Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Diagrams / tables | Clinical features | Uses by pathologists | Prognostic factors | Molecular / cytogenetics description | Sample pathology report | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Koopman B, van Kempen LC. PI3K / PIK3CA. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/molecularPI3K.html. Accessed April 25th, 2024.
Definition / general
- Phosphatidylinositol 3 kinase catalytic subunit alpha (PIK3CA) is 1 of 4 catalytic subunits of phosphatidylinositol 3 kinase (PI3K) (OMIM: Phosphatidylinositol 3-Kinase, Catalytic, Alpha; PIK3CA [Accessed 15 September 2022])
- PIK3CA: proto-oncogene at 3q26.32; 1,068 amino acids with 9.3 kb mRNA (OMIM: Phosphatidylinositol 3-Kinase, Catalytic, Alpha; PIK3CA [Accessed 15 September 2022], NIH: Homo Sapiens Phosphatidylinositol-4,5-Bisphosphate 3-Kinase Catalytic Subunit Alpha (PIK3CA), mRNA [Accessed 15 September 2022])
Essential features
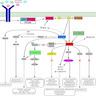
- PI3Ks promote cell growth, proliferation and survival through the PI3K / AKT / mTOR signaling pathway (Nat Rev Drug Discov 2014;13:140)
- PIK3CA, which encodes a catalytic subunit of PI3Ks, is one of the most recurrently mutated oncogenes in cancer (N Engl J Med 2018;379:2052)
- Alpelisib, in combination with fulvestrant, is an FDA approved PI3K inhibitor used to treat hormone receptor (HR)+ / HER2- breast cancers with activating PIK3CA mutations (Clin Cancer Res 2021;27:1842)
- Germline PIK3CA mutations are associated with PIK3CA related overgrowth syndrome (PROS), which encompasses clinical entities including macrodactyly; fibroadipose hyperplasia or overgrowth (FAO); hemihyperplasia multiple lipomatosis (HHML); congenital lipomatous overgrowth, vascular malformations, epidermal nevi, scoliosis / skeletal and spinal (CLOVES) syndrome; and megalencephaly (Am J Med Genet A 2015;167A:287, Genet Med 2017;19:989)
Terminology
- Phosphatidylinositol 3 kinase, catalytic, alpha (OMIM: Phosphatidylinositol 3-Kinase, Catalytic, Alpha; PIK3CA [Accessed 15 September 2022])
- Phosphatidylinositol 4,5 bisphosphate 3 kinase catalytic subunit alpha (HGNC: Symbol Report for PIK3CA [Accessed 15 September 2022])
- PI3Kα (GeneCards: PIK3CA Gene - Phosphatidylinositol-4,5-Bisphosphate 3-Kinase Catalytic Subunit Alpha [Accessed 15 September 2022])
- p110α (GeneCards: PIK3CA Gene - Phosphatidylinositol-4,5-Bisphosphate 3-Kinase Catalytic Subunit Alpha [Accessed 15 September 2022])
Pathophysiology
- PIK3CA is one of the most recurrently mutated oncogenes in cancer and activates the PI3K / AKT / mTOR signaling pathway (N Engl J Med 2018;379:2052)
- Biology:
- Phosphatidylinositol 3 kinases (PI3Ks) are grouped into 3 classes (I - III) based on the type and composition of the catalytic and regulatory subunits (OMIM: Phosphatidylinositol 3-Kinase, Catalytic, Alpha; PIK3CA [Accessed 15 September 2022])
- Main regulatory subunits of PI3Ks are encoded by PIK3R1 (p85α / p55α / p50α), PIK3R2 (p85β) and PIK3R3 (p55γ); catalytic subunits are encoded by PIK3CA (p110α), PIK3CB (p110β) and PIK3CD (p110δ) (Nat Rev Genet 2006;7:606)
- Class IA PI3Ks are activated by tyrosine kinase receptors
- Class IB PI3Ks are activated by G protein coupled receptors; activation results in a downstream signaling cascade towards generation of the highly versatile, membrane bound phospholipid signaling molecules phosphatidylinositol 4,5-bisphopshate (PIP2) and phosphatidylinositol 3,4,5 triphosphate (PIP3) that activate AKT (Nat Rev Drug Discov 2014;13:140)
- Regulatory subunits are encoded by PIK3R1 (p85α / p55α / p50α), PIK3R2 (p85β) and PIK3R3 (p55γ) and catalytic subunits are encoded by PIK3CA (p110α), PIK3CB (p110β) and PIK3CD (p110δ) (Nat Rev Genet 2006;7:606)
- Through the PI3K / AKT / mTOR signaling pathway, class I PI3Ks promote cell growth, proliferation and survival (Nat Rev Drug Discov 2014;13:140)
- Function and mechanism of action of class II and class III PI3Ks remain poorly understood (Clin Microbiol Rev 1988;1:377, Nat Commun 2019;10:1566)
- Activating mutations:
- PIK3CA is among the most recurrently mutated oncogenes in cancer (N Engl J Med 2018;379:2052)
