Table of Contents
Definition / general | Essential features | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Case reports | Microscopic (histologic) description | Microscopic (histologic) images | Sample pathology report | Differential diagnosis | Additional references | Practice question #1 | Practice answer #1 | Practice question #2 | Practice answer #2Cite this page: Thiryayi SA, Turashvili G. Exogenous hormones. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/uterusexogenoushor.html. Accessed October 6th, 2025.
Definition / general
- Exogenous hormones taken for various reasons may have an effect on the morphology of endometrium, endometrial stroma and myometrial lesions, such as leiomyomas
Essential features
- Exogenous hormones taken for various indications may affect women of any age
- Morphologic changes are secondary to the effect of exogenous hormones on estrogen or progesterone receptors in the endomyometrium
- Subsequent estrogenic or progestogenic effects are variable, ranging from benign (decidual, secretory, inactive or mixed patterns, polyps, stromal changes) to preneoplastic (endometrial hyperplasia) or neoplastic (endometrial carcinoma) changes
- Appearance of myometrial lesions, such as leiomyomas, may also be affected
- Clinical history of recent use of exogenous hormones is critical for accurate morphologic assessment, including endometrial dating
Epidemiology
- Females of any age
Sites
- Endometrium, myometrium
Pathophysiology
- Morphologic changes arise secondary to the effect of exogenous hormones on estrogen or progesterone receptors in the endomyometrium (Expert Rev Clin Pharmacol 2020;13:1103, Menopause 2018;25:1033, Contraception 2015;91:360)
- Contraceptives:
- Progestogen only: oral, subdermal implant, intramuscular injection or intrauterine device (IUD) containing a progestin (e.g., levonorgestrel or medroxyprogesterone acetate)
- Results in thickening of cervical mucus, thinning of endometrium (low and high dose) or inhibition of follicular development and ovulation (high dose)
- Combined: estrogen (ethinyl estradiol) and progestogen (progestin)
