Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Practice question #1 | Practice answer #1 | Practice question #2 | Practice answer #2Cite this page: Kapur RP. Hirschsprung disease. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/colonhirschsprung.html. Accessed September 8th, 2025.
Definition / general
- Absent enteric ganglion cells in submucosal and myenteric plexuses in distal rectum and variable length of contiguous intestine, causing functional obstruction and colonic dilation proximal to affected segment (eMedicine: Hirschsprung Disease [Accessed 9 January 2020])
Essential features
- Diagnostic: Absent ganglion cells, submucosal nerve hypertrophy, absent calretinin immunoreactive mucosal innervation and increased cholinergic mucosal innervation (AChE histochemistry or ChT immunohistochemistry)
- Intraoperative:
- Levelling biopsy: identification of ganglion cells
- Frozen section of proximal resection margin: exclude features of transition zone (partial circumferential aganglionosis, myenteric hypoganglionosis, submucosal nerve hypertrophy)
Terminology
- Also called congenital aganglionic megacolon
- Short segment (~80% of patients): aganglionic portion limited to rectosigmoid colon; M:F ≈ 4:1
- Long segment (~20% of patients): aganglionic segment extends proximal to the sigmoid colon, may extend into small bowel; M:F approaches 1:1 as length of aganglionic segment increases; some regard long segment as proximal to splenic flexure and “colonic” as aganglionosis extending to somewhere between sigmoid colon and splenic flexure
- Very (ultra) short segment: aganglionic portion restricted to < 2 cm of the distal most rectum; more common in boys; potentially more difficult to document because this portion of rectum is normally hypoganglionic
- Total colonic aganglionosis (TCA): uncommon; M:F ≈ 1:1; clinical and genetic differences; rectal biopsy may lack submucosal nerve hypertrophy; more difficult to diagnose and manage (Pediatr Surg Int 2016;32:221)
- Pan-intestinal aganglionosis: very rare; aganglionosis of entire small and large intestines
- Skip area (or skip lesion): aganglionic segment is interrupted by a focus or segment of ganglionic bowel; proximal aganglionic portion frequently includes appendix + / - adjacent cecum and distal ileum
- Zonal colonic aganglionosis: interstitial aganglionic segment flanked proximally and distally by ganglionic bowel; distal rectum innervated normally; pathogenesis (likely secondary disruption as opposed to malformation) considered different from classic Hirschsprung disease
