Table of Contents
Definition / general | Essential features | CPT coding | Sites | Diagrams / tables | Cytology description | Cytology images | Sample pathology report | Additional references | Practice question #1 | Practice answer #1 | Practice question #2 | Practice answer #2 | Practice question #3 | Practice answer #3Cite this page: Lin LH, Brandler TC. International system for reporting serous fluid cytopathology. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/cytopathologyinternationalsystem.html. Accessed September 16th, 2025.
Definition / general
- Effusion cytopathologic evaluation can be challenging due to multiple, different processes affecting serous cavities, ranging from benign (infectious, autoimmune) to malignant processes (primary or metastatic neoplasms)
- The international system standardized the way to report cytopathology diagnoses of serous fluids, which includes 5 different categories: nondiagnostic (ND), negative for malignancy (NFM), atypia of undetermined significance (AUS), suspicious for malignancy (SFM) and malignant (MAL)
Essential features
- The international system for reporting serous fluid cytopathology includes 5 categories with different risk of malignancy: nondiagnostic (ND), negative for malignancy (NFM), atypia of undetermined significance (AUS), suspicious for malignancy (SFM) and malignant (MAL) (Chandra: The International System for Serous Fluid Cytopathology, 1st Edition, 2020)
- For an adequate diagnosis, effusion cytopathology should be interpreted with clinical and radiologic information and correlated with ancillary techniques if needed (immunostains, molecular, flow cytometry)
- Nondiagnostic classification and adequacy evaluation depends on fluid volume, quantity of cells and quality of the preparation
- Atypia of undetermined significance and suspicious for malignancy are categories of uncertainty and can be used as preliminary diagnoses while ancillary tests are performed, in cases where there is insufficient material for ancillary testing or in cases where ancillary testing results are noncontributory or inconclusive
