Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Sample pathology report | Differential diagnosis | Additional references | Practice question #1 | Practice answer #1 | Practice question #2 | Practice answer #2Cite this page: Dominguez Gonzalez C, Glass C. Aortic aneurysms. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/heartcoronaryarteritis.html. Accessed September 17th, 2025.
Definition / general
- Progressive, irreversible, localized dilatation of the aortic wall (involving all 3 layers) exceeding the expected aortic diameter by > 1.5 fold
Essential features
- Majority of cases are asymptomatic until rupture, which is fatal (> 80% estimated mortality) (J Vasc Surg 2018;68:612)
- Aneurysm size and growth rate are the best predictors of risk of rupture (Gen Thorac Cardiovasc Surg 2019;67:1)
- Intervention is recommended for diameter > 5.0 - 5.5 cm or growth > 0.5 cm/year
- Risk factors include male sex, advanced age, smoking, hypertension, atherosclerosis, bicuspid aortic valve (BAV) and connective tissue syndromes (Circ Res 2019;124:607)
- Characteristic histologic findings include disruption of elastic lamellae, loss of smooth muscle cells, inflammation infiltration, increased proteolysis of extracellular matrix (Cardiovasc Pathol 2016;25:247)
Terminology
- Thoracic aortic aneurysm (TAA): aortic aneurysm (AA) located within the chest cavity
- Abdominal aortic aneurysm (AAA): aortic aneurysm located within abdominal cavity
- Pseudoaneurysm: false aneurysm; a rupture of the arterial wall contained by the tunica adventitia or a blood clot
- Aortic root dilation / aortic root aneurysm: aortic aneurysm located at the aortic root
- Aortic dissection: tear of the inner layer of the aortic wall, can involve multiple layers
- Ectasia: dilatation of the aorta that does not measure > 1.5 times the diameter of normal aorta
ICD coding
- ICD-10
- I71 - aortic aneurysm and dissection
- I71.0 - dissection of aorta