- Transition zone: ganglionic but neuroanatomically abnormal, bowel located proximal to aganglionic segment; typically < 5 cm in short segment aganglionosis; may be longer in long segment aganglionosis; may cause persistent obstructive symptoms if not resected with aganglionic segment (Am J Surg Pathol 2016;40:1637)
Epidemiology
- Average: 1 in 5,000 live births (0.75 in Caucasians; 2.8 in Asians) (Clin Genet 2020;97:114)
- Male predominance for short segment aganglionosis (3 - 4.5:1)
- ~80% sporadic
- ~18% have other congenital anomalies; some with specific syndromes
- ~12% have major chromosomal variants; ~10% associated with Down syndrome
- About 5% have neurologic abnormalities that may be serious
- Occasionally associated with intestinal atresia and malrotation, anorectal malformations and other abnormalities of the GI tract
- 5% have a sibling with disease; variation in risk depending on sex, segment length and familiality
- Patients usually present as neonates; 80% present before their first birthday but up to 10% of cases are diagnosed in adulthood
Sites
- Classic Hirschsprung disease invariably includes aganglionosis of the distal rectum and variable length of contiguous bowel
- Short segment, long segment, total colonic and pan-intestinal forms as defined above
Pathophysiology
- Absence of enteric ganglia causes a loss of intrinsic excitatory and inhibitory innervation; net effect is spastic contraction of aganglionic segment, including internal anal sphincter, with impaired peristalsis and defecation
- Bowel proximal to the aganglionic segment undergoes muscular hypertrophy and dilation
- Abnormal extrinsic innervation (enlarged cholinergic nerves) in aganglionic rectum + / - more proximal aganglionic left colon may contribute to spasticity
- Numerous alterations in expression of various receptors, channels, cytoskeletal proteins, neurotrophic factors and other molecules described in ganglionic bowel but functional significance unclear (Pediatr Dev Pathol 2020;23:40)
Etiology
- Proximate cause is failure of neural crest derived neuroglial progenitors to colonize the entire length of the intestinal tract (Dev Biol 2016;417:158)
- Complex genetic basis with at least 24 susceptibility genes; variable penetrance for most genetic alterations; combinatorial effects of low penetrance gene variants (N Engl J Med 2019;380:1421)
