Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Prognostic factors | Case reports | Treatment | Clinical images | Microscopic (histologic) description | Positive stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Practice question #1 | Practice answer #1 | Practice question #2 | Practice answer #2Cite this page: Schukow CP, Sulejmani P, Ahmed A. Erythema migrans. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/skinnontumorerythemachronicummigrans.html. Accessed July 21st, 2025.
Definition / general
- Erythema migrans is the most common manifestation of Lyme disease
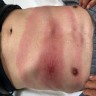
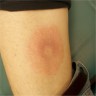
- Classically presents as erythematous, annular, homogeneous patches with central punctum
- Characteristic bullseye appearance with central clearing may be present in only around 9% of cases (Clin Dermatol 2006;24:509)
- Usually caused by spirochete bacteria Borrelia burgdoferi, which is most frequently transmitted by the tick Ixodes scapularis
- Reference: Infect Dis Clin North Am 2015;29:211
Essential features
- Primary erythema migrans is the first stage of Lyme disease (early localized disease)
- No serology is recommended if only primary erythema migrans is present given the low true seropositivity for early localized disease (< 40%)
- Histology of erythema migrans commonly shows nonspecific superficial and deep perivascular infiltrates with or without periadnexal involvement
- Prognosis is excellent with > 90% of patients returning to their previous health status when prompt antimicrobial treatment is initiated
- Reference: Nat Rev Dis Primers 2016;2:16090
Terminology
- Erythema chronicum migrans
ICD coding
Epidemiology
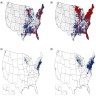
- An estimated 300,000 cases of Lyme disease occur annually in the U.S. (Infect Dis Clin North Am 2015;29:211)
- Up to 95% of cases occur in New England, the Middle Atlantic and North Central regions
- Incidence of erythema migrans is likely underestimated due to unnoticed skin lesions, a lack of systemic or local symptoms and unreported cases due to false negative serologic testing
- 2 peaks in age distribution
- 5 - 14 years old
- 45 - 54 years old
- Majority of cases occur in late spring and summer due to an increase in outdoor activity
Sites
- Erythema migrans lesions can occur on almost every part of the body, including the trunk, all extremities, groin, axilla and buttocks (Infect Dis Clin North Am 2015;29:211)
- Mucous membranes, palms and soles are exempt from developing erythema migrans lesions
- Some patients may present with multiple annular secondary lesions
- These contain viable spirochetes due to hematogenous or lymphatic spread from the original tick bite
- In children, the most common regions affected are the head and neck; in adults, typically the extremities or pelvis area (J Autoimmun 2015;57:82)
Pathophysiology
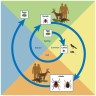
- Borrelia burgdoferi (spirochetes) replicate in the midgut of Ixodes scapularis (tick) and subsequently migrate to the salivary glands (Biophys J 2014;106:763)
- As the Ixodes tick feeds on human skin the B. burgdoferi spirochetes are transported into the dermis
- This process of spirochete migration from the tick's midgut to the dermis takes at least 36 - 48 hours of direct feeding / tick attachment (Infect Dis Clin North Am 2015;29:211)
- At the end of the blood meal, a small punctum on the skin at the site of spirochete inoculation becomes present
- In the dermis, spirochetes replicate rapidly locally and may travel into the bloodstream where they can spread diffusely
- Spirochetes remain bound to the extracellular matrix during replication and may adhere
- Innate and adaptive immune responses are activated and release proinflammatory cytokines which result in localized erythema and swelling
- Bullseye appearance is likely a result of spirochete replication / spreading, phagocytic cell activation / migration and deactivation of host phagocytes once the bacteria are cleared
- Other Ixodes species may less commonly transmit Lyme disease, including I. pacifcus and I. ricinus
- As the Ixodes tick feeds on human skin the B. burgdoferi spirochetes are transported into the dermis
Etiology
- Usually transmitted to humans by a bite from Ixodes ticks infected with B. burgdorferi (Biophys J 2014;106:763)
- Rarely may be due to other tick borne pathogens, such as Amblyomma americanum (Am J Med 2017;130:231)
