Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Prognostic factors | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Peripheral smear description | Peripheral smear images | Positive stains | Negative stains | Flow cytometry description | Flow cytometry images | Molecular / cytogenetics description | Molecular / cytogenetics images | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Aqil B. AML with BCR::ABL1. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/bonemarrowneoplasticAMLBCRABL1.html. Accessed April 26th, 2024.
Definition / general
- Acute myeloid leukemia (AML) with BCR::ABL1 fusion is a de novo AML in which BCR::ABL1 is detected at initial diagnosis without evidence of chronic myeloid leukemia (CML)
Essential features
- BCR::ABL1 fusion is detected at the time of diagnosis in a de novo AML with BCR::ABL1
- No CML morphologic features prior to or at diagnosis or posttherapy
- Morphologic spectrum, includes AML with minimal differentiation, without maturation or with monocytic differentiation
- Additional chromosomal aberrations are infrequently seen
- Cryptic deletions within immunoglobulin and T cell receptor accompanied by losses of the IKZF1 or CDNK2A / B genes
Terminology
- Acute myeloid leukemia with t(9;22)(q34.1;q11.2)
ICD coding
- ICD-O: 9912/3 - acute myeloid leukemia with BCR::ABL1
- ICD-11: 2A60.0 & XH6FZ7 - acute myeloid leukemia with recurrent genetic abnormalities & acute myeloid leukemia with BCR::ABL1
Epidemiology