- Coding variants and large copy number variants associated with highest risk but less frequently implicated than noncoding variants
- Coding or noncoding mutations RET proto-oncogene appear to play some role in the majority cases
Diagrams / tables
Clinical features
- Most common presentation: failure to pass meconium within 48 hours of birth
- Other findings may include abdominal distension, bilious emesis, explosive defecation in response to manual anal dilatation, bowel perforation and enterocolitis
- Older children may have chronic constipation and failure to thrive
- Down syndrome patients more commonly have associated enterocolitis
- 80% male; usually sporadic (1 per 5,000 live births); 5% have affected sibling
- 10% have Down syndrome; another 5% have other serious neurologic impairment
- Complications: proximal innervated colon may become massively distended (15 cm in diameter) with muscular wall hypertrophy and rupture / perforation, usually near cecum or appendix; also acute intestinal obstruction, enterocolitis with fluid and electrolyte imbalance
- Mortality: currently 5 - 10%
- Eur J Pediatr Surg 2008;18:140
Diagnosis
- Screening tests
- Anorectal manometry - absent rectoanal inhibitory reflex (RAIR, relaxation of internal anal sphincter in response to balloon dilatation of the rectum)
- Contrast enema - ratio of rectal diameter to sigmoid diameter is < 1
- Rectal biopsy, either suction or incisional
- Suction rectal biopsy: most common, does not require sedation in neonate or young infant, samples mucosa and submucosa; no direct visualization of rectum or anal canal; recommend multiple biopsies at different sites (e.g., 2, 3 and 4 cm above the anal verge)
- Incisional biopsy: requires anesthesia, some prefer this for patients > 1 year of age, may sample myenteric plexus in addition to mucosa and submucosa; direct visualization of dentate line
- Pediatr Dev Pathol 2020;23:8
Case reports
- Newborn with volvulus, intestinal malrotation and total colonic aganglionosis (J Neonatal Perinatal Med 2019)
- 2 day old girl with colonic atresia and Hirschsprung disease (Arch Iran Med 2015;18:322)
- 10 day old boy with Hirschsprung disease and a distal skip area (Fetal Pediatr Pathol. 2019;38:437)
- 21 year old man and 22 year old woman who presented with Hirschsprung disease as young adults (Ann Med Surg (Lond) 2019;48:59)
