Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Prognostic factors | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Immunofluorescence description | Immunofluorescence images | Positive stains | Negative stains | Electron microscopy description | Electron microscopy images | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #2 | Board review style question #2 | Board review style answer #2Cite this page: Mirza R, Gupta RK. Light chain proximal tubulopathy. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/kidneylcpt.html. Accessed May 13th, 2024.
Definition / general
- Acute or chronic involvement of proximal tubules of kidney in monoclonal light chain disease
- Characterized by intracytoplasmic accumulation of nephrotoxic light chains within proximal tubular epithelial cells in crystalline or noncrystalline form, causing tubular injury
Essential features
- Light chain proximal tubulopathy (LCPT) is a rare variant of monoclonal light chain associated renal disease
- May be associated with acute or chronic renal failure, proteinuria or Fanconi syndrome
- Characterized by intracytoplasmic deposition of light chains in the cytoplasms of proximal tubular epithelial cells and may be crystalline or noncrystalline variant
- Kappa or lambda (never both) staining of proximal tubular epithelial cells on routine or pronase digested immunofluorescence is pathognomonic
- Electron microscopy shows crystals of various shapes in crystalline variant and numerous, large or abnormal looking (mottled) lysosomes with or without mitochondrial swelling in noncrystalline variant
- References: J Am Soc Nephrol 2016;27:1555, Mod Pathol 2011;24:1462
Terminology
- Proximal tubulopathies, monoclonal light chain related
- LCPT, crystalline variant
- LCPT, noncrystalline variant
ICD coding
Epidemiology
- Real incidence and prevalence are still unknown
- Age: Mostly adults, median age: 60 years (range: 39 - 87 years) (J Am Soc Nephrol 2016;27:1555)
- Gender: M > F (63% men) (J Am Soc Nephrol 2016;27:1555)
- Race: can affect all races; more common in Caucasian population as per few studies (J Am Soc Nephrol 2016;27:1555)
- Strong association with some form of overt or clinically silent hematolymphoid neoplasm
Sites
- Kidney, proximal tubular (PT) epithelium
Pathophysiology
- Main pathogenesis occurs as a consequence of imbalance in interaction of tubulopathic light chains and lysosomes within the proximal tubular cells due to excess light chain production
- In monoclonal renal disease, the excess light chains (particularly their variable domains) resist degradation and start to deposit locally, either in crystalline or noncrystalline form
- These pathologic light chains are more commonly of kappa type and specifically belong to the VK1 subgroup
- References: Mod Pathol 2011;24:1462, Arch Pathol Lab Med 2014;138:1365
Etiology
- Monoclonal gammopathy from a hematolymphoid neoplasm, causing excess light chain production of one type, either kappa (K) or lambda (L), with K > L
- Hematolymphoid neoplasm may be some form of overt plasma cell dyscrasia or B cell lymphoma or covert disease (elevated monoclonal light chain in blood or urine in absence of symptoms)