- AML with BCR::ABL1 is a rare entity and accounts for 0.5 - 3% of all AML cases
- It is seen more commonly in males (Haematologica 1996;81:423, Leuk Res 2004;28:579, Am J Clin Pathol 2007;127:642)
Sites
- Peripheral blood and bone marrow are mostly involved
- Extramedullary site involvement is rare (Leuk Res 2008;32:1476)
Pathophysiology
- Philadelphia (Ph) chromosome and the chimeric BCR::ABL1 fusion gene result from t(9;22)(q34;q11) or its variants, which encodes a constitutively active tyrosine kinase with oncogenic properties (Br J Haematol 2013;161:541)
- 3 different breakpoint cluster regions in the BCR gene (M-bcr, m-bcr and µ-bcr) are reported: 8.5 kb hybrid mRNA (b2a2 or b3a2) encodes the 210 kDa protein (p210), 7.5 kb hybrid mRNA (e1a2) encodes the 190 kDa protein (p190) and 9 kb hybrid mRNA (c3a2) encodes the 230 kDa protein (p230) (Genomics 1995;27:67, Oncogene 2002;21:8652)
- ~70 - 80% cases of AML with BCR::ABL1 harbor p210 transcripts (Am J Clin Pathol 2007;127:642)
Etiology
- Unknown
Clinical features
- Present with leukocytosis and anemia or thrombocytopenia
- Patients with AML with BCR::ABL1 in comparison to CML myeloid blast phase (CML MBP) have lower peripheral blood basophil percentage, absolute basophil count and are, less commonly, found to have splenomegaly (Am J Clin Pathol 2007;127:642)
Diagnosis
- Essential diagnostic criteria
- Myeloid neoplasm with > 20% blasts expressing myeloid immunophenotype (based on immunohistochemistry or flow cytometry) in the peripheral blood or bone marrow