- Biopsy findings in an infant with trisomy 21 and very short segment Hirschsprung disease (Pediatr Dev Pathol 2016;19:87)
Treatment
- Surgical: excision of aganglionic segment and transition zone (ganglionic but neuroanatomically abnormal bowel immediately proximal to the aganglionic segment) with a pullthrough procedure to anastomose ganglionic bowel just superior to the anal canal
- One stage (resection and anastomosis) or two stage (ostomy then later resection and anastomosis)
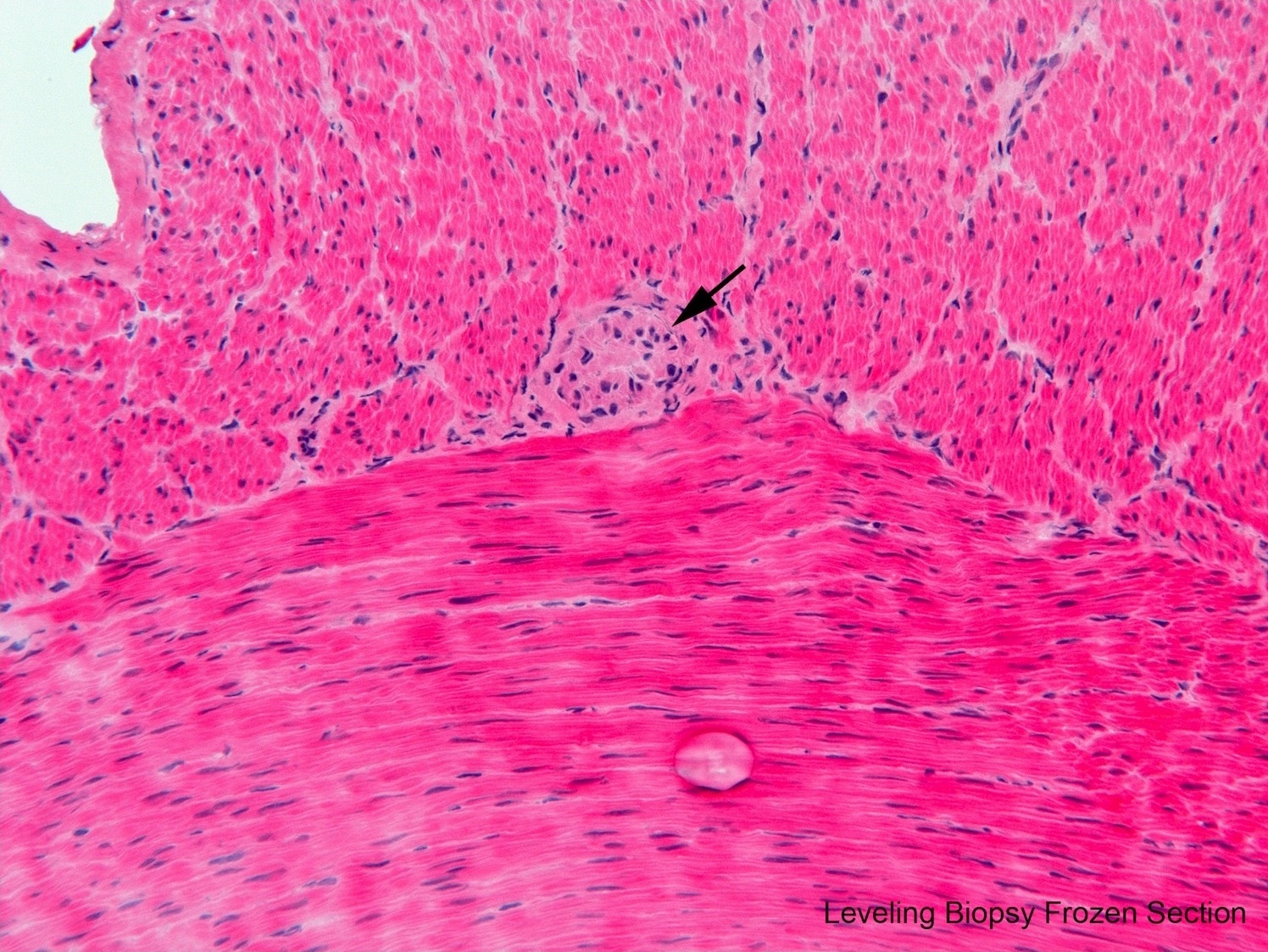
- Levelling biopsy with intraoperative frozen section should be performed during either procedure to insure ganglionic bowel proximal to aganglionic segment is used for anastomosis
- Biopsy is seromuscular or full thickness
- Identification of myenteric ganglion cells is the key; rebiopsy of more proximal bowel is necessary until ganglion cells are located
- 5 - 10 well oriented sections is usually sufficient
- H&E is satisfactory; some prefer Diff-Quik, Giemsa or toluidine blue
- Use of Giemsa, Diff-Quik or toluidine blue stains in addition to routine H&E has been advocated to improve diagnostic accuracy at frozen section
- Presence of ganglion cells in seromuscular biopsy does not exclude transition zone
- To reduce likelihood of transition zone pullthrough or ostomy, intraoperative frozen section of a full circumference (donut) section from the proximal resection margin (or ostomy site) is recommended (Pediatr Dev Pathol 2020;23:23)
- Histological features of transition zone include:
- Partial circumferential aganglionosis (⅛ or more of circumference devoid of ganglion cells)
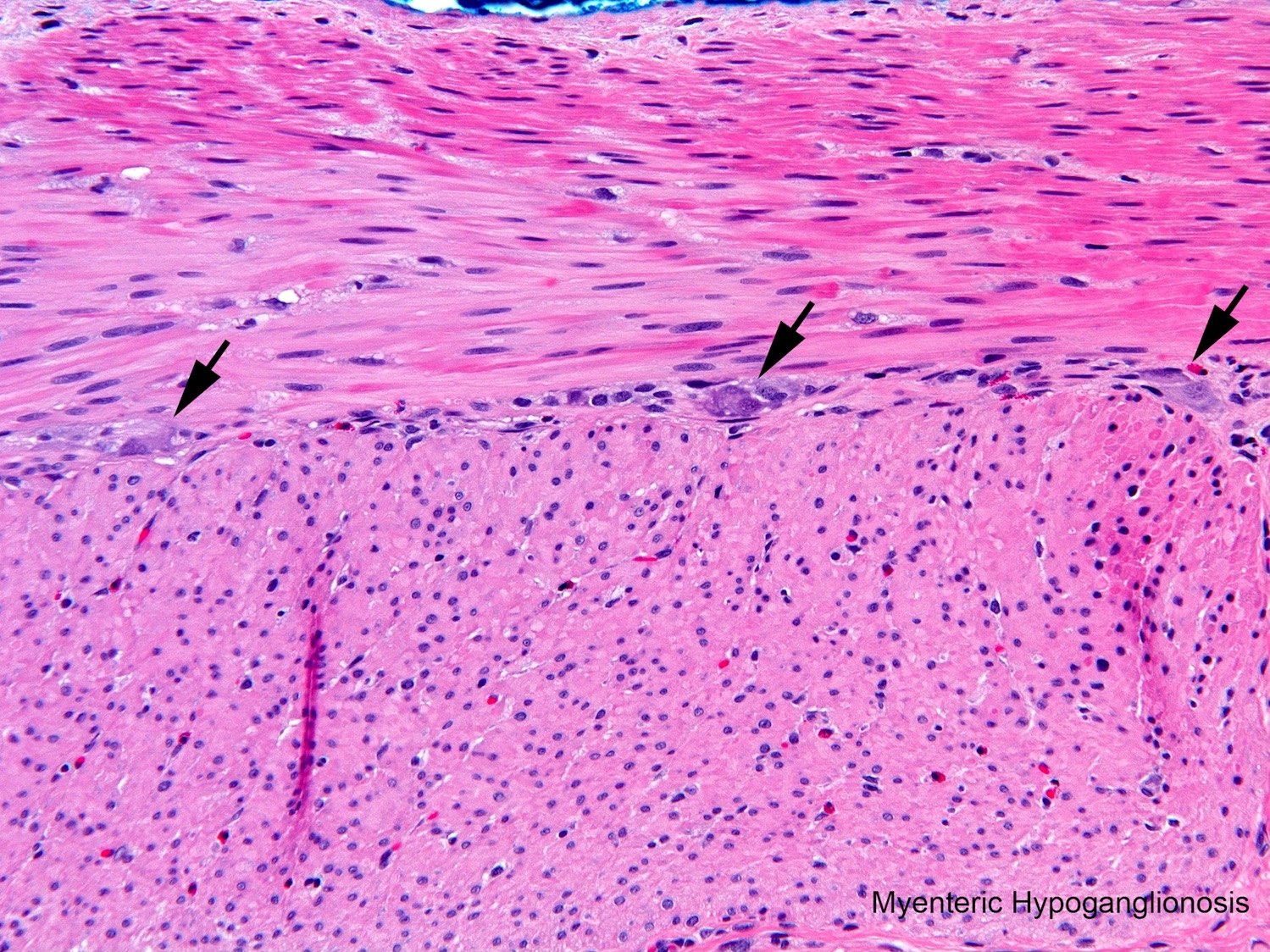
- Myenteric hypoganglionosis (⅛ or more of circumference predominantly tiny ganglia with 1 or 2 ganglion cells and minimal neuropil)
- Submucosal nerve hypertrophy (e.g., > 2 submucosal nerves > 40 µm diameter in a 400x field)
- Histological features of transition zone include:
- Surgery successful in most patients
- Treatment failures may be due to transition zone pullthrough, strictures at anastomosis site, twists proximal to the anastomosis, excessive retained aganglionic distal rectum, post-surgical loss of ganglion cells proximal to anastomosis or poorly understood physiological abnormalities in the ganglionic bowel
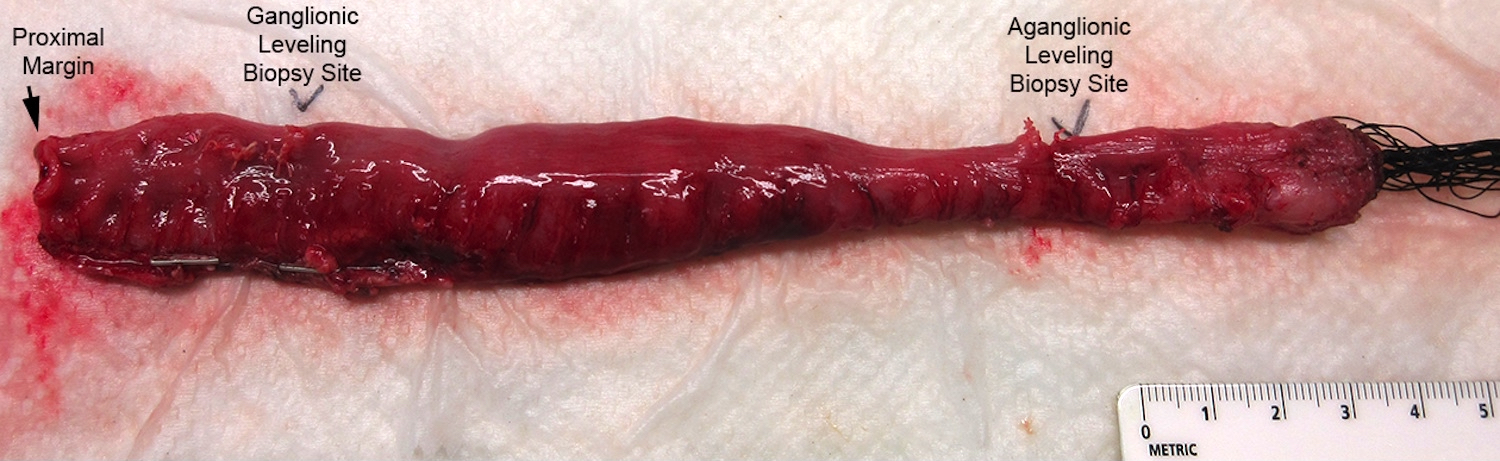
Gross description
- Aganglionic segment typically narrow with dilated proximal ganglionic bowel and funnel shaped transition near interface
