Table of Contents
Definition / general | Essential features | Terminology | ICD coding | WHO classification (2022) - details | ICC classification (2022) | WHO classification (2016) | Epidemiology | Diagrams / tables | Diagnosis | Laboratory | Prognostic factors | Microscopic (histologic) images | Peripheral smear images | Sample pathology report | Additional references | Practice question #1 | Practice answer #1 | Practice question #2 | Practice answer #2Cite this page: Pakasticali N, Zhang L. MDS-general. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/bonemarrowneoplasticmdsgeneral.html. Accessed August 26th, 2025.
Definition / general
- Myelodysplastic neoplasms (MDS), previously called myelodysplastic syndromes, are clonal hematopoietic stem cell neoplasms characterized by ineffective marrow hematopoiesis that results in morphologic dysplasia and peripheral cytopenia; there is increased risk of transformation to acute myeloid leukemia (AML)
- This topic discusses myelodysplastic syndromes / neoplasms based on the WHO 2022 classification; differences between WHO 2022 and the ICS classification system are noted in Diagrams / tables below
Essential features
- The definition of cytopenia comes from clonal cytopenia of undetermined significance (CCUS), MDS and myelodysplastic / myeloproliferative neoplasm (MDS / MPN)
- 2 main categories are accepted for subclassification of MDS
- MDS with defining genetic abnormalities, which is encompassed by MDS-5q, MDS-SF3B1 and MDS with biallelic TP53
- MDS, morphologically defined, consisting of MDS with low blasts, MDS with increased blasts and MDS, hypoplastic
- There is also a third category named MDS of childhood
- The previous subclassification of MDS-U has been eliminated from the new version of WHO classification
- Clarifications for the 2022 WHO MDS diagnoses
- Criteria for cytopenia
- Anemia: hemoglobin < 13 g/dL in men, < 12 g/dL in women
- Leukopenia: absolute neutrophil count < 1.8 x 109/L
- Thrombocytopenia: low platelet count < 150 x 109/L
- Threshold for dysplasia remains at 10% dysplastic cells
- Criteria for cytopenia
- Reference: Blood 2016;127:2391
Terminology
- Per the fifth edition of WHO published in 2022, the terminology of myelodysplastic neoplasms has been introduced to replace the previous term of myelodysplastic syndromes (MDS), in parallel to myeloproliferative neoplasm and to emphasize the neoplastic course of the disease; however, the abbreviation of MDS is kept for myelodysplastic neoplasm
- Increased blasts is a term that has been adopted to replace excess blasts as previously used in the fourth edition of WHO classification
ICD coding