- I71.1 - thoracic aortic aneurysm, ruptured
- I71.2 - thoracic aortic aneurysm, without rupture
- I71.3 - abdominal aortic aneurysm, ruptured
- I71.4 - abdominal aortic aneurysm, without rupture
- I71.5 - thoracoabdominal aortic aneurysm, ruptured
- I71.6 - thoracoabdominal aortic aneurysm, without rupture
- I71.8 - aortic aneurysm of unspecified site, ruptured
- I71.9 - aortic aneurysm of unspecified site, without rupture
- A52.01 - syphilitic aneurysm of aorta
- S25.09 - other specified injury of thoracic aorta
- S35.09 - other injury of abdominal aorta
- I71 - aortic aneurysm and dissection
Epidemiology
- It is estimated that 1 - 2% of the population have an AA, increasing to 10% of individuals older than 65 years (Cardiovasc Pathol 2016;25:432)
- According to the Centers for Disease Control and Prevention (CDC), AA rupture accounted for 9,317 deaths in 2020
- There is higher prevalence in men, White populations, individuals with hypertension, with tobacco use, with advanced age (Circulation 2009;119:2202, J Vasc Surg 2010;52:539)
- TAA frequently occurs as a manifestation of connective tissue disorders (Marfan, Loeys-Dietz, Ehlers-Danlos, familial TAA)
- AAAs are often associated with atherosclerosis (Circulation 2010;121:e266)
Sites
- Thoracic aortic aneurysm
- Sinus of Valsalva
- Aortic root
- Ascending aorta
- Aortic arch
- Descending aorta
- Combined
- Abdominal aortic aneurysm: most common
- Suprarenal aorta
- Infrarenal aorta
- Combined
- Integrated (with iliac arteries)
- Thoracoabdominal aortic aneurysm
- Type I (from left subclavian artery [LSA] to celiac artery [CA])
- Type II (from LSA to iliac bifurcation [IB])
- Type III (from sixth intercostal space to IB)
- Type IV (from subdiaphragmatic segment to IB)
- Type V (from sixth intercostal space to renal artery [RA])
- Reference: Semin Vasc Surg 2021;34:18
Pathophysiology
- Medial degeneration, led by 3 interconnected processes (Cardiovasc Pathol 2016;25:432)