- Resection specimen from Soave pullthrough procedure lacks muscularis propria distally; sleeve of submucosa and mucosa at distal end
- Resection specimen from Swenson or Duhamel pullthrough procedure is full thickness throughout
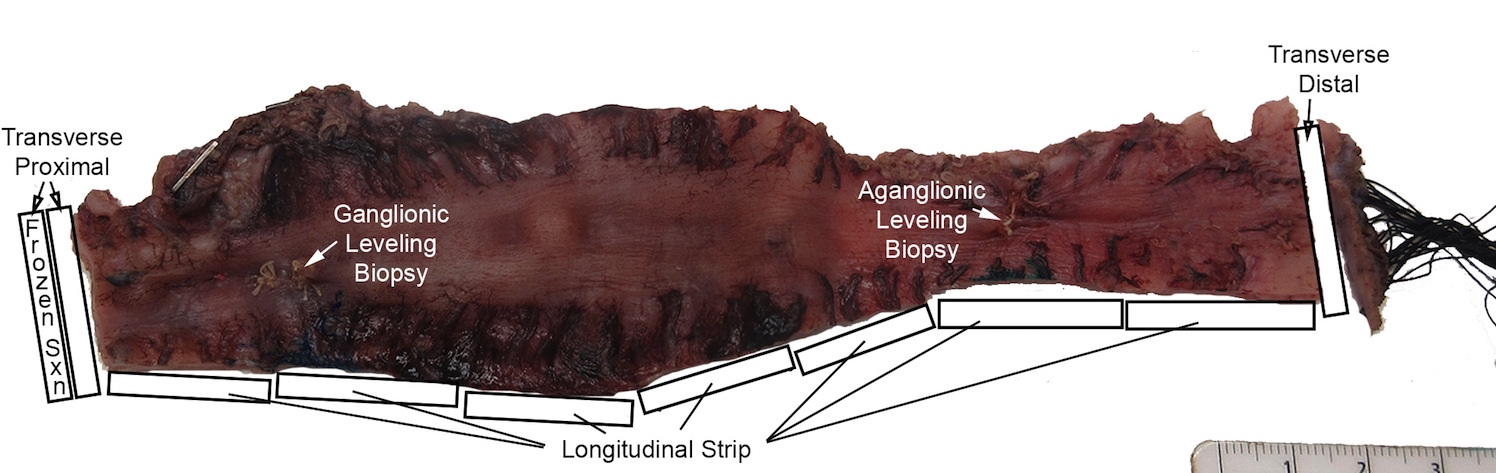
- Histological sampling of resection should include:
- Full circumference adjacent to proximal margin (confirm normal neuroanatomy)
- Full circumference at distal margin (confirm aganglionosis)
- One or more longitudinal strip of entire resection or closely spaced transverse sections along the length of the resection (establish length of aganglionic segment)
- J Pediatr Surg 2019;54:2017
Gross images
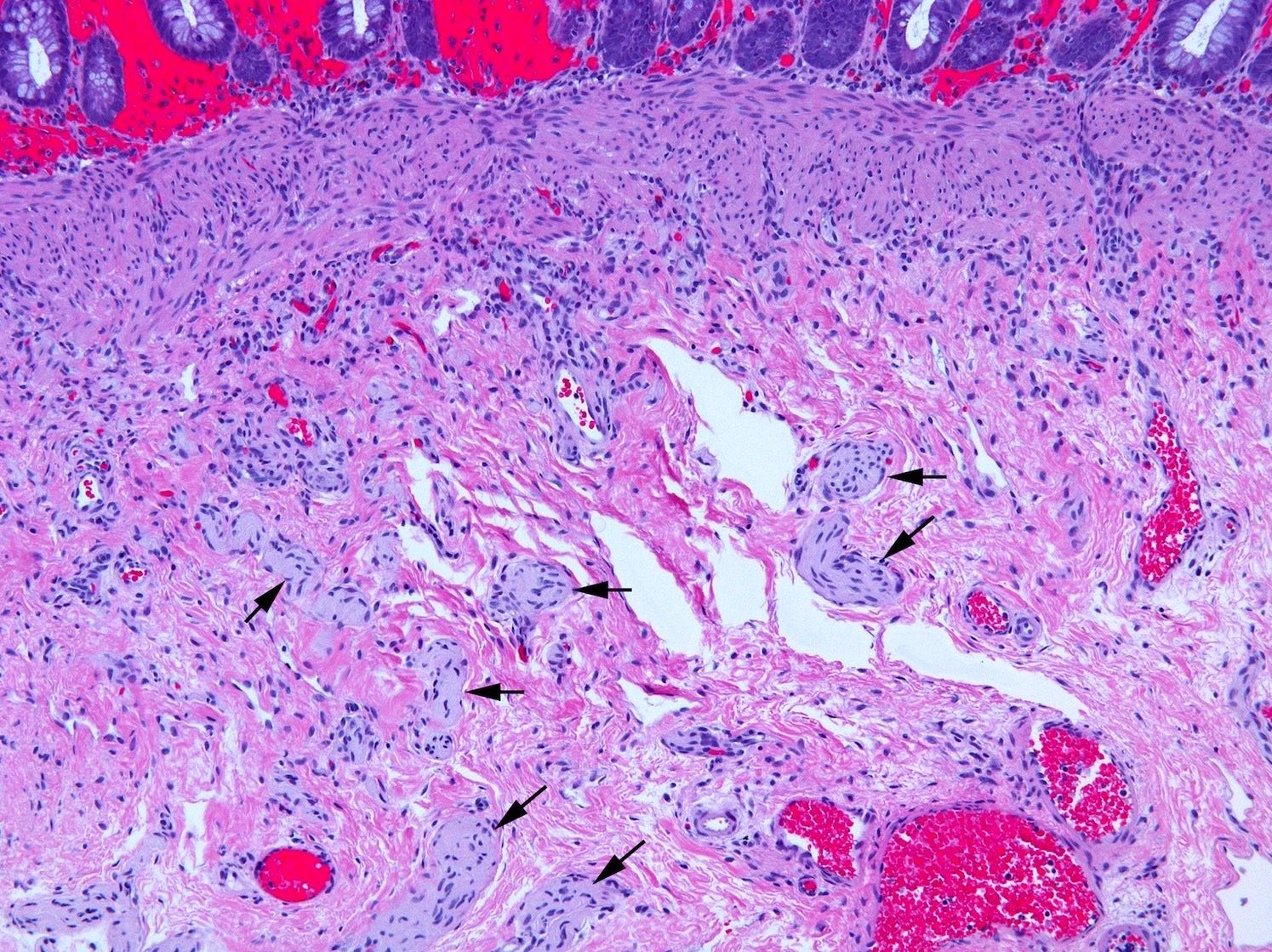
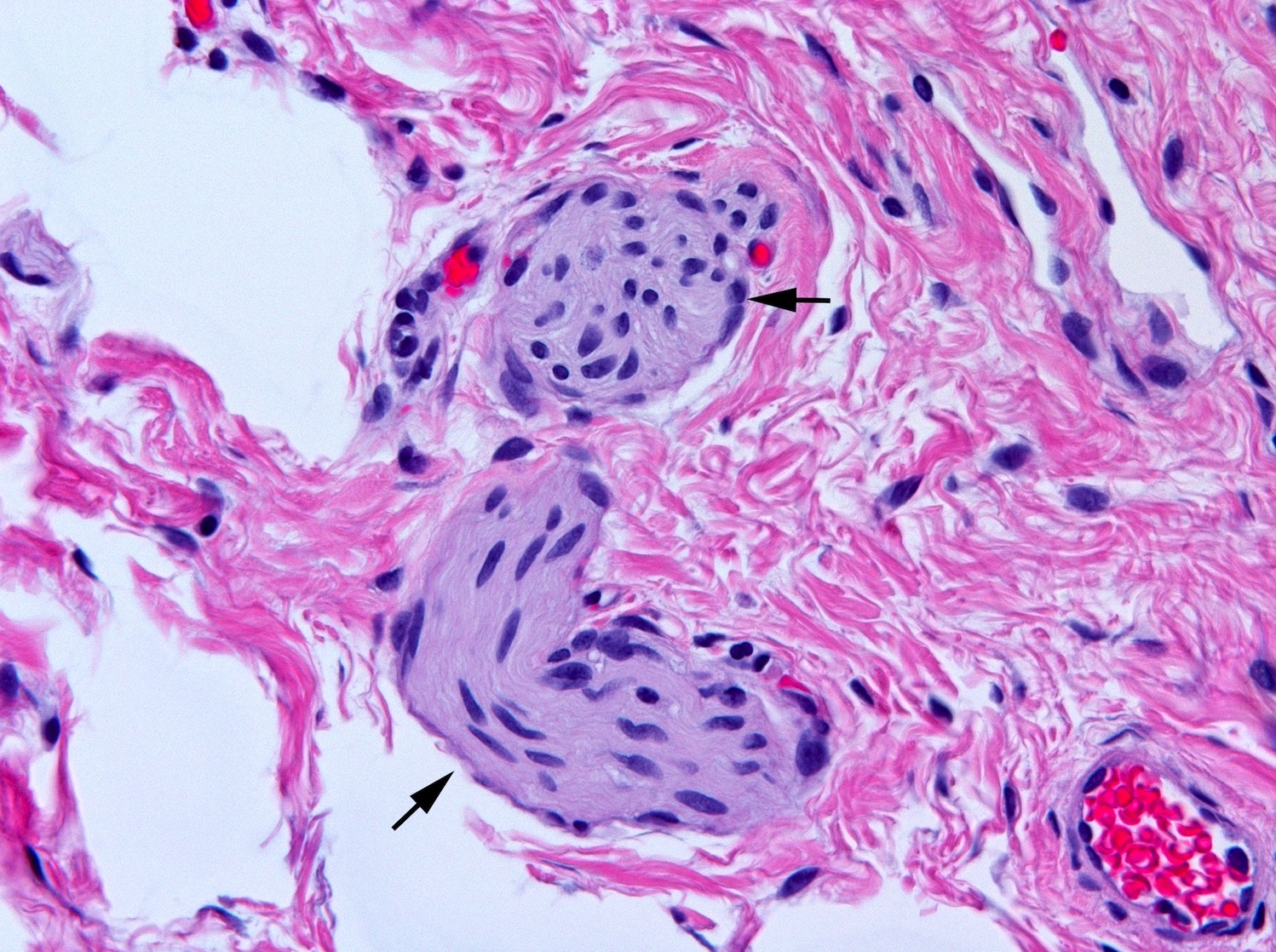
Microscopic (histologic) description
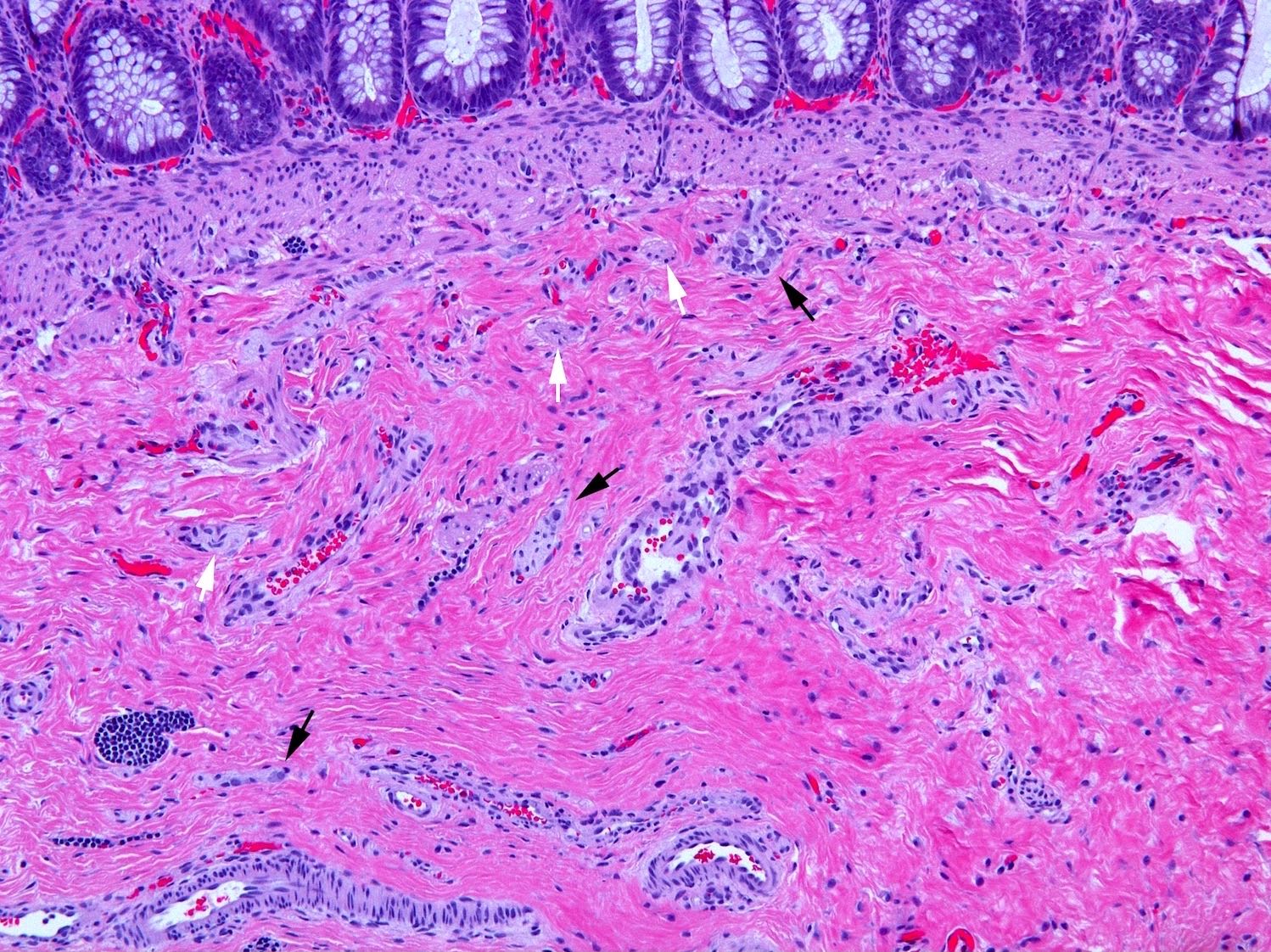
- Aganglionic segment
- Complete absence of submucosal and myenteric ganglion cells
- Submucosal and myenteric nerve hypertrophy in aganglionic rectum; gradually lost in more proximal aganglionic colon; may not be prominent in total colonic aganglionosis; usually assessed subjectively but in neonate nerves > 40 µm in diameter are abnormal; enlarged nerves have extrinsic features including Glut1 positive perineurium but occasional Glut1+ nerves are also present in normal rectum
- Transition zone (any of the following)
- Partial circumferential aganglionosis (⅛ or more of circumference devoid of ganglion cells)
- Myenteric hypoganglionosis (⅛ or more of circumference predominantly tiny ganglia with 1 or 2 ganglion cells and minimal neuropil)
- Submucosal nerve hypertrophy (e.g., > 2 submucosal nerves > 40 µm diameter in a 400x field)
- Ganglia in newborns are smaller than in older children and nucleoli and Nissl substance may be lacking, a situation even more pronounced in premature infants
- Myenteric ganglia in the transition zone may be ectopically situated within the muscularis externa or serosa
- Associated nerve hypertrophy is a helpful clue but is not diagnostic
- Active mucosal inflammation (enterocolitis)
- Stercoral ulcers (sharply demarcated shallow ulcers with mucosal inflammation due to pressure of feces on obstructed colon)
- Fibromuscular dysplasia of submucosal or seromuscular arteries in aganglionic segment or transition zone (Pediatr Dev Pathol 2018;21:363)
Microscopic (histologic) images
Contributed by Raj P. Kapur, M.D., Ph.D.