CPT coding
- 88108 - concentrated cytology specimen (e.g. centrifugation)
- 88112 - selective enhanced cytology specimen (e.g. liquid based slide preparation)
- 88173 - fine needle aspiration
- 88305 - cell block
- 88342 - immunohistochemistry, first stain
- 88341 - immunohistochemistry, additional stains
- 88180 - flow cytometry
Sites
- Pleura, peritoneum, pericardium
Diagrams / tables
Cytology description
- The international system standardized reporting of serous fluid cytology includes 5 categories with different malignancy risks (J Am Soc Cytopathol 2020;9:469, Chandra: The International System for Serous Fluid Cytopathology, 1st Edition, 2020)
- Cytology specimens must be interpreted in concert with clinical and radiologic information
- Ancillary studies can be utilized for further characterization (immunostains, flow cytometry, molecular) (Cancer Cytopathol 2015;123:258, Cancer Cytopathol 2018;126:590)
- Categorization is recommended whenever possible
- If the diagnosis is uncertain, a descriptive interpretation should be included
- Categories (Chandra: The International System for Serous Fluid Cytopathology, 1st Edition, 2020):
- Nondiagnostic (ND):
- Risk of malignancy: 17% (± 8.9%) (Diagn Cytopathol 2019;47:1145)
- Adequacy of specimen depends on:
- Volume of fluid: nondiagnostic category can only be used after adequate amount of fluid has been processed (there is no specific cutoff but volume < 50 mL might not be enough to exclude malignancy; higher volumes of liquid yield lower rates of nondiagnostic cases) (Cytopathology 2011;22:179)
- Cellularity: a specimen can be nondiagnostic if there are not enough cellular elements of interest to classify the sample in another diagnostic category; there is no definitive criteria of number of mesothelial cells for adequacy and the criteria might vary depending on the pathologic process (e.g. a specimen with prominent acute inflammation and scant mesothelial cells may be adequate if the clinical suspicion is empyema)