- Excessive extracellular matrix (ECM) degradation
- Disruption of elastin and collagen homeostasis
- Increase in matrix metalloproteinase activity leading to extensive proteolysis
- Inflammation
- Inflammatory cell infiltration and activation of proteases (Circ Res 2019;124:607)
- Neovascularization
- Smooth muscle cell (SMC) apoptosis
- Significant loss or disorganization of smooth muscle cells within the intima media
- Excessive extracellular matrix (ECM) degradation
- Degradation of the aortic wall → weakening of the aortic wall → dilation of the aorta → increased aortic wall stress → further wall weakening and risk of rupture
Etiology
- Degenerative (Vasc Med 2022;27:88)
- Hypertension and atherosclerosis accelerate medial degeneration
- Smoking and hypercholesterolemia
- Familial / genetic
- Marfan, Ehlers-Danlos, Loeys-Dietz and familial TAA
- Anatomic
- Bicuspid aortic valve (BAV)
- Infectious
- Hematogenous spread of infectious microemboli, preexisting intimal defect infection or direct inoculation of the aortic wall (Anesthesiol Clin 2022;40:671)
- Staphylococcus and Streptococcus, fungal infections, syphilis
- Hematogenous spread of infectious microemboli, preexisting intimal defect infection or direct inoculation of the aortic wall (Anesthesiol Clin 2022;40:671)
- Inflammatory
- Giant cell arteritis, Takayasu arteritis, Kawasaki disease, Behçet syndrome (Anesthesiol Clin 2022;40:671)
- Others
- Trauma
- Dissection
- Angioplasty
- Drug eluting stents
Clinical features
- Risk factors: smoking, older age, male sex, family history of AA, hypertension, atherosclerosis, connective tissue syndromes (Cardiovasc Pathol 2016;25:432)
- Most cases are asymptomatic until rupture
- Can present as nonpositional angina pectoris, back pain, diffuse abdominal pain, tenderness on palpation, abdominal bruit or edema (Anesthesiol Clin 2022;40:671)
- Symptomatic AAs are at an increased risk of rupture