- Detection of BCR::ABL1 at the time of initial diagnosis
- Lack of CML features prior to or at diagnosis or after therapy
- Desirable diagnostic criteria
- Presence of t(9;22)(q34.1;q11.2) on conventional karyotyping
- Determination of the BCR::ABL1 transcript subtype and establishment of a baseline level of BCR::ABL1 transcript for monitoring treatment response
Laboratory
- Leukocytosis
- Anemia or thrombocytopenia
Radiology description
- FDG avid extramedullary lesions may be seen (Leuk Res 2008;32:1476)
Prognostic factors
- AML with BCR::ABL1 has an unfavorable prognosis with a median survival of < 9 months (Haematologica 1996;81:423, Am J Clin Pathol 2007;127:642)
Case reports
- 28 year old woman presented with leukocytosis and was diagnosed with AML with e1a2 BCR::ABL1 fusion (Rev Bras Hematol Hemoter 2017;39:379)
- 68 year old man with a history of dementia presented with leukocytosis and was diagnosed with AML with BCR::ABL1 (Hemasphere 2020;4:e484)
- 77 year old woman who presented with pancytopenia underwent a bone marrow biopsy and was diagnosed with AML with BCR::ABL1 (Leuk Res Rep 2020;15:100233)
- 78 year old man with no prior history of CML presented with leukocytosis and was diagnosed with AML with BCR::ABL1 (BMC Cancer 2019;19:50)
Treatment
- Standard AML induction chemotherapy (anthracycline / cytosine arabinoside containing regimen) response is poor (Leukemia 1998;12:1881)