- ICD-10: D46 - myelodysplastic syndromes
WHO classification (2022) - details
- Myelodysplastic neoplasms with defining genetic abnormalities
- Myelodysplastic neoplasm with low blasts and 5q deletion (MDS-5q)
- Anemia, commonly macrocytic, transfusion dependent, with or without other cytopenias; thrombocytopenia is uncommon, instead, 33% of patients present with thrombocytosis (platelet count ≥ 450 x 109)
- Blasts < 5% in bone marrow and < 2% in peripheral blood
- Normo or hypercellular marrow
- Dysplasia involving megakaryocytes with characteristic features (megakaryocytes, intermediate in size, with single lobation), with or without dysplasia involving other lineages (see Microscopic (histologic) images)
- Often associated with erythroid hypoplasia
- Ring sideroblasts can be seen
- Detection of 5q deletion, isolated or with one other cytogenetic aberration but not including monosomy 7 or 7q deletion; not fulfilling diagnostic criteria of AML, MDS with biallelic TP53 inactivation, MDS with increased blasts or MDS / MPN
- MDS-5q with SF3B1 mutation (20% of the cases) are included in this category, as the SF3B1 mutation is likely a secondary event in this context
- Myelodysplastic neoplasm with low blasts and SF3B1 mutation (MDS-SF3B1)
- Detection of SF3B1 mutation with variant allele frequency (VAF) ≥ 5%; lower than the threshold is not considered diagnostic
- Cytopenia involving ≥ 1 lineages, most commonly anemia, without thrombocytosis (presence of thrombocytosis and SF3B1 without del(5q) belongs to MDS / MPN with SF3B1 mutation)
- Blasts < 5% in bone marrow and < 2% in peripheral blood
- Erythroid preponderance, mild left shifted, with megaloblastoid maturation or dysplasia; single or multilineage dysplasia can be observed in the subcategory, without impact on diagnosis or prognosis (Blood 2015;126:233, Blood 2020;136:157)
- Ring sideroblasts are often identified; however, an absence of ring sideroblasts in the setting does not exclude the diagnosis
- If SF3B1 mutation analysis is not available, demonstration of ring sideroblasts comprising ≥ 15% of erythroid precursors can be used as a surrogate
- Absence of 5q deletion, monosomy 7 / 7q deletion or complex karyotype
- Not fulfilling diagnostic criteria of AML, MDS with low blasts and 5q deletion, MDS with biallelic TP53 inactivation, MDS with increased blasts or any MDS / MPN type
- Myelodysplastic neoplasm with biallelic TP53 inactivation (MDS-biTP53)
- Detection of ≥ 1 TP53 mutations, meeting molecular diagnostic criteria of biallelic TP53 mutations (see Diagnosis)
- Cytopenia, dysplasia and < 20% blasts or 30% erythroblasts in bone marrow
