Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Diagrams / tables | Clinical features | Interpretation | Uses by pathologists | Tissue handling | Prognostic factors | Microscopic (histologic) description | Microscopic (histologic) images | Positive staining - normal | Positive staining - disease | Negative staining | Sample pathology report | Practice question #1 | Practice answer #1Cite this page: Mason AD, Mistry A, Shiller M. Folate receptor alpha (FRα). PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/stainsfra.html. Accessed September 23rd, 2025.
Definition / general
- Folate is an essential vitamin required for one carbon metabolism, a metabolic pathway that supports the synthesis of DNA, RNA and enzyme cofactors
- Folate receptor alpha (FRα) is a glycosyl phosphatidylinositol (GPI) anchored cell surface protein, encoded by the FOLR1 gene and required for uptake of folate into cells
Essential features
- VENTANA FOLR1 (FOLR1-2.1) RxDx Assay is the FDA approved companion diagnostic to identify FRα positive (≥ 75% of viable tumor cells with moderate [2+] or strong [3+] membrane staining) patients who may be eligible for mirvetuximab soravtansine (Elahere) in platinum resistant epithelial ovarian, fallopian tube and primary peritoneal cancer
- Scoring of FRα expression using the FOLR1 (FOLR1-2.1) antibody in epithelial ovarian cancer is evaluated by membrane (partial and complete) expression only