- Quality of preparation: poor cell preservation, degeneration, hemorrhage, artifacts and contaminants can impair interpretation
- Explanation for the lack of adequacy should be provided in the report
- If the specimen meets criteria for other categories (e.g. significant atypia), it should not be classified as nondiagnostic, regardless of volume / cellularity
- Negative for malignancy (NFM):
- Risk of malignancy: 21% (± 0.3%) (Diagn Cytopathol 2019;47:1145)
- Most fluids (> 80%) fall within the negative of malignancy category (Diagn Cytopathol 1987;3:8, Diagn Cytopathol 1999;20:350)
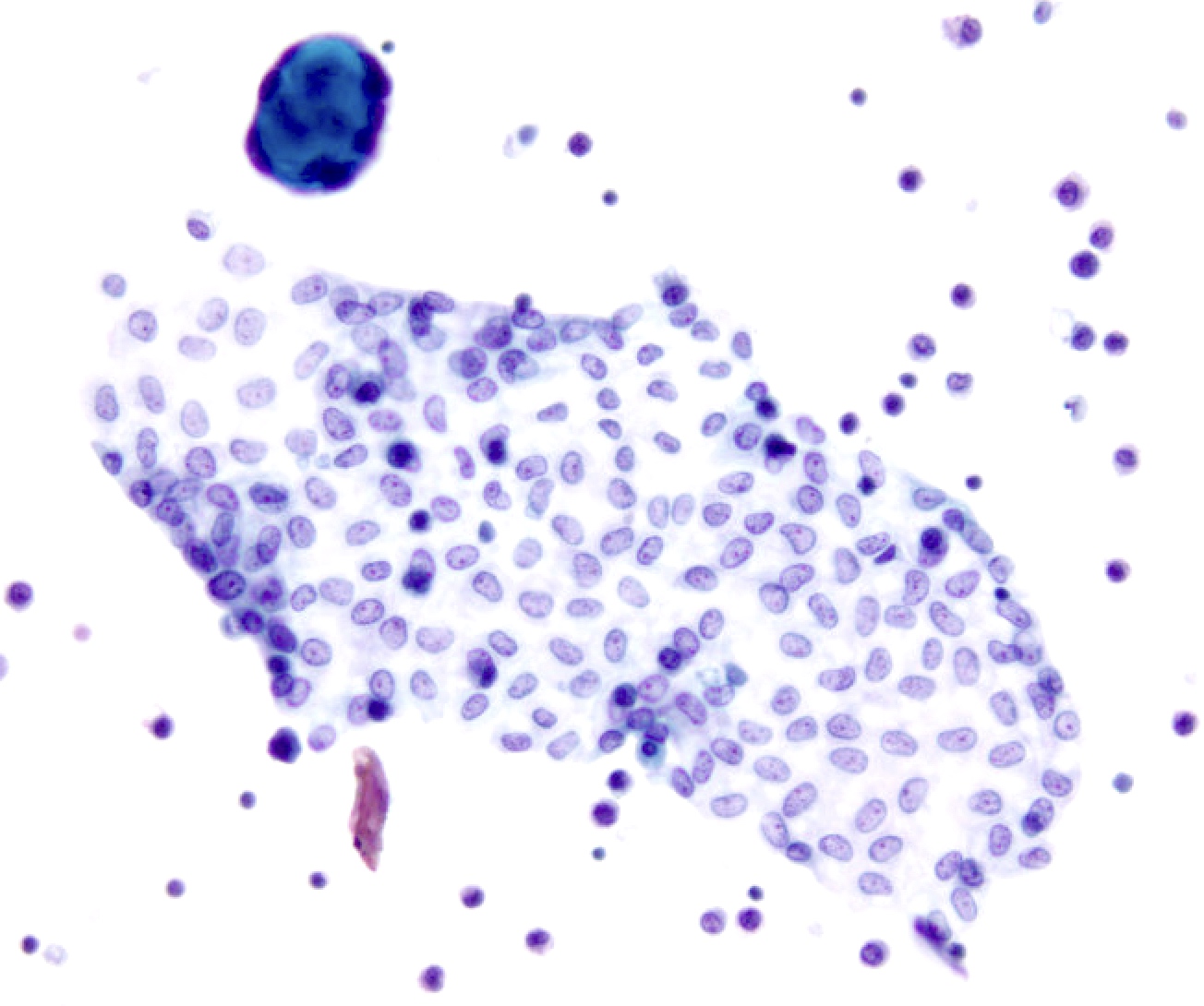
- Benign and reactive components: mesothelial and inflammatory cells
- Reactive mesothelial cells are typically single cells or small clusters and can exhibit binucleation, multinucleation and reactive cellular atypia; mesothelial cells can present as flat sheets in peritoneal lavages
- Benign cellular and noncellular findings are included in the negative for malignancy category, such as collagen balls, psammoma bodies, ciliary tufts, endosalpingiosis, endometriosis, asbestos bodies, lupus erythematosus cells, microorganisms, among others; a comment should be added explaining the finding (Acta Cytol 1992;36:466, Cancer 2004;102:87, Acta Cytol 1985;29:310)
- Unexpected second population of cells should not be present in the negative for malignancy category (expected cells include mesothelial or inflammatory cells; unexpected second population of cells often indicates malignancy)
- Majority of cases can be signed out based only on morphology but ancillary testing might be needed to rule out malignancy in particular scenarios:
- Highly atypical mesothelial cells or increased histiocytes / lipophages: immunohistochemistry to rule out adenocarcinoma
- Highly atypical mesothelial cells: clinical and radiologic correlation, immunostains and molecular to rule out mesothelioma