- In addition, single agent tyrosine kinase inhibitors (TKI) alone are also not effective; however, a single case report indicated achieving complete molecular remission in a patient treated with dasatinib and chemotherapy (Ann Hematol 2016;95:1211, Medicine (Baltimore) 2018;97:e12949)
- Venetoclax and TKI combination regimens have shown good response in some studies (Acta Haematol 2020;143:567)
- Outcome of patients < 50 years of age with TKI pretreated BCR::ABL1 positive AML receiving allogeneic stem cell transplant is relatively favorable (Am J Hematol 2018;93:31)
- Survival status postallogeneic transplantation appears similar to intermediate risk AML, with one report demonstrating 3 year overall survival of 73% (Bone Marrow Transplant 2021;56:232)
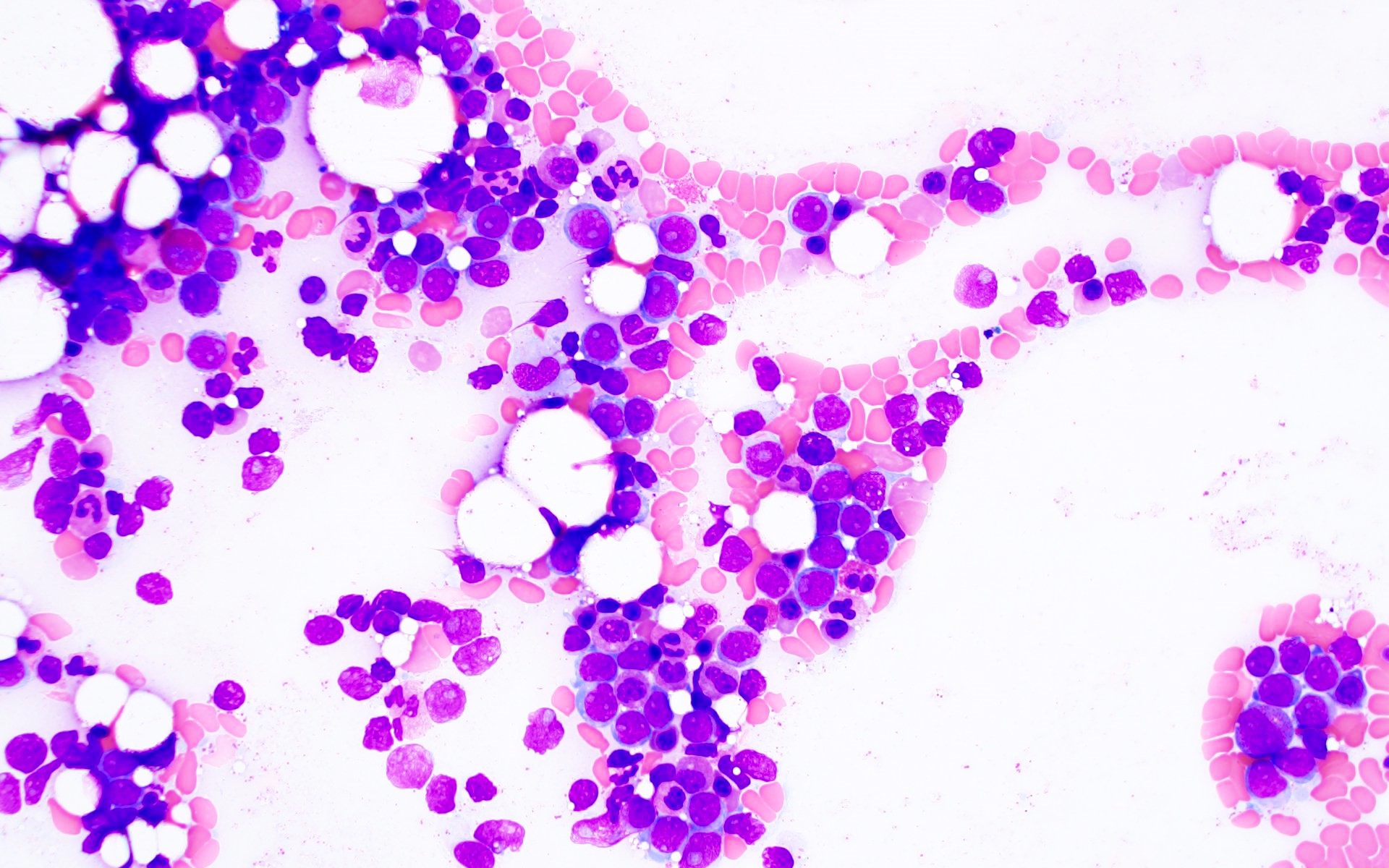
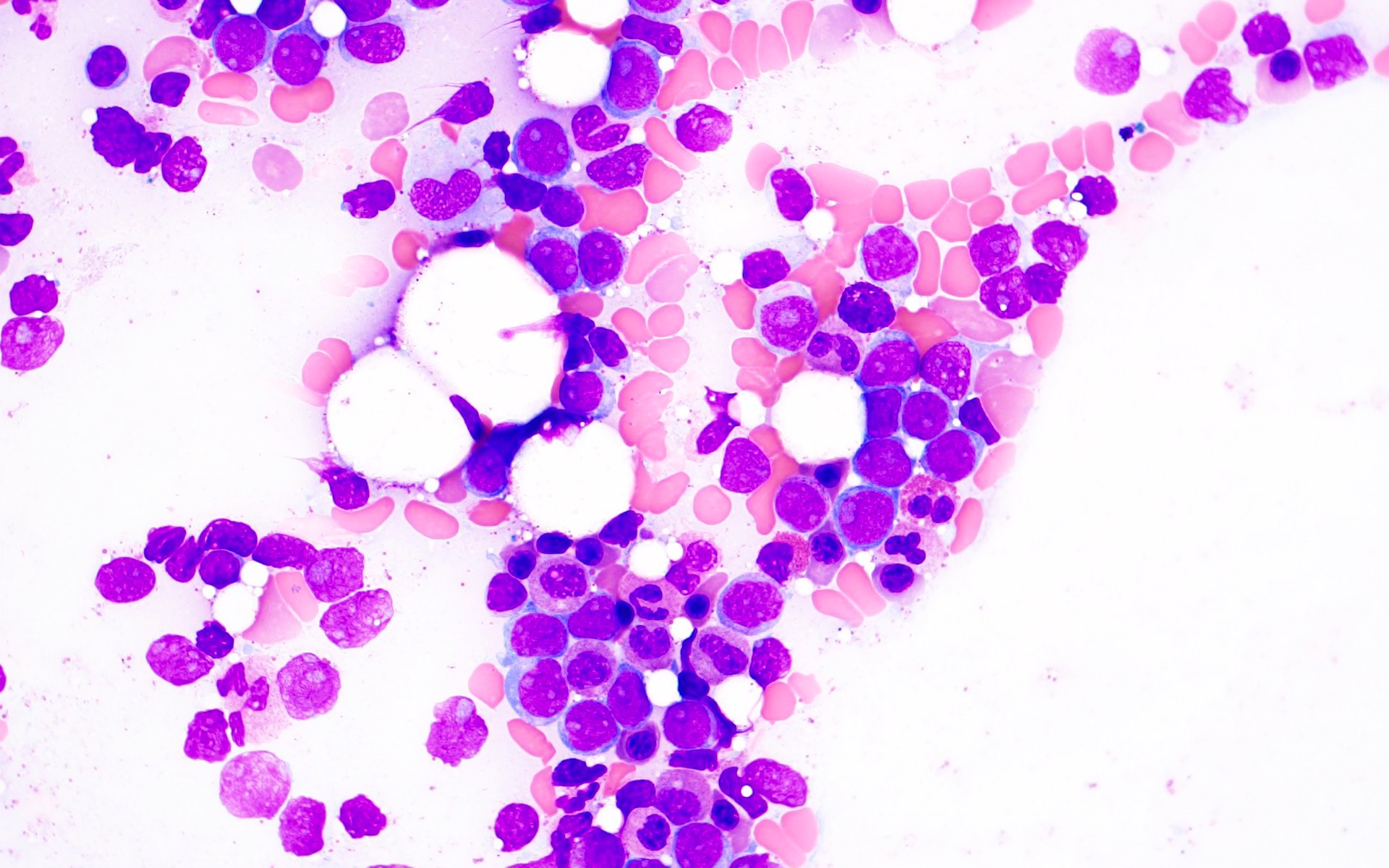
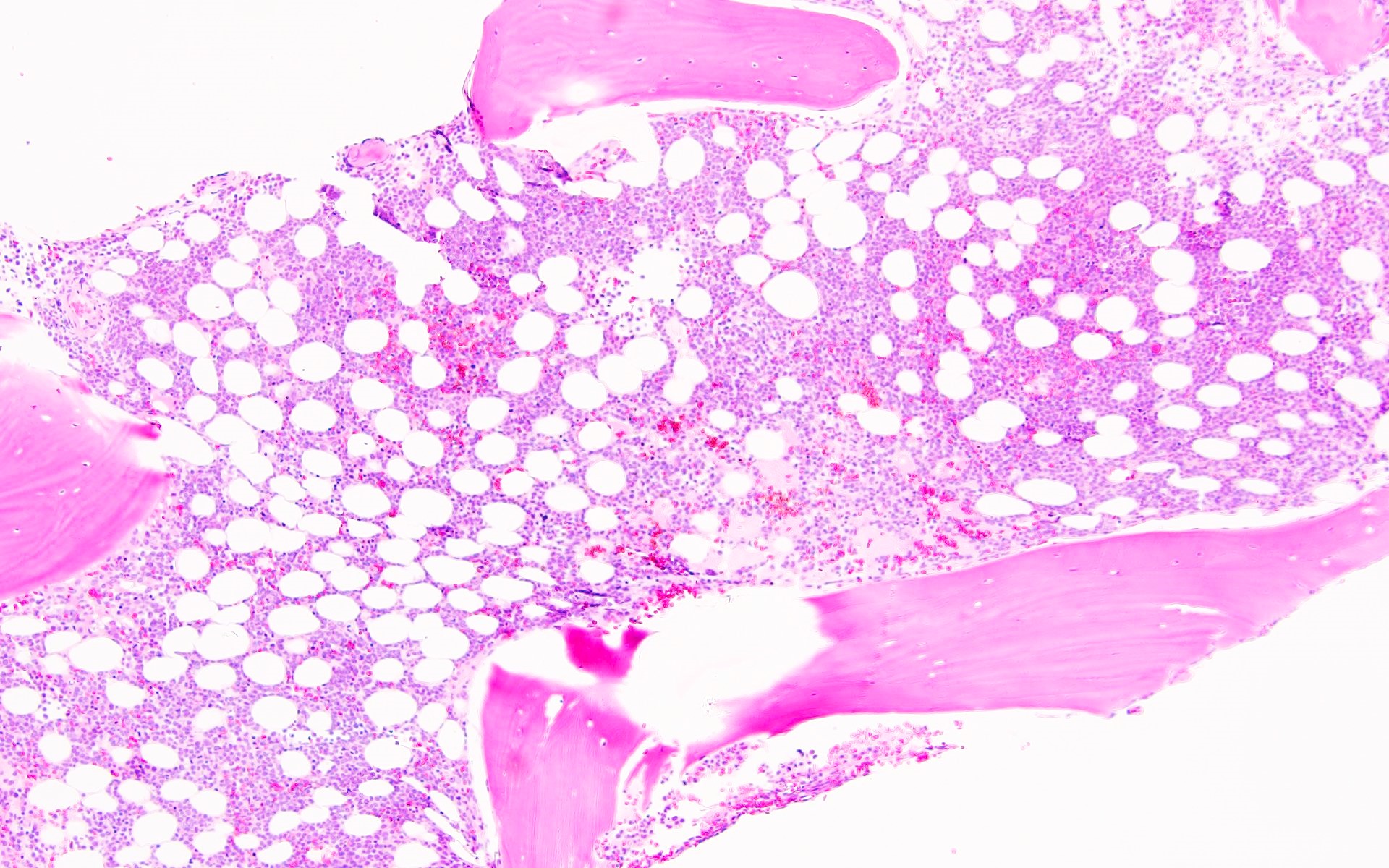
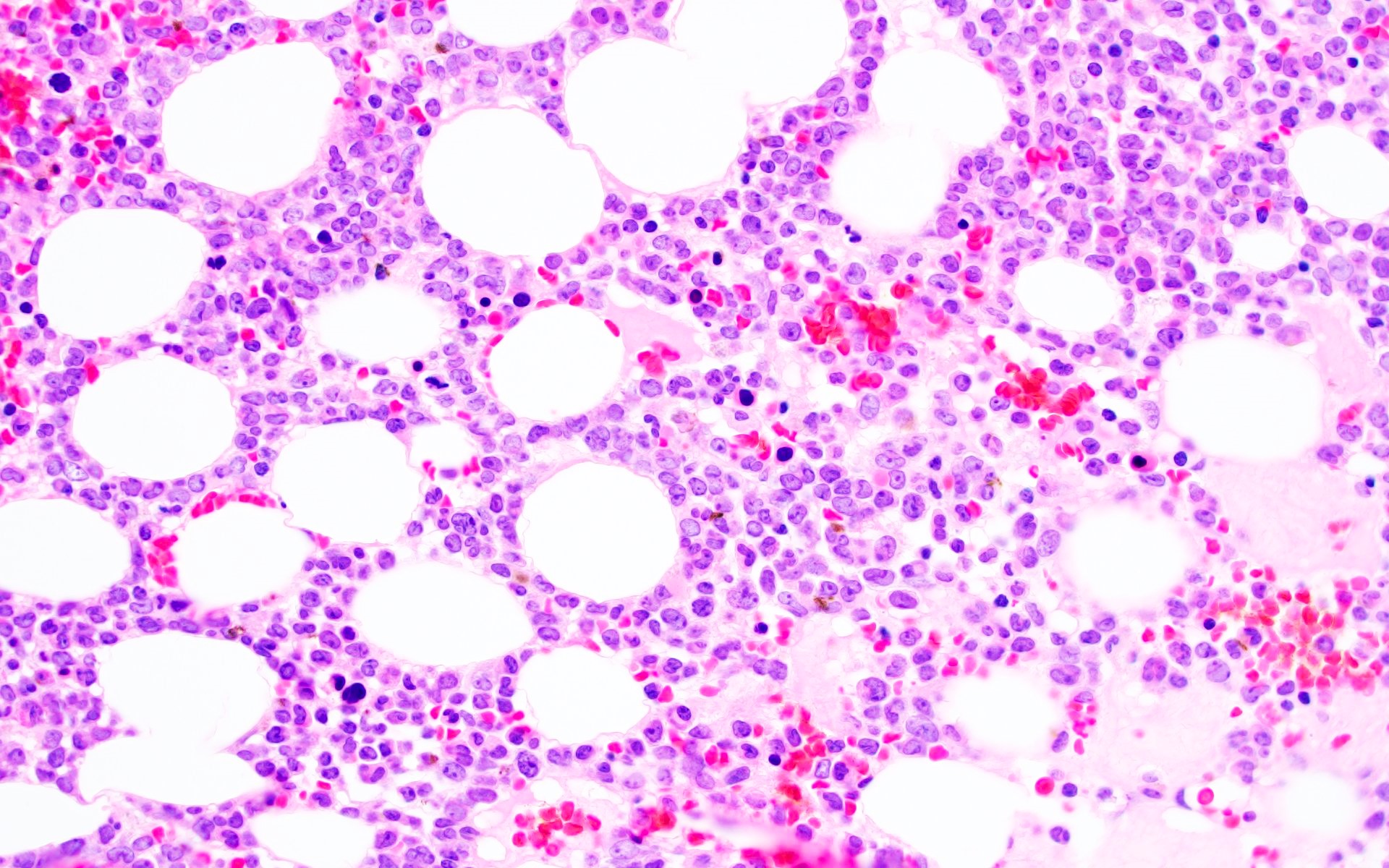
Microscopic (histologic) description
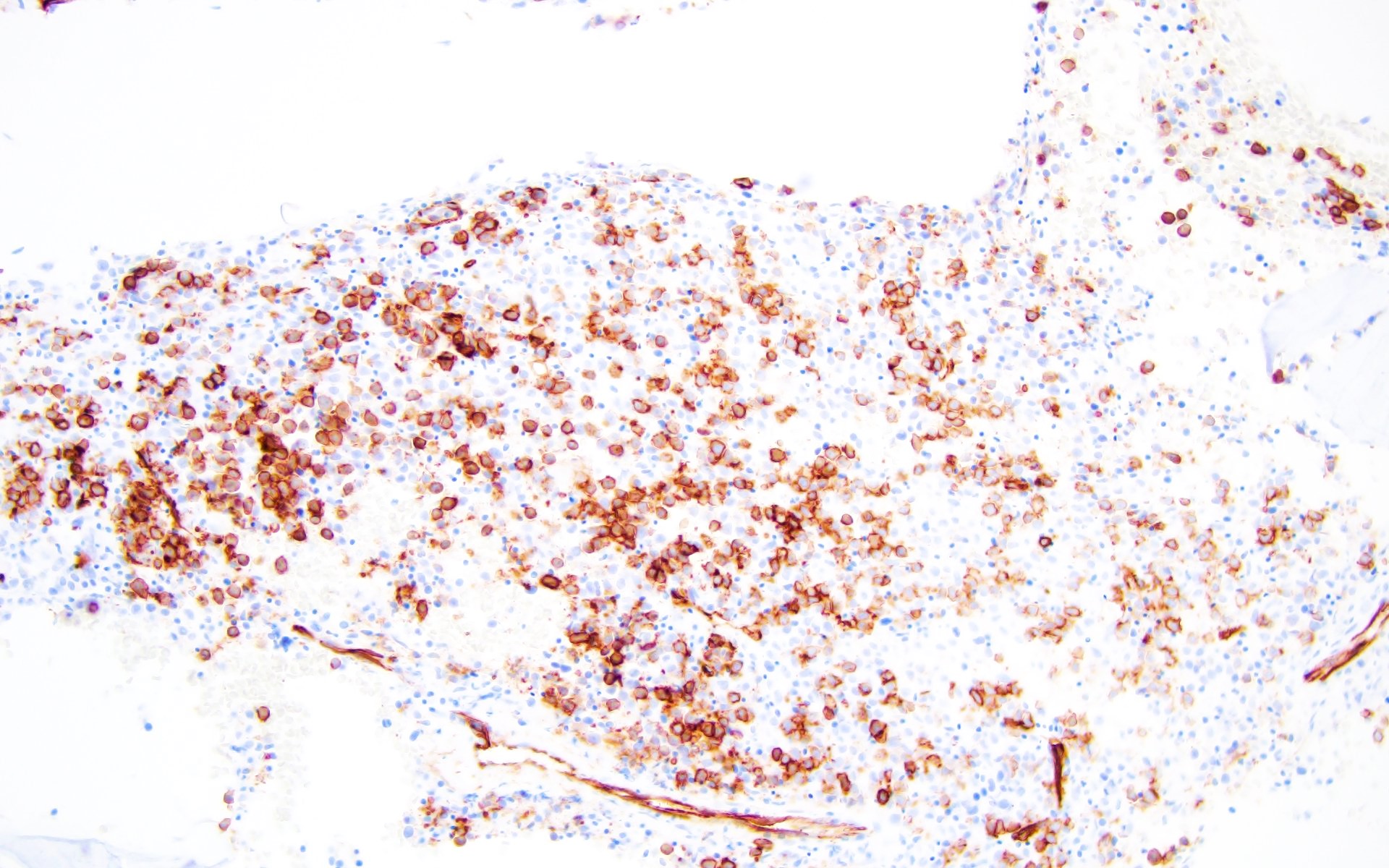
- AML with BCR::ABL1 has a broad morphologic spectrum, which includes AML with minimal differentiation (FAB M0), without maturation (FAB M1) or with monocytic differentiation (FAB M5) (Haematologica 1996;81:423, Leuk Lymphoma 2013;54:138)
- AML with BCR::ABL1 demonstrates a marrow cellularity of 80%, which is lower than the 98% median marrow of CML MBP (Am J Clin Pathol 2007;127:642)
- In comparison to CML MBP, the bone marrow in AML with BCR::ABL1 shows lower myeloid/erythroid ratio at diagnosis (median: 2.0 versus 4.8) and a lower bone marrow aspirate basophil percentage (median: 0% versus 4.5%) (Am J Clin Pathol 2007;127:642)