- Increased folate requirement due to rapid cell division in some epithelial tumors is believed to drive FRα overexpression (Nat Rev Clin Oncol 2020;17:349)
- Epithelial ovarian cancer (EOC) is most often represented by high grade serous ovarian cancer (HGSOC), though other histologic subtypes such as clear cell, endometrioid and low and high grade mucinous do occur
- The most common presentation for epithelial ovarian cancer is advanced stage; the disease process is characterized by multiple recurrences with decreasing disease free intervals with subsequent recurrences
- As such, therapies to improve disease free intervals are needed to improve survival in patients with epithelial ovarian cancer
Terminology
- Alternative names: FR1, folate receptor alpha (FRα), FOLR1
Pathophysiology
- FRα is a glycosyl phosphatidylinositol (GPI) anchored cell surface protein that binds and internalizes folate via endocytosis, thereby providing folate for metabolism
- Folate is a water soluble vitamin essential for one carbon metabolism, the metabolic pathway required for DNA and RNA synthesis that in turn supports cell growth and proliferation
- Minimal FRα expression is present in most normal adult tissues, as they utilize an alternative folate transporter for folate uptake
- Tumors shown to overexpress FRα are often of epithelial origin: ovarian, breast, lung, uterus, kidney and brain
- Reference: Nat Rev Clin Oncol 2020;17:349
Clinical features
- VENTANA FOLR1 (FOLR1-2.1) RxDx Assay is the FDA approved companion diagnostic to identify FRα positive (≥ 75% of viable tumor cells with moderate [2+] or strong [3+] membrane staining) patients who may be eligible for mirvetuximab soravtansine in platinum resistant epithelial ovarian, fallopian tube and primary peritoneal cancer
- Accelerated FDA approval of mirvetuximab soravtansine for FRα positive (≥ 75% of viable tumor cells with moderate [2+] or strong [3+] membrane staining) patients with platinum resistant epithelial ovarian, primary peritoneal and fallopian tube cancer was based on the results of the SORAYA trial; full approval was based on results from the MIRASOL trial (J Clin Oncol 2023;41:2436, N Engl J Med 2023;389:2162)
- FRα testing has been incorporated into national ovarian cancer guidelines (NCCN: NCCN Guidelines - Ovarian Cancer / Fallopian Tube Cancer / Primary Peritoneal Cancer [Accessed 27 May 2025])
- Mirvetuximab soravtansine has also been investigated in combination with bevacizumab with differing FRα expression cutoffs (Gynecol Oncol 2023;170:241)
Interpretation
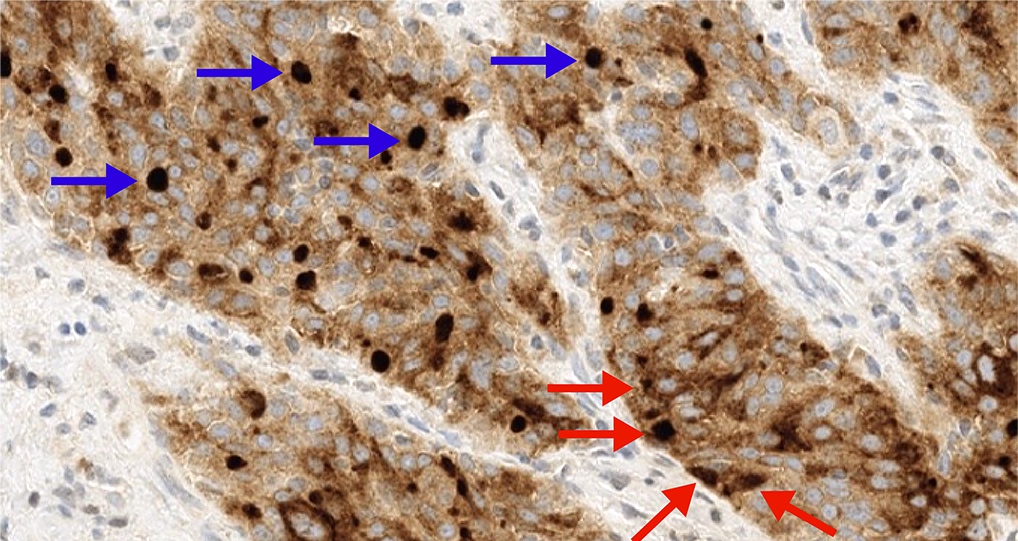
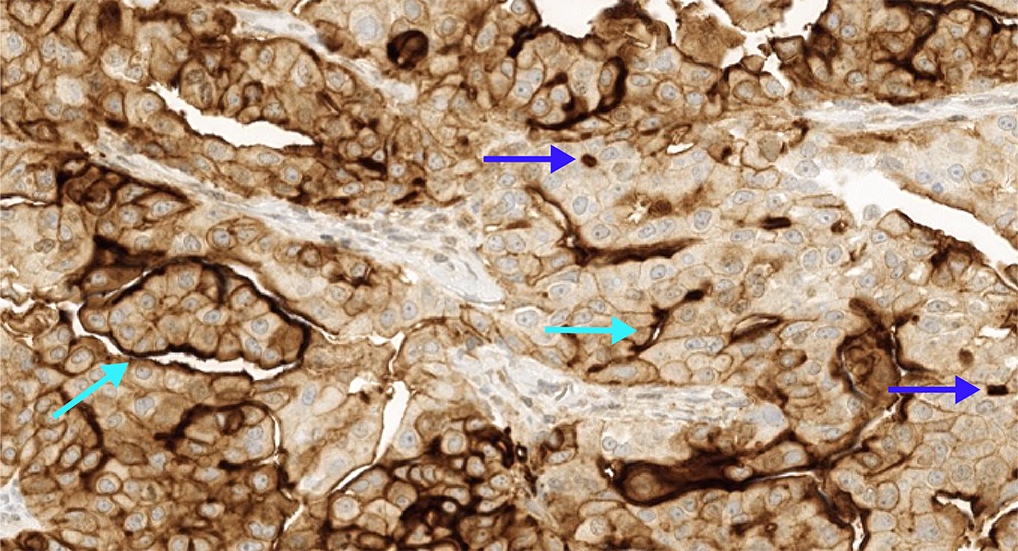
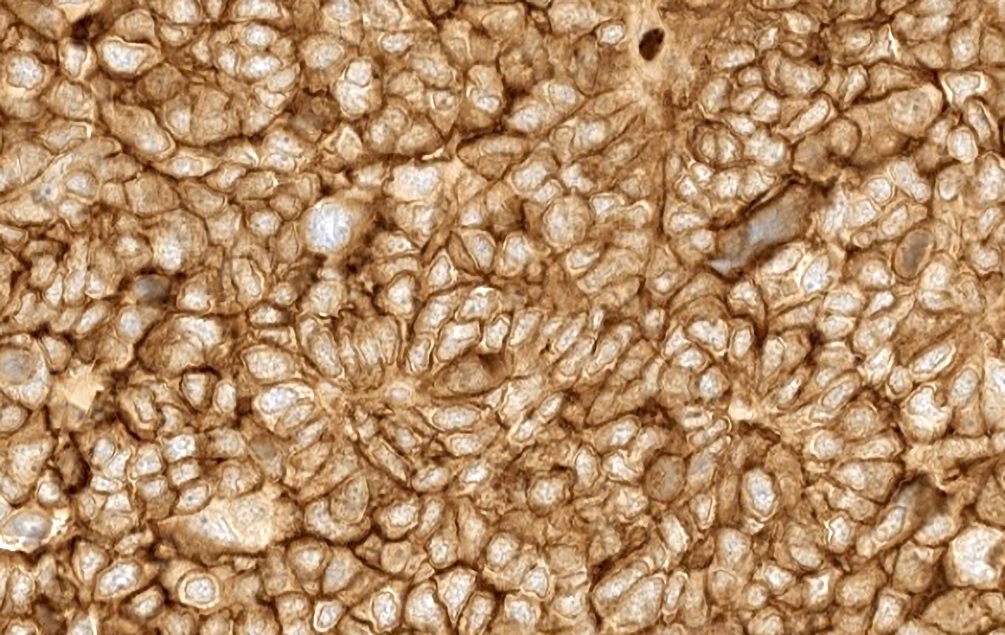
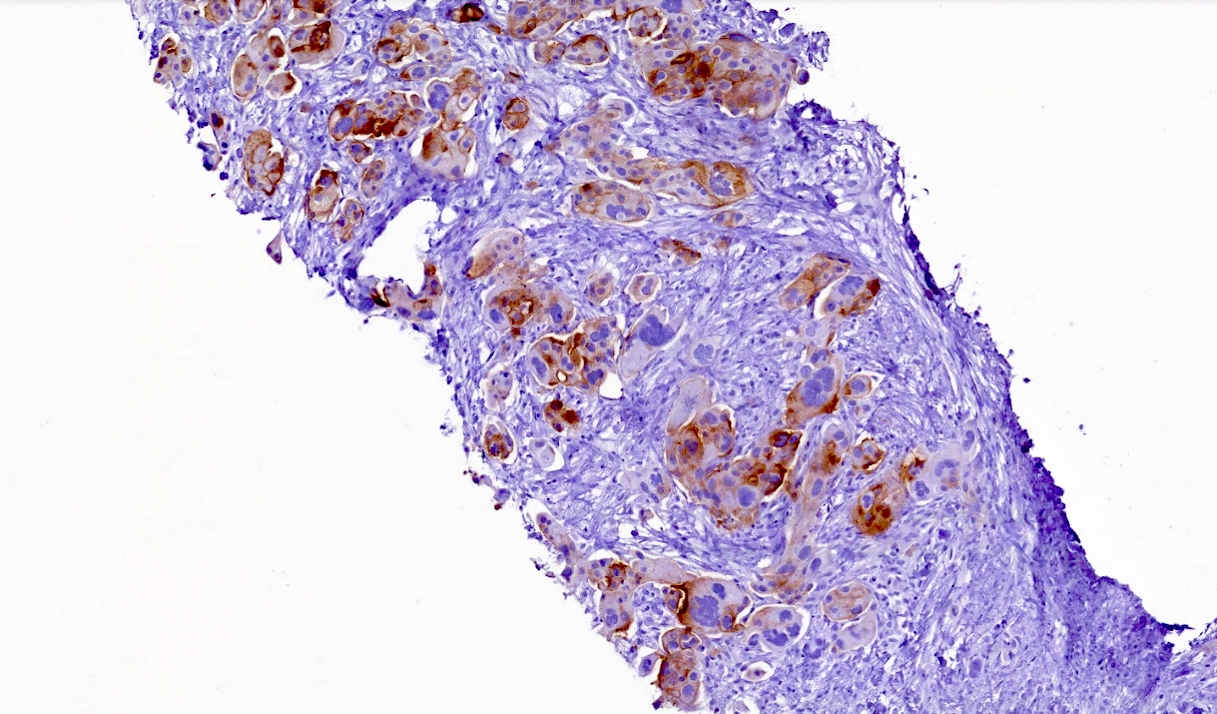
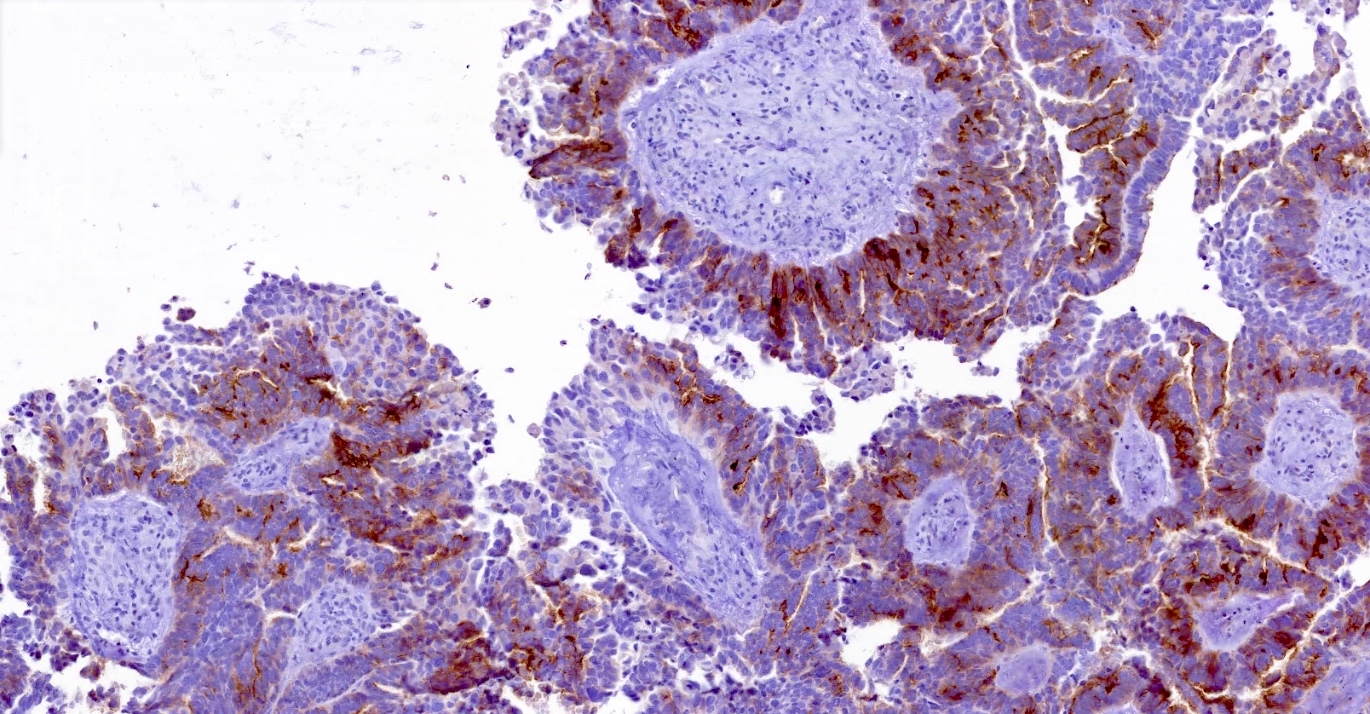