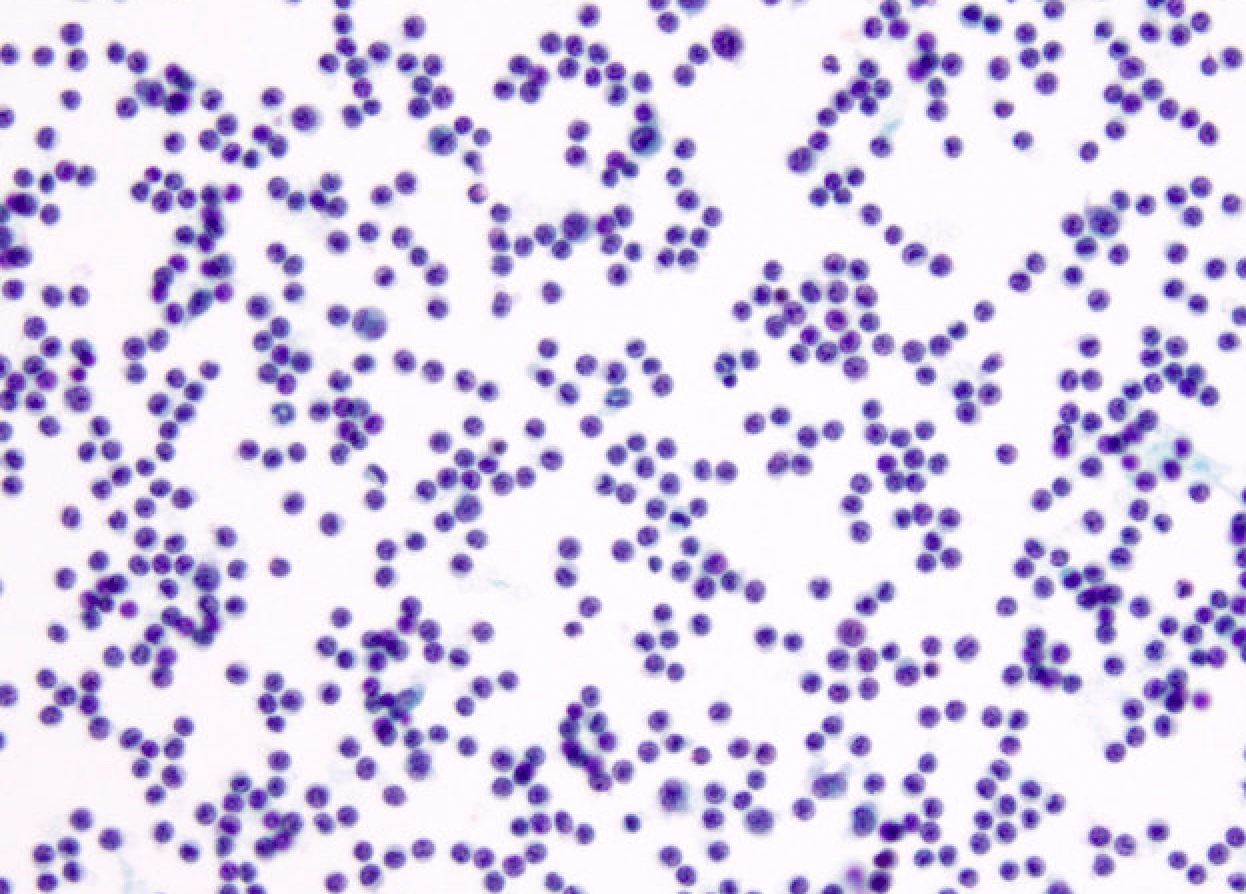
- Lymphocytic effusions: flow cytometry or immunostains to rule out lymphoma
- Atypia of undetermined significance (AUS):
- Risk of malignancy: 66% (± 10.6%) (Diagn Cytopathol 2019;47:1145)
- Limited nuclear or architectural atypia with overlapping features between reactive changes and malignant appearance but features are closer to benign processes
- Degree of atypia is sufficient for the specimen not to be classified as nondiagnostic
- Heterogenous group of conditions with variable criteria (Diagn Cytopathol 2019;47:1145, J Am Soc Cytopathol 2015;4:44)
- Can be used in preliminary reports before confirmatory ancillary tests; ancillary testing can lead to diagnostic upgrade (malignant or suspicious for malignancy) or downgrade (negative for malignancy)
- If ancillary tests are noncontributory or insufficient material is available for additional testing, the final diagnosis remains as atypia of undetermined significance
- Scenarios that can fall in the AUS category:
- Limited atypia that does not meet criteria for suspicious for malignancy
- Specimen with atypia, likely benign; however, due to poor preservation or low cellularity cannot confidently rule out malignancy
- Peritoneal washing with cells from benign or borderline tumors of ovary
- Peritoneal washing with epithelial cells of indeterminate origin, exhibiting benign or low grade features
- Lymphocytic effusion, favoring reactive but indefinite for lymphoproliferative disorder (when there is no confirmatory flow cytometry)
- Suspicious for malignancy (SFM):
- Risk of malignancy: 82% (± 4.8%) (Diagn Cytopathol 2019;47:1145)
- Features suspicious but not definitive for malignancy
- In comparison to the atypia of undetermined significance category, the degree of suspicion of malignancy is higher
- Can be used in preliminary reports before confirmatory ancillary tests, which can lead to upgrade (malignant) or downgrade (negative for malignancy); if the ancillary tests are noncontributory or insufficient material is available for additional testing, the final diagnosis remains as suspicious for malignancy