- Ruptured AA can cause severe / diffuse abdominal pain, dyspnea, shock and a palpable / pulsatile abdominal mass
- Median yearly growth rate of AAs is 0.1 - 0.4 cm/year (J Transl Int Med 2016;4:35)
- Diameter has an exponential effect on risk of rupture
Diagnosis
- Computed tomography (CT) is the gold standard for evaluation of AA size and morphology (Circulation 2022;146:e334)
- Ultrasound (US) is the main screening, diagnostic and monitoring tool (Br J Radiol 2018;91:20170306)
- U.S. Preventive Services Task Force (USPSTF) recommends US screening for men 65 - 75 years who ever smoked (Ann Vasc Surg 2019;54:298)
- CT scan with contrast is preferred for intervention planning
- Magnetic resonance imaging (MRI) used as alternative during pregnancy
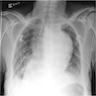
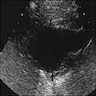
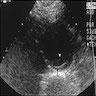
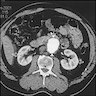
Radiology description
- Chest radiographs: widening of the mediastinum or bulging of the ascending aorta (J Vasc Interv Radiol 2008;19:S2)
- Ultrasound: dilatation of the aorta of > 1.5 times the normal diameter (J Vasc Interv Radiol 2008;19:S2)
- CT / MRI: dilatation of the aortic lumen; the walls may be thin or thickened by the presence of a mural thrombus (Emerg Med Clin North Am 2021;39:745)
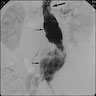
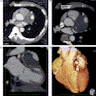
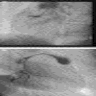
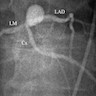
Radiology images
Prognostic factors
- Without intervention, AA will continue to expand and eventually rupture
- Without immediate intervention, rupture is fatal
- Mortality rate of surgical rupture repair is estimated to be 43 - 46% (J Vasc Surg 2021;73:39)
- Risk factors for dissection / rupture (PLoS One 2022;17:e0270585, Bioengineering (Basel) 2020;7:79, Gen Thorac Cardiovasc Surg 2019;67:1)
- > 5 - 7 cm diameter