- Dwarf megakaryocytes are rarely seen (Leuk Lymphoma 2013;54:138)
Microscopic (histologic) images
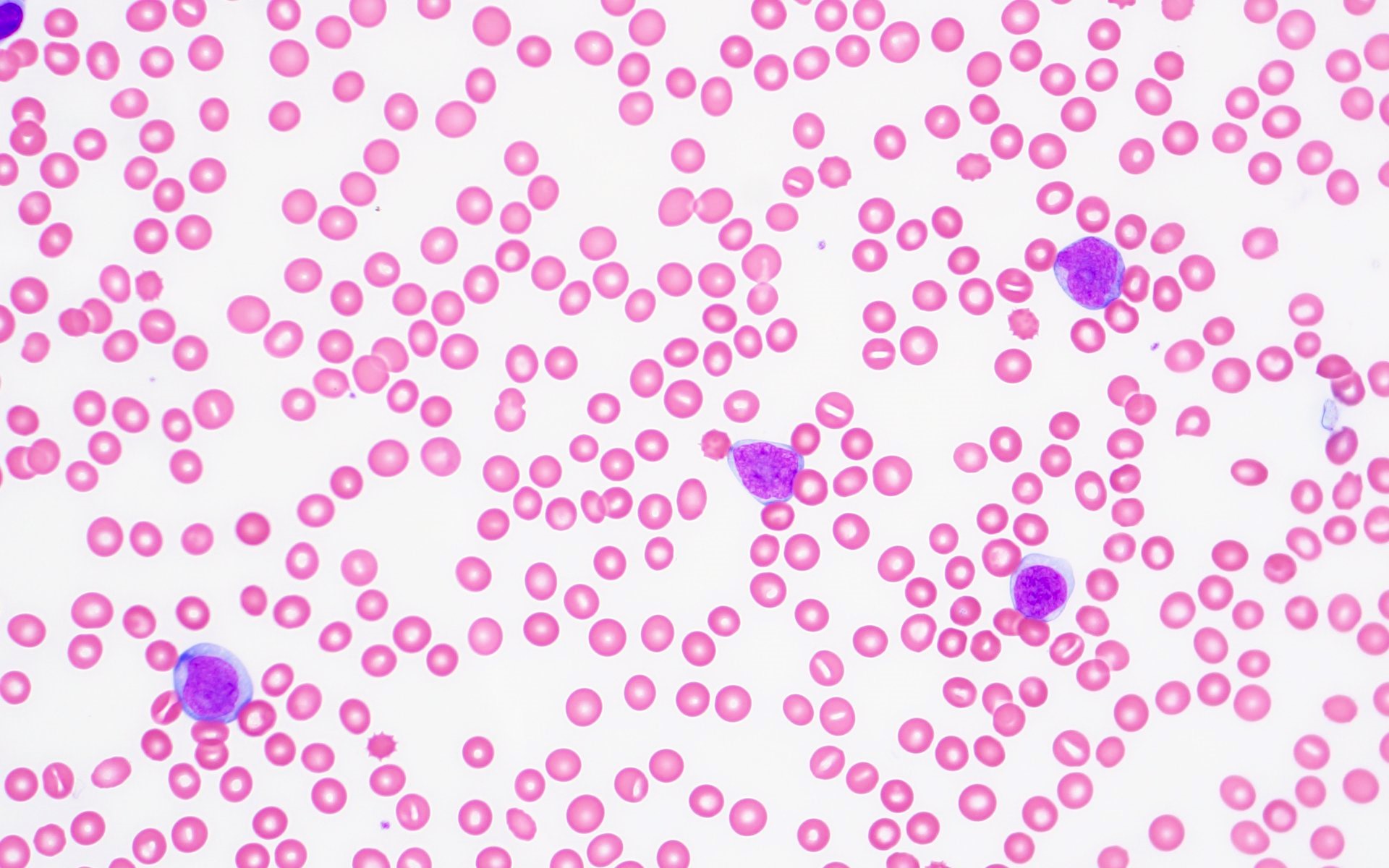
Peripheral smear description
- Leukocytosis
- Variable anemia, with normocytic or macrocytic red blood cells
- Variable thrombocytopenia
- Left shifted granulocytes may be seen with circulating blasts, which may be ≥ 20% by definition
- Blasts may be myeloblasts or monoblasts / promonocytes
- CML MBP cases have a higher peripheral blood basophil percentage (median: 2.5%) than AML with BCR::ABL1 (median: 0%) and a higher peripheral blood absolute basophil count (Am J Clin Pathol 2007;127:642)
Positive stains
- CD34, HLA-DR and myeloid antigens (CD117, CD13 and CD33)
- Expression of CD7, CD19 and TdT may be seen (Leukemia 1998;12:1881, Am J Clin Pathol 2007;127:642)
Negative stains
- Myeloperoxidase may be positive or negative depending on the type of blasts
Flow cytometry description
Flow cytometry images
Molecular / cytogenetics description
- Fluorescence in situ hybridization (FISH) and chromosome analysis identified t(9;22)(q34.1;q11.2) that defines this subtype of AML
- Additional chromosomal aberrations, such as trisomy 8, an additional Ph chromosome, trisomy 19 and isochromosome 17q seen in CML MBP are infrequently seen in AML with BCR::ABL1 (Am J Clin Pathol 2007;127:642)