- Suppresses release of gonadotropins, thereby inhibiting follicular development and preventing ovulation
- Progestogen only: oral, subdermal implant, intramuscular injection or intrauterine device (IUD) containing a progestin (e.g., levonorgestrel or medroxyprogesterone acetate)
- Hormone replacement therapy (HRT):
- Estrogen only: leads to endometrial proliferation with increased risk for endometrial hyperplasia and carcinoma
- Combined: progestogens have a protective effect on endometrium by downregulating estrogen receptors and thus limiting response to estrogens
- Androgens:
- Danazol: weak androgen and weak progestin effects due to main metabolite, ethisterone
- Gestrinone: combined androgenic, antiestrogenic and antiprogestogenic effects
- Tibolone: estrogenic and progestogenic activity with weak androgenic effect
- Testosterone: androgenic effect on endometrium
- Selective estrogen receptor modulators (SERM): competitive inhibitors of estrogen binding to estrogen receptors
- Tamoxifen (first generation): estrogen antagonist (breast) or agonist (endometrium), effect depends on estradiol concentration, dose and duration of use as well as menopausal status
- Clomiphene (first generation): estrogenic or antiestrogenic activity, competitively binding to hypothalamic estrogen receptors and causing an increase in follicle stimulating hormone (FSH) and luteinizing hormone (LH), which induces ovulation
- Raloxifene (second generation): estrogen antagonist in uterus
- Bazedoxifene (third generation): estrogen antagonist in uterus
- Selective progesterone receptor modulators (SPRM): bind to progesterone receptors with agonist, antagonist or mixed agonist / antagonist activity
- Gonadotropin releasing hormone (GnRH) agonists: reduce gonadotropin levels which suppresses ovulation and secretion of ovarian hormones causing hypoestrogenism
Etiology
- Exogenous hormones, including estrogens, progestogens and androgens
- Hormone-like medications:
- Selective estrogen receptor modulators: tamoxifen, clomiphene, raloxifene, bazedoxifene (when combined with conjugated estrogen termed tissue selective estrogen complex)