- Rapid growth rate: > 0.5 cm in 6 months
- Longer aneurysm segment
- Diastolic pressure > 105 mmHg
- High peak wall stress (hypertension, atherosclerosis)
- Asymmetry
- Tobacco / cocaine use
- Connective tissue disorder: Marfan, Ehlers-Danlos, bicuspid aortic valve
- Vascular inflammation: giant cell arteritis, Takayasu arteritis, syphilis
- Family history of AA or aortic dissection
- Symptomatic aneurysm
- Advanced age
Case reports
- 25 year old man with Marfan syndrome and giant aortic root aneurysm (J Invasive Cardiol 2021;33:E231)
- 37 year old man with large thoracic aortic aneurysm (Vasc Health Risk Manag 2022;18:1)
- 58 year old man with mycotic abdominal aortic aneurysm (J Med Case Rep 2022;16:44)
- 79 year old man with 10 cm abdominal aortic aneurysm (Perm J 2019;23:18.218)
- 84 year old woman with thoracic aortic aneurysm (BMC Gastroenterol 2020;20:63)
Treatment
- American College of Cardiology (ACC) / American Heart Association (AHA) guidelines (2022) recommends repair for AA ≥ 5.0 - 5.4 cm and surveillance for smaller diameter lesions (Circulation 2022;146:e334)
- Intervention: endovascular aneurysm repair versus open surgical repair
- Earlier intervention may be recommended (Trends Cardiovasc Med 2020;30:500)
- Growth rate: > 0.5 cm in 6 months
- Connective tissue syndromes / vascular inflammation
- Family history of aortic dissection
- Women
- Cross sectional aortic area/height ratio > 10 cm2/m
- Earlier intervention may be recommended (Trends Cardiovasc Med 2020;30:500)
- Society for Vascular Surgery (2018) recommended surveillance (J Vasc Surg 2018;67:2)
- > 2.5 - 2.9 cm: rescreen after 10 years
- 3.0 - 3.9 cm: 3 year interval
- 4.0 - 4.9 cm: 1 year interval
- 5.0 - 5.4 cm: 6 month interval
- Intervention: endovascular aneurysm repair versus open surgical repair
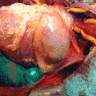
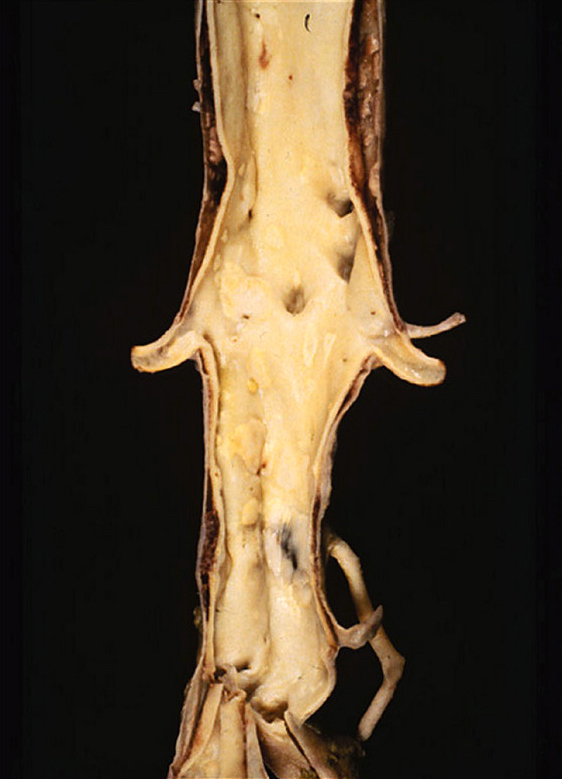
Gross description