- Del(7q) / -7 abnormalities can be seen (Am J Clin Pathol 2007;127:642, Leuk Lymphoma 2013;54:138, Leukemia 1998;12:1881)
- Cryptic deletions within immunoglobulin and T cell receptor genes are seen and are frequently accompanied by losses of the IKZF1 or CDNK2A / B genes (BMC Genomics 2010;11:41, Br J Haematol 2013;161:541)
- RUNX1 mutation is common in AML with BCR::ABL1 and occurs in ~40% of cases (Genes Chromosomes Cancer 2021;60:426)
- Mutations of NPM1, FLT3 or DNMT3A are not commonly detected (Genes Chromosomes Cancer 2021;60:426)
- Other mutated genes include ASXL1, BCOR, IDH1 / IDH2 and SRSF2; each of these occur in 10 - 15% of cases (Leuk Lymphoma 2013;54:138, Leukemia 2017;31:2211, Genes Chromosomes Cancer 2021;60:426)
Sample pathology report
- Peripheral blood, bone marrow aspirate and core biopsy:
- Acute myeloid leukemia with BCR::ABL1 (see comment)
- Comment: The cytogenetic and FISH studies showed t(3;9;22) resulting in rearrangement of BCR::ABL1. The overall findings are consistent with acute myeloid leukemia with BCR::ABL1. The peripheral blood neutrophils show no significant shift to immaturity and the marrow myeloid to erythroid ratio is not elevated. Given these findings, a de novo acute myeloid leukemia with BCR::ABL1 is favored over blast phase of chronic myeloid leukemia with BCR::ABL1.