- FRα expression can be membranous and cytoplasmic, though only membrane staining patterns are evaluated
- Positive membrane staining patterns include circumferential (partial and complete), apical and dot-like expression
- Dot-like staining appears as dark brown secretions filling the gland lumen and should be differentiated from cellular cytoplasmic expression, which is not included
- Positivity for FOLR1 is ≥ 75% of viable tumor cells with moderate (2+) or strong (3+) membrane staining for mirvetuximab soravtansine eligibility
- Do not include cytoplasmic or nuclear expression (Roche Diagnostics: VENTANA® FOLR1 (FOLR1-2.1) RxDx Assay [Accessed 27 May 2025])
Uses by pathologists
- VENTANA FOLR1 (FOLR1-2.1) RxDx Assay is a qualitative immunohistochemical assay using the mouse monoclonal anti-FOLR1 clone FOLR1-2.1 intended for use in the assessment of FRα in formalin fixed, paraffin embedded (FFPE) epithelial ovarian cancer, including primary peritoneal cancer and primary fallopian tube cancer (EOC) tissue specimens by light microscopy
- OptiView DAB IHC Detection Kit is used for staining on a BenchMark ULTRA instrument (Roche Diagnostics: VENTANA® FOLR1 (FOLR1-2.1) RxDx Assay [Accessed 27 May 2025])
- FRα expression in epithelial ovarian cancer is independent of the presence or absence of other oncogenic drivers (such as TP53, BRCA1/2, PALB2 and other genes in the homologous recombination repair pathway) and mutations in the Lynch syndrome family of genes