- Aortic aneurysms are defined as a focal dilatation of at least 50% of the normal arterial diameter
- AAA typically > 3 - 3.5 cm (J Am Coll Cardiol 2022;80:e223)
- TAA typically > 4.0 - 5.0 cm (J Am Coll Cardiol 2022;80:e223)
Gross images
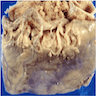
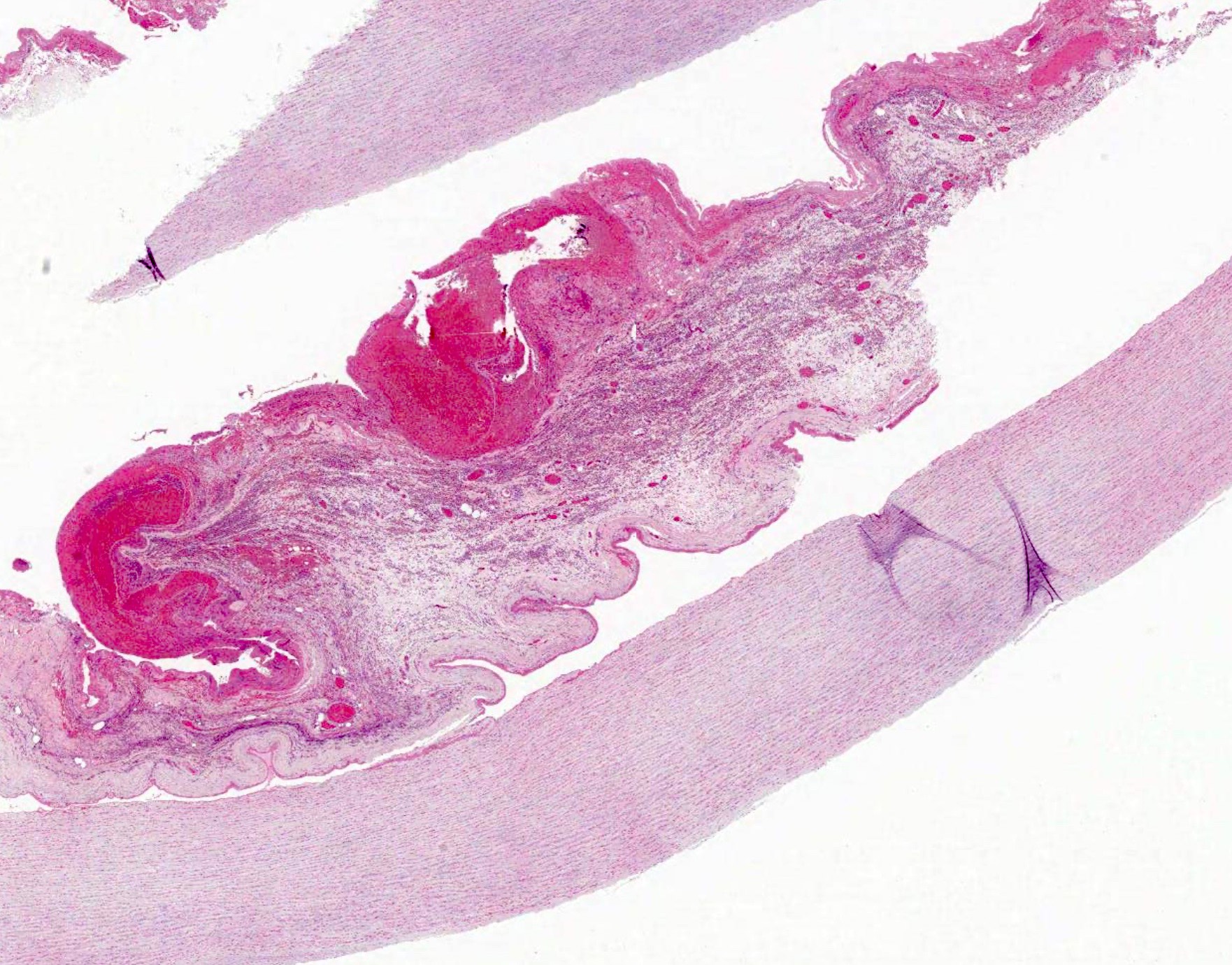
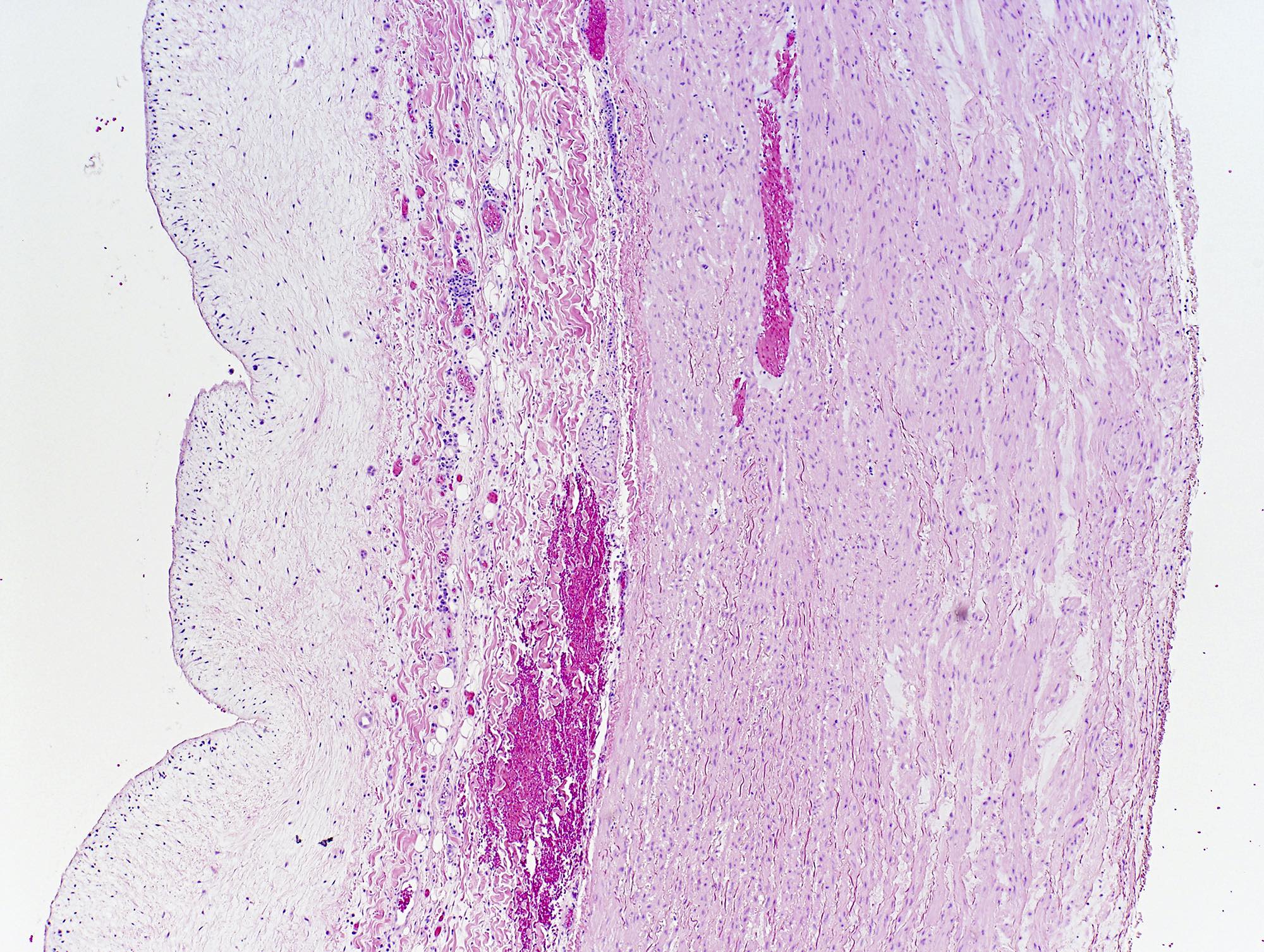
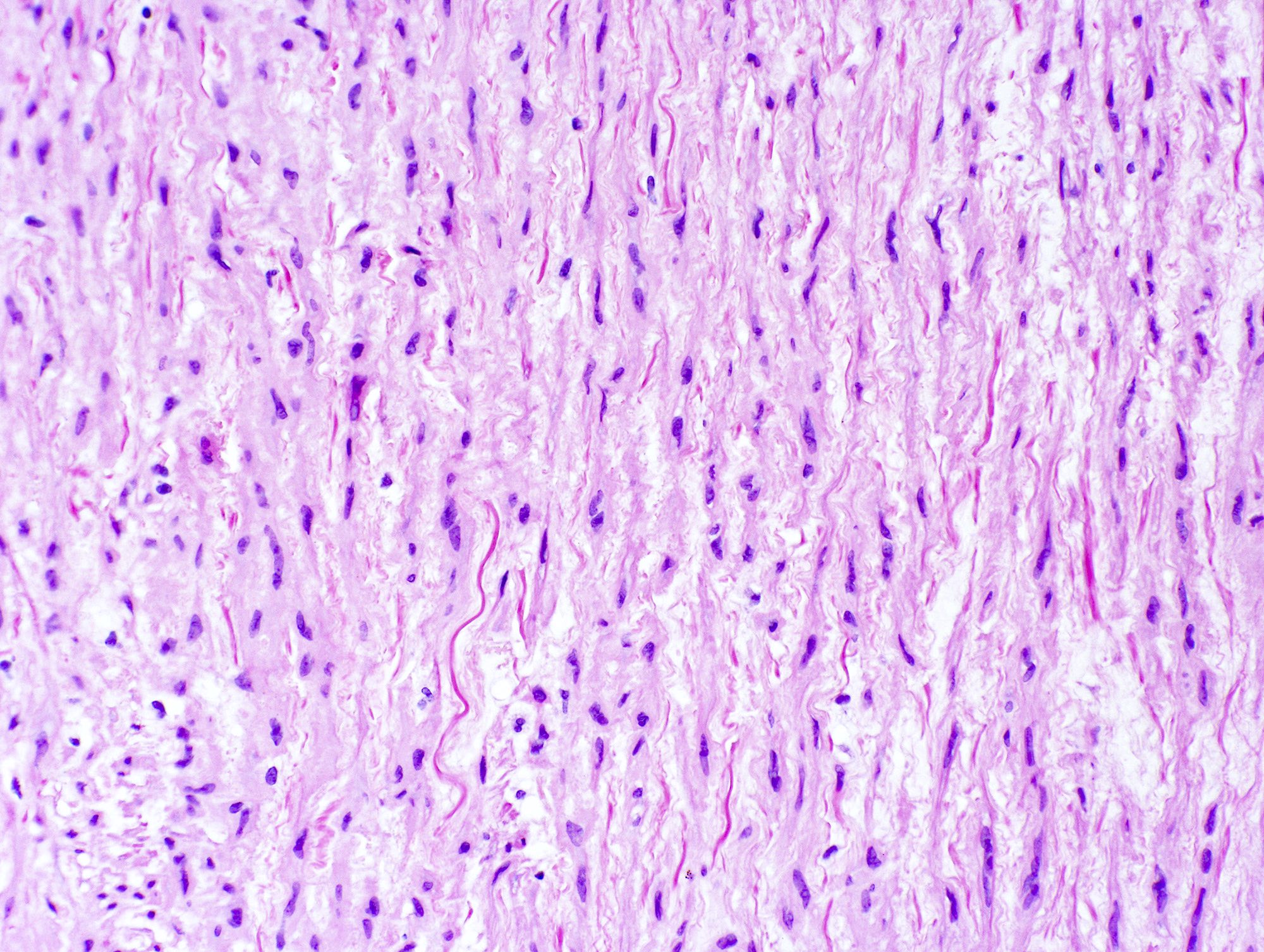
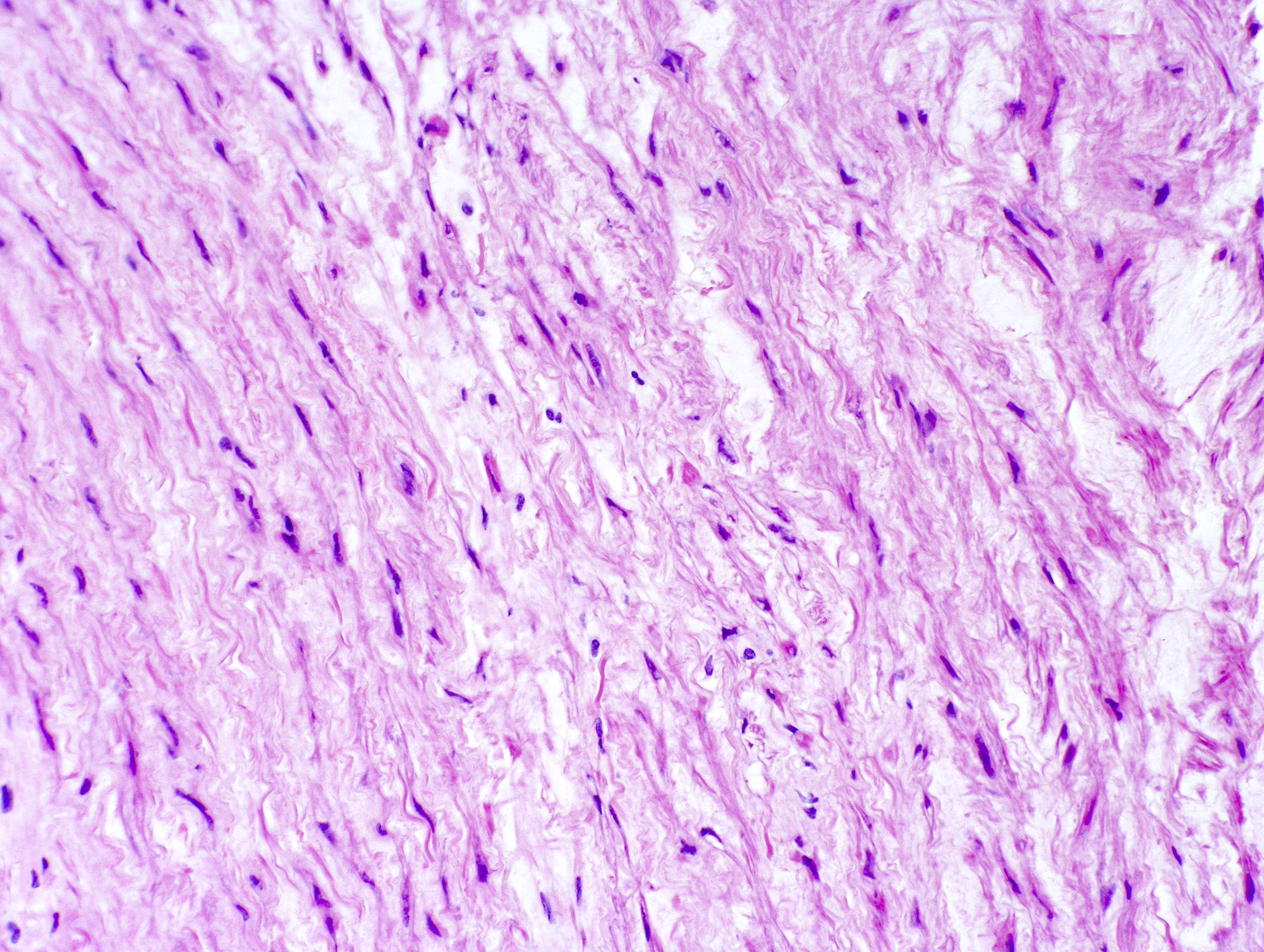
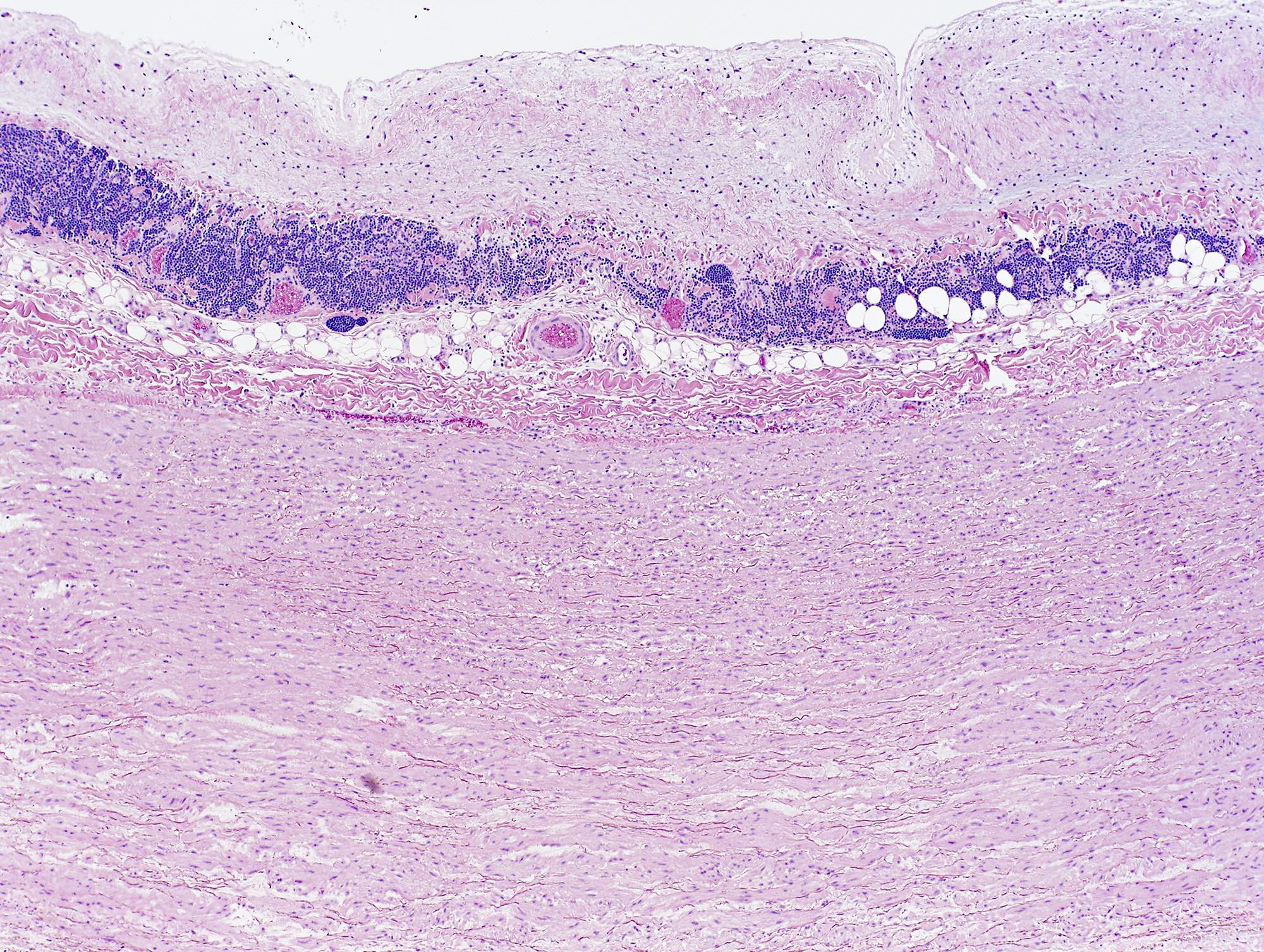
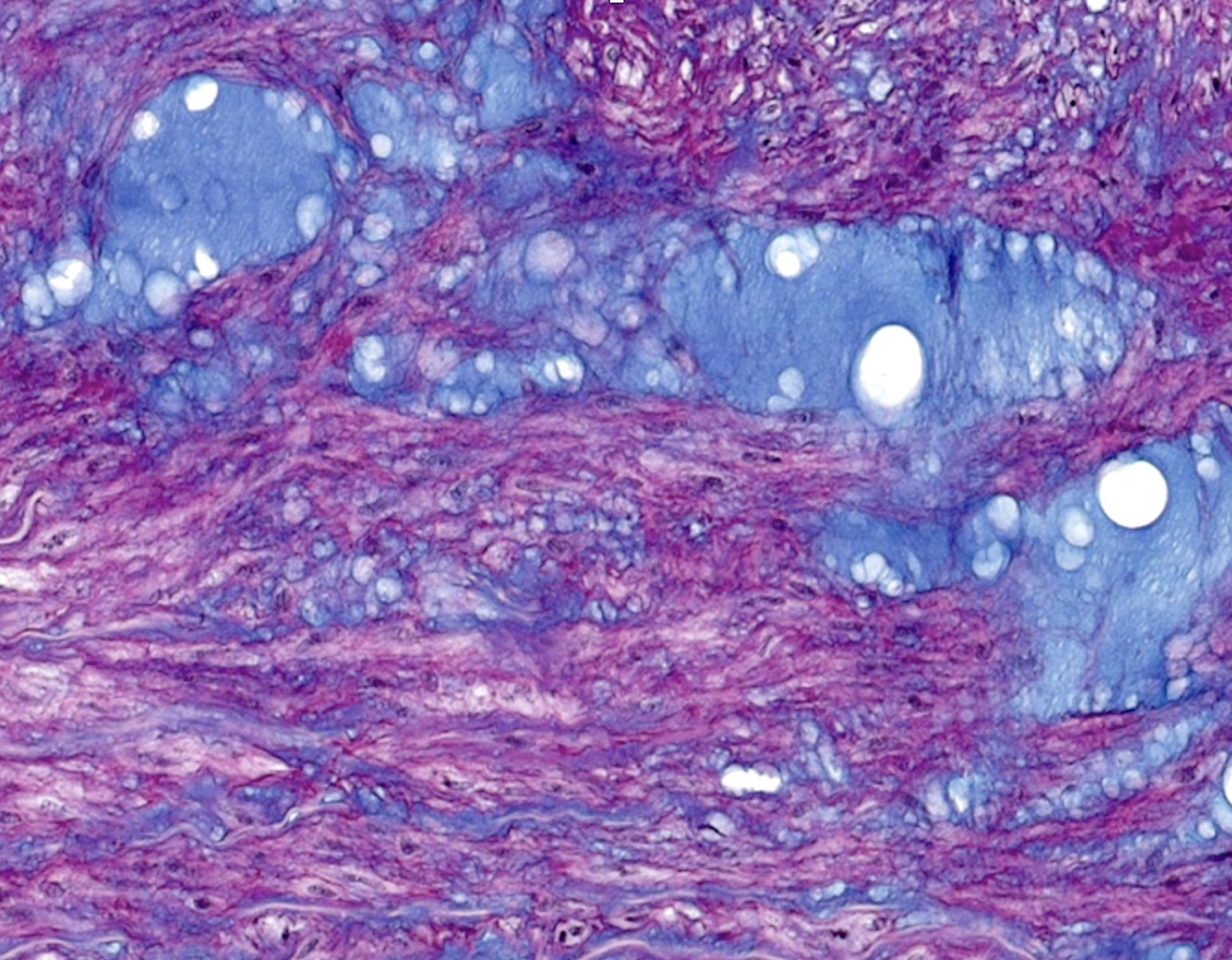
Microscopic (histologic) description
- Medial degeneration (Histopathology 1990;16:557)
- Loss / disorganization of elastic lamellae
- Loss of smooth muscle cells
- Mucoid extracellular matrix accumulation (MEMA)
- Medial fibrosis
- Border between tunica media and tunica intima may be obscured
- Inflammatory reaction (JVS Vasc Sci 2021;2:260)
- Lymphocyte and macrophage infiltration
- Medial neovascularization
- Increased proteolysis
- Increasing proteoglycan deposition
- Atherosclerotic lesions (Cardiovasc Pathol 2015;24:267)
- Lipid deposits, foam cells, cholesterol clefts, eosinophilic debris, calcifications or neovascularization
- Increase of various matrix metalloproteinases (MMPs) and cadherin (Histopathology 1990;16:557)
- Increased collagenase / elastase activity
- There may be luminal fibrin thrombus present (Cardiovasc Pathol 2015;24:267)
Microscopic (histologic) images
Sample pathology report
- Pathology is usually not included for diagnosis and diagnosis does not need to be reported but can be included in the microscopic description
- Abdominal aorta, endovascular aneurysm repair (EVAR):
- Abdominal aortic aneurysm (6.2 cm) (see comment)
- Comment: Microscopic examination reveals multifocal, extensive intralamellar and translamellar MEMA (mucoid extracellular matrix accumulation). There is also extensive elastic fiber disorganization and elastic fiber fragmentation along the tunica media. Morphologic findings, including frequent, band-like smooth muscle nuclei loss and extensive smooth muscle disorganization along the tunica media, are worrisome and along with the rest of the findings, reach the threshold for classification of severe medial degeneration.