- Scenarios that can fall into the SFM category:
- Greater degree of atypia but cells are limited in number or by preservation of specimen
- Large number of bland appearing epithelial cells (e.g., suspicious for breast lobular carcinoma)
- Mucinous material with epithelial cells with limited atypia
- Atypical lymphoid cells, suspicious for lymphoma (when there is no confirmatory flow cytometry or flow cytometry confirms lymphoma but cytomorphology is not clear enough to term positive for malignancy)
- Malignant (MAL):
- Risk of malignancy: 99% (± 0.1%) (Diagn Cytopathol 2019;47:1145)
- Definitive evidence of malignancy
- Subclassified into:
- Primary (MAL-P): majority are mesothelioma; other neoplasms such as primary lymphoma and primary mesenchymal tumors can also occur
- Secondary (MAL-S): majority are metastatic adenocarcinoma; other metastatic neoplasms such as squamous cell carcinoma, neuroendocrine tumors, melanoma, lymphoma, mesenchymal and germ cell tumors can also occur
- In the majority of cases, immunostains can aid in the differential diagnosis (please refer to the Immunostaining panels in effusion cytology topic)
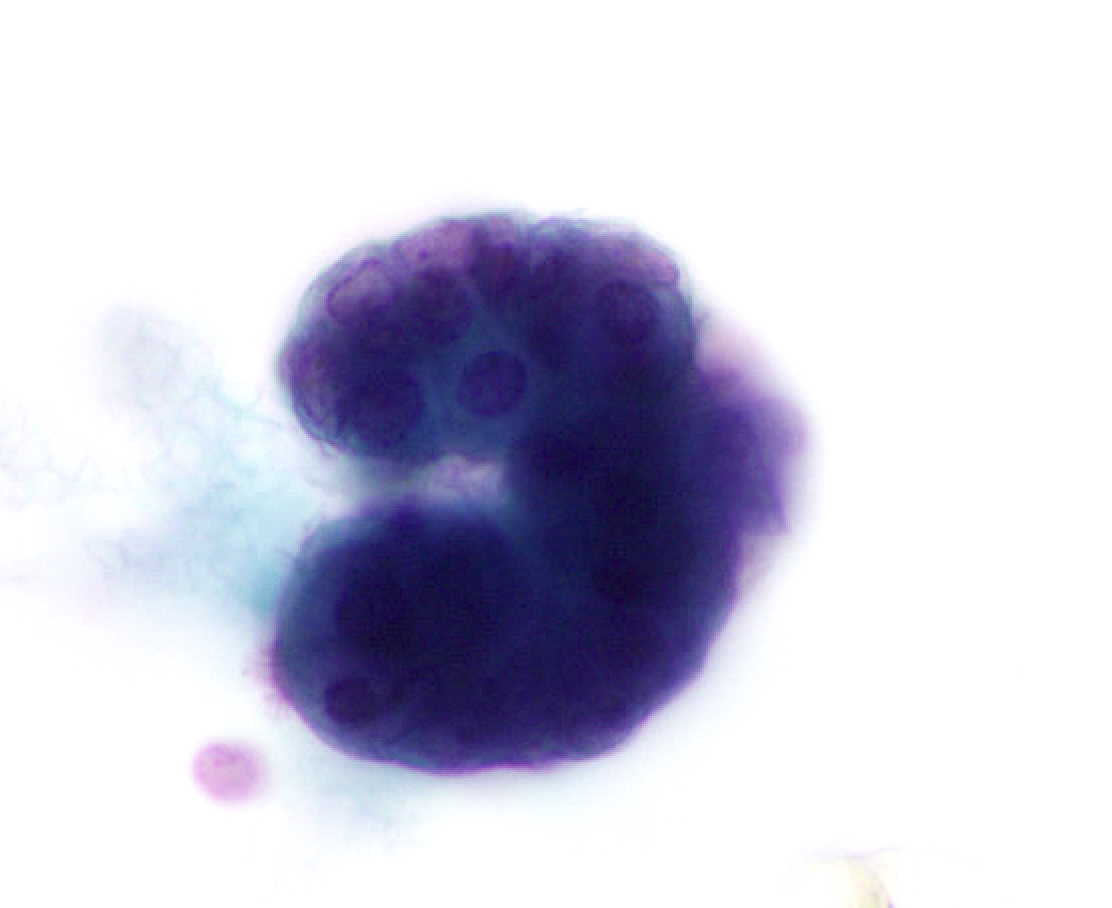
- Cytologic and architectural features of mesothelioma (Cytojournal 2015;12:26, Arch Pathol Lab Med 2018;142:893, Cancer Cytopathol 2014;122:70)
- Highly cellular smears composed of mesothelial cells (cells with dense cytoplasm with pale rim / ectoplasm [skirts], displaying a narrow space in between cells when grouped [window])
- Architecture atypia: large tissue fragments or cell clusters (papillary, morules, spheres) with numerous cells per cluster; can also present as single cells or mixture of architectures
- Cytologic atypia: nuclear enlargement and pleomorphism, irregular nuclear contours, macronucleoli, frequent binucleation or multinucleation, atypical mitosis) with intermediate to high grade features; cytologic atypia of mesothelioma can overlap with reactive mesothelial cells