- Abnormal light chain gets reabsorbed and likely inadequately excreted by proximal tubules, causing tubular injury
- Reabsorbed light chains may or may not form crystals
- References: Mod Pathol 2011;24:1462, Arch Pathol Lab Med 2014;138:1365, J Am Soc Nephrol 2016;27:1555
Diagrams / tables
Clinical features
- Plasma cell dyscrasia
- B cell lymphoma
- Monoclonal gammopathy of undetermined significance (MGUS)
- Monoclonal gammopathy of renal significance (MGRS)
- Fanconi syndrome
- Acute renal failure
- Chronic renal failure
- Proteinuria
- Osteomalacia
- References: Mod Pathol 2011;24:1462, Arch Pathol Lab Med 2014;138:1365, J Am Soc Nephrol 2016;27:1555
Diagnosis
- Diagnosis is by renal biopsy using different modalities - light microscopy (LM), immunofluorescence microscopy (IF) and electron microscopy (EM)
- Immunofluorescence of paraffin sections by pronase digestion is recommended when routine IF is negative
- In challenging cases, immunoelectron microscopy may be helpful
Laboratory
- Increased urinary protein on routine urinalysis
- Monoclonal M spike detected on serum or urine electrophoresis
- Elevated free light chain assay in serum or urine (kappa or lambda)
- Detection of monoclonal B cells or plasma cells by flow cytometry, fluorescent in situ hybridization or bone marrow biopsy
- Increased serum creatinine and blood urea nitrogen
- Renal tubular acidosis in Fanconi syndrome
- References: Arch Pathol Lab Med 2014;138:1365, J Am Soc Nephrol 2016;27:1555, Nat Rev Nephrol 2019;15:45