- Abdominal aorta, endovascular aneurysm repair (EVAR):
Differential diagnosis
- Aortic dissection:
- There is a distinct intimal wall tear as well as separation of the arterial layers
- Pseudoaneurysm:
- Local hematoma in vessel not containing any layer of the vessel wall
- Myocardial infarction:
- Electrocardiogram (ECG) changes, elevated cardiac enzymes, ischemic changes in myocardium
- Acute cholecystitis:
- Imaging US / CT can show gallstones, edema and fat stranding surrounding gallbladder
- Gastritis and peptic ulcer disease:
- Abdominal pain usually related to positional changes and eating habits
- Pancreatitis:
- Elevated amylase / lipase levels, CT shows pancreatic edema / fat stranding, duct changes
- Bowel obstruction / ischemic bowel:
- Imaging will show bowel obstruction or reduced flow to the bowel
- Appendicitis:
- Presents with fever, McBurney point tenderness
- CT shows enlarged appendix with inflammatory signs
- Musculoskeletal pain:
- Associated with point tenderness and does not radiate
- Pulmonary embolism:
- Imaging shows central filling defect
- Pain tends to be pleuritic
Additional references
Practice question #1
A 70 year old man dies after the sudden onset of back pain. The autopsy pathologist (gross image shown above) notes severe medial degeneration when examining a section of the suprarenal aorta. What specific change is most likely to also be seen in this tissue sample?
- Fibrinoid necrosis of vessel walls
- Inflammation limited to the adventitia with scarring
- Significant loss of smooth muscle cells
- Well formed granulomas with eosinophilic presence
Practice answer #1