Positive stains
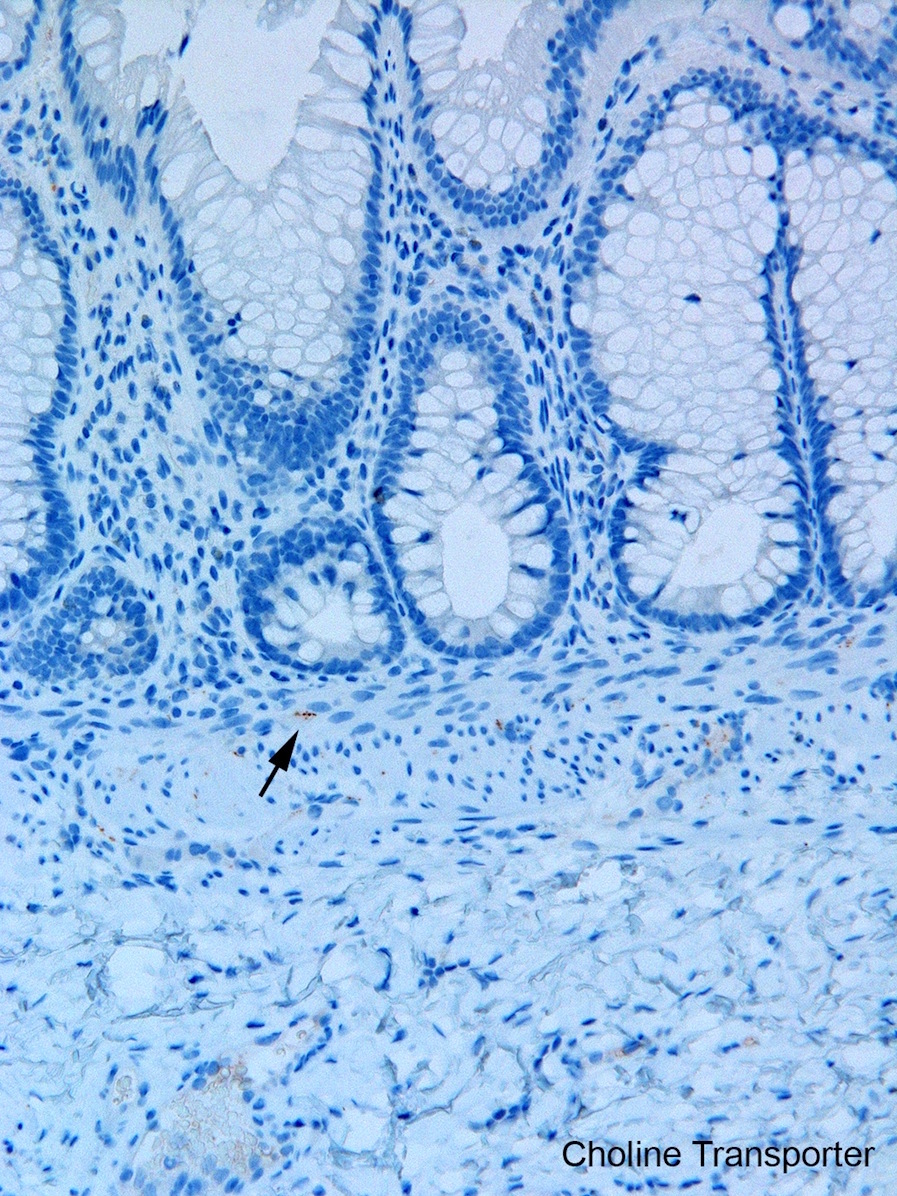
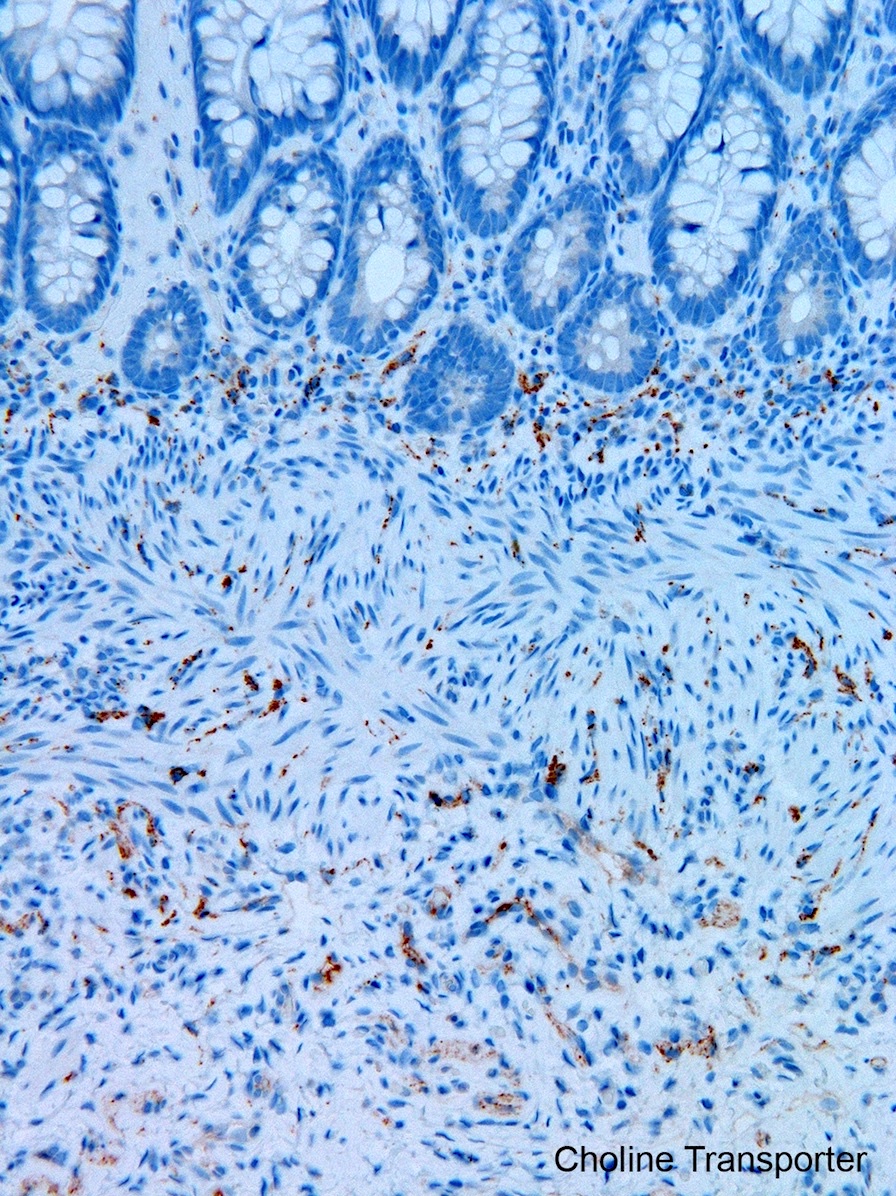
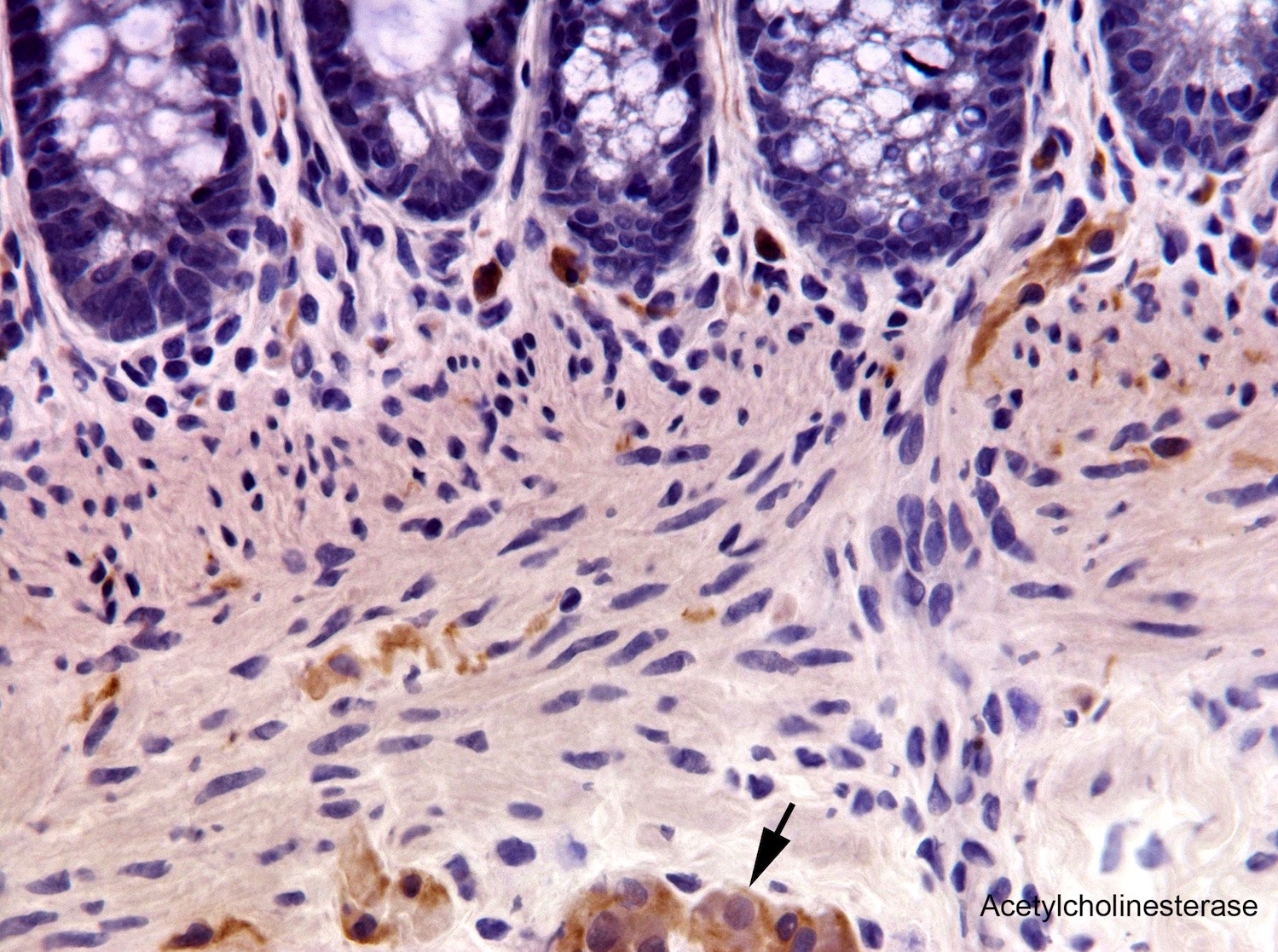
- Most diagnostically useful are acetylcholinesterase histochemistry (AChE) or choline transporter immunohistochemistry; both demonstrate increased cholinergic innervation in lamina propria and muscularis mucosa
- AChE requires frozen sections and special methodology (Fetal Pediatr Pathol 2016;35:399)
- Choline transporter immunohistochemistry: formalin fixation and paraffin embedding; relatively new and has not been validated in many laboratories; may be more challenging to interpret than AChE (Pediatr Dev Pathol 2017;20:308)
Negative stains
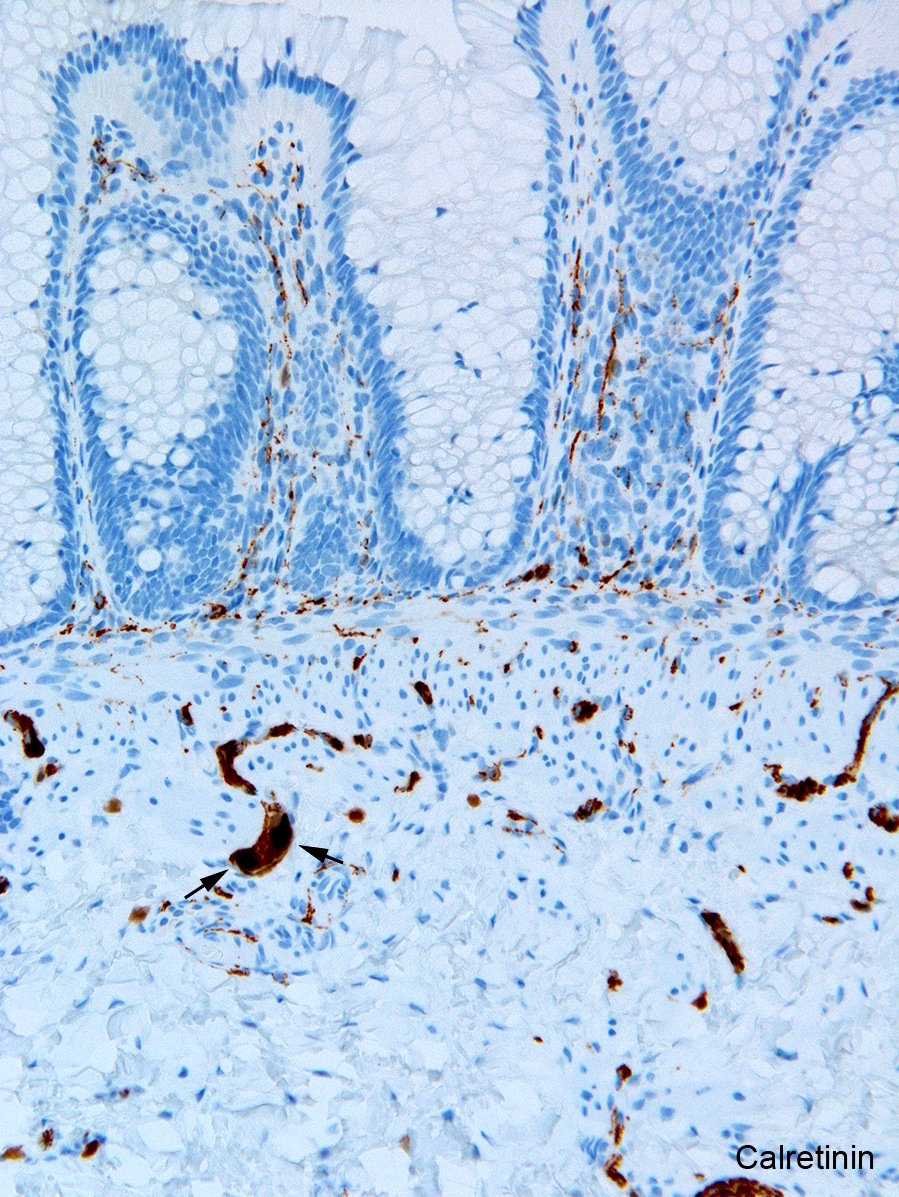
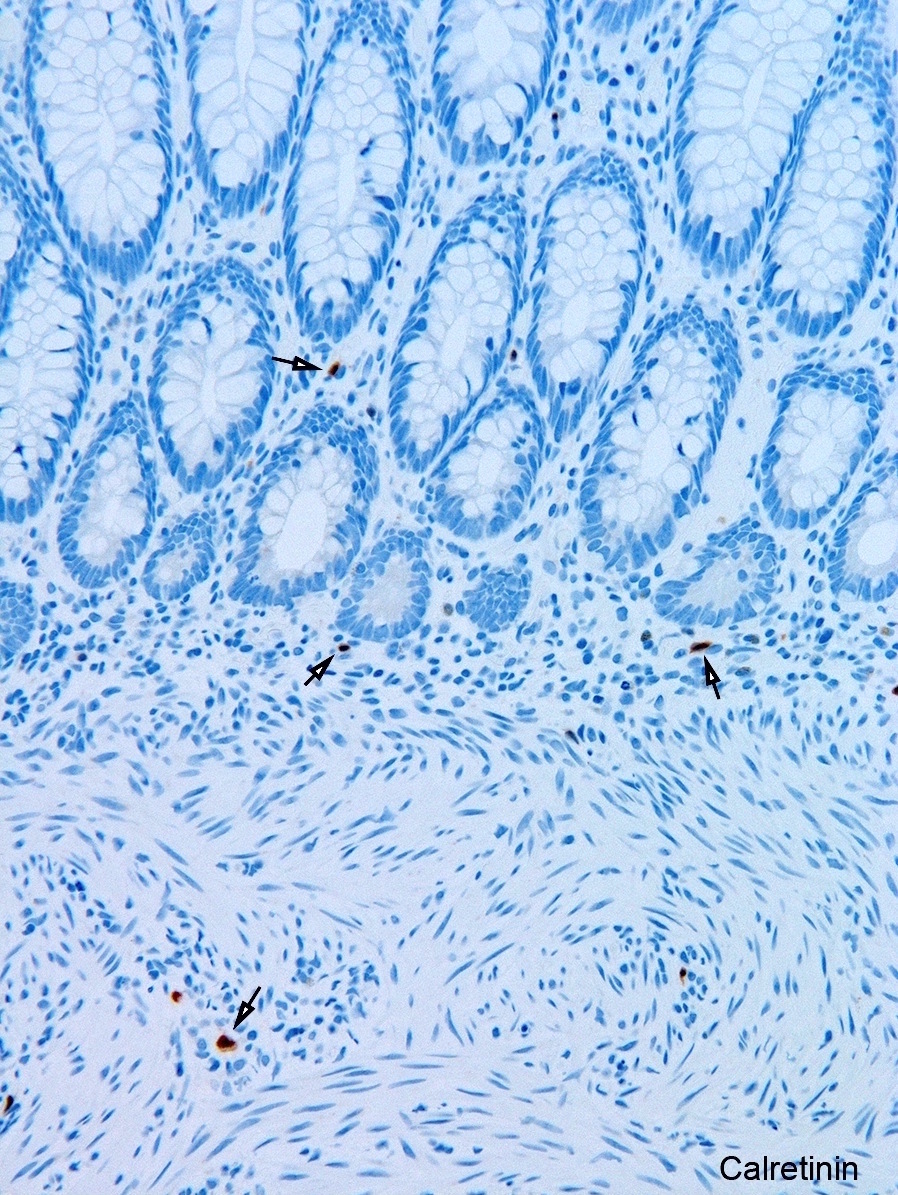
- Calretinin immunohistochemistry
- Most widely used ancillary method
- Run routinely with all rectal biopsies to exclude aganglionosis
- Absence of calretinin staining in muscularis mucosae and lamina propria of aganglionic segment
- Negative result pertains only to mucosa; submucosal nerves retain calretinin positive neurites are retained in submucosal nerves
- Immunoreactive mucosal nerves may be present in rectal biopsy from patient with very short segment aganglionosis due to descending fibers from transition zone (Pediatr Dev Pathol 2014;17:28)
- Many antibodies available to identify neuronal cell bodies (e.g., Hu C/D, Phox2B); seldom used in practice as H&E is sufficient
Electron microscopy description
- Not clinically relevant
- Hypertrophic nerves in aganglionic segment resemble extrinsic peripheral nerves (perineurium, endoneurial collagen, non-myelinating Schwann cells)
Molecular / cytogenetics description
- Complex genetics as described above; see Etiology
- Formal testing generally not indicated but karyotype, genomic array or mutational analysis may be pursued for syndromic cases (e.g., Waardenburg-Shah syndrome, Down syndrome, multiple endocrine neoplasia type 2A)
Sample pathology report
- Rectal biopsy
- Rectum, suction biopsies: findings diagnostic of Hirschsprung disease
