Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Cytology images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Practice question #1 | Practice answer #1 | Practice question #2 | Practice answer #2Cite this page: Agostini-Vulaj D. Intraductal papillary mucinous neoplasm (IPMN). PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/pancreasipmn.html. Accessed July 29th, 2025.
Definition / general
- Grossly visible noninvasive mucinous epithelial neoplasm arising from main pancreatic duct or branch ducts, usually > 5 mm, composed of various cell types with various cytologic and architectural atypia (Virchows Arch 2005;447:794, Pancreatology 2017;17:738)
- Further classified as:
- Low grade (encompasses low or intermediate grade dysplasia) (Am J Surg Pathol 2015;39:1730)
- High grade (Am J Surg Pathol 2015;39:1730)
- With an associated invasive carcinoma (Am J Surg Pathol 2015;39:1730)
- Further classified as:
- 1 of 3 precursor lesions of pancreatic adenocarcinoma (see also mucinous cystic neoplasm)
Essential features
- Grossly visible (> 5 mm) cystic pancreatic neoplasm, usually in head of pancreas
- > 90% 5 year survival with complete resection
- Roughly 33% of cases have an associated invasive carcinoma
- Further subtyped into gastric, intestinal and pancreaticobiliary types based on epithelium (note: intraductal oncocytic papillary neoplasm (IOPN) is now considered a distinct and separate entity)
Terminology
- Low grade to intermediate grade dysplasia previously termed: intraductal papillary mucinous adenoma
- High grade dysplasia previously termed: intraductal papillary mucinous carcinoma, noninvasive
- With an associated invasive carcinoma previously termed: intraductal papillary mucinous carcinoma, invasive
- Other previous terms include: mucin producing tumor, mucinous duct ectasia, ductectatic mucinous cystadenoma / cystadenocarcinoma, villous adenoma or papillary adenoma / carcinoma
ICD coding
- ICD-10: D13.6 - benign neoplasm of pancreas
Epidemiology
- Rising incidence likely secondary to better characterization of this entity along with use of radiologic imaging studies (Clin Gastroenterol Hepatol 2010;8:213)
- More common in men, usually ages 60 - 70 years (Hum Pathol 2012;43:1)
Sites
- Main duct intraductal papillary mucinous neoplasm (IPMN) mostly involves head of pancreas, 33% in body and tail (Hum Pathol 2012;43:1)
- Branch duct IPMN mostly involves head of pancreas or uncinate process, with multiple distinct lesions seen in ~33% of cases (Hum Pathol 2012;43:1)
Pathophysiology
Etiology
- No well established factors; cigarette smoking implicated in one series (Adv Anat Pathol 1999;6:65)
- Reported in Peutz-Jeghers syndrome and familial adenomatous polyposis (Gut 2002;51:446, Am J Pathol 1999;154:1835)
Clinical features
- Clinically divided into main duct IPMN, branch duct IPMN and mixed IPMN (most mixed type IPMN present and behave as main duct IPMN) (Hum Pathol 2012;43:1)
- Main duct IPMN tends to be symptomatic, with symptoms related to duct obstruction (pancreatitis) (Hum Pathol 2012;43:1)
- Signs and symptoms include epigastric pain, weight loss, jaundice, diabetes, pancreatitis (Arch Pathol Lab Med 1996;120:981)
- Branch duct IPMN tends to be asymptomatic and is generally discovered incidentally on imaging (Hum Pathol 2012;43:1)