C. Significant loss of smooth muscle cells. Significant loss of smooth muscle cells is a characteristic process of medial degeneration in aortic aneurysm. Answer A is incorrect because fibrinoid necrosis can be seen in necrotizing vasculitis. Answer D is incorrect because granulomas with eosinophilic infiltrate can be seen in eosinophilic granulomatosis with polyangiitis. Answer B is incorrect because inflammation limited to the adventitia with scarring is more commonly seen in atherosclerosis.
Comment Here
Reference: Aortic aneurysms
Comment Here
Reference: Aortic aneurysms
Practice question #2
A 55 year old man is seen for follow up imaging for a 4.2 cm thoracic aortic aneurysm found 4 months ago. Computed tomography (CT) with contrast shows a focal dilation along the descending aorta, measuring 4.9 cm. What is the most recommended next step for this patient's management?
- Elective aortic aneurysm repair
- Follow up CT in 6 months
- Follow up ultrasound in 6 months
- Follow up ultrasound in 12 months
Practice answer #2
A. Elective aortic aneurysm repair. Although the diameter of the aneurysm has not reached > 5.0 cm (general threshold recommended by American College of Cardiology [ACC] / American Heart Association [AHA] guidelines 2022 for intervention), the aneurysm has grown > 0.5 cm in 6 months. Thus, this patient's aneurysm growth rate meets the guidelines for recommending intervention. Answers B and C are incorrect because although screening every 6 months is recommended for men with AAA of diameter 4.0 - 4.9 cm, according to ACC / AHA guidelines 2022 for intervention, a repair is recommended for an AAA that has grown > 0.5 cm in 6 months as there is an increased risk of rupture. Answer D is incorrect because this is the surveillance recommendation for patients with AAA with a diameter of 3.0 - 3.9 cm, whereas this patient requires repair due to the risk of rupture.
Comment Here
Reference: Aortic aneurysms
Comment Here
Reference: Aortic aneurysms