Tissue handling
- VENTANA FOLR1 (FOLR1-2.1) RxDx Assay is a qualitative immunohistochemical assay using the mouse monoclonal anti-FOLR1 (clone FOLR1-2.1)
- Intended for use in the assessment of FRα protein in tumor cells in the FFPE epithelial ovarian, fallopian tube or primary peritoneal cancer tissues
- Assay includes antibody detection using the OptiView DAB IHC Detection Kit performed on a VENTANA BenchMark ULTRA instrument
- Fixation recommendation: tissue fixed in 10% neutral buffered formalin between 6 and 72 hours
- Alcohol formalin acetic acid (AFA), 95% alcohol, Prefer, zinc formalin and Z-5 fixatives are not recommended due to loss or variability of specific expression
- Recommended control tissue: benign fallopian tube
- Evaluation requires a minimum of ~100 viable tumor cells
- Not validated for cytology specimens, including pleural and peritoneal fluids
- Not validated for decalcified specimens
- Avoid performing on stored precut unstained slides that are older than 2 months
- Testing must be performed according to standardized analytically validated protocols (Roche Diagnostics: VENTANA® FOLR1 (FOLR1-2.1) RxDx Assay [Accessed 27 May 2025])
Prognostic factors
- In a study of 91 ovarian cancer patients, high levels of FRα expression was associated with chemotherapy resistance, decreased overall survival and decreased disease free intervals