- Next generation sequencing analysis or Sanger sequencing covering at least exons 4 - 11 of TP53 gene is required for detection of biallelic TP53 alterations, coupled with a technique to detect copy number status, usually fluorescence in situ hybridization (FISH) with a probe set specific for the TP53 locus on 17p13.1 (Leukemia 2014;28:241, Blood 2013;122:3616)
- Detection of ≥ 2 TP53 mutations, usually affecting both alleles that can be considered multihit status (Nat Med 2020;26:1549)
- After exclusion of constitutional changes, a TP53 VAF > 49% may be regarded as presumptive (not definitive) of copy loss on the trans allele or copy neutral loss of heterozygosity (LOH)
- In the presence of one TP53 mutation, evidence of TP53 copy loss or copy neutral LOH is required as concurrent 17p LOH, suggestive of biallelic TP53 alterations (Nat Med 2021;27:1239, Nat Commun 2020;11:4980)
- Characterized by increased blast counts, higher risk of leukemic transformation and higher risk of mortality
- Myelodysplastic neoplasm with low blasts and 5q deletion (MDS-5q)
- Myelodysplastic neoplasms, morphologically defined
- Myelodysplastic neoplasm with low blasts (MDS-LB)
- Low blasts are defined as < 5% in bone marrow and < 2% in peripheral blood
- Cytopenia involving ≥ 1 lineages
- Dysplastic changes in ≥ 1 lineages, involving at least 10% of cells
- Blasts < 5% in bone marrow and < 2% in peripheral blood
- Need to exclude folate and vitamin B12 deficiency
- No fulfilling diagnostic criteria of MDS with defining genetic alterations or hypoplastic MDS
- Myelodysplastic neoplasm, hypoplastic (h-MDS)
- Hypocellular bone marrow (adjusted for age of the patient); drug / toxin exposure or pertinent nutritional deficiency need to be excluded
- Cytopenia involving ≥ 1 lineages
- Dysplasia involving myeloid or megakaryocytic lineages
- Blasts < 5% in bone marrow and < 2% in peripheral blood
- Not meeting criteria for MDS with defining genetic abnormalities or MDS with increased blasts
- Myelodysplastic neoplasm with increased blasts (MDS-IB)
- Cytopenia involving ≥ 1 lineages
- Dysplastic changes in ≥ 1 lineages, involving at least 10% of cells
- Blasts ≥ 5% in bone marrow or ≥ 2% in peripheral blood
- No fulfilling diagnostic criteria of MDS with biallelic TP53 inactivation or AML
- Subclassification
- MDS with increased blasts 1 (MDS-IB1): 5 - 9% blasts in bone marrow or 2 - 4% blasts in peripheral blood, without significant reticulin fibrosis
- MDS with increased blasts 2 (MDS-IB2): 10 - 19% blasts in bone marrow or 5 - 19% blasts in peripheral blood, without significant reticulin fibrosis; or with presence of Auer rods
