal cytology is reported.
Answer D is incorrect because rescreening of a minimum of 10% negative Paps prior to sign out (prospective QA) is required. Thorough rescreening of all (100%) negative Paps prior to sign out is neither required nor practical.
Answer B is incorrect because gynecologic Pap proficiency testing is a mandatory external QA mechanism.
Answer A is incorrect because correlational QA and review of follow up surgical pathology on all cases diagnosed as high grade dysplasia (HSIL) or higher by cytology is required. Correlational QA and review of low grade dysplasia with follow up surgical pathology diagnosis is not required.
Comment Here
Reference: Quality assurance for cytopathology
Comment Here
Reference: Quality assurance for cytopathology
Practice question #2
What is the ASC/SIL ratio from the following data collected from the cytology laboratory in a tertiary hospital?
| AGC (%) | 0.2% |
| ASCUS (%) | 10.2% |
| ASC-H (%) | 0.4% |
| LSIL (%) | 4.4% |
| HSIL (%) | 0.4% |
| Malignant (%) | 0.1% |
- 1
- 2.1
- 2.3
- 25.5
Practice answer #2
B. 2.1. ASC/SIL ratio is a calculated ratio of percentage of ASCUS and ASC-H diagnosed cases divided by the percentage of LSIL, HSIL and malignant cases; so here in this scenario it will be: "10.2 + 0.4" divided by "4.4 + 0.4 + 0.1". Answer C is incorrect because ASC/SIL ratio is not the ratio of percentage of ASCUS and LSIL cases. Answer D is incorrect because ASC/SIL ratio is not the ratio of percentage of ASCUS and HSIL cases. Answer A is incorrect because ASC/SIL ratio is not the ratio of percentage of ASC-H and HSIL cases.
Comment Here
Reference: Quality assurance for cytopathology
Comment Here
Reference: Quality assurance for cytopathology