Diagrams / tables
Clinical features
- 3 stages of Lyme disease are: (1) early localized, (2) early disseminated and (3) late disseminated (J Autoimmun 2015;57:82)
- Primary erythema migrans is the first stage (early localized disease) (J Cutan Pathol 2016;43:32)
- Presents in ~70 - 95% of patients within the first 3 weeks of inoculation
- Lesions have either a central clearance with ovoid expansion or a targetoid appearance
- Size of lesions can be highly variable, although > 5 cm is required according to the CDC for a diagnosis of primary erythema migrans (Infect Dis Clin North Am 2015;29:211)
- Local pruritus and burning are not uncommon
- Represents local cutaneous spirochete infection in the area of tick bite
- Secondary erythema migrans presents as multiple lesions with similar annular appearance / characteristics, although their morphology may vary (Indian J Dermatol 2013;58:167)
- Frequently affected sites include the face and extremities
- More likely to be accompanied by systemic symptoms, such as malaise, lymphadenopathy and fever
- Suggests disseminated spirochetes in the blood or lymph
Diagnosis
- Erythema migrans is typically a clinical diagnosis (e.g., characteristic skin lesion appearance, epidemiologic / exposure history) (Infect Dis Clin North Am 2015;29:211)
- As only ~25% of U.S. patients with erythema migrans recall a preceding tick bite, this history is not essential
- Serology may be negative in early disease, and patients with the charactersitic erythema migrans rash and suggestive history should be treated clinically
- In later stages of disease (e.g., cardiac manifestations), 2 tier confirmation with quantitative screening for serum antibodies to B. burgdorferi via enzyme of antibody immunoassays followed by Western blot in positive or equivocal specimens is recommended
- PCR positivity for Borrelia in skin samples may be positive in approximately 77% of cases (PLoS One 2015;10:e0136600)
- Culture positivity with isolation of B. burgdorferi is the gold standard for confirming the diagnosis via laboratory studies
- However, this is the least practical for diagnosis as it requires special media and takes a long time for growth
- Positive serology and quantitative polymerase chain reaction (PCR) analyses of blood and skin samples are also supportive
Laboratory
- IgM seropositivity for B. burgdorferi (or other infectious agents) (J Cutan Pathol 2016;43:32)
- IgG seronegativity for the same etiologic agent of erythema migrans is more likely in acute infections
- Seropositivity may be more likely in males than females and less likely in children / adolescents (PLoS One 2012;7:e41321)
- B. burgdorferi can be isolated from leading margins of erythema migrans lesions and cultured (PLoS One 2015;10:e0136600)
- Quantitative PCR analysis on tissue / culture is supportive
- Routine laboratory tests (e.g., complete blood counts, liver enzyme assays, sedimentation rate) are often not helpful (except in cases of coinfection with other infectious agents) (Infect Dis Clin North Am 2015;29:211)
Prognostic factors
- Prognosis is excellent with > 90% of patients returning to their previous health status when prompt antimicrobial treatment is initiated (Eur J Clin Microbiol Infect Dis 2019;38:201)
- Erythema migrans lesions often resolve spontaneously (usually within 4 weeks) even when no treatment is administered (Infect Dis Clin North Am 2015;29:211)
- Untreated patients may experience recurrent primary erythema migrans, secondary lesions or both
- Some patients may experience evanescent lesions
- Manifestations of later stages of Lyme disease may develop in patients that do not receive treatment
- These include joint symptoms, neurologic abnormalities and cardiac involvement
- 20% of untreated patients have no subsequent manifestation of Lyme disease after the resolution of erythema migrans lesions
- New lesions of erythema migrans occurring after successful antimicrobial treatment of a prior episode of Lyme disease are likely due to repeat tick bites (Infect Dis Clin North Am 2015;29:211)
- Human immune systems are not fully protected against reinfection
- Strain variability of B. burgdorferi is likely contributive
- Patients with later stages of Lyme disease are unlikely to develop reinfection due to a greater developed immune response
Case reports
- 10 year old boy with an erythematous annular plaque on his leg and lymphangitic spread (Indian Dermatol Online J 2017;8:124)