- Multiple studies support expression in high grade disease, specifically high grade serous ovarian cancer, which has aggressive overall behavior (Mol Oncol 2012;6:360, Gynecol Oncol 2008;108:619, Oncologist 2019;24:425)
Microscopic (histologic) description
- FOLR1 staining can be membranous and cytoplasmic, though only membrane staining patterns are evaluated
- Positive membrane staining patterns include circumferential (partial and complete), apical and dot-like expression
- Dot-like staining appears as dark brown secretions filling the gland lumen and should be differentiated from cellular cytoplasmic expression, which is not included
- Do not include cytoplasmic or nuclear expression
- Reference: Roche Diagnostics: VENTANA FOLR1 (FOLR1-2.1) RxDx Assay Interpretation Guide for Epithelial Ovarian, Fallopian Tube, or Primary Peritoneal Cancer [Accessed 27 May 2025]
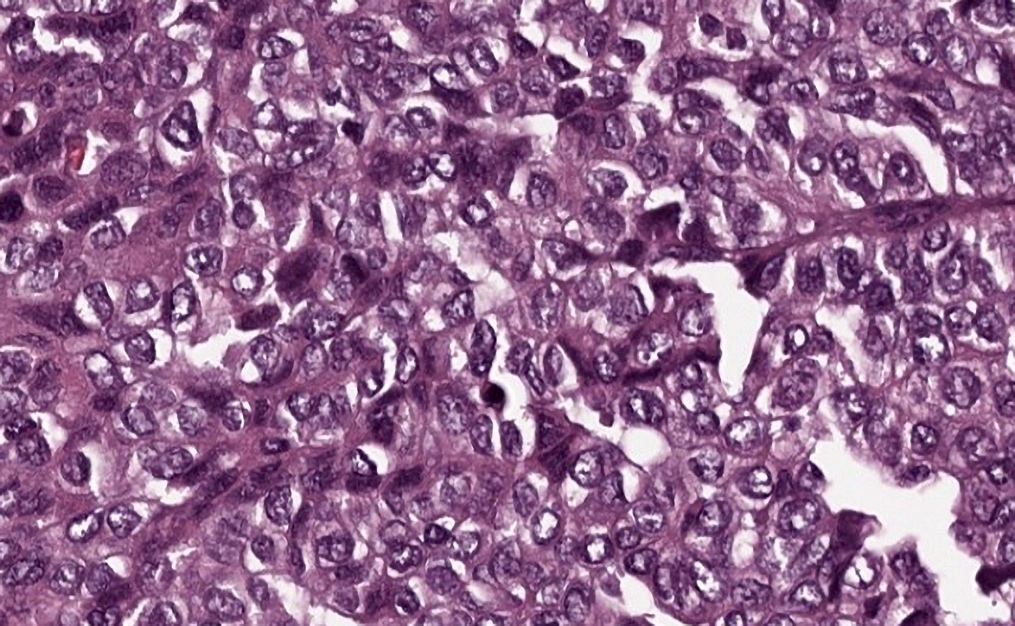
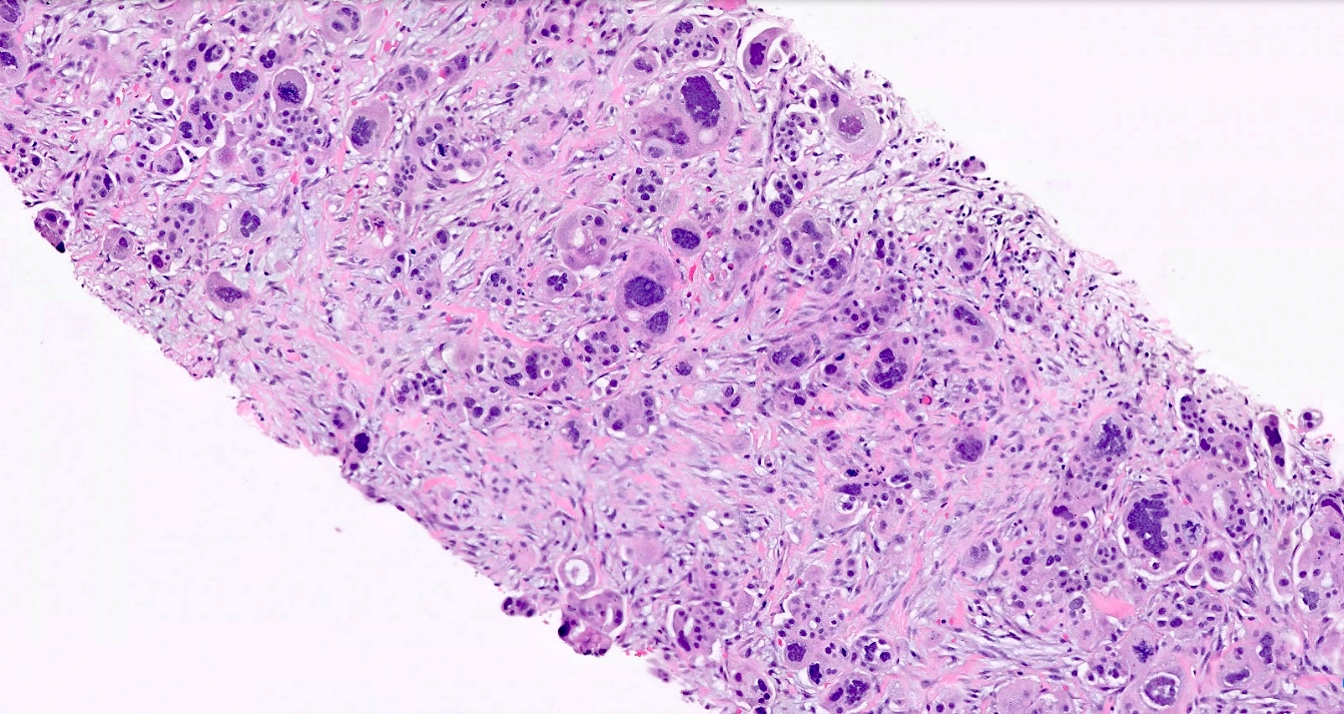
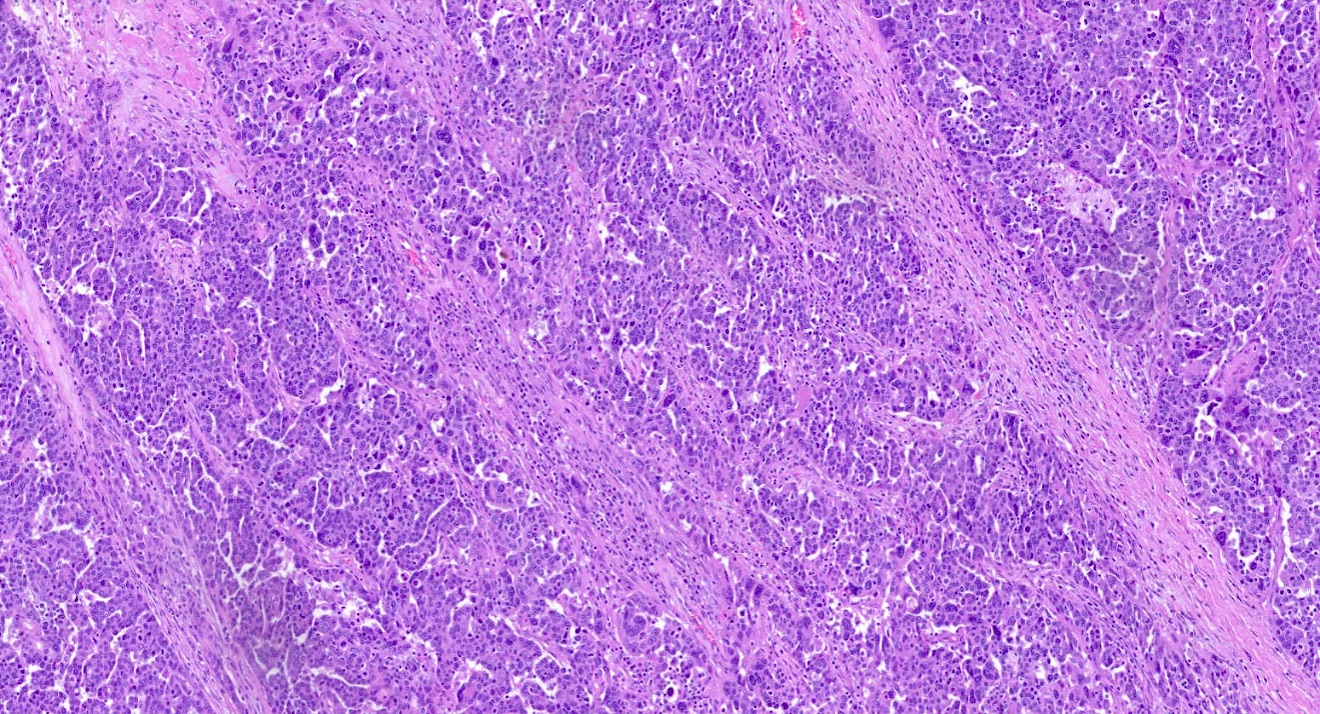
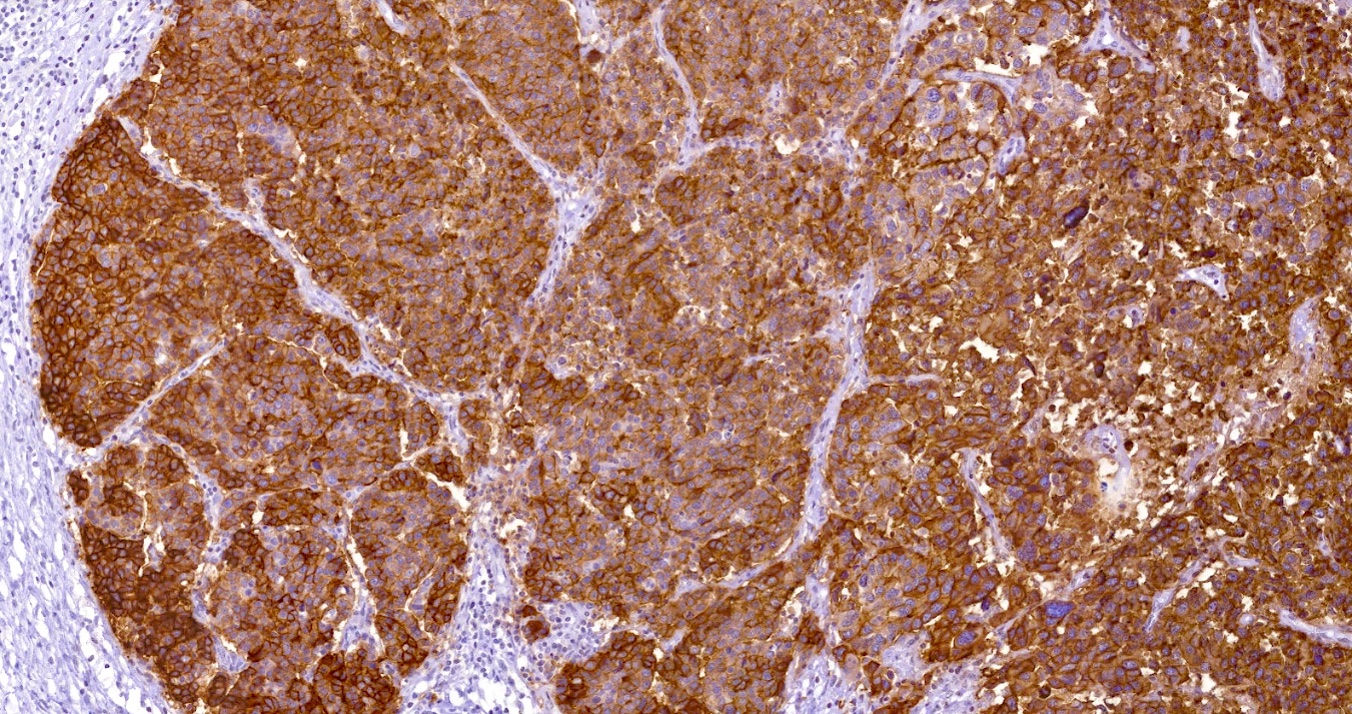
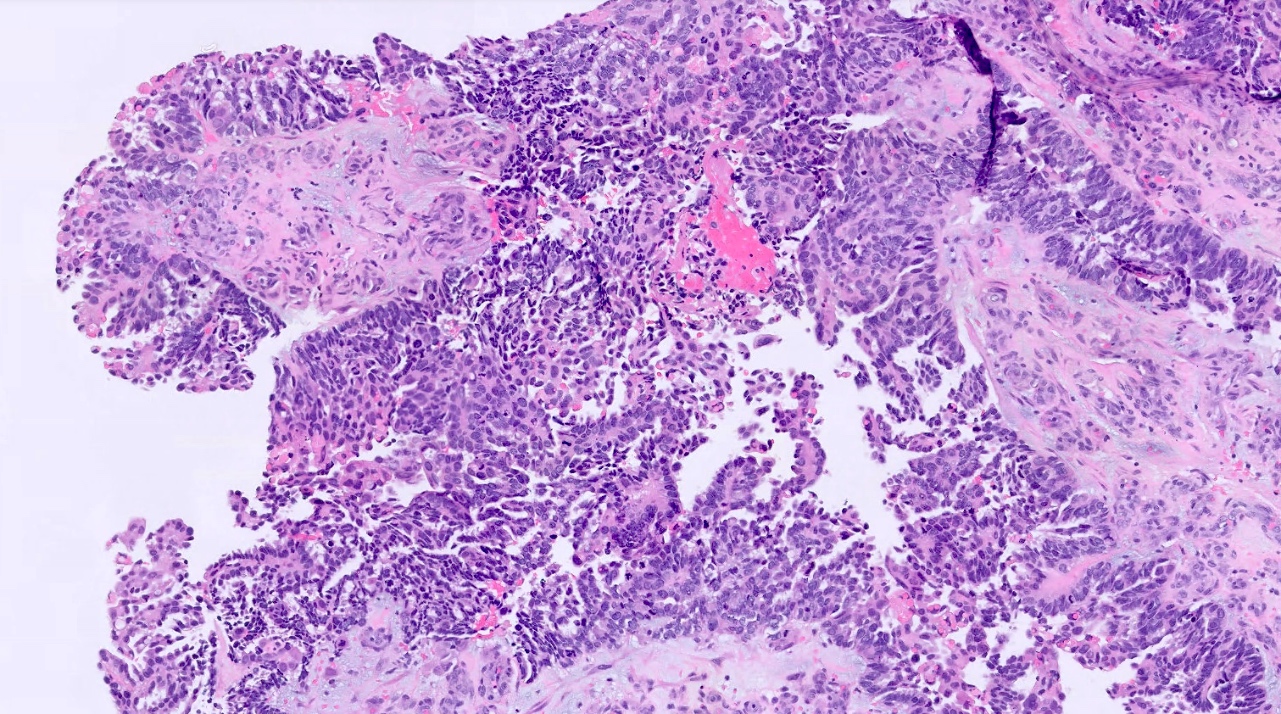
Microscopic (histologic) images
Positive staining - normal
- Fallopian tube
- Kidney (4/4)
- Larynx (1/3)
- Adrenal gland (1/4)
- Reference: Roche Diagnostics: VENTANA® FOLR1 (FOLR1-2.1) RxDx Assay [Accessed 27 May 2025]
Positive staining - disease
- Clear cell carcinoma (kidney) (1/2)
- Endometrioid adenocarcinoma (ovary) (9/16)
- Serous adenocarcinoma (ovary) (39/42)