Prognostic factors
- Good control (complete or partial remission) of the hematological disease often results in recovery of renal and tubular function
- Many patients develop chronic kidney disease if early diagnosis is not made and sometimes in spite of treatment (J Am Soc Nephrol 2016;27:1555)
- Patients with Fanconi syndrome often have an indolent course
- In treated patients, those who receive stem cell treatment had the best prognosis
Case reports
- 53 year old woman with moderate proteinuria and renal failure (BMC Nephrol 2018;19:322)
- 64 year old man who presented with acute renal failure and Fanconi syndrome (BMJ Case Rep 2020;13:e234361)
- 66 year old woman with known history of K-restricted multiple myeloma, acute kidney injury and crystalline structures in urine sediment (Clin Nephrol 2020;93:203)
Treatment
- Usually directed towards the underlying hematolymphoid neoplasm
- Conventional chemotherapy
- Monoclonal antibodies (rituximab, daratumumab)
- Immunomodulatory drugs (thalidomide)
- Glucocorticoids
- Autologous stem cell transplant
- Reference: J Am Soc Nephrol 2016;27:1555
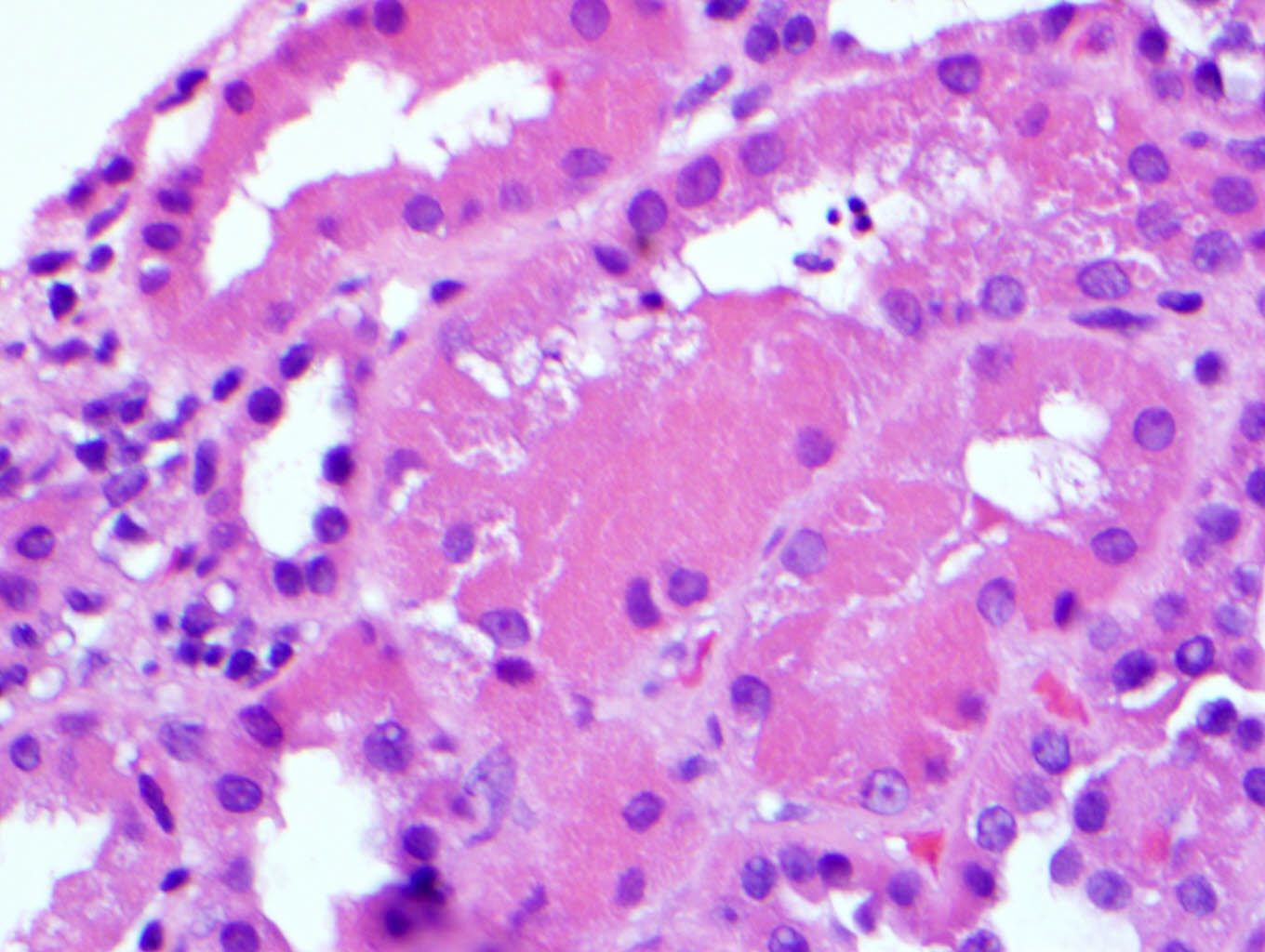
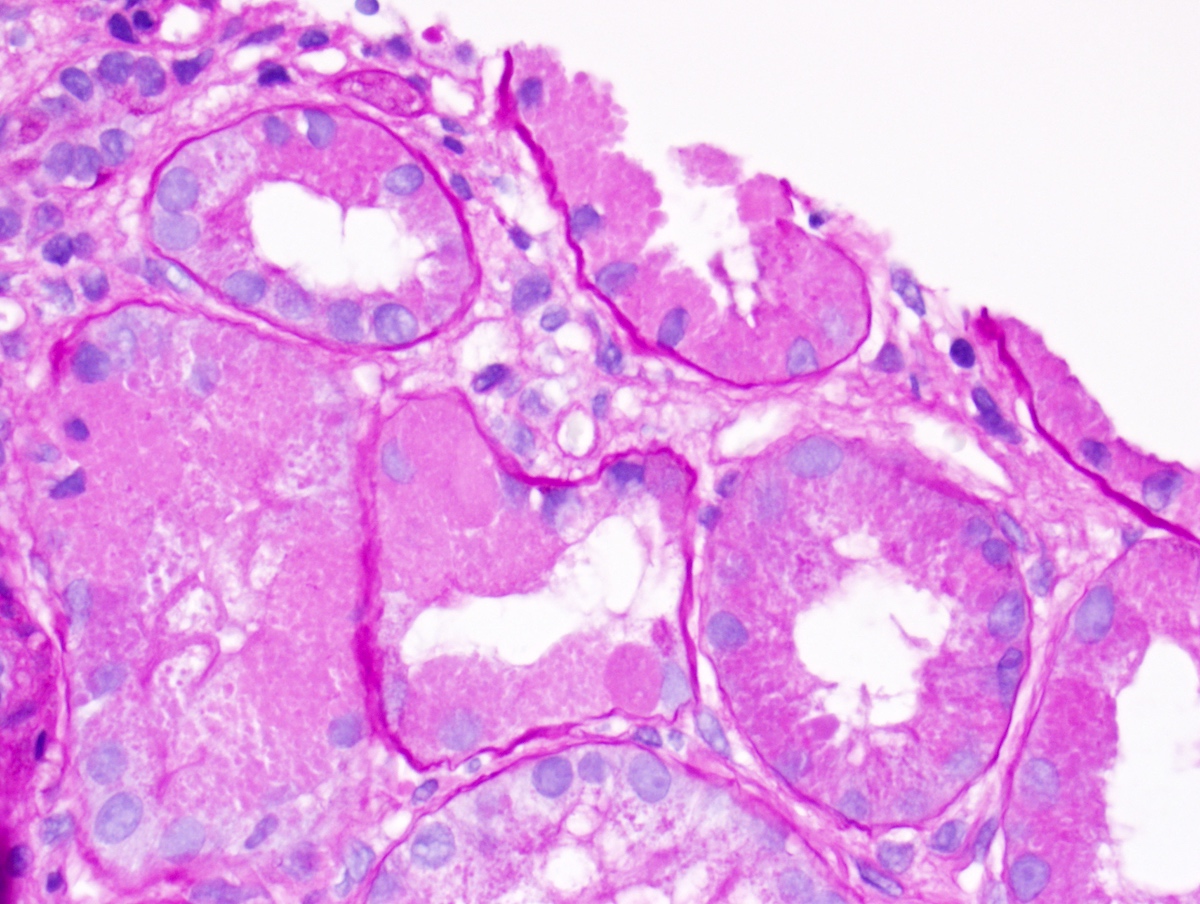
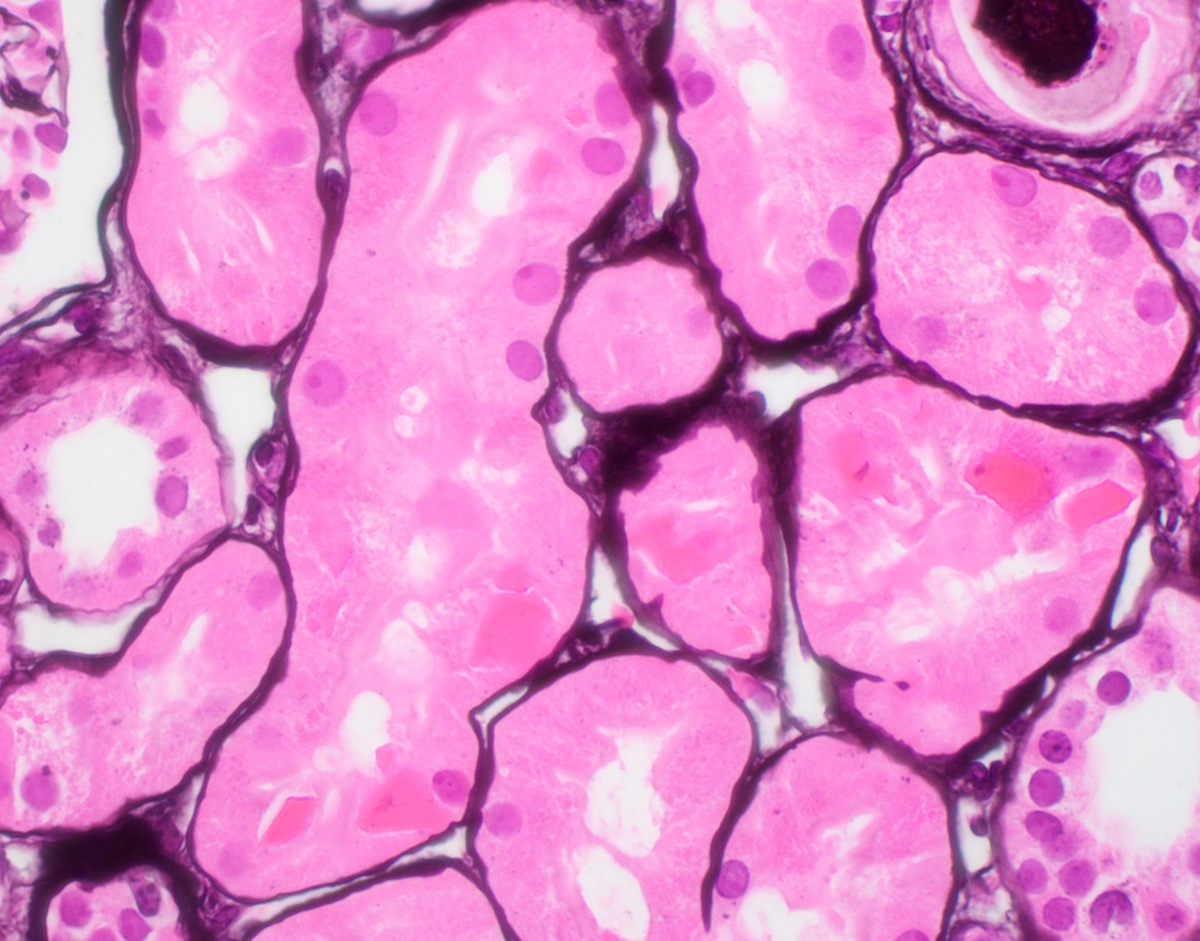
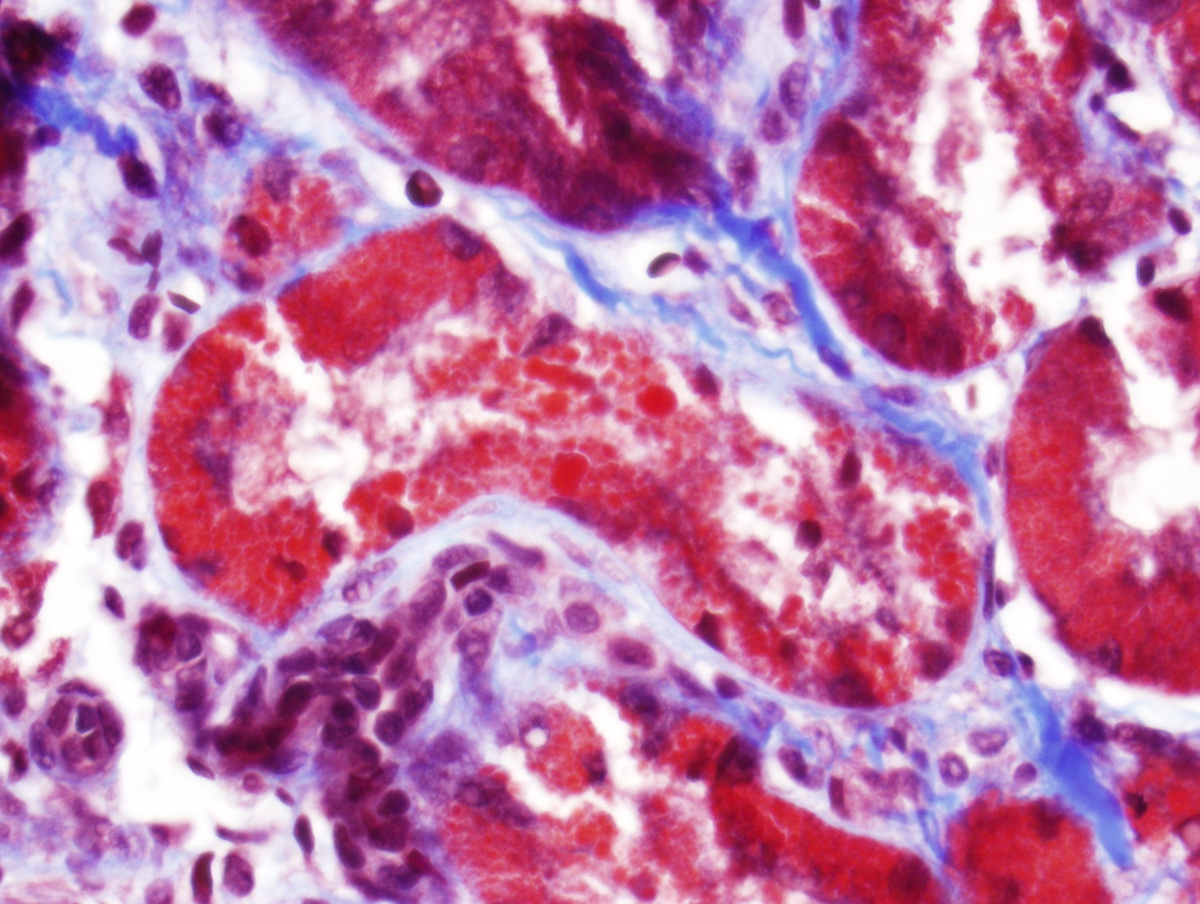
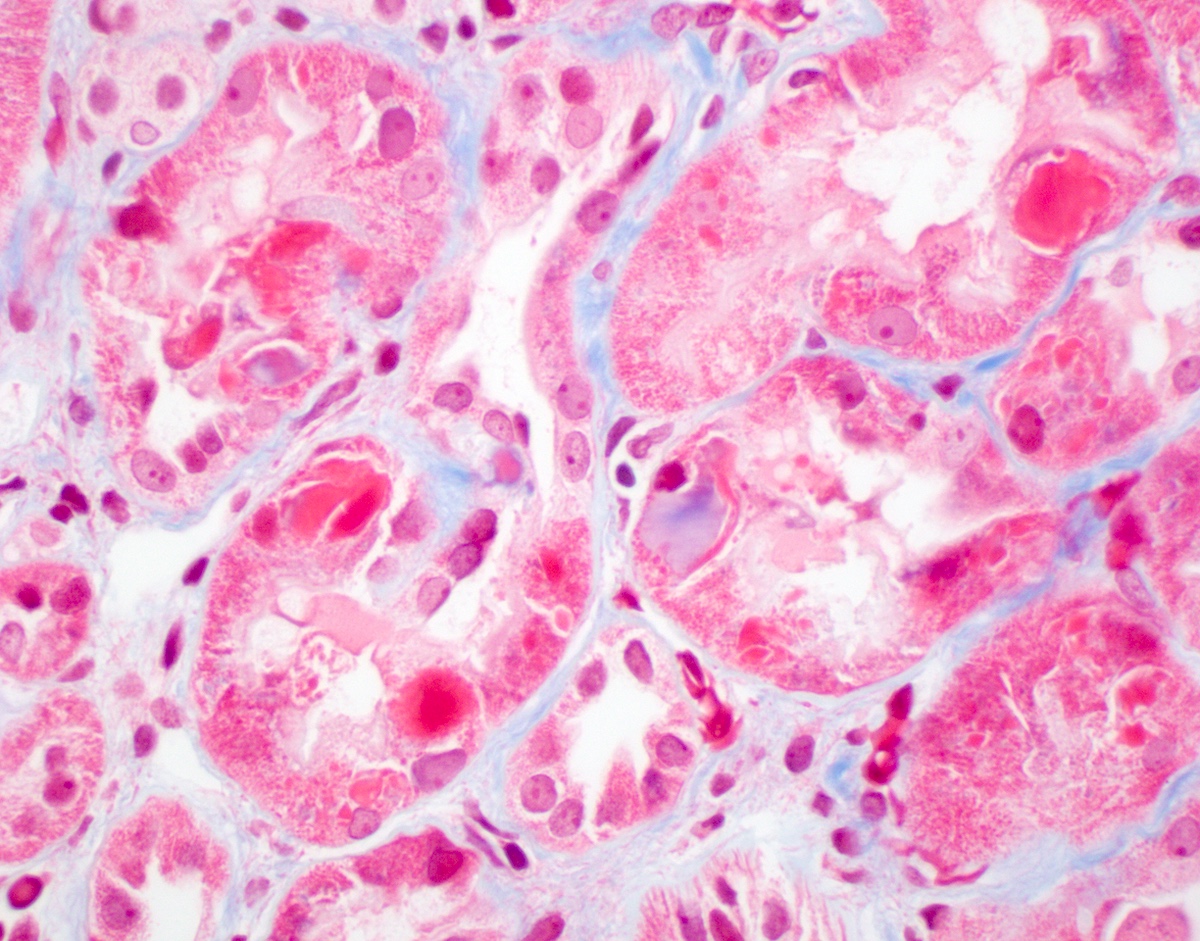
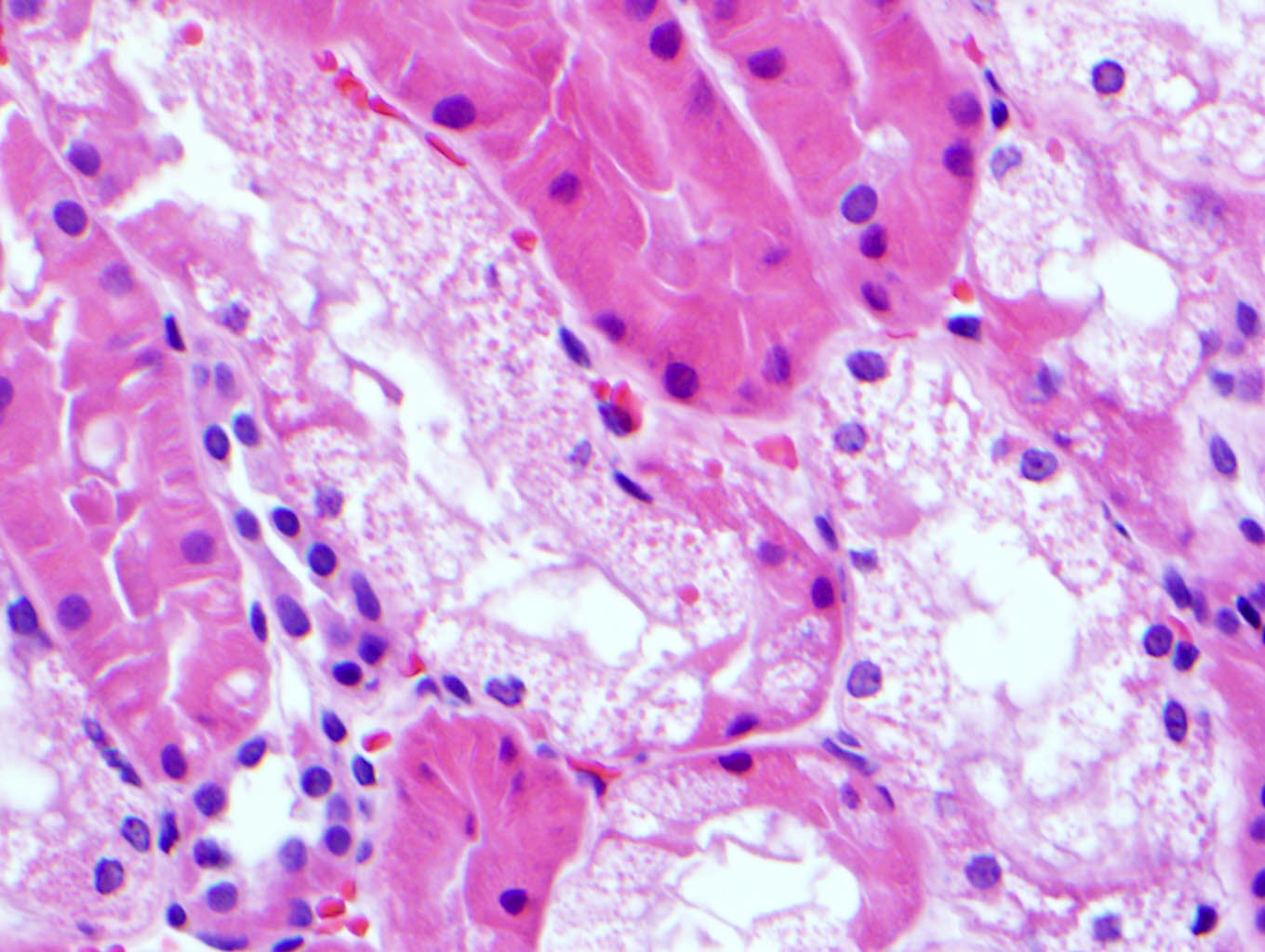
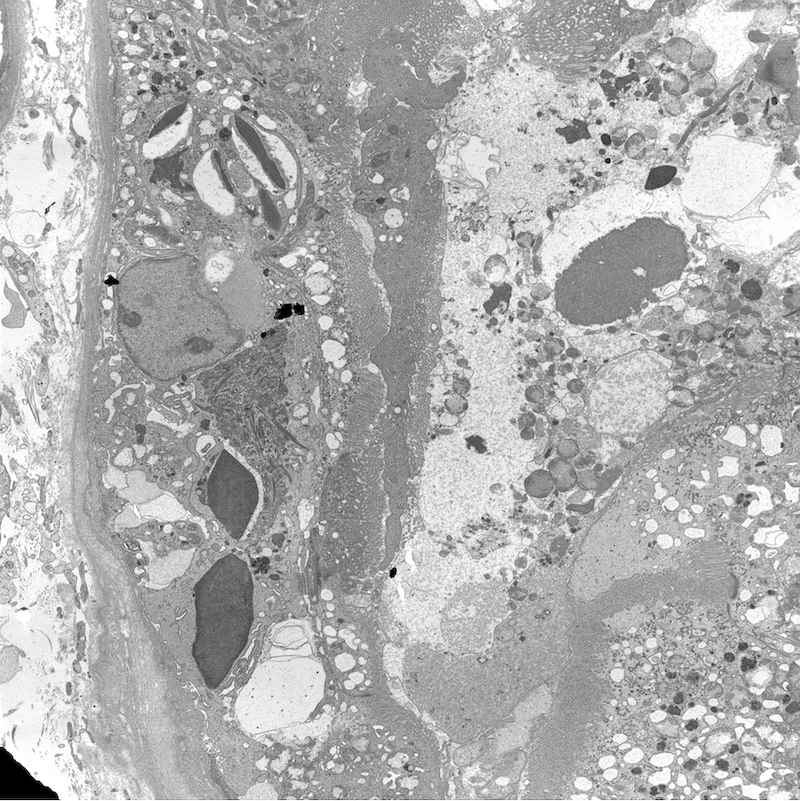
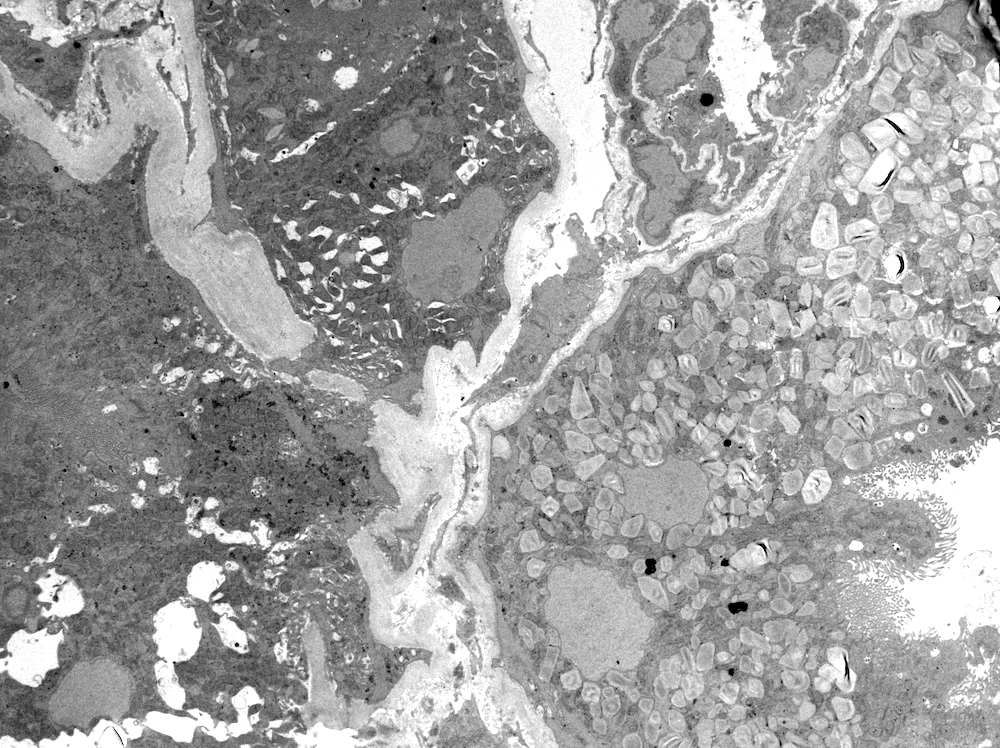
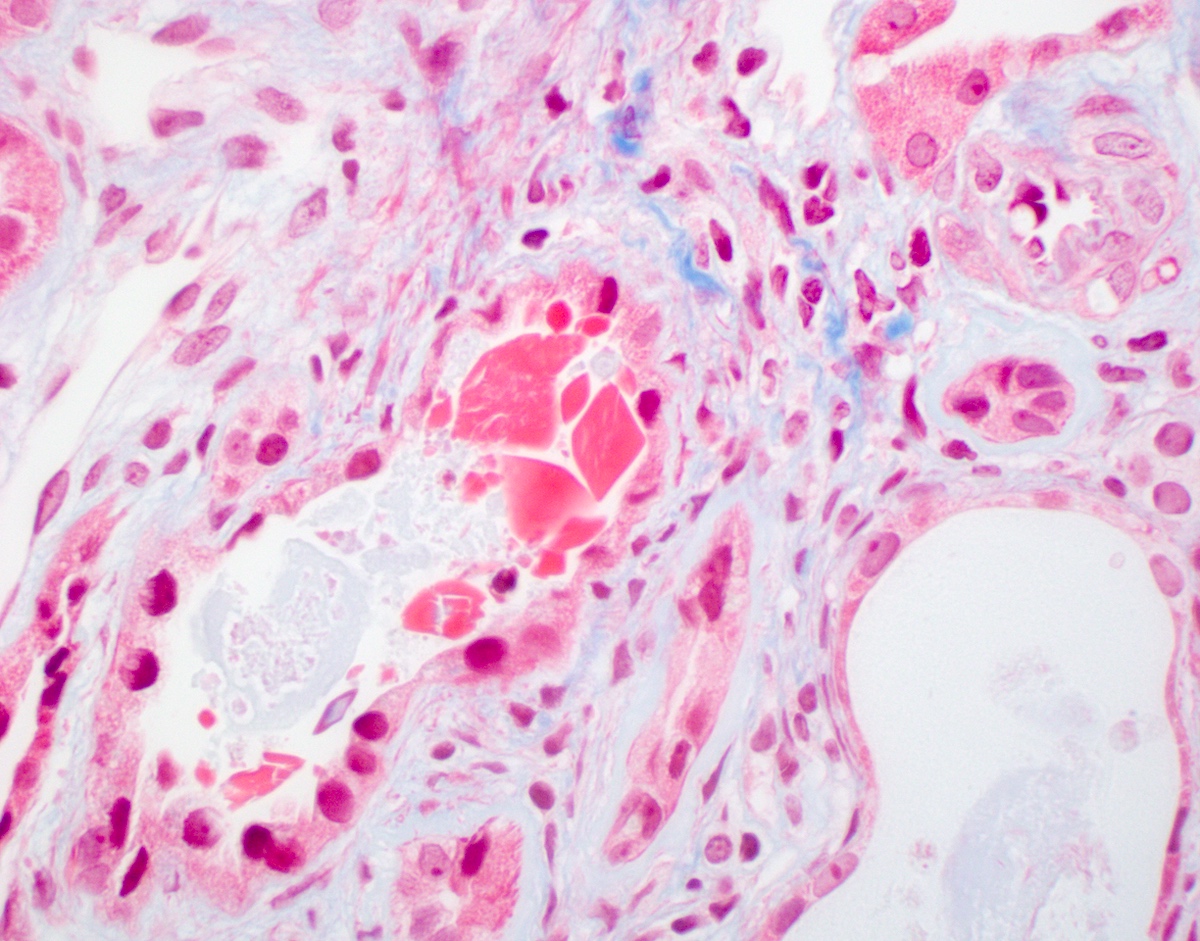
Microscopic (histologic) description