- Selective progesterone receptor modulators: ulipristal acetate, mifepristone, asoprisnil
- Gonadotropin releasing hormone agonists: buserelin, goserelin, leuprolide (Lupron), nafarelin
Clinical features
- Indications for estrogen or progestogen therapy include contraception, irregular vaginal bleeding, infertility, polycystic ovarian syndrome, hirsutism, postmenopausal symptoms, adjuvant treatment for breast and endometrial carcinoma, gender dysphoria and congenital abnormal sexual development
- Androgens:
- Danazol: treatment of endometriosis, menorrhagia, symptomatic leiomyomas
- Gestrinone: treatment of endometriosis
- Tibolone: menopausal symptoms, endometriosis, prevention of osteoporosis
- Testosterone: gender affirming hormone therapy for gender transition
- Selective estrogen receptor modulators:
- Tamoxifen: treatment of hormone receptor positive breast cancer in premenopausal women, breast cancer prevention in patients with ductal carcinoma in situ
- Clomiphene: ovulation induction
- Raloxifen: treatment and prevention of postmenopausal osteoporosis
- Bazedoxifene: treatment of osteoporosis
- Selective progesterone receptor modulators:
- Ulipristal acetate: used for management of endometriosis and preoperative treatment of uterine leiomyomas (recommendation in November 2020 by European Medicines Agency to restrict use due to rare risk of serious liver injury) (European Medicines Agency: Ulipristal acetate 5mg medicinal products [Accessed 9 December 2021])
- Mifepristone: mainly for medical termination of early pregnancy; also for the treatment of leiomyomas and pain secondary to endometriosis
- Gonadotropin releasing hormone agonists: used for ovulation induction, treatment of endometriosis, pre-endometrial ablation and preoperative treatment of large leiomyomas
Diagnosis
- Histologic examination of biopsy or resection specimens
Case reports
- 32 year old woman with uterine fibroids treated with ulipristal acetate prior to in vitro fertilization (Eur Rev Med Pharmacol Sci 2016;20:202)