- Softer signs: metachromatic / 2 tone cytoplasm, large variation in size (from normal to gigantic), pseudokeratotic cells (pyknotic, eosinophilic or orangeophilic cells), May-Grünewald-Giemsa stain showing increased perinuclear lipid vacuoles (confirmed by oil-red O stain) and pink / red granular background
- Ancillary techniques: immunostains or FISH (p16 / CDKN2A) (please refer to the Immunostaining panels in effusion cytology topic)
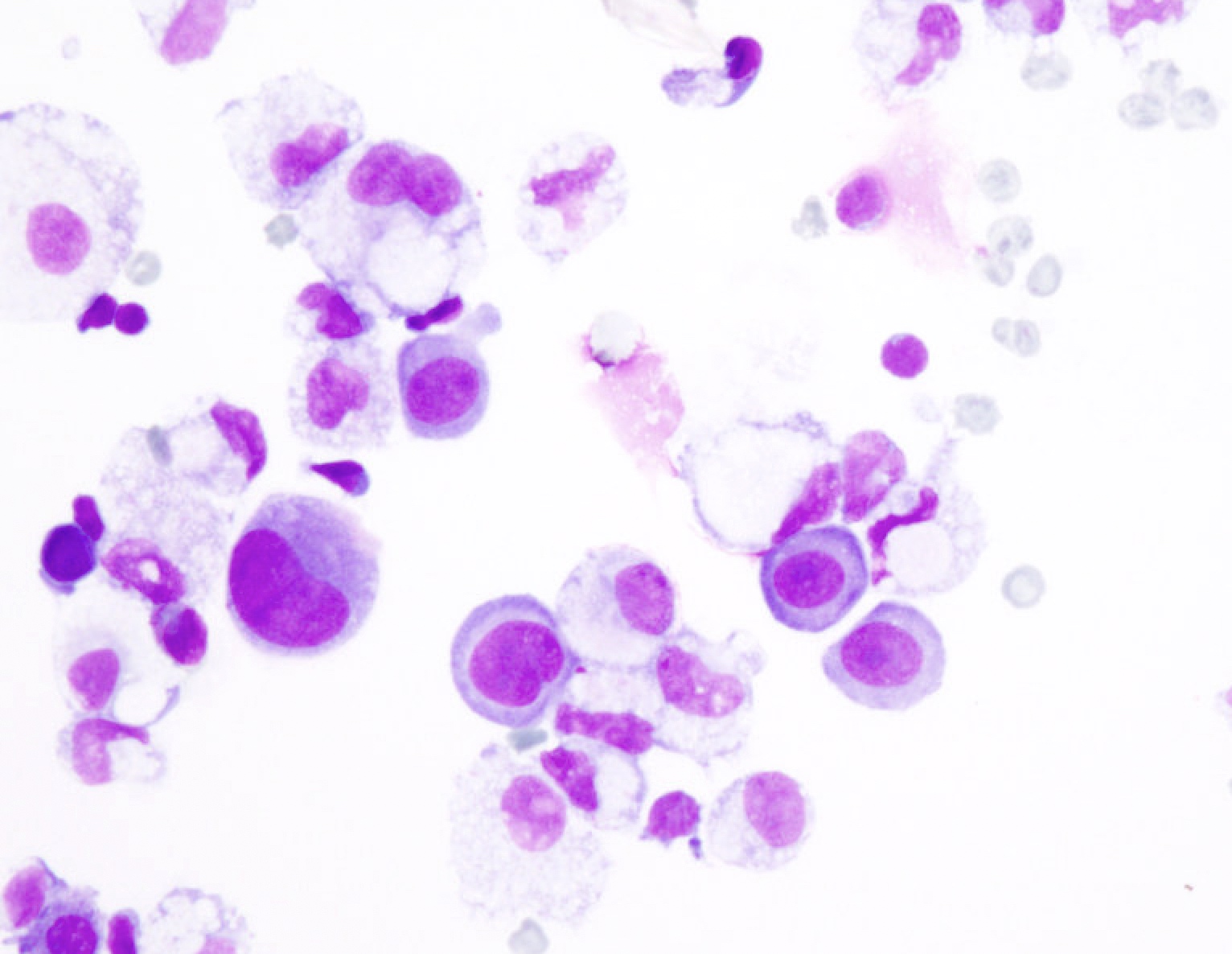
- Cytologic and architectural features of metastatic adenocarcinoma (Adv Anat Pathol 2006;13:174, Surg Pathol Clin 2018;11:523)
- Highly cellular smears
- Second population of cells
- 3 dimension clusters with smooth contours, papillary and glandular formations, single signet ring cells
- Highly atypical cells (nuclear enlargement, increased N/C ratio, variable pleomorphism, irregular nuclear contours, coarse chromatin); atypia can be subtle, particularly in breast lobular and gastric carcinomas
- Intracellular mucin vacuoles or extracellular mucin
- Commonly display tumor groups surrounded by retraction artifact on cell block
- Ancillary techniques: immunostains (lineage specific and site specific) (please refer to the Immunostaining panels in effusion cytology topic)
- Nondiagnostic (ND):
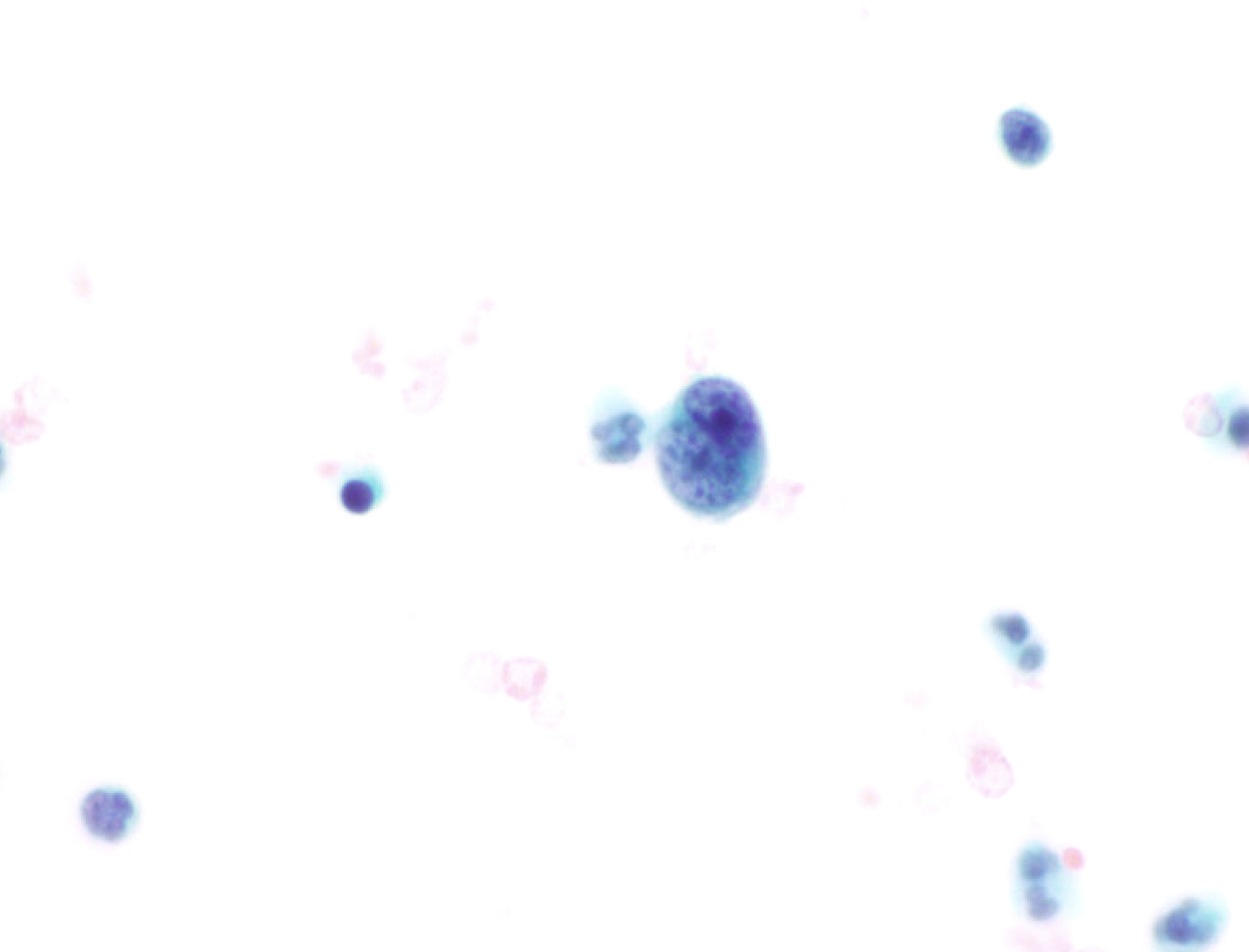
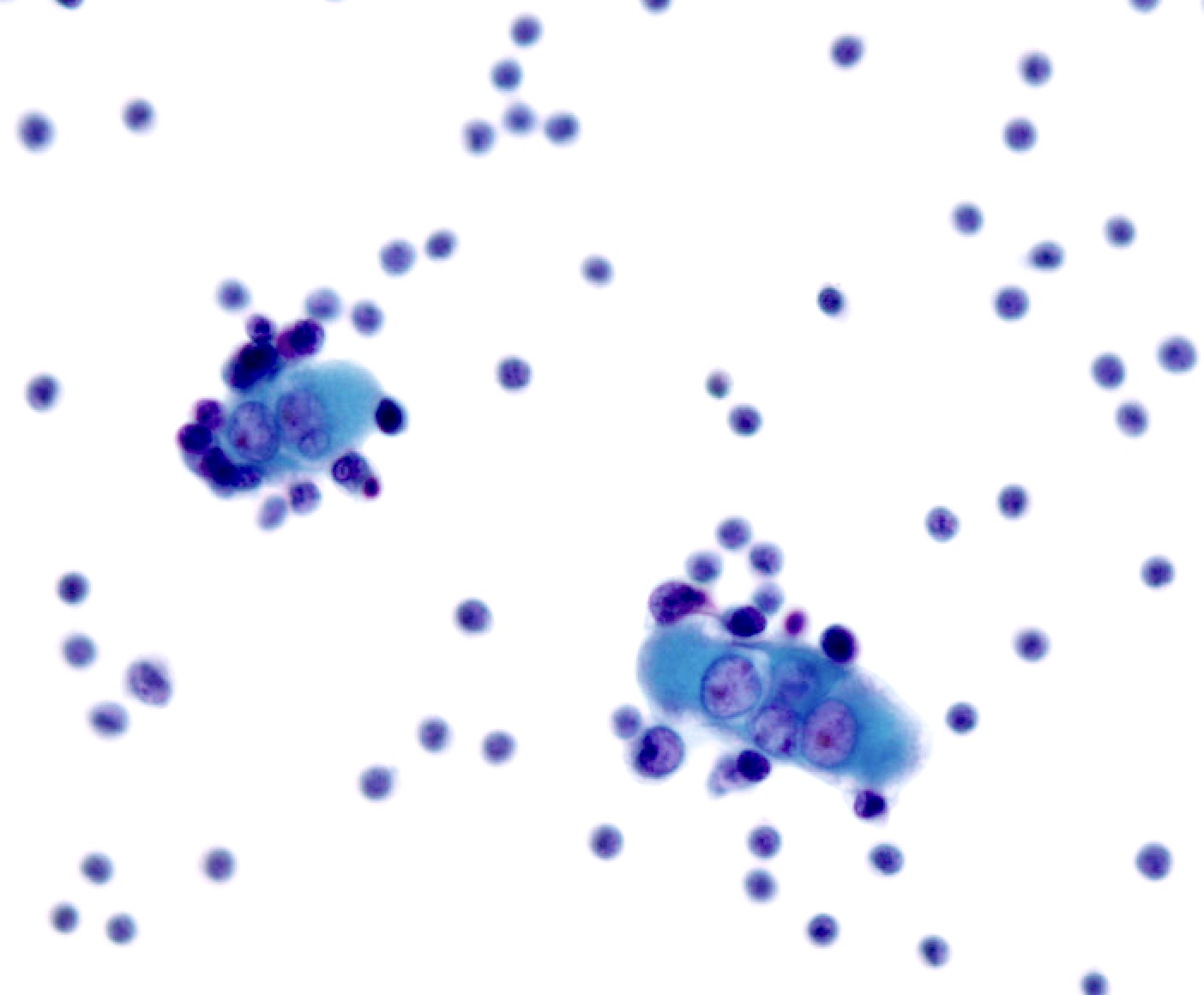
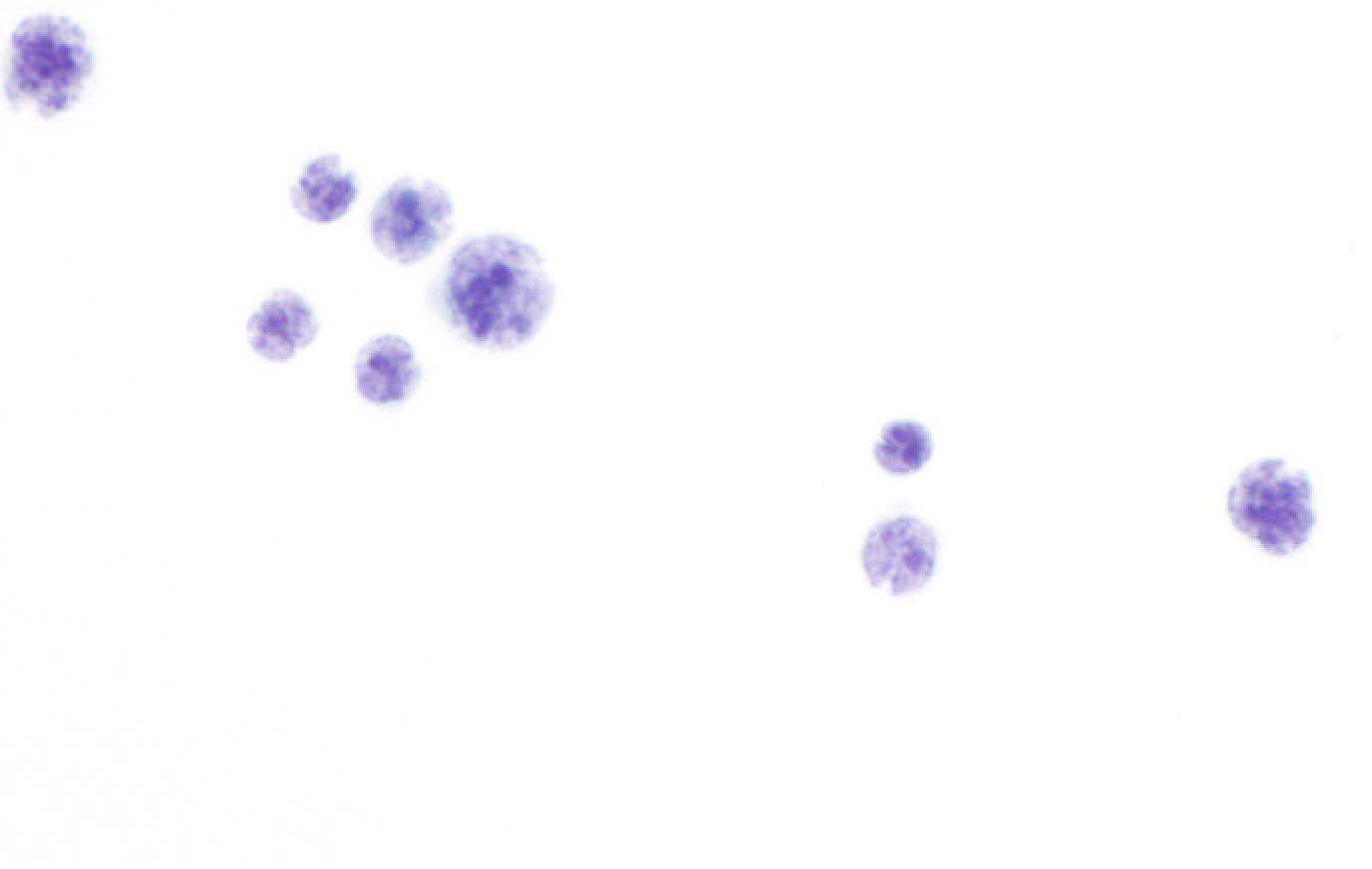
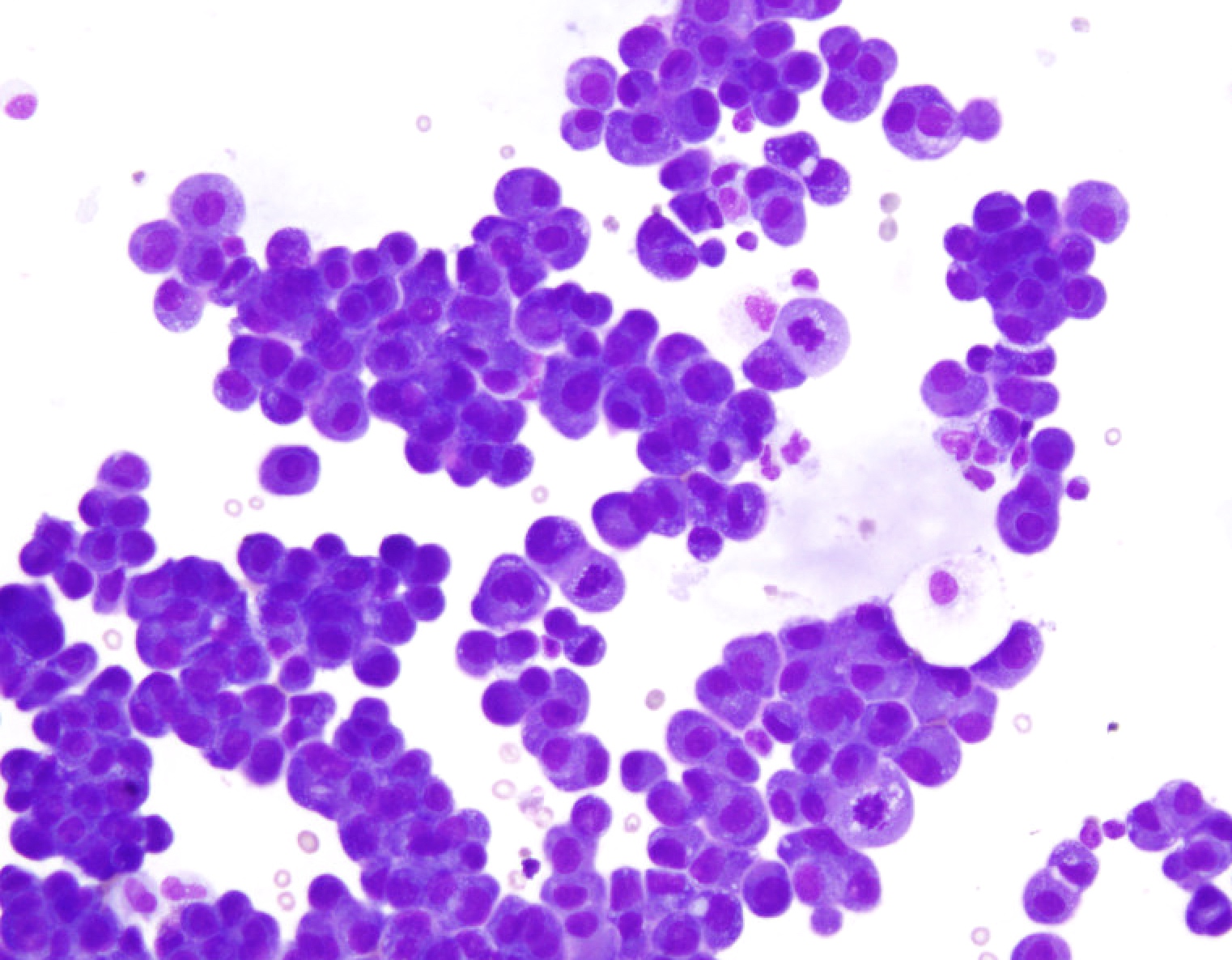
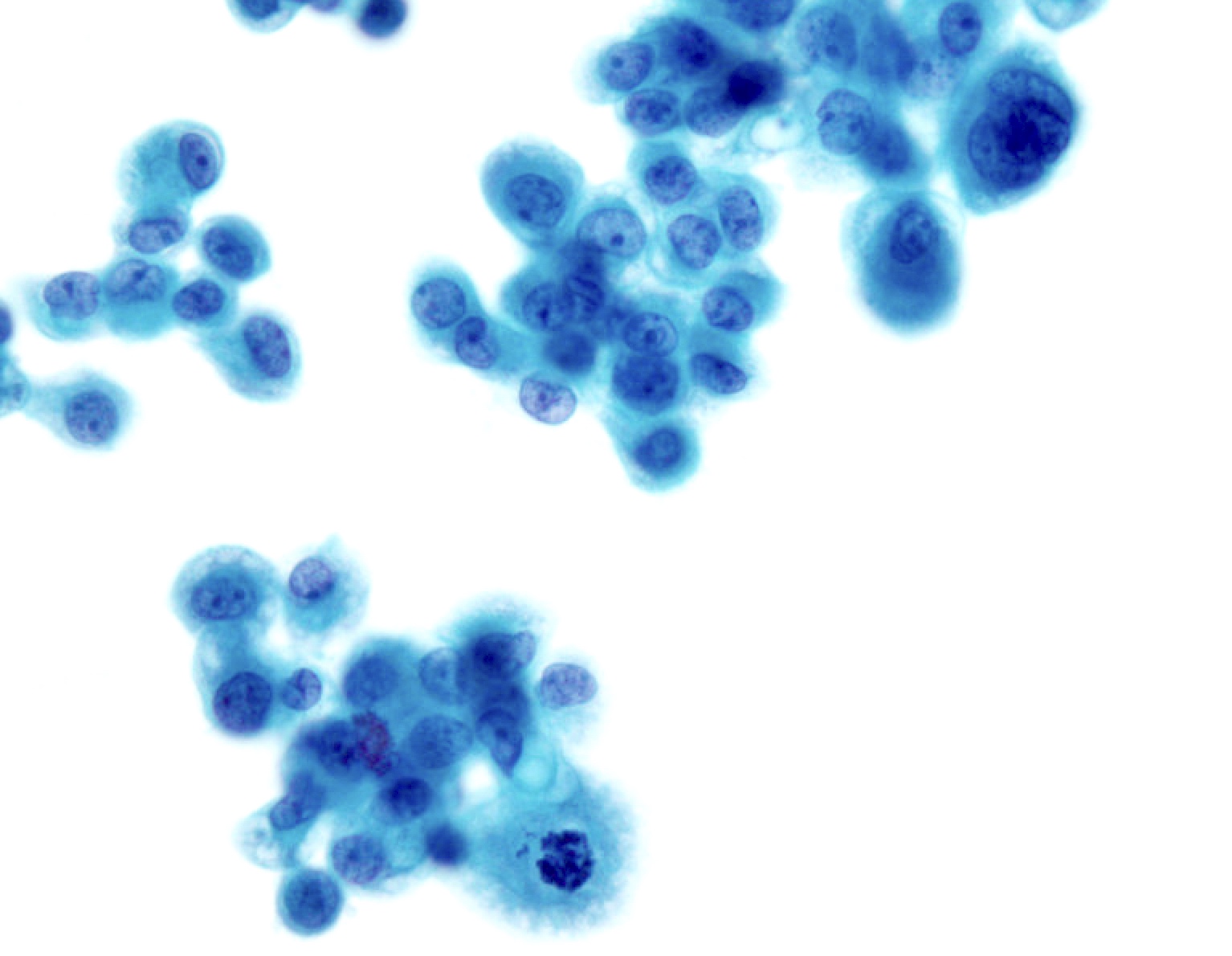
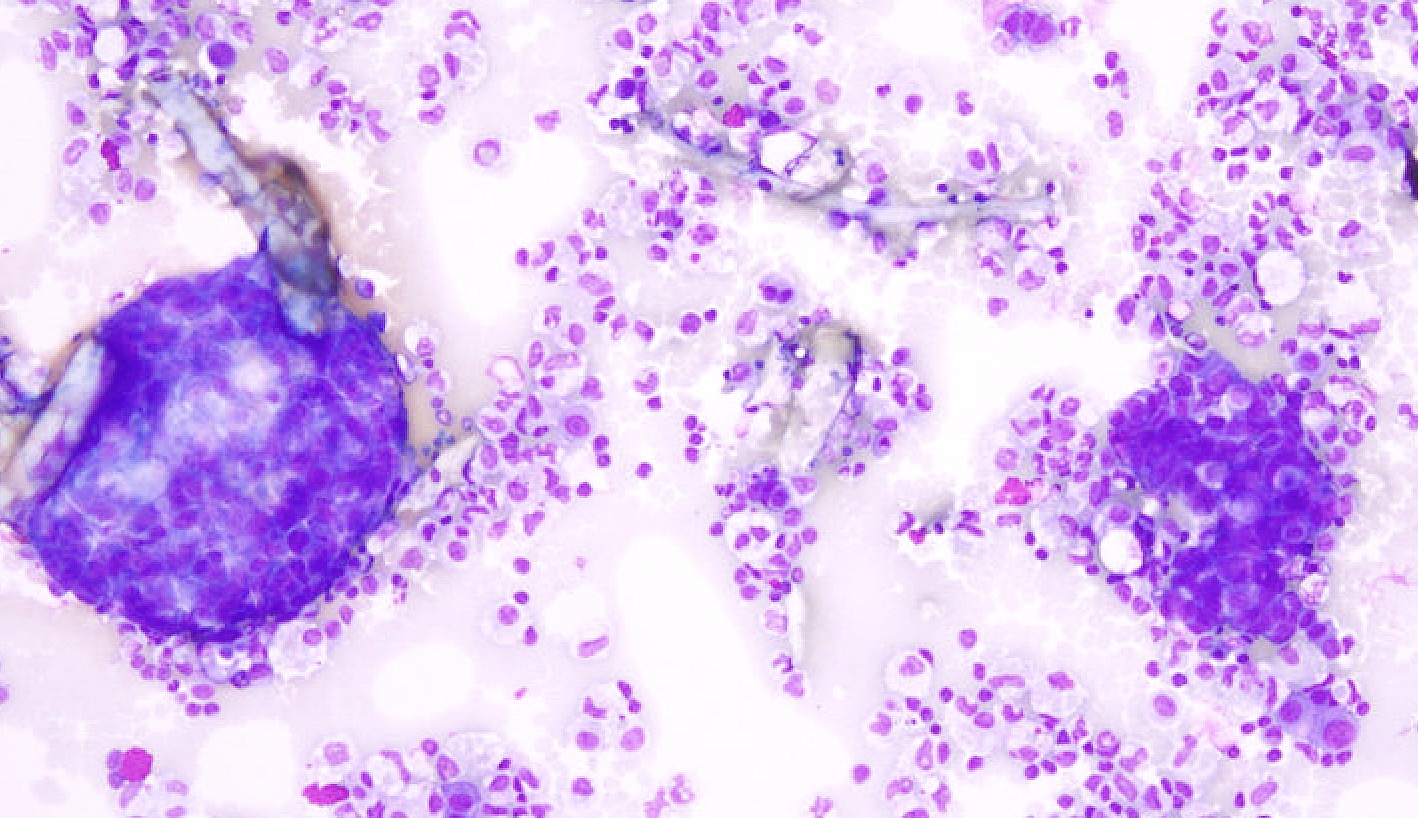
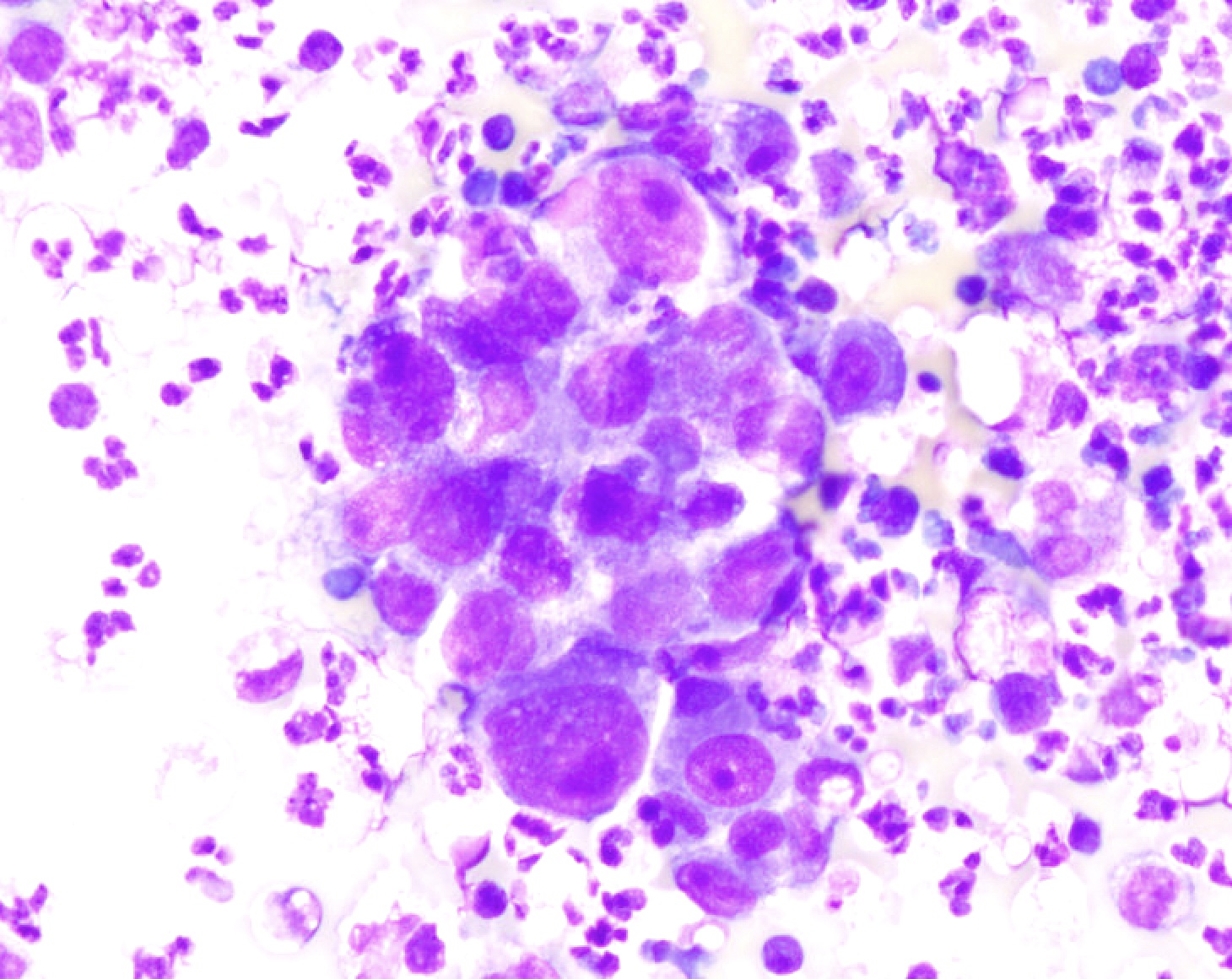
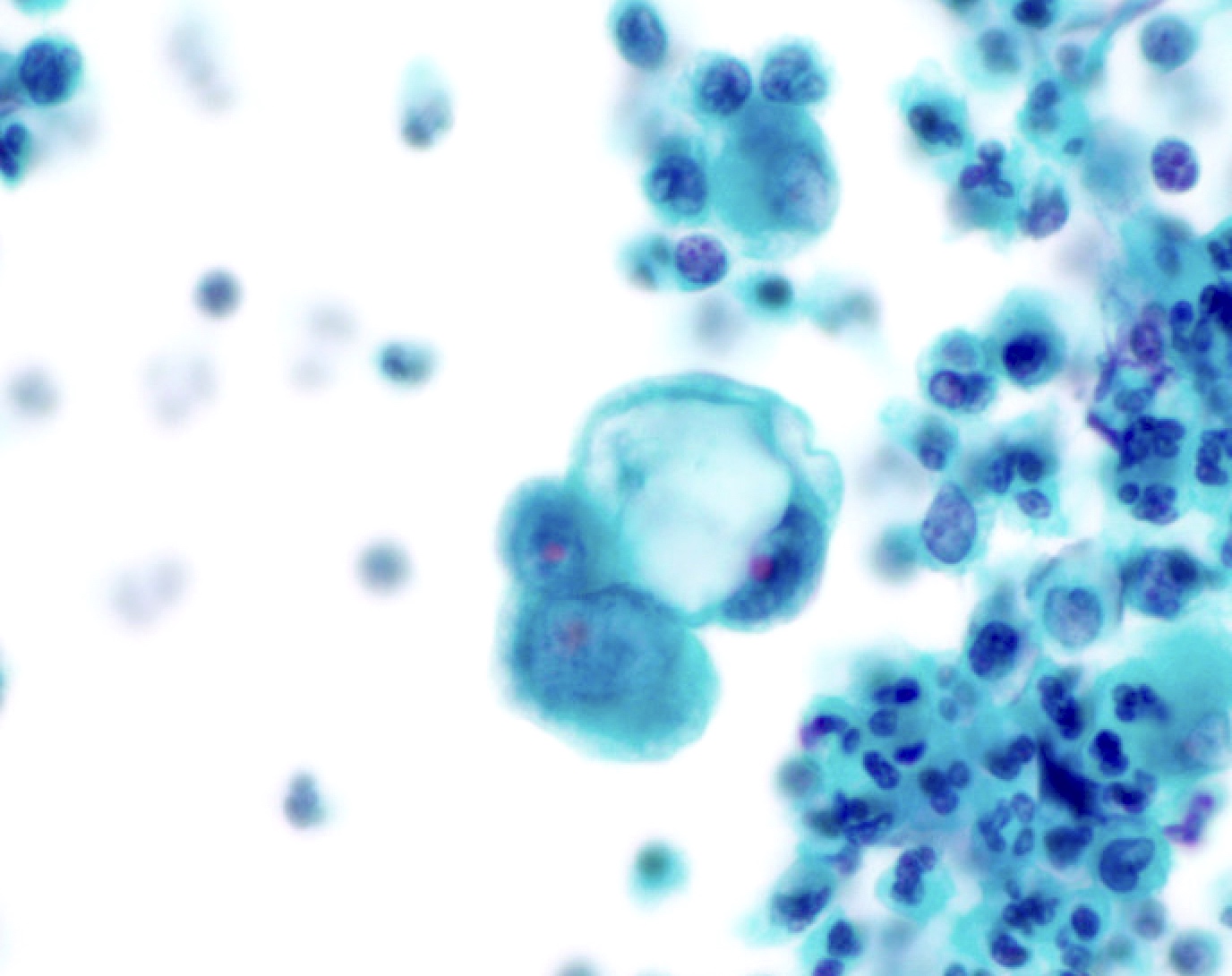
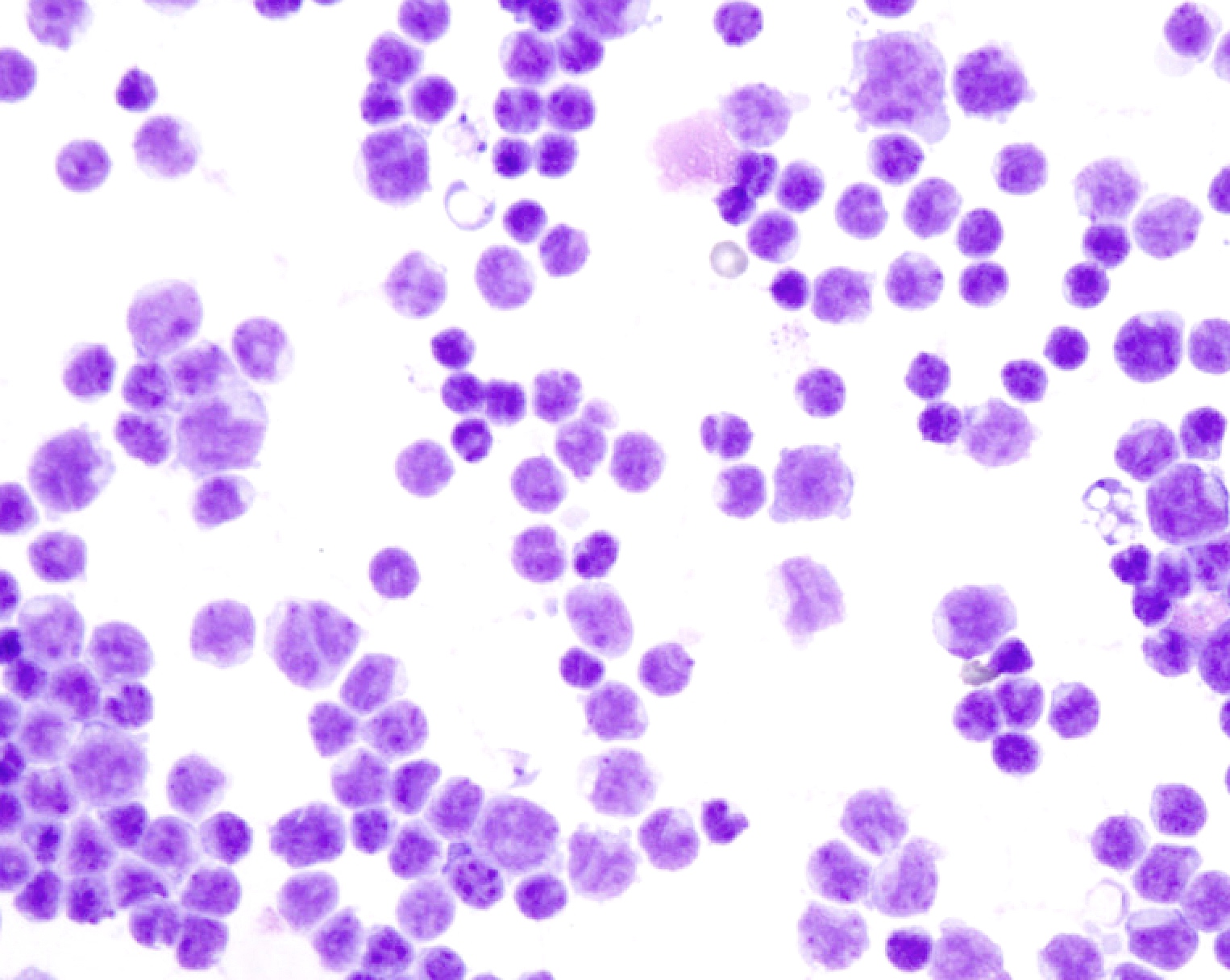
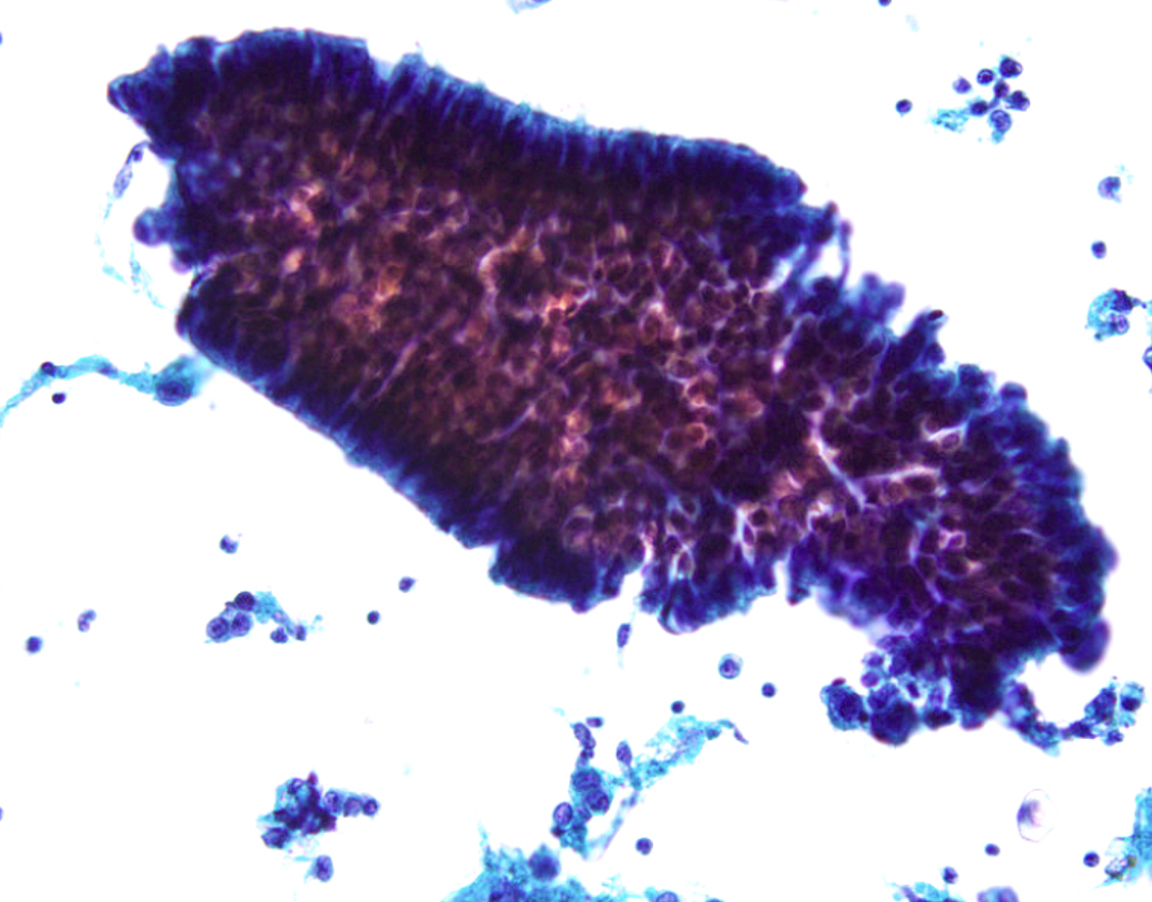
Cytology images
Contributed by Lawrence Hsu Lin, M.D., Ph.D. and Tamar C. Brandler, M.D., M.S.
Sample pathology report
- Lung, right, pleural effusion, thoracocentesis:
- Evaluation limited by extensive hemorrhage