- No identifiable ganglion cells
- Submucosal nerve hypertrophy
- Absent calretinin immunoreactive mucosal innervation
- Rectum, suction biopsies: findings diagnostic of Hirschsprung disease
- Pullthrough resection
- Rectosigmoid colon, Soave resection (12.5 cm): Hirschsprung disease with the following features:
- Aganglionosis (distal 4 cm)
- No significant neuromuscular pathology at the proximal surgical margin
- Rectosigmoid colon, Soave resection (12.5 cm): Hirschsprung disease with the following features:
- Resection synoptic report
- Can be used for diagnosis or as comment after diagnosis
- Online resource with dropdown menus for report preparation: https://hematogones.com/surg-path/hirsch
Differential diagnosis
- Clinical
- Chronic idiopathic pseudo-obstruction
- Clinical condition with heterogeneous etiologies, most affect small and large intestine, some primary (e.g., visceral myopathy; myenteric hypoganglionosis), some secondary (e.g., hypothyroidism)
- Megacystis microcolon intestinal hypoperistalsis syndrome (MMIHS)
- Bladder and intestinal dysmotility
- Typically presents pre- or perinatally
- Mutations in genes for smooth muscle contractile proteins
- X-linked intestinal pseudo obstruction
- Meconium ileus
- Especially with perforation, consider cystic fibrosis
- Anatomic intestinal obstruction (e.g., atresia, volvulus, stenosis)
- Anal achalasia
- Spasticity of internal anal sphincter with absent RAIR and reduced nitrergic innervation of sphincter muscle (Pediatr Surg Int 2013;29:855)
- Chronic idiopathic pseudo-obstruction
- Histological (clinical mimics with different diagnostic findings in rectal biopsy)
- Intestinal neuronal dysplasia type B
- Controversial pattern of submucosal hyperganglionosis (Arch Pathol Lab Med 2019;143:235)
- Neuroglial hamartoma syndromes (multiple endocrine neoplasia type 2B, neurofibromatosis, PTEN-hamartoma syndromes)
- Ganglioneuromatous hyperplasia of submucosal and/or myenteric plexuses, + / - mucosal ganglioneuromas
- Investigate clinical history and extra enteric findings
- Confirm with molecular genetic testing