- Peripheral blood: The peripheral blood smear shows pancytopenia. There is macrocytic anemia with anisopoikilocytosis including ovalocytes, teardrop forms and rare circulating nucleated red blood cells. Occasional circulating blasts are noted. The blasts are intermediate to large in size with high nuclear to cytoplasmic ratio and dispersed chromatin. Neutrophils show toxic changes but without significant shift to immaturity. Eosinophils are present but not increased. Platelets are decreased in number with unremarkable morphology.
- Bone marrow aspirate: The bone marrow aspirate smears show increased blasts showing similar morphology to those seen in the peripheral blood smear. The erythroid precursors are decreased but show progressive mature with unremarkable morphology. Megakaryocytes are present with unremarkable morphology.
- Bone marrow core biopsy: The bone marrow core biopsy shows hypercellular marrow (~80% cellular). Myeloid precursors are increased with a shift to immaturity including frequent blasts. The blasts are intermediate to large in size with dispersed chromatin, present scattered and in aggregates throughout the biopsy. Erythroid precursors are relatively decreased but show progressive maturation. Megakaryocytes are decreased with unremarkable morphology.
- Cytogenetic analysis reported an abnormal male karyotype: 46,XY,t(3;9;22)(p21; q34.1; q11.2)[3]/48, idem,+8,+12[17]. The t(3;9;22) is a 3 way variant translocation resulting in BCR::ABL1 fusion. Gains of chromosomes 8 and 12 in related abnormal clone 2 represent clonal evolution.
- FISH analysis was positive for the BCR::ABL1 fusion with a signal pattern consistent with a 3 way translocation (1F2R2G, 66%).