- Woman in her late 30s treated with ulipristal acetate for uterine fibroids, diagnosed with paratubal endometriosis showing a morphology similar to progesterone receptor modulator associated endometrial changes (Pathol Res Pract 2017;213:79)
- 44 year old woman with posterior reversible encephalopathy syndrome after leuprolide injection for uterine leiomyoma (Obstet Gynecol Sci 2019;62:69)
- 53 year old woman on tamoxifen with atypical endometrial stromal cells in an endometrial polyp and osteoclastic-like giant cells in leiomyoma (Acta Biomed 2019;90:572)
Microscopic (histologic) description
- Contraceptives:
- Progestogen only: 3 morphologic patterns of response, dependent on dose and duration of progestin as well as endogenous estrogen levels, which may be coexistent and overlapping (Expert Rev Clin Pharmacol 2020;13:1103)
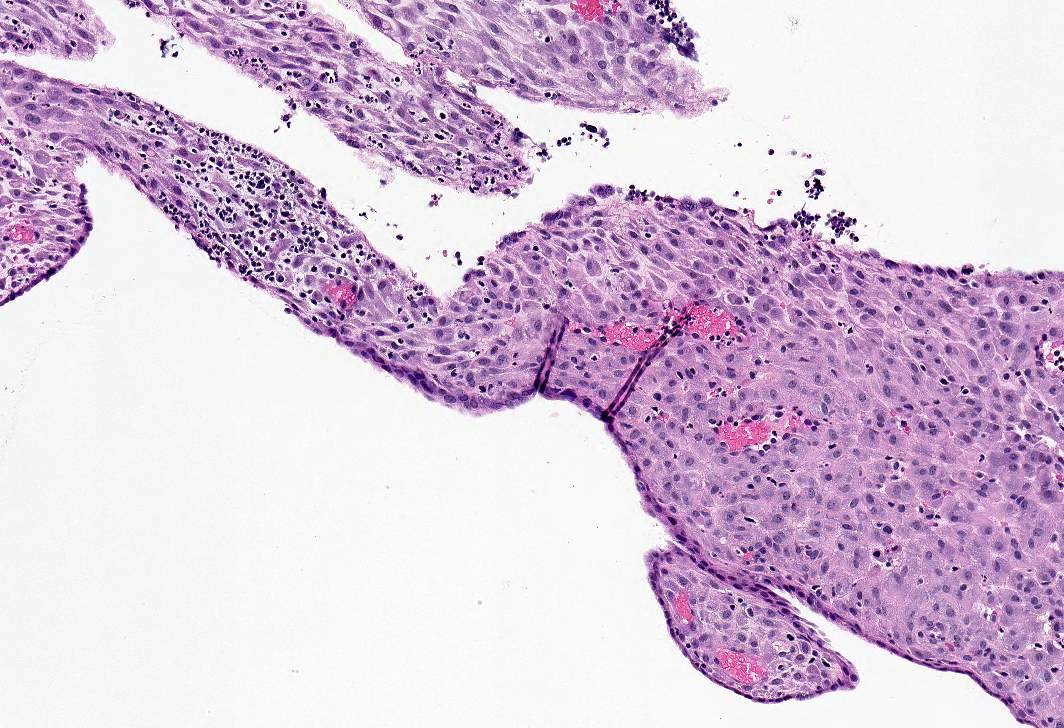
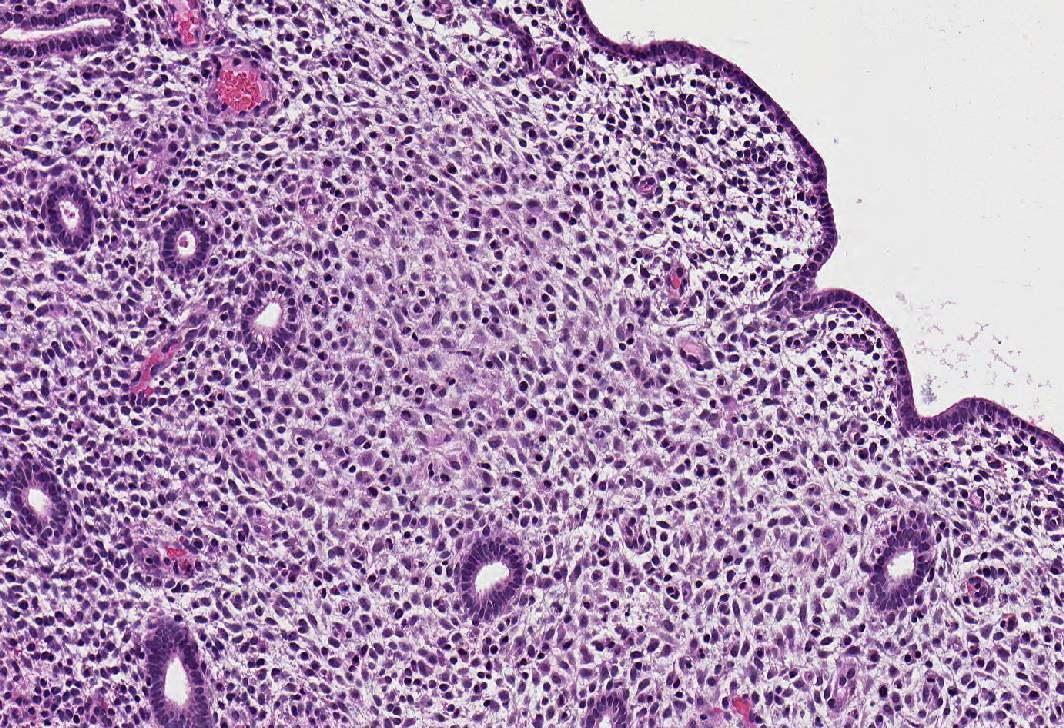
- Decidual (pregnancy-like) pattern:
- Abundant tissue, frequently polypoid
- Mostly inactive glands, rarely with marked secretory features (Arias-Stella-like)
- Decidualized stroma (large cells, abundant cytoplasm, prominent cell borders) with occasional stromal mitoses and lymphocytes
- Ectatic venules with or without markedly thickened spiral arterioles
- In advanced cases, thrombosis of venules, breakdown and bleeding
- Stromal lymphocyte and mononuclear cell infiltrate, especially with levonorgestrel IUD; may mimic chronic endometritis but no plasma cells or gland infiltration
- IUD also associated with stromal macrophages, reactive surface papillary epithelial pattern, less often stromal edema, hemosiderin, calcification and necrosis
- Neutrophils may be associated with tissue necrosis in areas of breakdown
- Rarely, decidual cells with vacuolated cytoplasm and eccentric nuclei resulting in signet ring cell appearance
- Rare pseudosarcomatous stromal features with nuclear pleomorphism, hyperchromasia and prominent nucleoli
- Secretory (luteal phase-like) pattern:
- Scant to moderate tissue
- Mildly tortuous secretory glands lined by columnar cells with basal nuclei and scant dense eosinophilic intraluminal secretions
- Predecidual stromal cells with pale cytoplasm, ovoid nuclei and scattered mitoses