- Oncogenic activation of PIK3CA is often the result of missense mutations in exon 9 (helical domain) or exon 20 (catalytic domain) (J Clin Oncol 2010;28:1075)
- Hotspot mutations E542K, E545K and H1047R account for > 50% of all oncogenic mutations in PIK3CA (AACR: AACR Project GENIE Data [Accessed 15 September 2022])
- Other PI3K activating mutations have been reported (Breast Cancer Res 2020;22:45)
- Mechanisms of activation vary by mutation and include disrupting the inhibitory interface with regulatory subunits (E542K / E545K) or enhancing the interaction with the cell membrane (H1047R) (Cell 2017;170:605)
Clinical features
- Missense PIK3CA mutations are frequently found in cancers of the endometrium (~46%), breast (~35%), cervix (~27%), bladder (~20%), colorectum (~20%), ovary (~10%) and lung (~6% in non-small cell subtype), among others (AACR: AACR Project GENIE Data [Accessed 15 September 2022])
- Alpelisib (Pigray), in combination with fulvestrant, is an FDA approved PI3K inhibitor used to treat HR+ / HER2- breast cancers with activating PIK3CA mutations (Clin Cancer Res 2021;27:1842)
- Presence of an activating PIK3CA mutation in a solid tumor other than breast cancer may render the patient eligible for targeted treatment in the context of a clinical trial with a PI3K inhibitor (NIH: ClinicalTrials.gov - PIK3CA [Accessed 4 October 2022])
- Other PI3K inhibitors (copanlisib, duvelisib, idelalisib, umbralisib) are FDA approved for other cancer types but usage does not depend on the presence of PI3K aberrations
- Somatic PIK3CA mutations (and, rarely, germline mutations) cause PIK3CA related overgrowth syndrome (PROS), which encompasses clinical entities including macrodactyly, FAO, HHML, CLOVES and megalencephaly (Am J Med Genet A 2015;167A:287, Genet Med 2017;19:989)
Uses by pathologists
- PIK3CA mutation analysis is used to identify HR+ / HER2- breast cancer patients who may benefit from combined alpelisib and fulvestrant treatment (Ann Oncol 2021;32:208)
- PIK3CA mutation analysis can support a diagnosis of PIK3CA related overgrowth syndrome (PROS) in patients with a suspicious clinical presentation (Am J Med Genet A 2015;167A:287)
- Mutations in PIK3CA can be detected with the FDA approved molecular tests
- QIAGEN Therascreen® PIK3CA RGQ PCR Kit (detects PIK3CA p.(C420R), p.(E542K), p.(E545A / D / G / K), p.(Q546E / R) and p.(H1047L / R / Y)),
- FoundationOne CDx or Liquid CDx (NGS panels that detect PIK3CA mutations)
- PIK3CA activating mutations can also be detected with other next generation sequencing based molecular diagnostic techniques that cover at least exons 4, 9 and 20
- There is no current role for PI3K immunohistochemistry as a validated proxy for PIK3CA mutation detection
Prognostic factors
- In early breast cancer, PIK3CA mutations are associated with improved outcome (Clin Cancer Res 2009;15:5049)
- In metastatic breast cancer treated with chemotherapy, PIK3CA mutations are associated with poor survival in HR+ / HER2- breast cancers but improved survival in triple negative breast cancers (Ann Oncol 2020;31:377)
- In other cancer types (including non-small cell lung cancer [NSCLC] and colorectal cancer), PIK3CA mutations often coexist with other driver mutations and adversely affect survival but not response rate in targeted therapy treatment (J Cancer Res Clin Oncol 2013;139:891, J Thorac Oncol 2015;10:1713)
Molecular / cytogenetics description
- HGNC ID: 8975
- Nucleotide resources:
- Confirmed PIK3CA driver mutations are single nucleotide missense mutations are concentrated in exons 4, 9 and 20 (Breast Cancer Res 2020;22:45)
Sample pathology report
- Clinical history: Patient with history of mammary carcinoma
- Microscopy: Liver needle biopsy of infiltrating lobular carcinoma demonstrating solid fields of cohesive tumor cells with round to round / oval partially polymorphic nuclei with focally prominent nucleoli and mitoses. Bland eosinophilic cytoplasm. Weak stromal response. Strong expression of CK AE1 / AE2, CK7 and GATA3. Negative immunostaining for CK20, E-cadherin, S100 and TTF1. Tumor is ER positive (100%) and PR positive (95%). HER2 score: 0.