- 58 year old woman with an expanding central chest plaque and interstitial granulomas on histology (JAAD Case Rep 2020;6:1236)
- 70 year old man with severe left eye pain and a 15 cm left subaxillary bullseye patch (BMJ Case Rep 2020;13:e231889)
Treatment
- Oral amoxicillin, doxycycline and cefuroxime axetil are recommended initial therapies to treat erythema migrans (Infect Dis Clin North Am 2015;29:211)
- Amoxicillin and cefuroxime axetil are preferred for pediatric patients (< 8 years old) and pregnant patients
- Alternative therapies include oral azithromycin, clarithromycin and erythromycin (Infect Dis Clin North Am 2015;29:211)
- These drugs have lower efficacy and are reserved for patients who are intolerant of tetracyclines, penicillins and cephalosporins
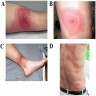
Clinical images
Microscopic (histologic) description
- Commonly nonspecific superficial and deep perivascular lymphohistiocytic infiltrate with or without periadnexal involvement (Am J Dermatopathol 2012;34:834)
- Central eosinophils and peripheral plasma cells are typically present
- Spongiosis, peripheral neutrophils, periadnexal without perivascular involvement and absence of plasma cells may all be seen in certain cases
- Accompanied focal interface dermatitis may be suggestive (Am J Dermatopathol 2020;42:745)
- Epidermal changes may occur centrally in conjunction with dermal changes typical of an arthropod bite reaction (lymphocytes, histiocytes, eosinophils, mast cells) while peripheral changes tend to be more lymphoplasmacytic without any epidermal changes
- Increased vascularity and even epidermal necrosis may be seen
- Warthin-Starry stain may identify organisms
- IHC and tissue PCR on formalin fixed, paraffin embedded (FFPE) tissue also described
Positive stains
- Warthin-Starry and IHC
Molecular / cytogenetics description
- No serology is recommended if only primary erythema migrans is present given the low true seropositivity for early localized disease (< 40%) (JAMA 2016;315:1767)
- If other symptoms are present or suspected later stages of Lyme disease, the CDC recommends 2 step serologic testing consisting of (step 1) enzyme immunoassay followed by (step 2) western blot analysis if IgM / IgG results for B. burgdorferi are positive or equivocal for step 1 (JAMA 2016;315:1780)
Sample pathology report
- Left upper extremity skin, biopsy:
- Gyrate erythema, most consistent with erythema migrans (see comment)
- Comment: Specimen shows superficial and deep mixed perivascular inflammatory infiltrates, including eosinophils, lymphocytes, histiocytes and plasma cells. Periadnexal involvement is detected as well as focal interface dermatitis. While the changes are nonspecific, the associated clinical history of annular erythematous rash with central punctum with a recent history of outdoor travel are most suggestive of the diagnosis above.
Differential diagnosis
- If an erythematous lesion develops in the setting of a suspected bite (Infect Dis Clin North Am 2015;29:211):
- Arthropod bite hypersensitivity reaction:
- Largest diameter is < 5 cm
- Can be associated with significant pruritus
- Lesions may fade spontaneously within 24 - 48 hours
- Lack of systemic symptoms
- Brown recluse spider bite:
- Focal necrosis and ulceration with or without the red, white and blue sign
- Preferentially occurs on extremities and spreads centrifugally
- Endemic to south central United States (such as southeastern Nebraska and southern Ohio)
- Arthropod bite hypersensitivity reaction:
- Other causes of erythematous lesions (Infect Dis Clin North Am 2015;29:211):
- Bacterial cellulitis:
- Homogenous band-like erythema that develops suddenly and is usually painful
- Patient often presents with leukocytosis, toxic appearing
- Distal lower extremities are preferentially affected
- History of recent trauma at the site of the lesion, underlying peripheral vascular disease or surgical history involving the venous or lymphatic systems
- Herpes simplex / zoster:
- Grouped vesicles on an erythematous base
- Oral, hand / finger, genital locations (Herpes simplex)
- Dermatomal distribution, prior history of chickenpox (Herpes zoster)
- Extremely painful
- Dermatophyte infection / tinea:
- Scaling, thin and irregularly raised borders with central clearing
- Resolves slowly (over a matter of weeks)
- Pruritic