- MDS with increased blasts and fibrosis (MDS-F): 5 - 19% blasts in bone marrow or 2 - 19% blasts in peripheral blood, with significant reticulin fibrosis (defined as grade 2 or 3)
- Myelodysplastic neoplasm with low blasts (MDS-LB)
- Myelodysplastic neoplasms of childhood
- Childhood myelodysplastic neoplasm with low blasts (cMDS-LB)
- Myeloid neoplasm with cytopenia and dysplasia arising in children and adolescents (< 18 years of age)
- Cytopenia involving ≥ 1 lineage
- Dysplastic changes in ≥ 1 lineage, involving at least 10% of cells
- Blasts < 5% in bone marrow and < 2% in peripheral blood
- Meeting at least 1 of the following criteria
- Detection of clonal cytogenetic or molecular abnormality; monosomy 7 is the most common cytogenetic abnormality
- Need to exclude other causes of cytopenia (nonneoplastic and some germline mutations)
- Subclassification
- cMDS-LB, hypocellular
- cMDS-LB, not otherwise specified
- Childhood myelodysplastic neoplasm with increased blasts (cMDS-IB)
- Myeloid neoplasm with cytopenia and dysplasia arising in children and adolescents (< 18 years of age)
- Cytopenia involving ≥ 1 lineages
- Dysplastic changes in ≥ 1 lineages, involving at least 10% of cells
- Blasts 5 - 19% in bone marrow or 2 - 19% in peripheral blood
- Need to exclude Down syndrome, juvenile myelomonocytic leukemia and AML with defining genetic abnormalities
- Childhood myelodysplastic neoplasm with low blasts (cMDS-LB)
ICC classification (2022)
- See differences from WHO (2022) in Diagrams / tables
WHO classification (2016)
- MDS with single lineage dysplasia (MDS-SLD)
- MDS with ring sideroblasts
- MDS with ring sideroblasts and single lineage dysplasia (MDS-RS-SLD)
- MDS with ring sideroblasts and multiple lineage dysplasia (MDS-RS-MLD)
- MDS with multilineage dysplasia (MDS-MLD)
- MDS with excess blasts, type I and II (MDS-EB-I and MDS-EB-II)
- MDS with isolated del(5q) [MDS del(5q)]
- MDS, unclassifiable (MDS-U)
- Provisional: refractory cytopenia of childhood (RCC)
Epidemiology
- Median age of MDS is 77 years at diagnosis; < 10% are < 50 years old
- Incidence of MDS increases progressively with age from 60 to 90 years of age (from 2.2 to 56.8 cases per 100,000) (Blood Rev 2019;34:1)
- M > F except in MDS with low blasts and 5q deletion (MDS-5q) where F > M (Am J Med 2012;125:S2)
Diagrams / tables
| Comparison of guidelines | WHO 4th edition, 2016 | WHO 5th edition, 2022 | ICC 2022 | |
| Name of the entity | Myelodysplastic syndrome (MDS) | Myelodysplastic neoplasms (MDS) | Myelodysplastic syndromes (MDS) | |
| Morphologically defined | ||||
| Lineage | MDS-SLD MDS-MLD | Subclassification using dysplastic lineages removed, replaced with MDS-LB (MDS with low blasts) | MDS, NOS-SLD MDS, NOS-MLD | |