- Ectatic vessels with or without thrombosis
- Appearance of glands and stroma inconsistent with days of normal menstrual cycle
- Inactive pattern:
- Scant tissue
- Widely spaced, small, inactive tubular glands lined by low columnar cells with scant cytoplasm and scant to absent luminal secretions
- Plump to spindled stromal cells with scant to moderate cytoplasm and inconspicuous mitotic activity
- More abundant stroma compared with physiologic postmenopausal atrophy
- Ectatic vessels
- Decidual (pregnancy-like) pattern:
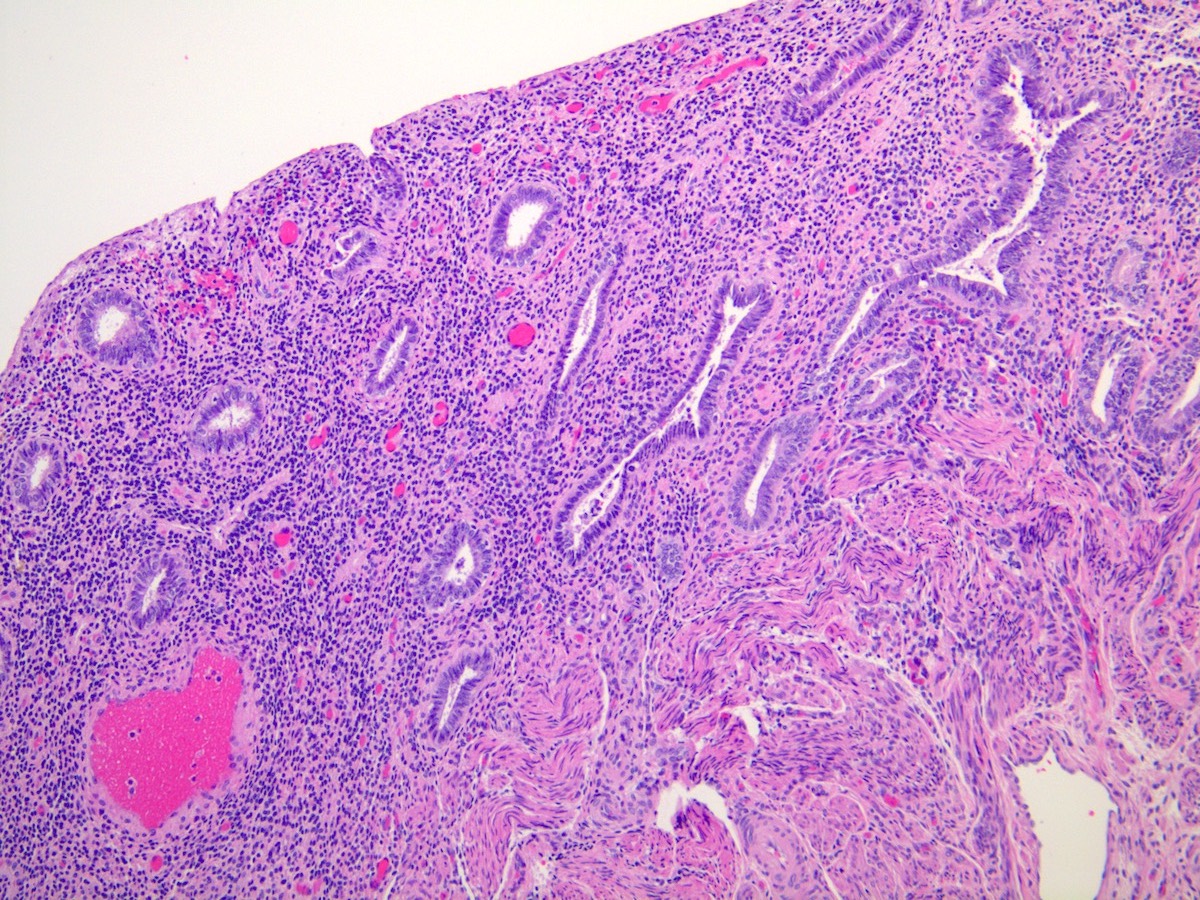
- Combined:
- Widely spaced, narrow, straight glands lined by cuboidal epithelial cells lacking nuclear pseudostratification or mitotic activity
- Sparsely cellular pseudodecidualized stroma
- Thin walled, ectatic stromal vessels, sometimes with thrombi
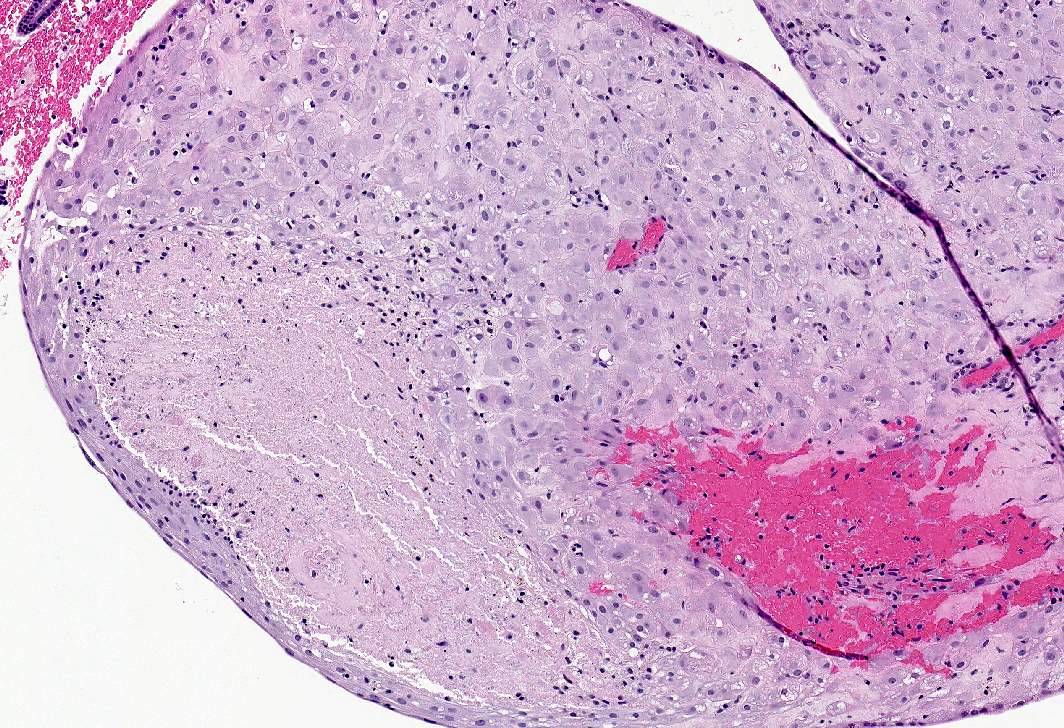
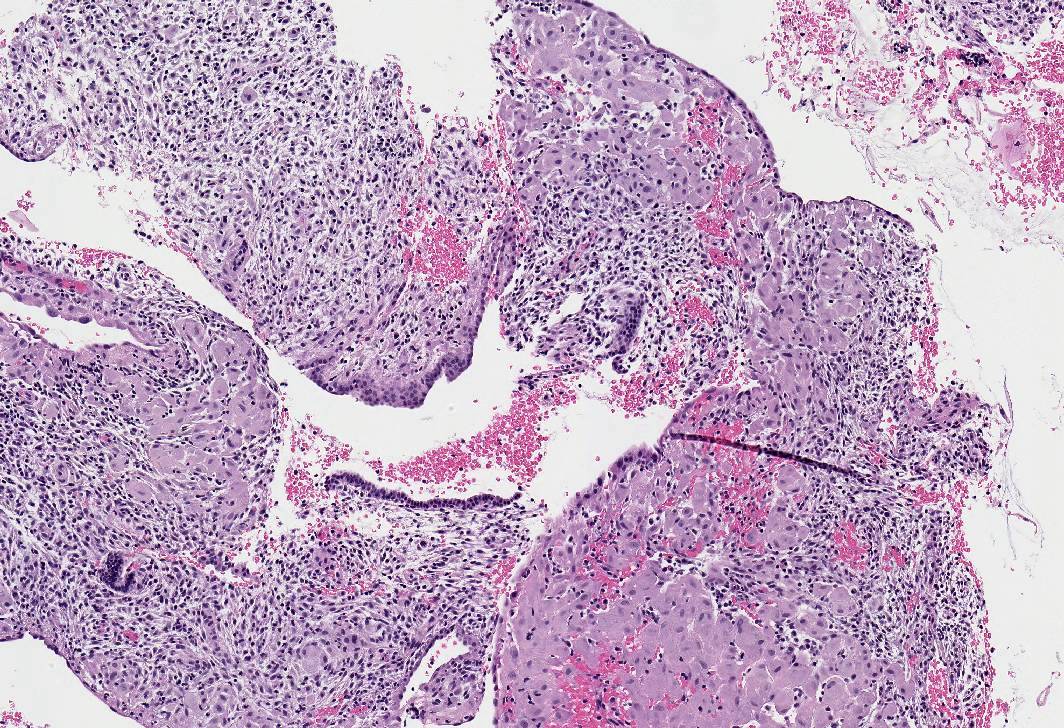
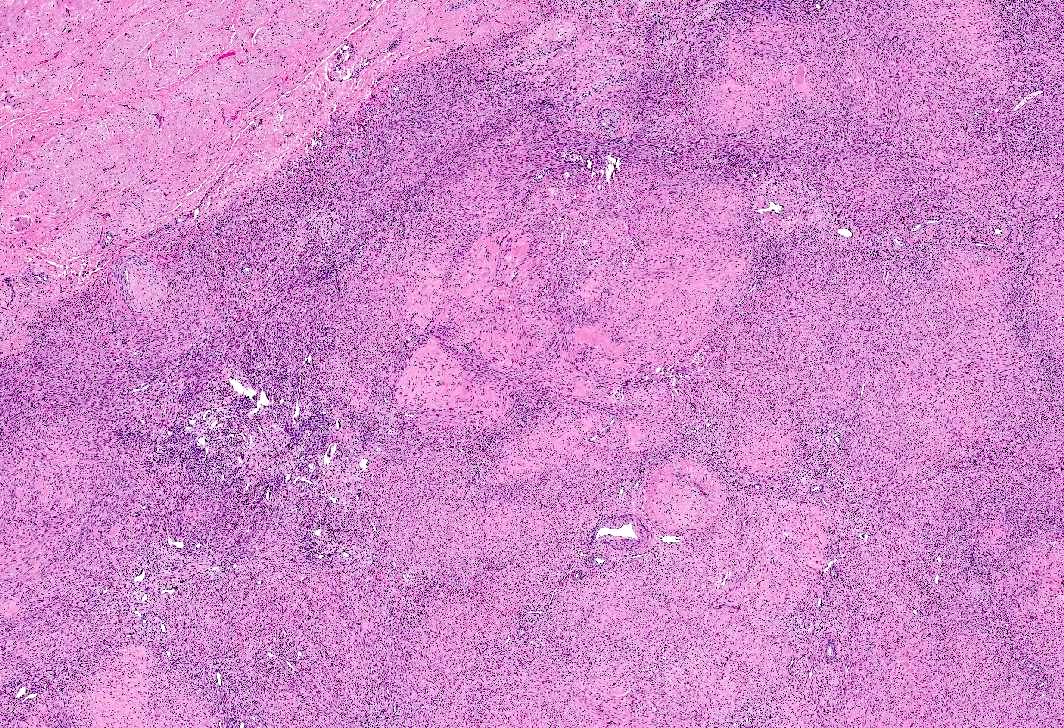
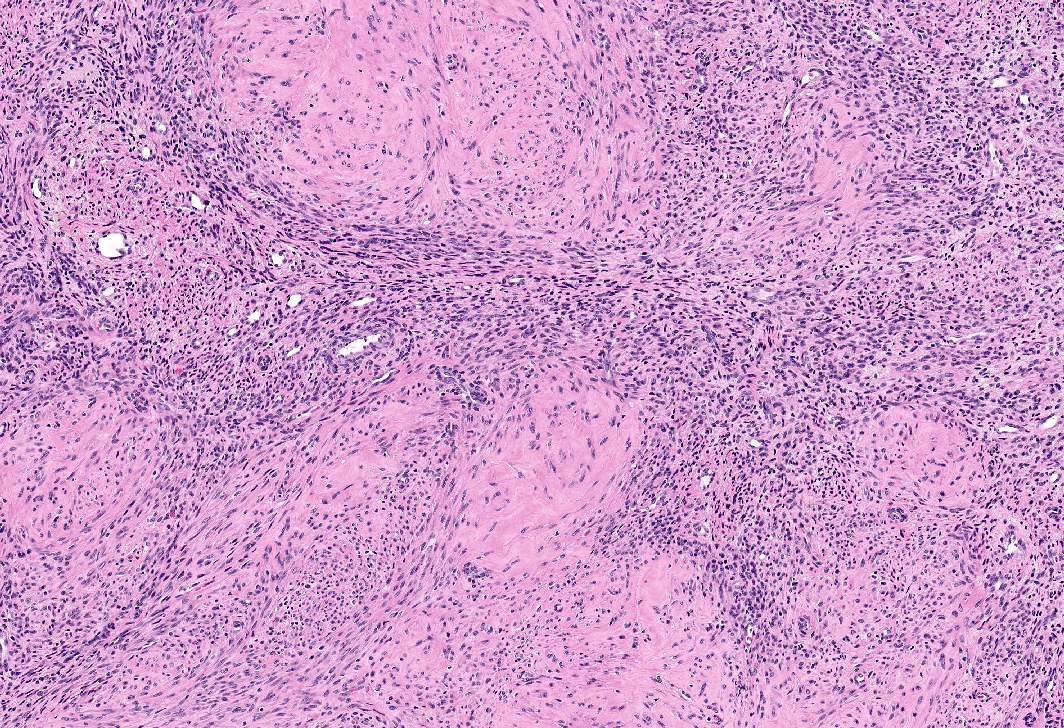
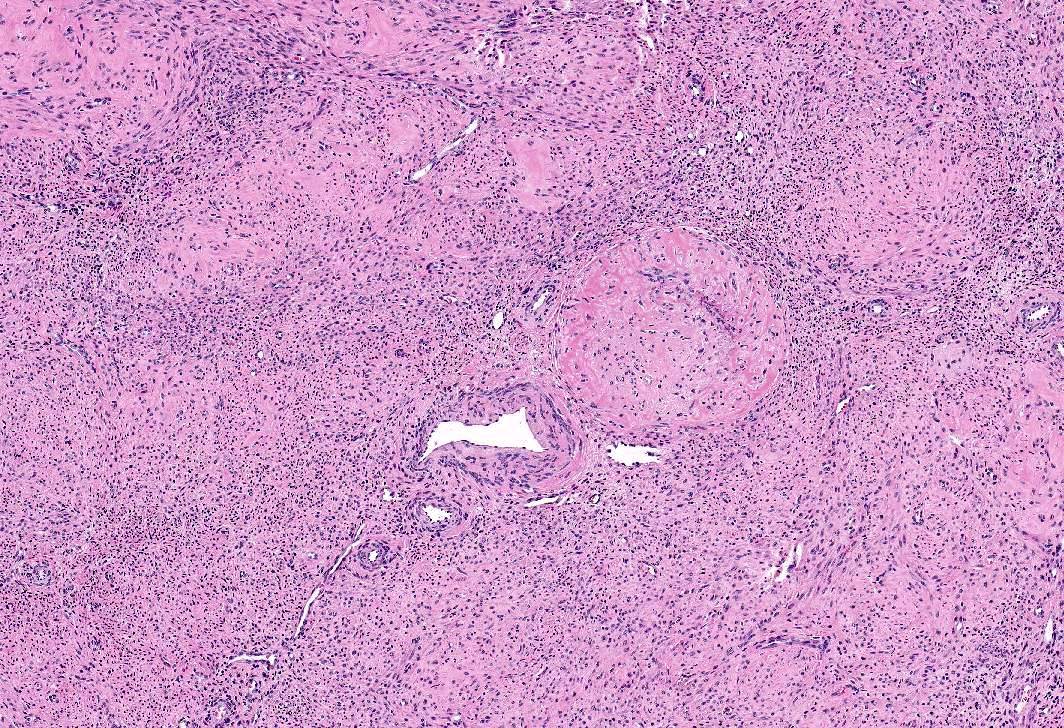
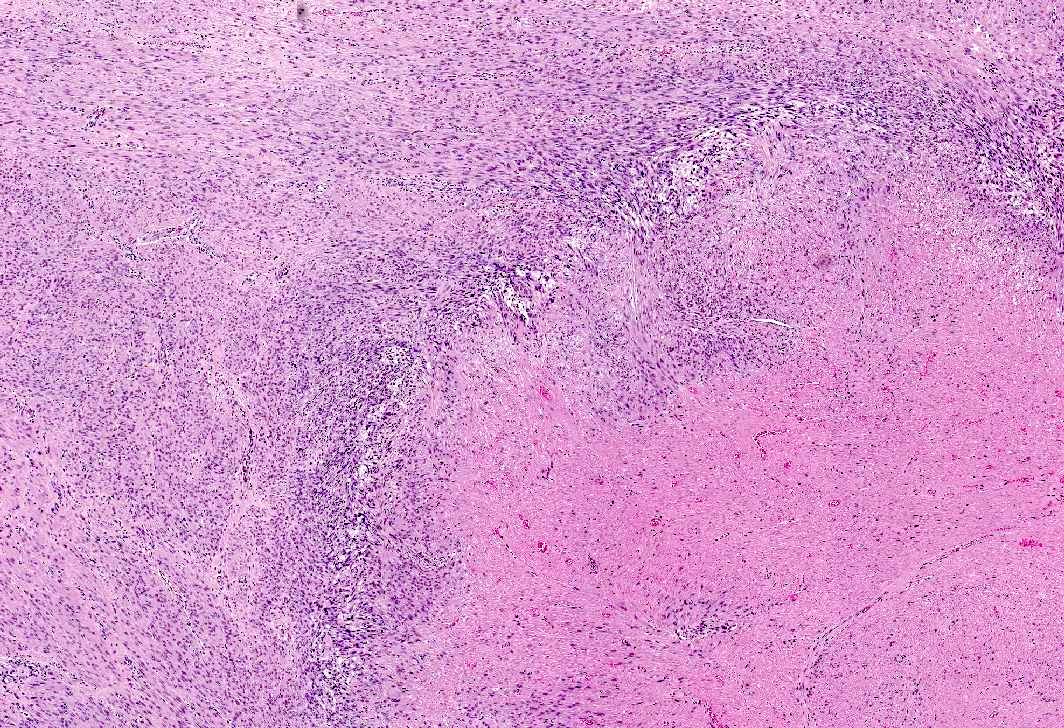
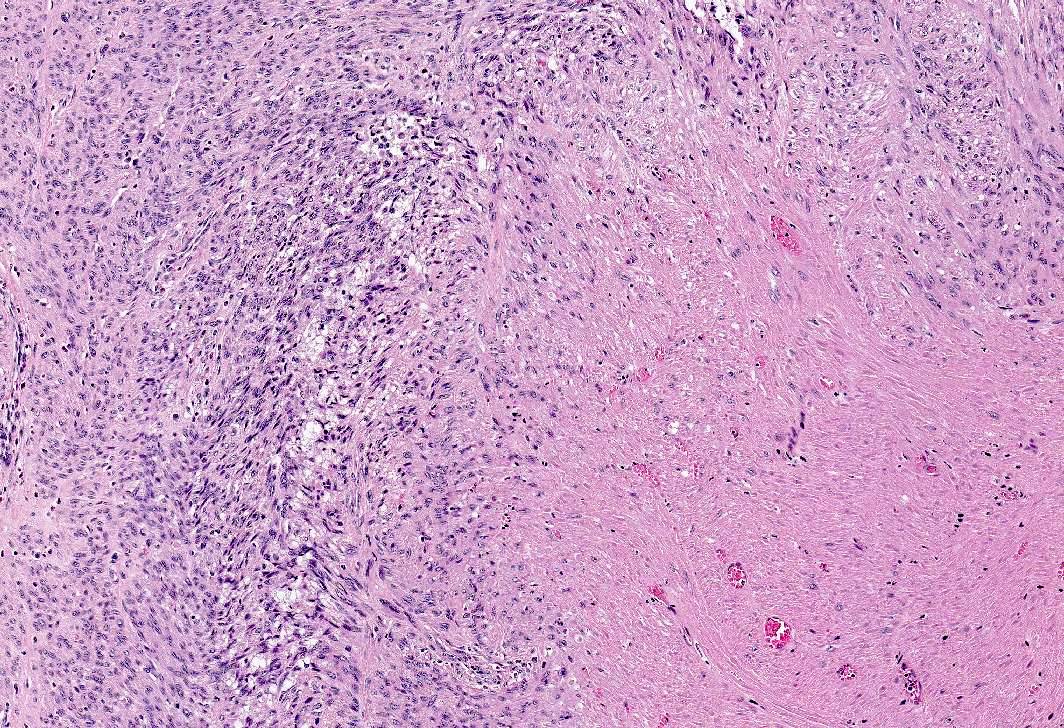
- Apoplectic leiomyomas (Am J Surg Pathol 2016;40:563):
- Related to progestational effect
- Multiple ovoid to stellate zones with central hemorrhage, necrosis, hyalinization or cystic change and hypercellular periphery
- Intracellular and extracellular edema of hydropic or alveolar type
- Spindled and rounded cells in hypercellular areas with eosinophilic cytoplasm and often nuclear pyknosis
- No to mild cytological atypia
- Increased mitotic activity may be found in hypercellular zone (0 – 14 mitoses/10 high power fields)
- Progestogen only: 3 morphologic patterns of response, dependent on dose and duration of progestin as well as endogenous estrogen levels, which may be coexistent and overlapping (Expert Rev Clin Pharmacol 2020;13:1103)
- Hormone replacement therapy:
- Estrogen only: weakly to markedly proliferative endometrium, stromal breakdown, squamous morules
- Progestogen only: scant literature, secretory pattern (mildly tortuous glands with basal nuclei and scant luminal secretions) not always present