- Nondiagnostic (see comment)
- Comment: The specimen is predominantly composed of blood. A repeat procedure is recommended if clinically indicated.
- Lung, right, pleural effusion, thoracocentesis:
- Satisfactory for evaluation
- Negative for malignancy (see comment)
- Comment: The specimen is predominantly composed of reactive mesothelial cells and histiocytes. Immunohistochemical stains were performed on the cell block and show that the mesothelial cells are reactive for calretinin and D2-40 and cells are nonreactive for BerEp4 and MOC31.
- Lung, right, pleural effusion, thoracocentesis:
- Satisfactory for evaluation
- Atypia of undetermined significance (see comment)
- Comment: The smears are composed of mixed population of mesothelial and inflammatory cells. There are a small cluster of cells with mild nuclear enlargement and mild hyperchromasia. The cell block consists predominantly of blood; therefore, immunostains could not be performed to further characterize the process. Additional sampling for further characterization should be considered if fluid reaccumulates, if clinically indicated.
- Lung, right, pleural effusion, thoracocentesis:
- Satisfactory for evaluation
- Suspicious for malignancy (see comment)
- Suspicious for carcinoma
- Comment: The smears are composed of a mixed population of mesothelial and inflammatory cells. There are few small clusters of cells with marked nuclear enlargement and hyperchromasia. The cell block consists predominantly of blood; therefore, immunostains could not be performed to further characterize the process. The overall findings are concerning for malignancy.
- Lung, right, pleural effusion, thoracocentesis:
- Satisfactory for evaluation
- Positive for malignancy (see comment)
- Metastatic adenocarcinoma