- Clear cell carcinoma (ovary) (5/8)
- Reference: Roche Diagnostics: VENTANA® FOLR1 (FOLR1-2.1) RxDx Assay [Accessed 27 May 2025]
Negative staining
- See Diagrams / tables regarding sensitivity of VENTANA FOLR1 (FOLR1-2.1) RxDx Assay determined by testing FFPE neoplastic tissues
Sample pathology report
- Right ovary, fallopian tube, right salpingo-oophorectomy:
- High grade serous ovarian carcinoma (see comment)
- Interpretation of folate receptor alpha / FOLR1 (VENTANA FOLR1-2.1): 85% (positive: ≥ 75% expression with 2+ or 3+ membrane staining)
- Comment: FOLR1 was assessed by manual qualitative immunohistochemistry on FFPE ovarian and fallopian tube tissue using the FDA approved clone FOLR1-2.1 (Ventana Medical Systems), OptiView DAB IHC Detection Kit on a VENTANA BenchMark ULTRA instrument using the VENTANA FOLR1 (FOLR1-2.1) RxDx Assay approved protocol. Results are reported as the percentage of viable tumor cells with moderate (2+) or strong (3+) membrane staining intensity. In this case, 85% of tumor cells demonstrated positive membranous staining (≥ 75% with 2+ or 3+ intensity), consistent with a positive result. This assay has not been validated for decalcified specimens or cytology specimens. All controls showed appropriate reactivity. Testing was performed in a CLIA certified laboratory for high complexity testing.
Practice question #1
Which of the following statements regarding FOLR1 (FOLR1-2.1) is correct?