- Combined:
- Sequential:
- Weakly proliferative (small tubular glands with occasional mitoses)
- Poorly developed secretory pattern during progestin administration phase
- Scant stroma
- Polyps
- Combined:
- Mostly inactive or atrophic endometrium
- Less commonly secretory pattern
- Rarely proliferative pattern
- Sequential:
- Androgens:
- Danazol:
- Secretory pattern with hypercellular stroma
- Inactive glands after prolonged therapy
- Sometimes vascular ectasia, glandular and stromal proliferative activity
- Tibolone: inactive endometrium
- Testosterone:
- Inactive to atrophic (50 - 74%), proliferative (18.5 - 40%) or secretory (4 - 7.5%) endometrium (Fertil Steril 2021;115:1312, Int J Gynecol Pathol 2019;38:520)
- Focal stromal decidual-like changes
- Transitional cell metaplasia of ectocervical and transformation zone epithelium or cervical atrophy (Obstet Gynecol 2021;138:51)
- Case reports of virilized paratubal mesonephric remnants resembling epididymis and prostate type glands involving cervical squamous epithelium (Int J Gynecol Pathol 2017;36:328)
- Danazol:
- Selective estrogen receptor modulators:
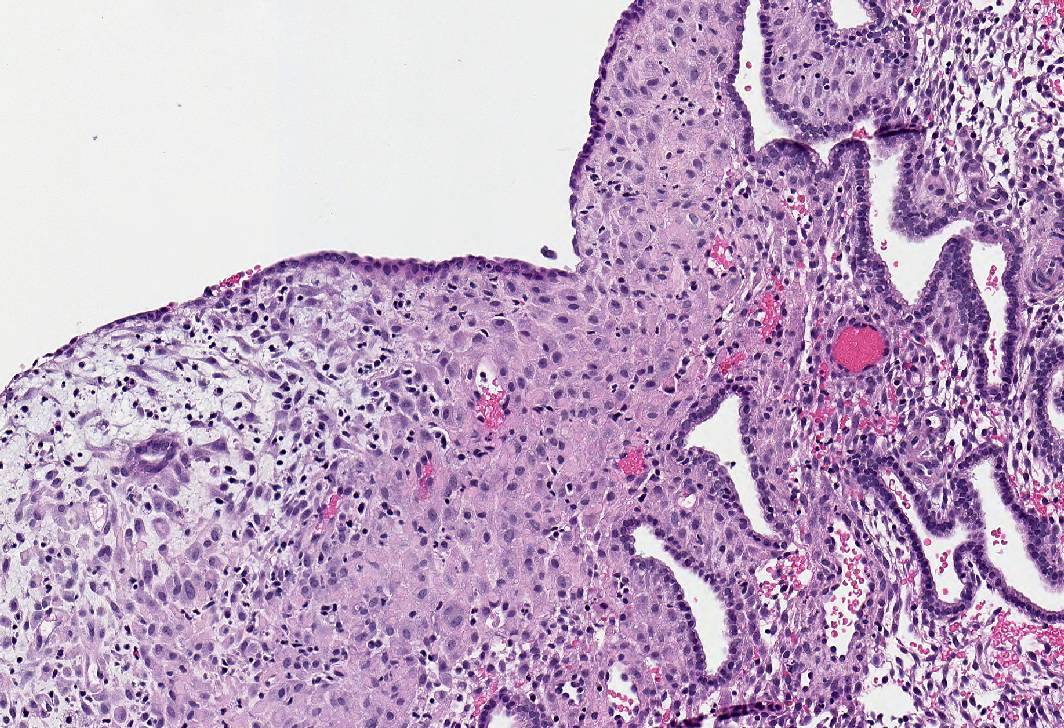
- Tamoxifen:
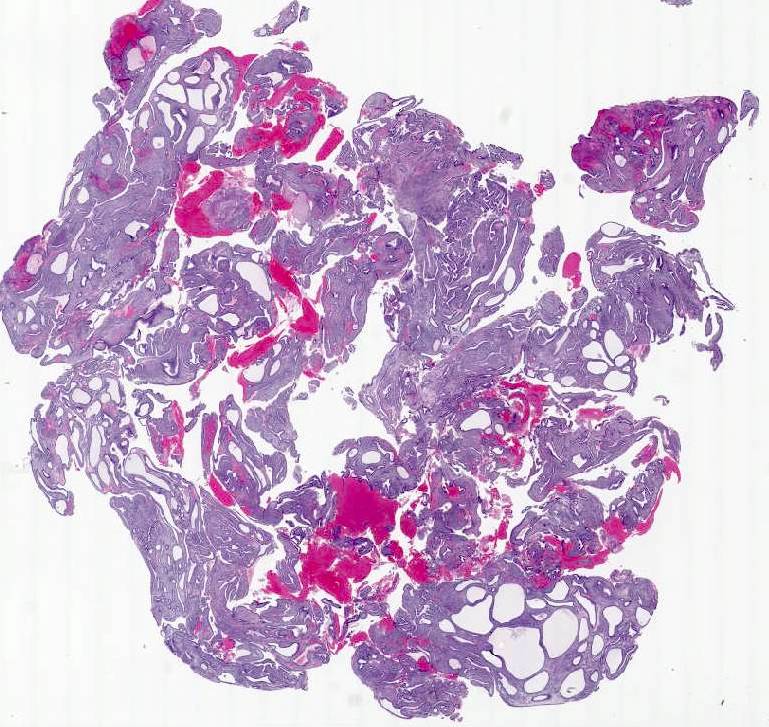
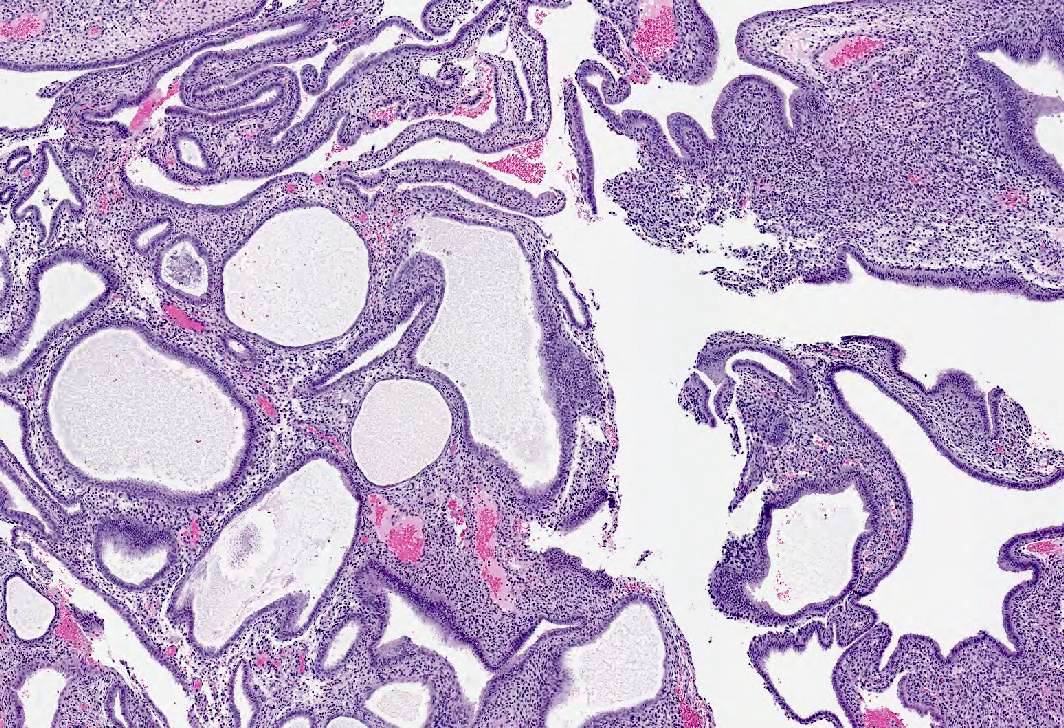
- Diffuse endometrial thickening; gross Swiss cheese appearance due to cystically dilated glands
- Polyps common in postmenopasual women after prolonged treatment
- Large, multiple, recurrent
- Staghorn shaped, cystic or hyperplastic glands, polarized along long axis of polyp with various epithelial metaplasias (mucinous, squamous, papillary eosinophilic and clear cell)
- Stroma myxoid, edematous or fibrous
- Glandular and stromal proliferative activity common
- Focal periglandular stromal condensation
- Endometrial hyperplasia and carcinoma
- Clomiphene: secretory pattern is subtle and difficult to appreciate
- Raloxifene: usually inactive endometrium
- Tamoxifen:
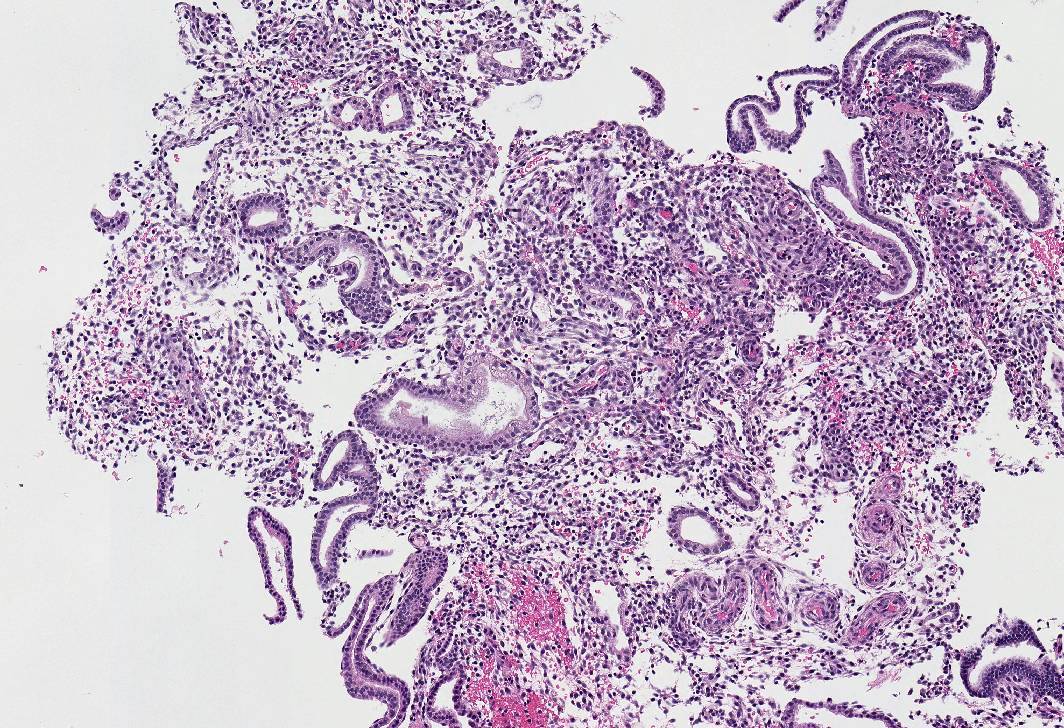
- Selective progesterone receptor modulators: progesterone receptor modulator associated endometrial change (Contraception 2015;91:360, Int J Gynecol Pathol 2018;37:575, Int J Gynecol Pathol 2020;39:146)