- Primary visceral myopathy
- Changes rarely diagnostic in muscularis propria of rectal biopsy
- Key features include muscle atrophy and fibrosis as well as myocyte vacuolar degeneration
- Mitochondriopathy
- Changes rarely diagnostic in muscularis propria of rectal biopsy
- Key features include muscle atrophy and fibrosis as well as eosinophilic megamitochondria in enteric ganglia
- Intestinal neuronal dysplasia type B
Additional references
- Pathogenesis oriented review (Nat Rev Gastroenterol Hepatol 2018;15:152)
- Normative data for rectal submucosal nerve thickness (J Pediatr Surg 2016;51:1585)
- Puri: Hirschsprung's Disease and Allied Disorders, 4th Edition, 2019
Practice question #1
A neonate with Hirschsprung disease, diagnosed by suction rectal biopsy, undergoes primary endorectal pullthrough surgery. During the operation, a leveling seromuscular biopsy from the proximal sigmoid colon is sent for frozen section diagnosis. Which of the following diagnoses and recommendations is appropriate, based on the microscopic field shown in the above image?
- No definitive ganglion cell, obtain another biopsy from an upstream location
- Ganglion cells present, recommend pullthrough resection at least 5 cm proximal to site of this biopsy
- Ganglion cells present, insist on rebiopsy of rectum to confirm Hirschsprung disease before resection
- Probably intestinal neuronal dysplasia, recommend additional upstream biopsies to define extent of dysplastic ganglia
- Inadequate sample to assess for ganglion cells, rebiopsy
Practice answer #1
B. Ganglion cells present, recommend pullthrough resection at least 5 cm proximal to site of this biopsy
Comment Here
Reference: Hirschsprung disease
Comment Here
Reference: Hirschsprung disease
Practice question #2
A rectal biopsy to exclude Hirschsprung disease may be considered inadequate if it demonstrates
- Submucosal nerve hypertrophy
- Absent calretinin immunoreactive mucosal innervation
- Crowded, coarse, acetylcholinesterase positive mucosal
- Transitional mucosa
- Absent submucosal ganglion cells
Practice answer #2