Differential diagnosis
- Acute myeloid leukemia with other recurrent cytogenetic abnormality:
- Presence of another cytogenetic abnormality, which is defining for de novo AML subtype in addition to t(9;22)(q34.1;q11.2), takes precedence over the diagnosis of AML with BCR::ABL1 (Br J Haematol 2011;152:713, Leuk Lymphoma 2013;54:138)
- Therapy related AML or AML with myelodysplasia related changes (AML MRC):
- BCR::ABL1 may be acquired secondarily in AML transforming from previous myelodysplastic neoplasm or in relapsed / refractory AML after therapy that previously lacked BCR::ABL1 (Mod Pathol 2018;31:1141)
- Chronic myeloid leukemia (CML), myeloid blast phase:
- CML MBP cases have a higher peripheral blood basophil percentage (median: 2.5%) than AML with BCR::ABL1 (median: 0%), a higher peripheral blood absolute basophil count and increased frequency of splenomegaly
- Bone marrow in AML with BCR::ABL1 shows lower myeloid/erythroid ratio at diagnosis (median: 2.0 versus 4.8) (Am J Clin Pathol 2007;127:642)
- Additional chromosomal alterations that are typical of myeloid blast phase of CML are less commonly seen in AML with BCR::ABL1
- Although genes reported to be altered in the transformed stages (BP), including TP53, RB1, MYC, CDKN2A, NRAS, KRAS, RUNX1 (also known as AML1), TET2, CBL, ASXL1, IDH1 and IDH2, are similar to AML with BCR::ABL1, no IKZF1 deletions together with cryptic deletions within the immunoglobulin and T cell receptor genes are usually seen (Leuk Lymphoma 2013;54:138, Genes Chromosomes Cancer 2021;60:426)
- Mixed phenotype acute leukemia (MPAL) with BCR::ABL1:
- AML with BCR::ABL1 may aberrantly express lymphoid antigens (CD7 and CD19)
- Additional cytogenetic abnormalities are observed less in MPAL with BCR::ABL1 (13 - 30%) as compared to AML with BCR::ABL1 (50 - 60%) and CML MBP (70 - 80%) (Int J Hematol 2011;94:552, Eur J Haematol 2014;93:297, Am J Clin Pathol 2007;127:642, Leuk Lymphoma 2013;54:138, Br J Haematol 2013;161:541, Genes Chromosomes Cancer 2021;60:426)
- Cryptic deletions within immunoglobulin and T cell receptor genes with losses of the IKZF1 or CDNK2A / B genes are also detected in MPAL with BCR::ABL1 as in AML with BCR::ABL1
- Mutations in TP53, NRAS, IDH2, FLT3, PHF6, ASXL1, ETV6 and DNMT3A occur in higher frequency in MPAL unlike AML with BCR::ABL1; in contrast, NPM1 mutations are not found in MPAL, similar to AML with BCR::ABL1 (Exp Hematol 2016;44:740, Nat Commun 2018;9:2670, Blood Adv 2018;2:3526)
Additional references
Board review style question #1
Which of the following is true of acute myeloid leukemia (AML) with BCR::ABL1?
- Detection of BCR::ABL1 can occur posttherapy
- It has no co-occurrence with t(8;21) / RUNX1::RUNX1T1
- It is a myeloid neoplasm with < 20% myeloid blasts
- There can be a prior history of myelodysplastic neoplasm or myeloproliferative / myelodysplastic neoplasm
Board review style answer #1
B. It has no co-occurrence with t(8;21) / RUNX1::RUNX1T1. The presence of another cytogenetic abnormality that is defining for another de novo AML subtype in addition to t(9;22)(q34.1;q11.2) takes precedence over the diagnosis of AML with BCR::ABL1. Answer A is incorrect because AML with BCR::ABL1 is a de novo AML in which BCR::ABL1 is detected at the time of initial diagnosis. Answer C is incorrect because, in the category of AML with defined genetic abnormality, AML with BCR::ABL1 and AML with CEBPA mutation are the only two subtypes that require > 20% blasts for the diagnosis per 2022, WHO 5 th edition. According to International Consensus Classification (Virchows Arch 2023;482:27), AML with BCR::ABL1 is the only subtype which require > 20% blasts. Answer D is incorrect because BCR::ABL1 can be acquired secondarily in AML arising from prior myelodysplastic neoplasm or myeloproliferative / myelodysplastic neoplasm. So, these cases are excluded from this category.
Comment Here
Reference: AML with BCR::ABL1
Comment Here
Reference: AML with BCR::ABL1
Board review style question #2
In comparison with chronic myeloid leukemia (CML) myeloid blast phase, AML with BCR::ABL1 shows which of the following findings?
- Cryptic deletions within the immunoglobulin and T cell receptor genes
- High frequency of splenomegaly
- Increased peripheral basophil percentage
- Presence of additional chromosomal aberrations (trisomy 8, trisomy 19 and isochromosome 17q)
Board review style answer #2
A. Cryptic deletions within the immunoglobulin and T cell receptor genes. Cryptic deletions within the immunoglobulin and T cell receptor genes, along with losses of the IKZF1 or CDNK2A / B genes are seen in AML with BCR::ABL1. Answers B and C are incorrect because splenomegaly, high myeloid to erythroid ratio in the bone marrow and increased basophils are seen in the myeloid blast phase of chronic myeloid leukemia rather than AML with BCR::ABL1. Answer D is incorrect because cytogenetic abnormalities, such as an isochromosome of the long arm of chromosome
17, gain of an extra copy of Ph, trisomy 19 and trisomy 8, which are known to occur frequently in the blast phase of chronic myeloid leukemia, are not usually seen in AML with BCR::ABL1.
Comment Here
Reference: AML with BCR::ABL1
Comment Here
Reference: AML with BCR::ABL1