- Dyssynchronous endometrium (secretory glands with mitotic activity and apoptosis)
- Large cystically dilated glands lined by flattened epithelium
- Edematous or fibroblastic stroma without predecidual change
- Abnormal blood vessels, including thin walled ectatic, thick walled clusters or anastomosing capillaries
- Gonadotropin releasing hormone agonists:
- Endometrial atrophy or secretory pattern if used in conjunction with a progestin
- Leuprolide (Lupron) related changes in leiomyomas
- Central geographic areas of edema and hydropic degeneration
- Subsequent hyaline degeneration
- Necrosis with surrounding hypercellularity (may vary from infarct type, coagulative or uncertain type)
- Lymphoid aggregates
Microscopic (histologic) images
Sample pathology report
- Endometrium, biopsy:
- Inactive endometrium with progestational effect (see comment)
- Comment: The clinical history of Mirena intrauterine device is noted.
- Uterus, myomectomy:
- Leiomyomas with degenerative changes (see comment)
- Comment: The leiomyomas exhibit degenerative changes including large areas of edema, hydropic to hyaline degeneration and necrosis associated with lymphoid aggregates. Most foci of necrosis appear infarct type, although some foci are difficult to differentiate from coagulative necrosis and would be best classified as uncertain type; however, given the recent history of Lupron therapy, these changes are most likely attributed to such therapy.
Differential diagnosis
- Endometrial dating:
- Clinical history of recent use of exogenous hormones is critical for accurate dating
- Chronic endometritis:
- Plasma cells (required) with or without lymphoid follicles and eosinophils
- Associated with endometrial (difficult to date) and stromal (spindling) changes, in the absence of structural abnormalities (endometrial polyp, submucosal leiomyoma)
- Smooth muscle tumor of uncertain malignant potential (STUMP):
- Smooth muscle tumor with unequivocal coagulative necrosis but lacking cytologic atypia or elevated mitotic activity may be classified as STUMP (Histopathology 2018;73:284)