- Conclusion: Liver needle biopsy reveals metastasis of infiltrating lobular carcinoma of the breast. ER positive (100%), PR positive (95%), HER2 negative.
- Reason for molecular analysis: PIK3CA mutation testing requested to aid with therapy management.
- Methodology: Extraction of DNA (Maxwell® CSC FFPE Plus DNA Kit) from formalin fixed paraffin embedded tissue containing at least 10% neoplastic cells. DNA was prepared for next generation sequencing using the TruSight Oncology 500 hybrid based capture assay (Illumina) of the complete coding region and splice sites of 523 genes, including PIK3CA. Sequencing was performed on a NovaSeq6000. Variant analysis is performed using the Illumina TruSight Oncology 500 local app. A variant allele frequency of > 5% can be detected when the tumor sample contains at least 10% neoplastic cells and when coverage of > 100 reads is obtained for at least 98% of the coding sequence. If a lower coverage is obtained, this will be indicated in the results. Interpretation of variants is performed according to the ACMG guideline (Genet Med 2015;17:405). Unless indicated otherwise, only pathogenic and likely pathogenic variants are reported. The laboratory is ISO-15189 certified (registration number ###).
- Mutation analysis result (example: no PIK3CA mutation detected):
- Neoplastic cells: 40%
- No pathogenic mutation detected in PIK3CA
- Clinical interpretation: Patients with breast cancer without an activating mutation in PIK3CA, in general, do not respond to targeted therapy with a PI3K inhibitor.
- Mutation analysis result (example: pathogenic PIK3CA mutation detected):
- Neoplastic cells: 40%
- Mutation in PIK3CA (NM_006218.3): c.1633G>A p.(Glu545Lys), 26% variant allele
- Clinical interpretation: Patients with breast cancer that harbors an activating mutation in PIK3CA may be eligible for targeted treatment with a PI3K inhibitor.
Board review style question #1
A 6 year old girl presents with an unusually large third toe of the left foot in the absence of other phenotypic abnormalities. Plain radiography reveals enlarged phalanges and metatarsals and an increase in soft tissue of the toe in question. A soft tissue biopsy shows an adipose tumor with a uniform structure, composed of lobularly divided adipose tissue consisting of nonatypical lipocytes, separated by irregularly situated, variably widened fibrotic septa but no cytonuclear atypia or lipoblasts. Molecular analysis using next generation sequencing shows a mutation in PIK3CA (NM_006218.3): c.1633G>A p.(E545K) with 16% variant allele frequency. What is your diagnosis?
- Acromegaly
- Maffucci syndrome
- Osteoid osteoma
- PIK3CA related overgrowth syndrome (PROS)
Board review style answer #1
D. PIK3CA related overgrowth syndrome (PROS). The clinical presentation of localized macrodactyly of the third toe of the left foot in the absence of other phenotypic abnormalities, in combination with a somatic, activating PIK3CA mutation, leads to a diagnosis of PROS.
Comment Here
Reference: PI3K / PIK3CA
Comment Here
Reference: PI3K / PIK3CA
Board review style question #2
Which of the following types of breast cancer are eligible for treatment with alpelisib plus fulvestrant?
- HER2 receptor negative (HER2-), BRCA1 mutation positive
- Hormone receptor negative (HR-), HER2 positive (HER2+), KRAS mutation positive
- Hormone receptor positive (HR+), HER2 negative (HER2-), PIK3CA mutation positive
- Triple negative, p53 overexpressed
Board review style answer #2
C. Hormone receptor positive (HR+), HER2 negative (HER2-), PIK3CA mutation positive. Alpelisib is a PI3K inhibitor with FDA approval for treating HR+ / HER2- breast cancers with a PIK3CA mutation, in combination with fulvestrant. HR- / HER+ positive breast cancers may be treated with HER2 targeted therapies (such as trastuzumab) and HER2 negative, BRCA1 mutation positive breast cancers may be treated with PARP inhibitors (such as olaparib).
Comment Here
Reference: PI3K / PIK3CA
Comment Here
Reference: PI3K / PIK3CA
Back to top