- Comment: The specimen is composed of highly cellular smears with a second population of markedly atypical cells arranged in large clusters. The cells exhibit nuclear enlargement, increased N/C ratio and hyperchromasia with prominent nucleoli. Immunostains show that the atypical cells are positive for MOC31, BerEP4, TTF1 and CK7 and negative for calretinin, D2-40, CK20 and p40. The overall findings and previous history of lung adenocarcinoma supports the diagnosis of metastatic lung adenocarcinoma.
- Lung, right, pleural effusion, thoracocentesis:
- Satisfactory for evaluation
- Positive for malignancy (see comment)
- Malignant mesothelioma
- Comment: The specimen is composed of highly cellular smears with predominant population of markedly atypical mesothelial cells in large clusters. The cells exhibit nuclear enlargement, increased N/C ratio and hyperchromasia with prominent nucleoli. Immunostains show that the cells are positive for calretinin and D2-40 and negative for MOC31 and BerEP4. Loss of BAP1 by immunohistochemistry and deletion of p16 / CDKN2 by FISH supports the above diagnosis.
Additional references
Practice question #1
Practice answer #1
C. Negative for malignancy
Comment Here
Reference: International system for reporting serous fluid cytopathology
Comment Here
Reference: International system for reporting serous fluid cytopathology
Practice question #2
Practice answer #2
C. Negative for malignancy
Comment Here
Reference: International system for reporting serous fluid cytopathology
Comment Here
Reference: International system for reporting serous fluid cytopathology
Practice question #3
Which of the following is the category for the above findings in a peritoneal washing according to the international system for reporting serous fluid cytopathology in a patient with concurrent surgical pathology with ovarian serous borderline tumor?
- Atypia of undetermined significance
- Malignant
- Negative for malignancy
- Nondiagnostic
- Suspicious for malignancy
Practice answer #3
A. Atypia of undetermined significance
Comment Here
Reference: International system for reporting serous fluid cytopathology
Comment Here
Reference: International system for reporting serous fluid cytopathology