- No history of Lupron therapy
- Conventional (spindle cell) leiomyosarcoma:
- 2 of 3 diagnostic features present, including coagulative necrosis, cytologic atypia, elevated mitotic count at ≥ 4 mitoses/mm2 or ≥ 10 mitoses/10 high power fields (field diameter = 0.55, 0.24 mm2 in area)
Additional references
Practice question #1
A 45 year old woman, who has been treated with Lupron for longstanding endometriosis and fibroids, underwent total hysterectomy. Most leiomyomas showed morphologic changes depicted in this figure. What additional features would be required to diagnose treatment related degenerative changes?
- Marked cytologic atypia and 15 mitoses per 10 high power fields
- Marked cytologic atypia and 5 mitoses per 10 high power fields
- Mild to absent cytologic atypia and 20 mitoses per 10 high power fields
- Mild to absent cytologic atypia and 2 mitoses per 10 high power fields
Practice answer #1
D. Mild to absent cytologic atypia and 2 mitoses per 10 high power fields
Comment Here
Reference: Exogenous hormones
Comment Here
Reference: Exogenous hormones
Practice question #2
A 32 year old woman has been on a combined oral contraceptive for 2 years. Which of the following statements is true regarding morphologic changes seen in her endometrial biopsy?
- Mildly tortuous glands lined by secretory endometrium and pseudodecidualized stroma, consistent with early secretory phase
- Straight glands lined by proliferative endometrium and proliferative type endometrial stroma, consistent with early proliferative phase
- Tortuous glands lined by secretory endometrium, with intraluminal secretions and pseudodecidualized stroma, consistent with late secretory phase
- Widely spaced, narrow, straight glands lined by inactive endometrium and pseudodecidualized stroma, consistent with changes associated with combined oral contraceptives
Practice answer #2
D. Widely spaced, narrow, straight glands lined by inactive endometrium and pseudodecidualized stroma, consistent with changes associated with combined oral contraceptives
Comment Here
Reference: Exogenous hormones
Comment Here
Reference: Exogenous hormones