| Excessive blast counts in bone marrow (5 - 9%) or in peripheral blood (2 - 4%) | MDS-EB-I# | MDS-IB1 | MDS-EB* | |
| Excessive blast counts in bone marrow (10 - 19%) or in peripheral blood (5 - 19%) | MDS-EB-II# | MDS-IB2 | MDS-AML** | |
| Others | MDS-U | cMDS (MDS of childhood) MDS-h (hypoplastic MDS) MDS-f (MDS with fibrosis, blasts 5 - 19% in bone marrow, 2 - 19% in peripheral blood) | MDS, NOS (without dysplasia) | |
| Defining genetic abnormalities | ||||
| SF3B1 mutation | MDS-RS-SLD MDS-RS-MLD (5% ring sideroblasts if SF3B1 mutated, 15% ring sideroblasts if SF3B1 wild type) | MDS-SF3B1 (MDS with low blasts and SF3B1 mutation or MDS with ring sideroblasts if SF3B1 wild type) | MDS-SF3B1 (MDS with mutated SF3B1) or MDS, NOS (with ring sideroblasts and SF3B1 wild type) | |
| del(5q) | MDS with isolated del(5q) | MDS-5q (MDS with low blasts and 5q deletion) [5q deletion alone or with 1 other genetic aberration other than del(7q) / -7] | MDS with del(5q) [del(5q) is isolated or with up 1 genetic aberration except del(7q) / -7 or multihit TP53] | |
| TP53 | Not included in classification | MDS-biTP53 (MDS with biallelic TP53 inactivation) | MDS with mutated TP53 Multihit TP53 Includes MDS, MDS / AML, AML | |
- WHO 4th edition, 2016
- MDS-SLD: MDS with single lineage dysplasia
- MDS-MLD: MDS with multilineage dysplasia
- MDS-RS-SLD: MDS with ring sideroblasts and single lineage dysplasia
- MDS-RS-MLD: MDS with ring sideroblasts and multilineage dysplasia
- MDS-EB-I: MDS with excess blast I
- MDS-EB-II: MDS with excess blast II
- MDS del(5q): MDS with isolated del(5q)
- MDS-U: MDS, unclassifiable
- RCC: refractory cytopenia of childhood
- WHO 5th edition, 2022
- MDS-LB: MDS with low blasts
- MDS-IB1: MDS with increased blasts I
- MDS-IB2: MDS with increased blasts II
- cMDS: MDS of childhood
- MDS-h: hypoplastic MDS
- MDS-f: MDS with fibrosis
- MDS-SF3B1: MDS with low blasts and SF3B1 mutation or MDS with RS if SF3B1 wild type
- MDS-5q: MDS with low blasts and 5q deletion
- MDS-biTP53: MDS with biallelic TP53 inactivation
- ICC 2022
- MDS, NOS-SLD: MDS, not otherwise specified with single lineage dysplasia
- MDS, NOS-MLD: MDS, not otherwise specified with multilineage dysplasia
- MDS-EB: MDS with excess blasts
- MDS-AML: myelodysplastic syndrome / acute myeloid leukemia
- MDS-SF3B1: MDS with mutated SF3B1
- MDS, NOS: MDS, not otherwise specified
- * ICC MDS-EB, MDS with excess blasts (blast count 5 - 9% in bone marrow, 2 - 4% in peripheral blood)
- ** ICC MDS-AML: myelodysplastic syndrome / acute myeloid leukemia (blast count 10 - 19% in bone marrow and 2 - 4% in peripheral blood)