- Ascitic fluid is a valid specimen type for FOLR1 testing in ovarian and related cancers
- Both membrane and cytoplasmic staining are evaluated for FOLR1 scoring
- FDA approval of mirvetuximab soravtansine was based on FRα positivity in platinum resistant ovarian and related cancers
- VENTANA FOLR1 (FOLR1-2.1) RxDx Assay is approved for use in sarcomas
Practice answer #1
C. FDA approval of mirvetuximab soravtansine was based on FRα positivity in platinum resistant ovarian and related cancers. Mirvetuximab soravtansine received accelerated FDA approval for the treatment of FRα positive, platinum resistant epithelial ovarian, fallopian tube and primary peritoneal cancers based on the SORAYA trial. Answer A is incorrect because cytology specimens are not validated for use with the VENTANA FOLR1 RxDx Assay; only resections and biopsies are valid specimen types. Answer B is incorrect because only membrane patterns of staining (partial or complete circumferential, apical and luminal dot-like patterns) are included in FOLR1 scoring, while any cytoplasmic staining is disregarded. Answer D is incorrect because the VENTANA FOLR1 RxDx Assay is approved for use in epithelial ovarian cancer (epithelial ovarian, fallopian tube and primary peritoneal cancer) and not sarcomas.
Comment Here
Reference: Folate receptor alpha (FRα)
Comment Here
Reference: Folate receptor alpha (FRα)