- Main light microscopic finding is some form of tubular pathology seen on kidney biopsy
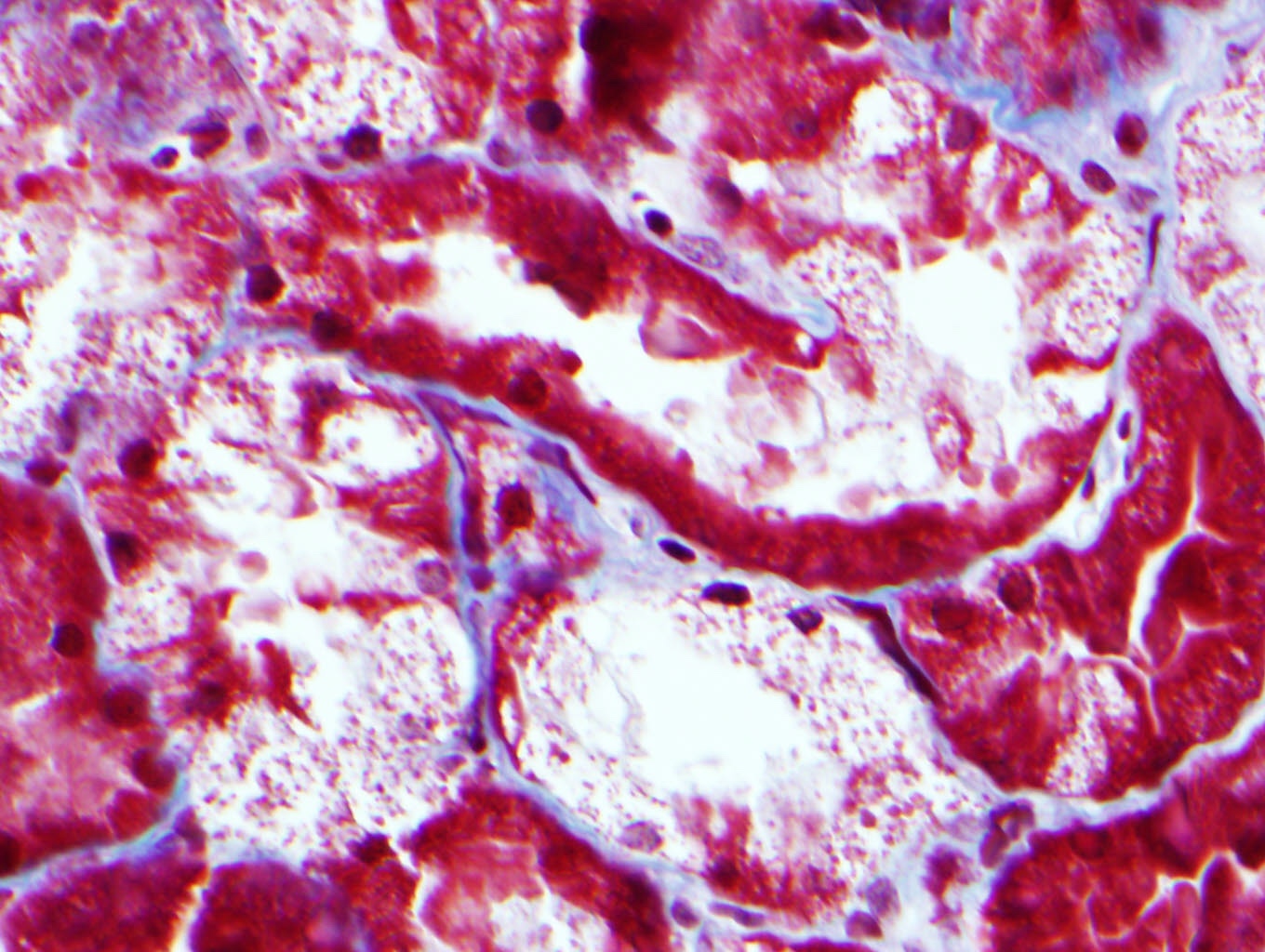
- Intracytoplasmic crystals of various shapes within tubular epithelial cells, which are PAS and trichrome positive (fuchsinophilic), mainly in crystalline variant
- PAS negative and trichrome negative eosinophilic finely granular cytoplasmic inclusions within proximal tubular epithelial cells, particularly in noncrystalline variant
- Acute tubular injury / necrosis, which may include tubular luminal dilatation, loss of brush border, epithelial cytoplasmic blebbing, epithelial cell desquamation or cytoplasmic vacuolization
- Tubulointerstitial inflammation, characterized by chronic inflammatory infiltrate (lymphocytes and plasma cells) in the interstitium, often with tubulitis
- Usually unremarkable glomeruli
- References: Mod Pathol 2011;24:1462, Arch Pathol Lab Med 2014;138:1365, J Am Soc Nephrol 2016;27:1555, BMC Nephrol 2020;21:146
Microscopic (histologic) images
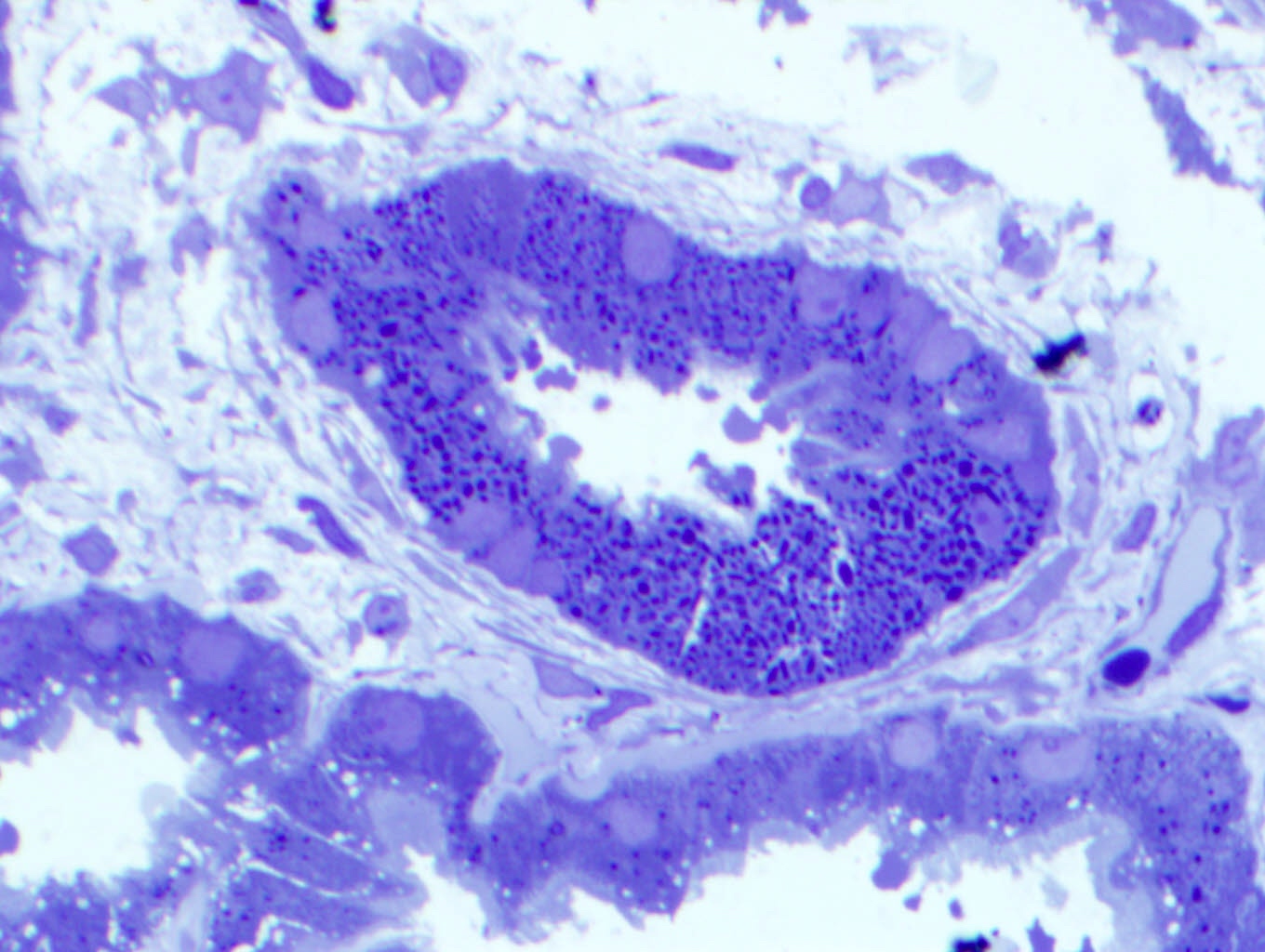
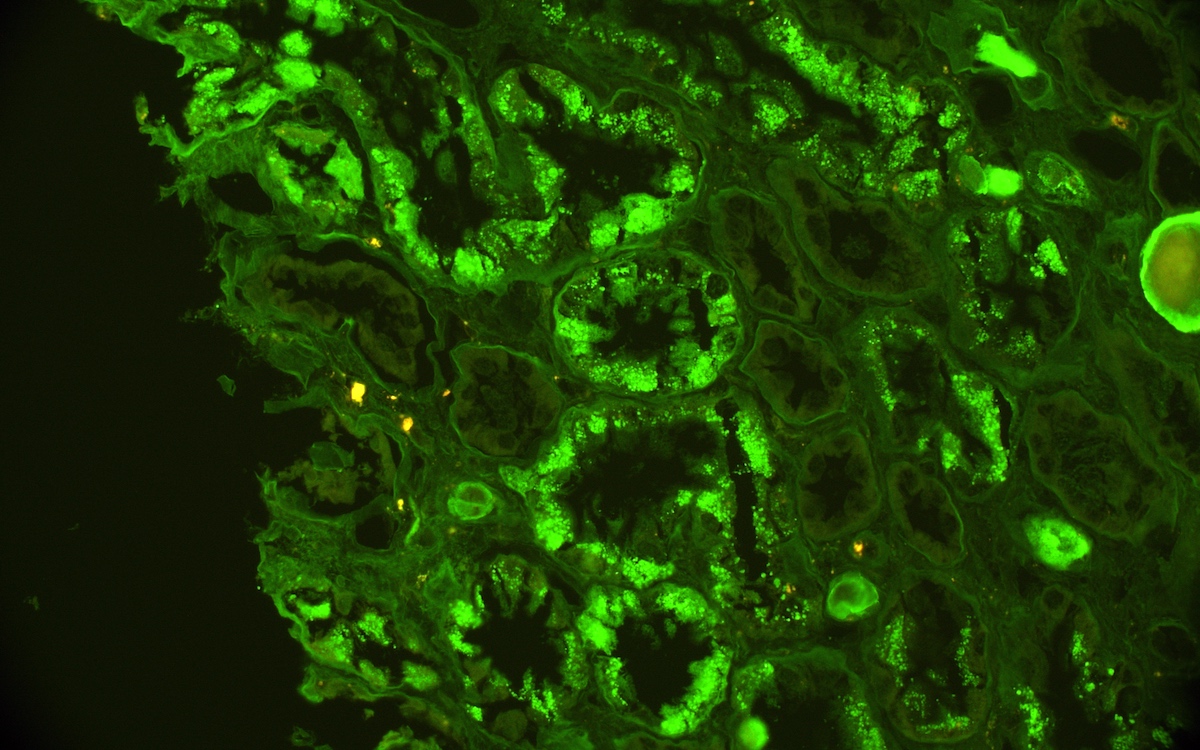
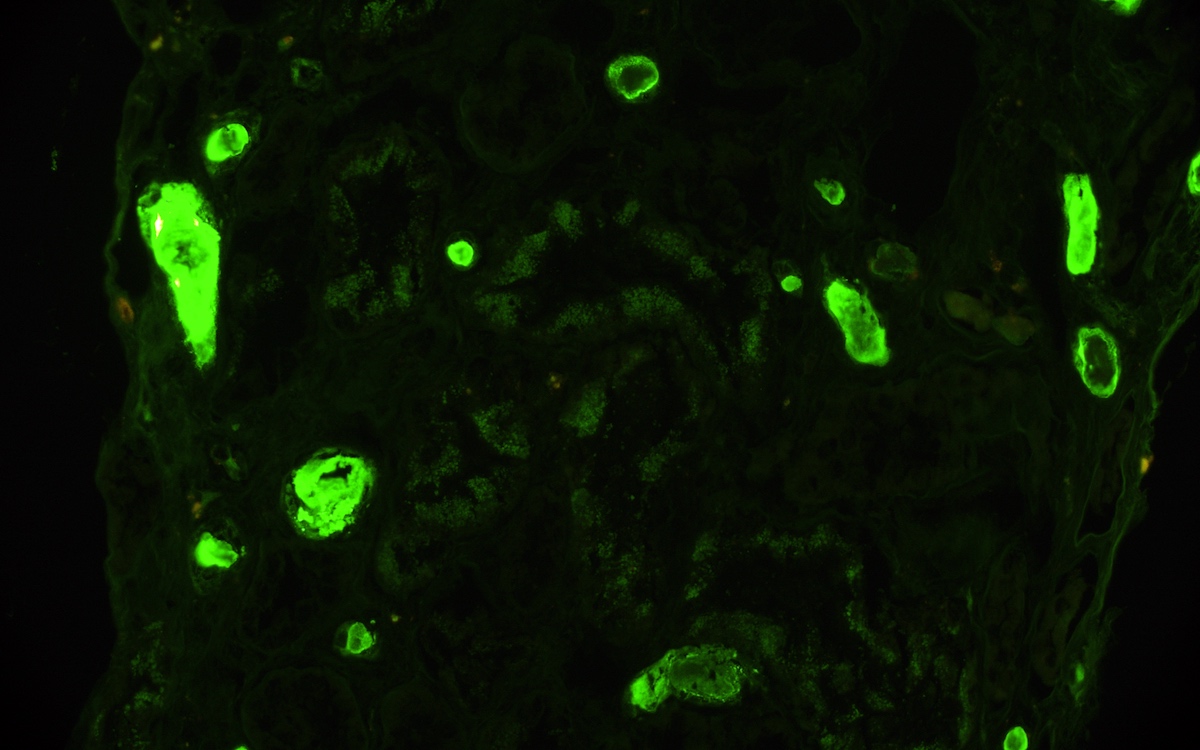
Immunofluorescence description
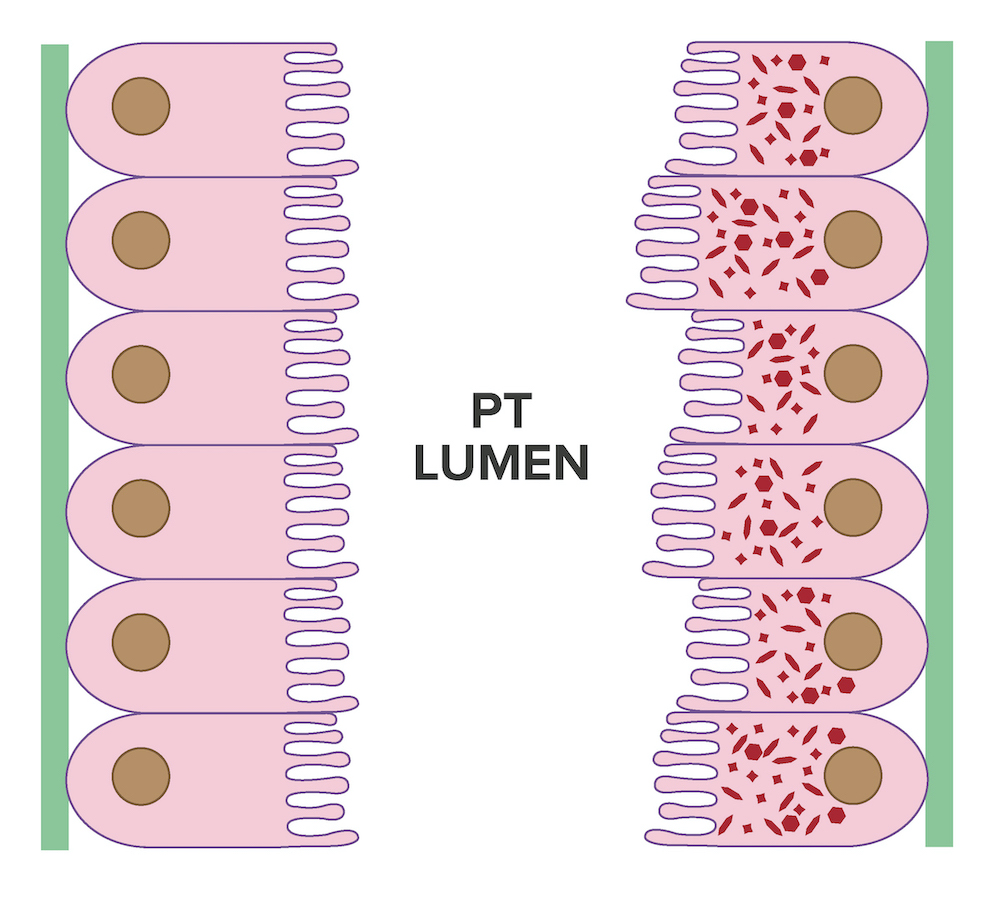
- Strong granular cytoplasmic positivity for either kappa or lambda light chain (never both) within proximal tubules on frozen section (routine) immunofluorescence
- Immunofluorescence (IF) after pronase digestion of paraffin sections is critically important in cases where routine immunofluorescence is nondiagnostic or negative (for both light chains)