- # WHO 4th edition, 2016: MDS-EB including MDS-EB and erythroid preponderance and MDS-EB and fibrosis
Diagnosis
- If a patient presented with sustained cytopenia and suspected MDS, an extensive clinical and laboratory workup is necessary to exclude other etiologies that could clinically or morphologically mimic MDS (e.g., nutritional deficiency [e.g., folate acid, vitamin B12, copper deficiency], infection [e.g., HIV], medication induced marrow suppression [e.g., cytoreduction therapy], autoimmune disorders [e.g., rheumatoid arthritis, SLE])
- Imaging study
- To detect hepatomegaly or splenomegaly, which is usually not present in MDS patients but often seen with primary myelofibrosis or chronic myelomonocytic leukemia
- Cytogenetic consulting
- For the patients with germline gene mutations (e.g., DDX41, GATA2, RUNX1) or family history of MDS or other bone marrow failure syndromes
- Reference: Blood 2016;127:2391
Laboratory
- A series of laboratory tests should be ordered
- Routine complete blood count (CBC) data with differential count
- Reticulocyte percentage: reticulocytopenia is commonly found in bone marrow failure, including MDS
- Serum vitamin B12, folate level: within normal limits
- Erythropoietin (EPO) levels are usually lower than normal serum ferritin; elevated in the presence of inflammatory conditions such as rheumatoid arthritis
- Serum iron levels and total iron binding capacity, along with serum ferritin, may be helpful to exclude iron deficiency anemia
- HIV screening
- Screening for paroxysmal nocturnal hemoglobinuria (PNH) and T cell large granular lymphocytic leukemia
- Microscopic examination of bone marrow and peripheral blood smears to identify morphologic dysplasia and enumerate blasts
- Chromosome analysis, e.g., conventional karyotyping, fluorescence in situ hybridization using specific probes, most commonly including del(5q) / -5, del(7q) / -7, +8, del(17p) / TP53 and del(20q)
- Mutation analysis (e.g., next generation sequencing myeloid targeted mutation profiling certain gene mutations are often seen in MDS cells including DNMT3A, TET2, ASXL1, TP53, RUNX1, SRSF2 and SF3B1)
- Optional flow cytometric analysis for immunophenotyping for monocyte subset, blasts or altered myeloid maturation (Blood 2014;123:3675)
- Immunohistochemical staining for assessment of blasts, abnormal localization of immature precursors, dysplastic megakaryocytes, p53 expression and altered trilineage distribution
Prognostic factors
- Myelodysplastic neoplasm with low blasts and 5q deletion (MDS-5q)
- Good prognosis cytogenetic subgroup in the Revised International Prognostic Scoring System (Blood 2012;120:2454)