- Typically not associated with systemic symptoms, targetoid appearance (Infect Dis Clin North Am 2015;29:211)
- Bacterial cellulitis:
- Figurate erythemas (J Dtsch Dermatol Ges 2021;19:963):
- Erythema annulare centrifugum (EAC):
- Classically, initial lesions present with urticaria-like papules that spread centrifugally with central clearing
- Persistent annular erythema that may resolve spontaneously
- Peak incidence mid adult life, M:F = 1:1
- Most common locations include trunk and proximal extremities
- 2 histologic variants: superficial and deep
- Superficial erythema annulare centrifugum tends to show spongiosis, parakeratosis and superficial perivascular lymphocytes and histocytes
- Lesions may demonstrate trailing scale and pruritus clinically
- Deep erythema annulare centrifugum does not show epidermal changes and has mid to deep dermal mononuclear infiltrates
- Lesions tend to be nonscaling and nonpruritic with cord-like borders
- Superficial erythema annulare centrifugum tends to show spongiosis, parakeratosis and superficial perivascular lymphocytes and histocytes
- Erythema gyratum repens (EGR):
- Mainly affects Caucasian males (M:F = 2:1) with average age of onset ~63 years old
- Clinically characterized by rapidly migrating (e.g., 1 centimeter / day), concentric, annular, erythematous bands with peripheral scale and pruritus
- May present with palmoplantar keratoderma, hypereosinophilia or ichthyosis
- Common locations include large areas of body (e.g., trunk, extremities), with acral and facial sparing
- Histology, although nonspecific, may show focal parakeratosis, spongiosis and superficial perivascular lymphocytes with eosinophils
- Clinical diagnosis and is strongly associated with underlying malignancy, such as lymphoma, lung, esophageal, breast, stomach and genitourinary cancer
- Erythema marginatum:
- Also known as erythema marginatum rheumaticum (EMR)
- Migratory annular and polycyclic erythematous lesions that exhibit fluctuance, fading and reappearing
- Cutaneous presentation of acute rheumatic fever (ARF), which is autoimmune disease secondary to group A beta hemolytic streptococcal pharyngitis
- Often has a 2 - 5 week latency period after initial pharyngitis infection
- Lesions favor proximal extremities and trunk, while sparing the face and acral surfaces
- Often copresents with other acute rheumatic fever signs, including carditis, polyarthritis, chorea and subcutaneous nodules
- Patients may also have fever, elevated inflammatory markers (e.g., C reactive protein) or prolonged PR interval on electrocardiogram
- Clinical diagnosis
- Presents more frequently in children than adults
- Erythema annulare centrifugum (EAC):
Additional references
Practice question #1
A 65 year old man presents with a rash. He states that he recently went on a weekend hiking trip and noticed a rash on his abdomen that has been slowly enlarging over the past 5 days. He endorses mild pruritus but denies any other associated symptoms. On physical exam, an erythematous, annular patch with central punctum and targetoid appearance is present on the patient’s abdomen. What is the most likely etiology of this patient’s condition?
- Amblyomma americanum
- Babesia canis
- Borrelia burgdorferi
- Ehrlichia chaffeesis
- Mycoplasma pneumoniae
Practice answer #1
C. Borrelia burgdorferi is a spirochete bacteria and is the most common pathogen responsible for Lyme disease. Early localized Lyme disease presents as erythema migrans, which are enlarging, bullseye, annular, erythematous lesions in sites of Ixodes scapularis (tick) bites. Clinical history is essential and typically includes a prior history of outdoor activity, namely in endemic regions such as the Northeast United States. A central punctum may be seen in the center of the lesion and ~25% of patients are unable to specify prior history of a known tick bite / blood feed.
Comment Here
Reference: Erythema migrans
Comment Here
Reference: Erythema migrans
Practice question #2
For how long must direct Ixodes scapularis blood feeding take place for spirochete inoculation in primary erythema migrans?
- 2 - 4 hours
- 6 - 12 hours
- 14 - 20 hours
- 24 - 32 hours
- 36 - 48 hours
Practice answer #2
E. 36 - 48 hours. The process of spirochete migration from the tick’s midgut to the dermis in erythema migrans takes at least 36 - 48 hours of direct feeding and tick attachment.
Comment Here
Reference: Erythema migrans
Comment Here
Reference: Erythema migrans