- Kappa only staining is overall more common, particularly in crystalline variant of LCPT (Arch Pathol Lab Med 2014;138:1365)
- Lambda only staining has been seen to be more common in noncrystalline variant of LCPT (Mod Pathol 2011;24:1462)
Immunofluorescence images
Positive stains
- Kappa ​light chain positive by routine or pronase immunofluorescence or lambda light chain positive by routine or pronase immunofluorescence; never both (J Am Soc Nephrol 2016;27:1555, Mod Pathol 2011;24:1462, BMC Nephrol 2020;21:146)
Negative stains
- Congo red
- Thioflavin T
- Reference: Kidney Int 2020;98:310
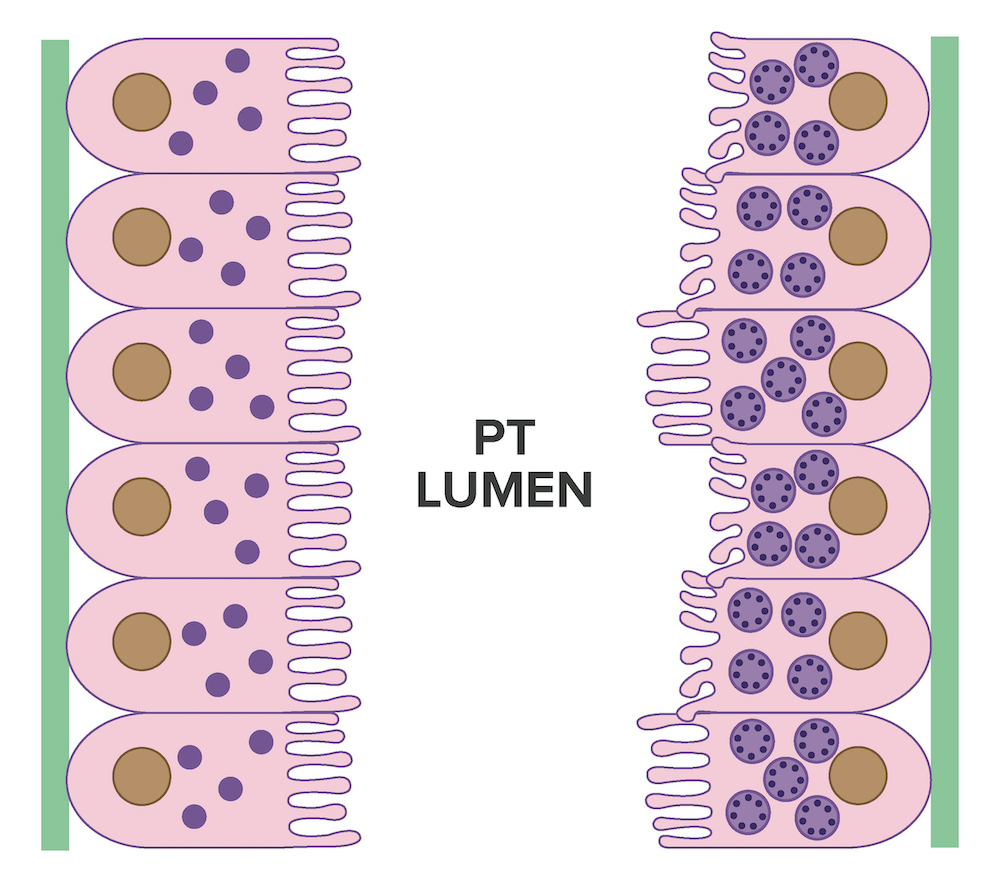
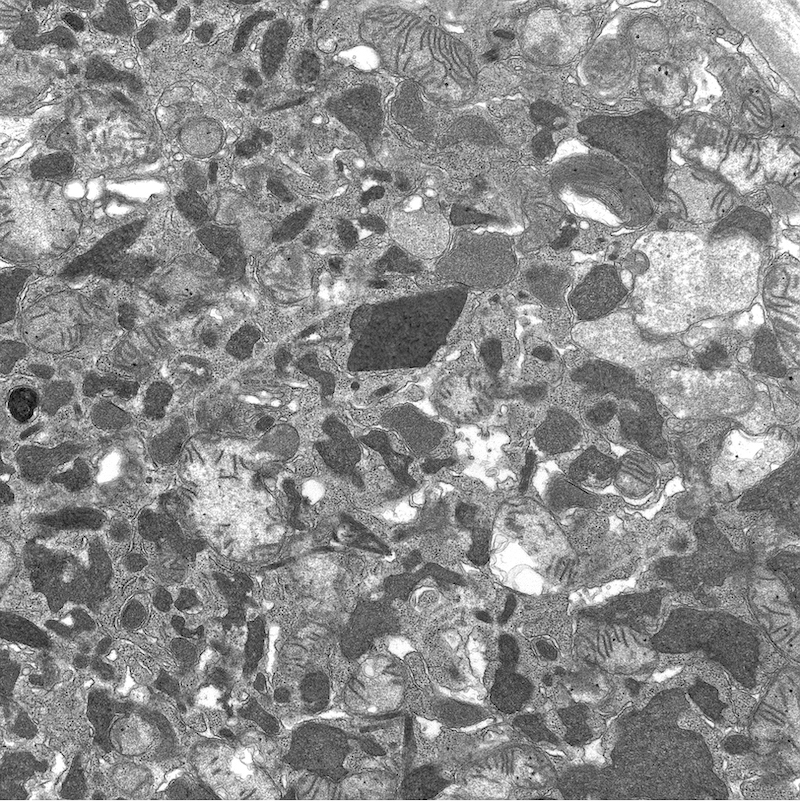
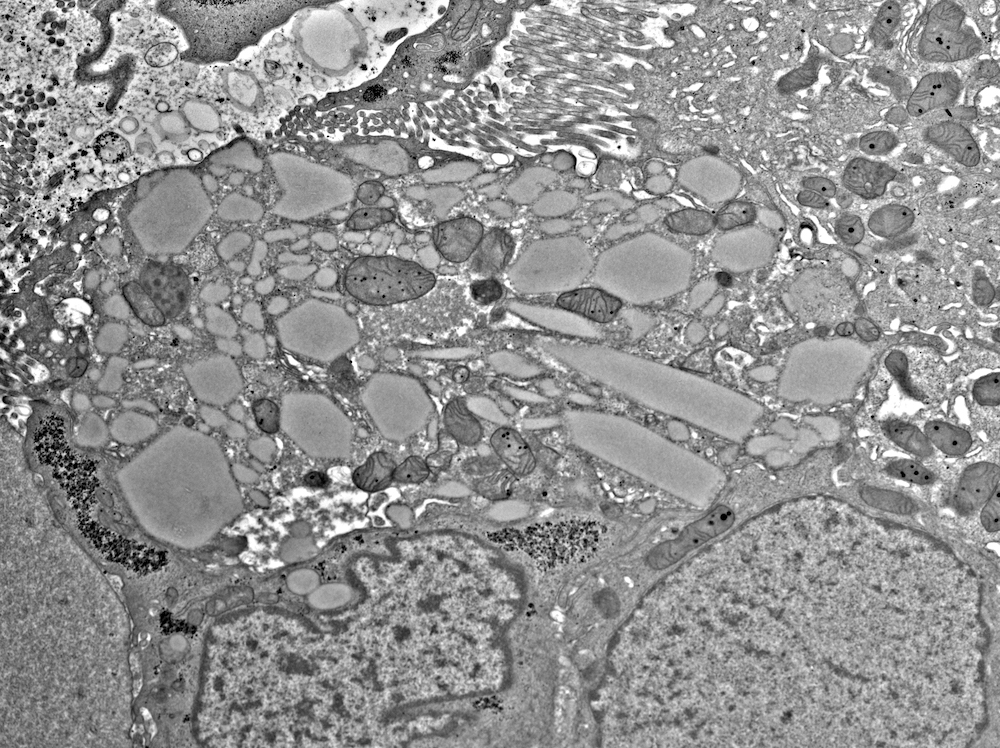
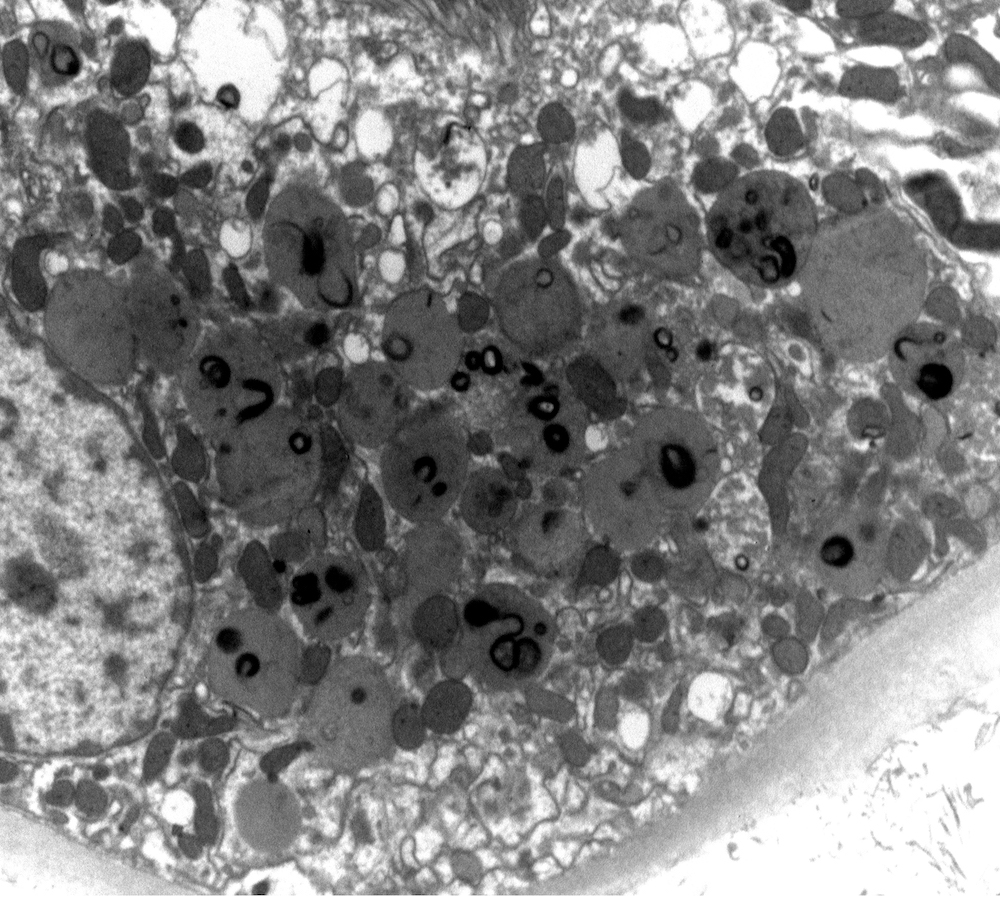
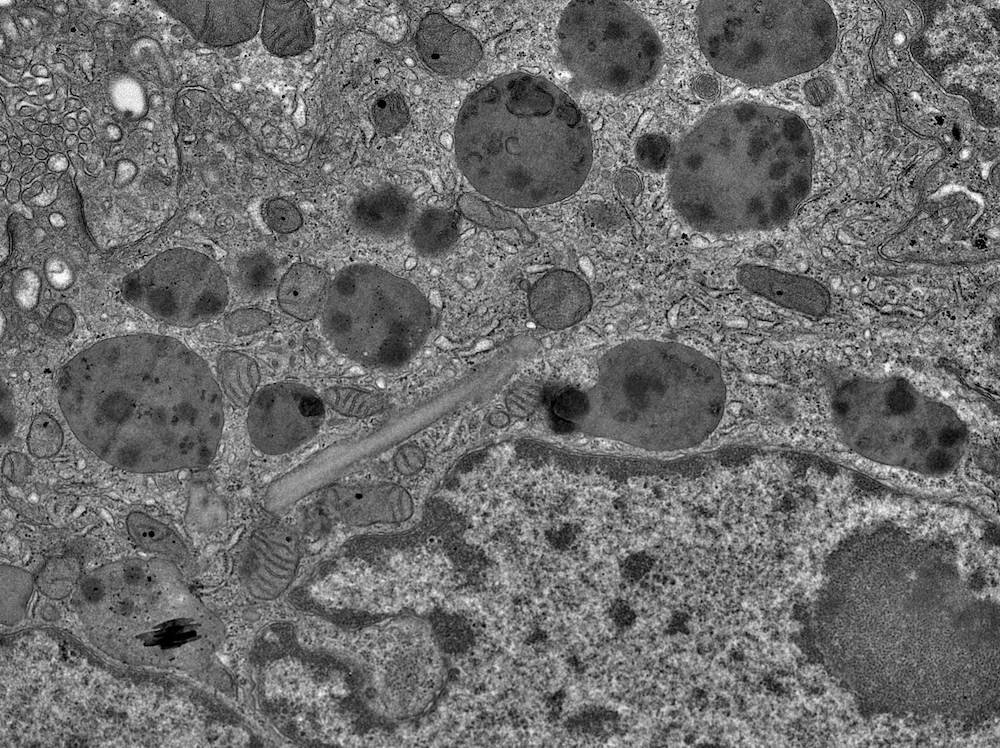
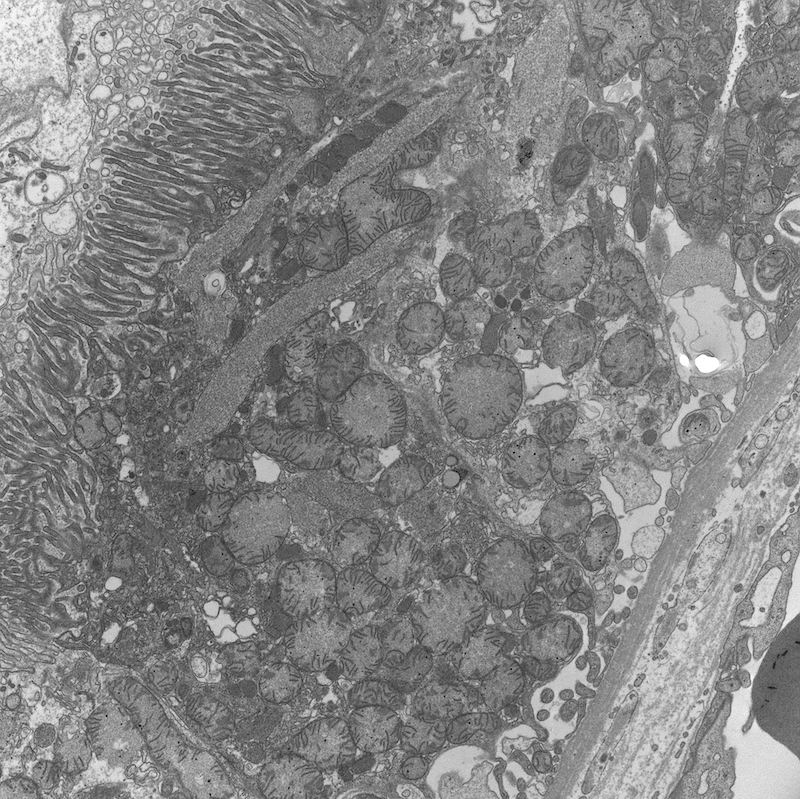
Electron microscopy description
- All findings restricted to tubular epithelial cells
- Intracytoplasmic electron dense or electron lucent crystals, usually with sharp edges (needle-like, rectangular, rhomboidal, polygonal or any other shape)
- Abnormal size, shape or number in intracytoplasmic lysosomes - increased number, large size, abnormal shape (including mottled lysosomes) and rarely, lysosomal packing, also known as lysosomal indigestion or constipation
- Mitochondrial swelling
- Tubular epithelial cell injury changes - segmental loss of brush border, cytoplasmic blebbing and vacuolization
- References: Mod Pathol 2011;24:1462, Arch Pathol Lab Med 2014;138:1365, BMC Nephrol 2020;21:146
Electron microscopy images
Sample pathology report