- Overexpressed p53 by IHC (≥ 1%, strong single) is associated with higher risk for AML transformation (Haematologica 2014;99:1041)
- Concurrent TP53, RUNX1, TET2 or single SF3B1 mutations may predict worse outcomes in MDS-5q (Leukemia 2016;30:1956, Blood 2020;136:157)
- Myelodysplastic neoplasm with low blasts and SF3B1 mutation (MDS-SF3B1)
- Favorable outcome among MDS types (Blood 2020;136:157)
- Concurrent mutations with TP53, RUNX1, EZH2 and FLT3 are associated with worse outcome (Blood 2020;136:157, Blood 2021;138:989)
- Presence of other molecular mutations (e.g., BCOR, BCORL1, NRAS, RUNX1, SRSF2 or STAG2) also alter clinical outcome when compared with single SF3B1 mutation
- Favorable outcome among MDS types (Blood 2020;136:157)
- Myelodysplastic neoplasm with biallelic TP53 inactivation (MDS-biTP53)
- Higher risk of leukemic transformation and higher risk of mortality, independent of Revised International Prognostic Scoring System risk categorization and independent of treatment (Nat Med 2020;26:1549)
- Similar adverse clinical outcomes are observed in MDS patients with mutated TP53 (VAF ≥ 40%) or complex cytogenetics
- Myelodysplastic neoplasm with low blasts (MDS-LB)
- Prognosis is usually linked to the degree of cytopenia (Blood 2012;120:2454)
- Myelodysplastic neoplasm, hypoplastic (h-MDS)
- Prognosis is better in this subtype of MDS (Leukemia 2022;36:1947)
- Myelodysplastic neoplasm with increased blasts (MDS-IB)
- Presence of fibrosis and CD7 expression were linked with worse outcome (Mod Pathol 2014;27:681, Blood 2002;100:3887)
- Childhood myelodysplastic neoplasm with low blasts (cMDS-LB)
- Patients with monosomy 7, 7q deletion or complex karyotype usually progress to AML and typically treated with hematopoietic stem cell transplantation (Blood 2018;131:1406)
- Normal karyotype or trisomy 8 are usually associated with a stable disease (Br J Haematol 2011;154:185)
- Patients with monosomy 7, 7q deletion or complex karyotype usually progress to AML and typically treated with hematopoietic stem cell transplantation (Blood 2018;131:1406)
- Childhood myelodysplastic neoplasm with increased blasts (cMDS-IB)
- Complex karyotype is associated with worse outcome (Nat Med 2021;27:1806)
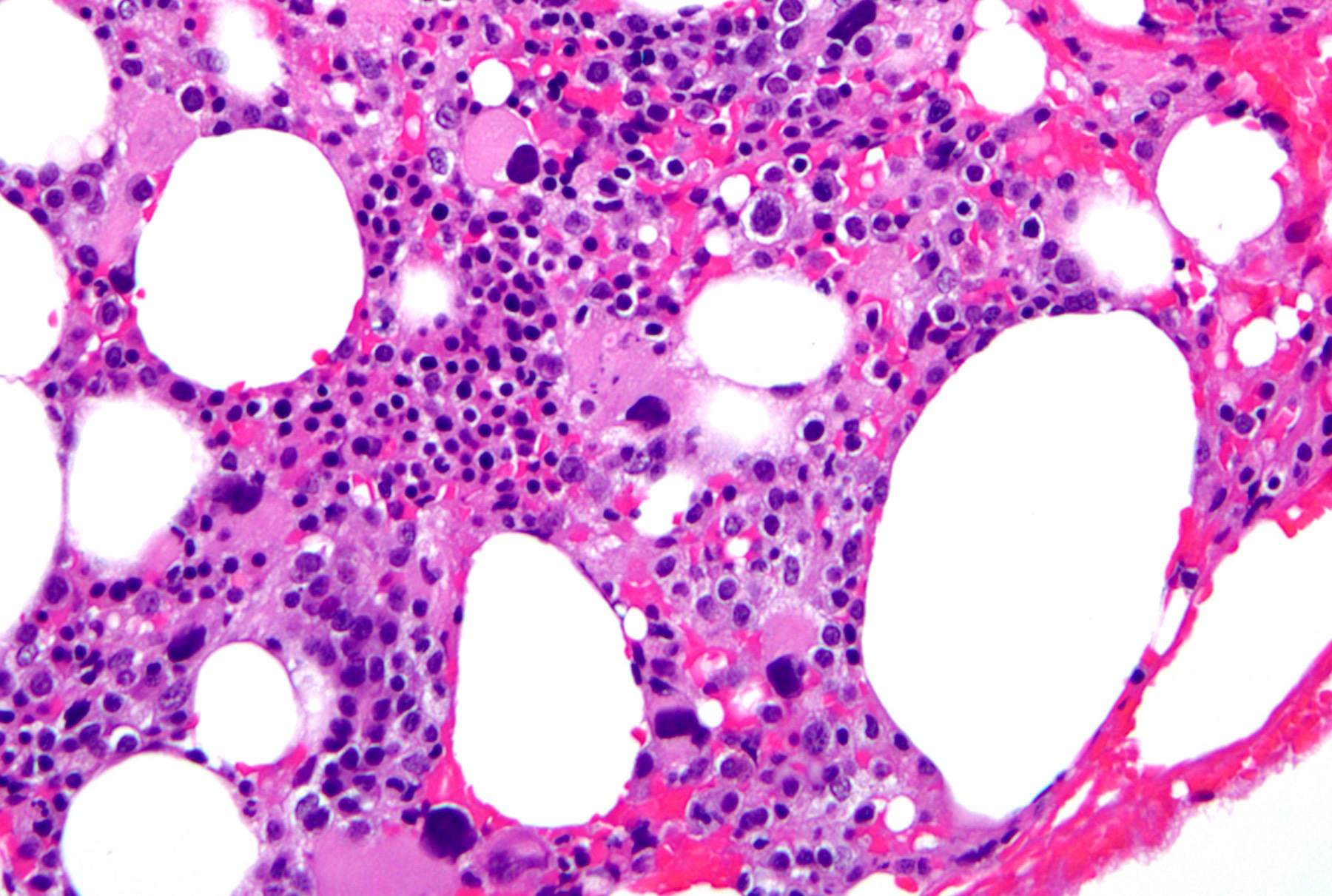
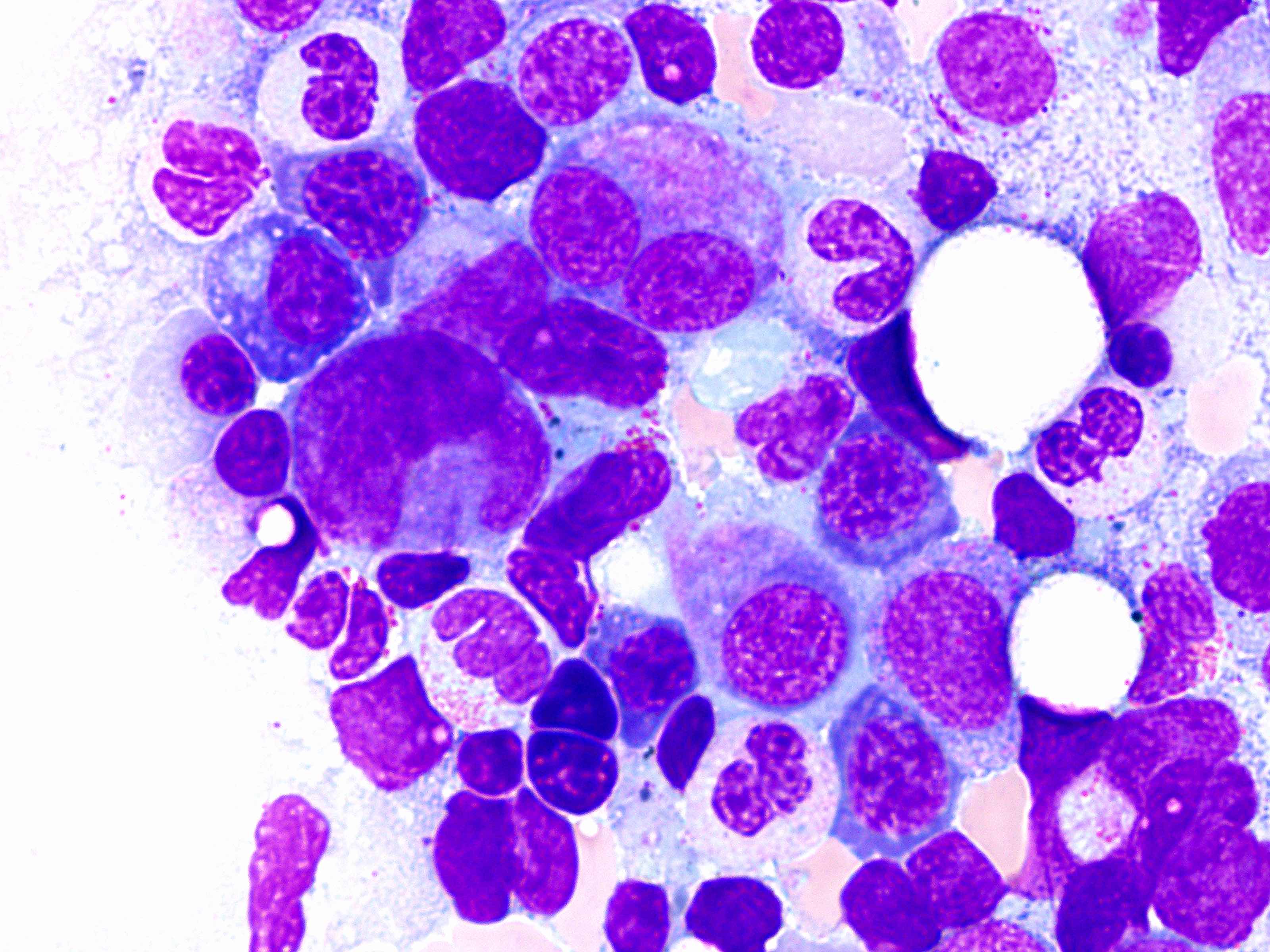
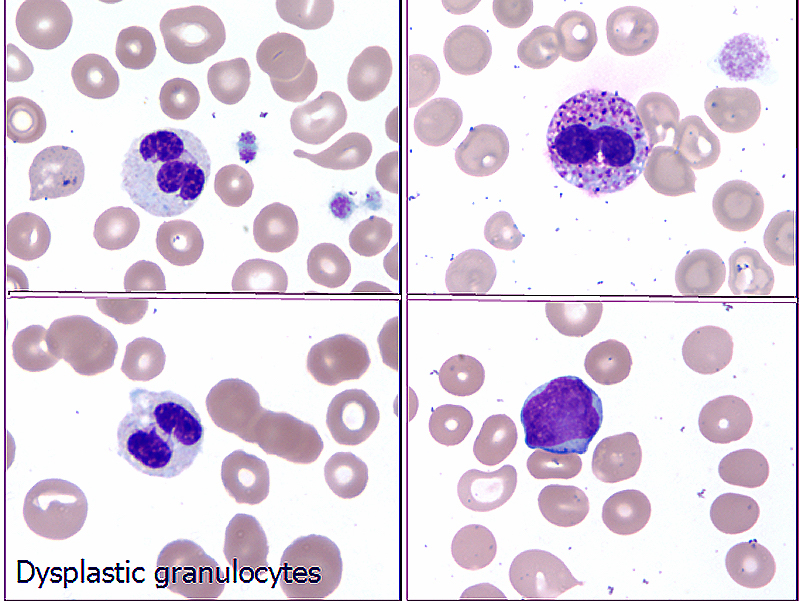
Peripheral smear images
Sample pathology report
- Bone marrow, right posterior iliac crest, biopsy and aspiration:
- Hypercellular marrow (90%) with trilineage dyspoiesis and increased blasts (7%) consistent with myelodysplastic syndrome with increased blasts (MDS-IB) (see comment)
- Comment: Immunochemistry with CD34 and CD117 confirms the presence of increased proportion of blasts. Mild diffuse reticulin fibrosis is noted. FISH MDS panel shows both del(5q) and del(7q). No BCR::ABL1 rearrangement is identified. Karyotyping shows 46,XY,del(5q)(27;q33),del(7q)(q22),+21[10]/46,XY[10] and NGS study reveals several tier 1 pathologic mutations involving ASXL1 (VAF of 27%), DNMTA (VAF of 33%) and RUNX1 (VAF of 6.5%). There are no JAK2, NPM1 or TP53 mutations detected.
Additional references
Practice question #1
Which of the following is a correct statement regarding myelodysplastic neoplasm (MDS) with low blasts and SF3B1 mutation?
- Absence of ring sideroblasts excludes the diagnosis
- Blasts are > 5% in bone marrow
- Detection of SF3B1 mutation with any percentage of variant allele frequency is considered diagnostic
- Favorable outcome among MDS types
- If SF3B1 mutation analysis is not available, demonstration of ring sideroblasts comprising ≥ 5% of erythroid precursors can be used as a surrogate
Practice answer #1
D. Favorable outcome among MDS types. Answer C is incorrect because detection of SF3B1 mutation with variant allele frequency (VAF) should be ≥ 5%. Lower than the threshold is not considered diagnostic. Answer A is incorrect because ring sideroblasts are often identified, however, an absence of ring sideroblasts in the setting does not exclude the diagnosis. Answer B is incorrect because blasts are < 5% in bone marrow and < 2% in peripheral blood in MDS-SF3B1. Answer E is incorrect because if SF3B1 mutation analysis is not available, demonstration of ring sideroblasts comprising ≥ 15% of erythroid precursors can be used as a surrogate (Blood 2020;136:157).
Comment Here
Reference: MDS-general
Comment Here
Reference: MDS-general
Practice question #2
Which of the following is correct regarding myelodysplastic neoplasm (MDS) with TP53 inactivation?
- Associated with low risk disease
- Presence of biallelic TP53 mutations is required for diagnosis
- Rarely transforms to acute myeloid leukemia
- Sensitive to conventional therapies
Practice answer #2
B. Presence of biallelic TP53 mutations is required for diagnosis. It is classified as MDS with biallelic TP53 inactivation. Answer A is incorrect because MDS with biallelic TP53 inactivation is associated with a worse prognosis. Answer C is incorrect because MDS with biallelic TP53 inactivation has a higher risk to transform to acute myeloid leukemia. Answer D is incorrect because MDS with biallelic TP53 inactivation is usually resistant to conventional therapies.
Comment Here
Reference: MDS-general
Comment Here
Reference: MDS-general