- Kidney, needle biopsy:
- Light chain proximal tubulopathy (see comment)
- Comment: Cytoplasmic accumulation of monoclonal kappa or lambda light chain (as the case may be) in the proximal tubules as confirmed by immunofluorescence fulfils criteria for LCPT diagnosis. Light microscopy and EM support the diagnosis. Congo red stain is negative. The findings are consistent with the patient’s documented history of X (if patient has a known diagnosis of any hematolymphoid neoplasm) OR clinical correlation with hematologic studies is recommended (if there is no known hematolymphoid diagnosis).
Differential diagnosis
- Monoclonal light or heavy chain deposition disease:
- Monoclonal light chain (LC) or rarely heavy chain (HC) deposition in powdery form along the tubular basement membrane, best visualized by immunofluorescence and electron microscopy
- Concurrent monoclonal LC or HC deposit in the glomerular basement membrane usually present
- Light chain cast nephropathy:
- Angulated sharp, often fractured LC casts filling tubular lumen
- Usually associated with tubular epithelial injury (acute tubular injury / acute tubular necrosis) and tubulointerstitial nephritis
- More commonly involves distal tubules and collecting ducts
- Amyloid cast proximal tubulopathy:
- Monoclonal light chain sharp looking casts filling tubular lumina and staining for Congo red
- Casts show characteristic amyloid type fibrillary structure (with diameter ranging from 8 - 12 nm) on electron microscopy
- Acute tubular injury with or without tubulointerstitial inflammation:
- Only tubular injury or interstitial inflammation without accumulation of monoclonal LC; for example, in allergic type tubulointerstitial nephritis
Additional references
Board review style question #1
A 54 year old woman presented with features of phosphaturia, glucosuria, proteinuria and proximal renal tubular acidosis. No significant medical history or family history is present. Her kidney biopsy showed kappa restricted staining pattern in proximal tubules on immunofluorescence. A microscopic image of the proximal tubules seen on kidney biopsy is shown above. No other histopathological changes are noted. What is the diagnosis?
- Interstitial nephritis
- Light chain proximal tubulopathy
- Light chain cast nephropathy
- Minimal change disease
Board review style answer #2
Board review style question #2
A 70 year old hypertensive man presented with recent onset of back pain, anemia, acute kidney injury and nonnephrotic proteinuria. A general body Xray showed few osteolytic lesions in vertebra and femur and blood tests showed hemoglobin of 8.5, ESR of 70, an abnormal M spike on serum protein electrophoresis and immunofixation result showing an abnormal IgG kappa protein. A kidney biopsy was done that showed acute tubular injury on light microscopy, restricted kappa staining in proximal tubules on immunofluorescence and increased number of mottled lysosomes on electron microscopy. What is the most likely underlying diagnosis in the patient?
- Ankylosing spondylosis
- Multiple myeloma
- Osteosarcoma
- Sarcoidosis
Board review style answer #2