- Peutz-Jeghers syndrome (autosomal dominant inherited syndrome, mutations in STK11 / LKB1I) is also associated with IPMN (Hum Pathol 2012;43:1)
Diagnosis
- Radiographic / endoscopic findings
- Fine needle aspiration
- Surgical specimen
Laboratory
- Pancreatic cyst fluid analysis can assist in determining type of cyst (Am J Gastroenterol 2008;103:2871, Gastrointest Endosc 2009;69:1095, Gastrointest Endosc 2016;83:140)
- Elevated CEA and identification of KRAS / GNAS mutations supports diagnosis of mucinous cyst
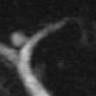
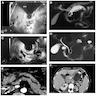
Radiology description
- CT:
- Main duct IPMN causes distention of main pancreatic duct
- Branch duct IPMN produces multilocular grape-like cystic appearance
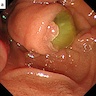
- ERCP (endoscopic retrograde cholangiopancreatography): pancreatic ductal filling defects may be seen / ductal dilation
- MRCP (magnetic resonance cholangiopancreatography): additional imaging option which does not produce radiation
- EUS (endoscopic ultrasound): can also allow FNA and cyst fluid analysis
- References: Diagn Interv Imaging 2016;97:1275, World J Gastroenterol 2016;22:9562
Radiology images
Prognostic factors
- Without an invasive carcinoma, has > 90% 5 year survival; those associated with an invasive carcinoma carry a worse prognosis (about half die of the disease) (Ann Surg 2016;263:162)
- Main duct IPMN: 60% have high grade dysplasia and 45% are associated with an invasive carcinoma (Hum Pathol 2012;43:1)
- Branch duct IPMN: most are low grade, 25% have high grade dysplasia and 20% are associated with an invasive carcinoma (Hum Pathol 2012;43:1)
- Invasive carcinoma associated with IPMN includes:
- Tubular (ductal) adenocarcinoma: seen in about half of cases, with slightly better prognosis than non IPMN associated pancreatic ductal adenocarcinoma
- Colloid carcinoma: seen in half of cases, with much better prognosis than pancreatic ductal adenocarcinoma (Ann Surg 2016;263:162)
Case reports
- 52 year old man with recurrent upper abdominal pain (Int J Clin Exp Med 2015;8:3332)
- 53 year old man with multiple pancreatic cystic lesions (World J Gastroenterol 2013;19:3358)
- 55 year old woman with abdominal pain and anemia; pancreatic masses are found on CT (World J Surg Oncol 2018;16:83)
- 68 year old man with cystic pancreatic mass with mural nodule (Int J Surg Case Rep 2017;40:69)
- 74 year old man with cystic pancreatic lesion on ultrasound (Intern Med 2018;57:1093)
Treatment
- Main duct IPMN: surgical resection indicated if main pancreatic duct > 10 mm, jaundice or presence of mural nodules (Pancreatology 2017;17:738)
- Branch duct IPMN: surgical resection indicated if symptomatic, presence of mural nodule ≥ 5 mm, suspicious / positive cytology, obstructive jaundice or main pancreatic duct ≥ 10 mm (Pancreatology 2017;17:738)
- See Diagrams / tables
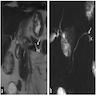
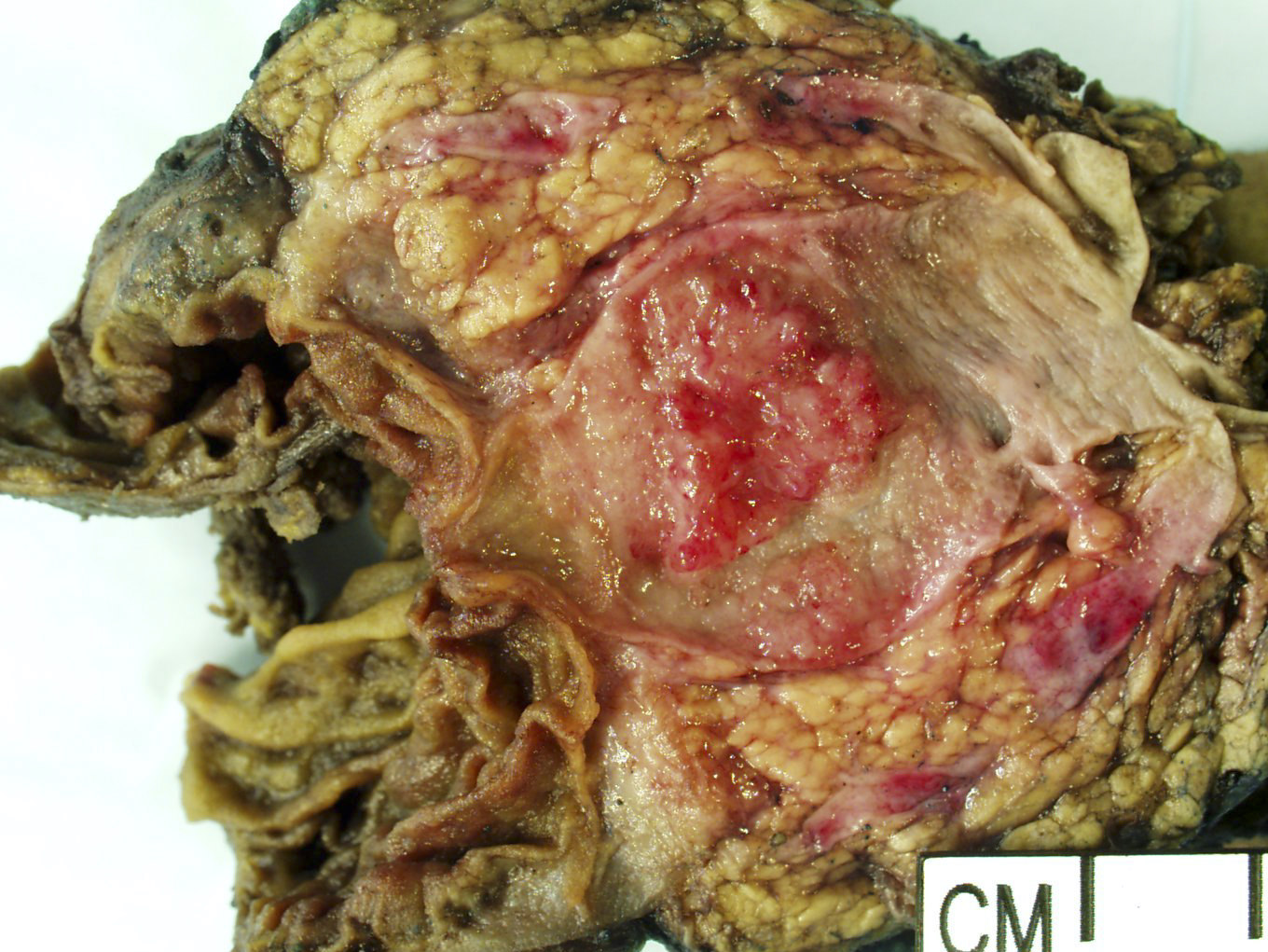
Gross description
- Main duct involvement:
- Usually diffusely dilated, tortuous and irregular, filled with mucin
- Typically arises in head and progresses along path of main duct, may involve entire pancreas; may involve major or minor papillae leading to mucin extrusion from ampulla
- Uninvolved pancreas is often pale and firm, reflecting extensive chronic obstructive pancreatitis
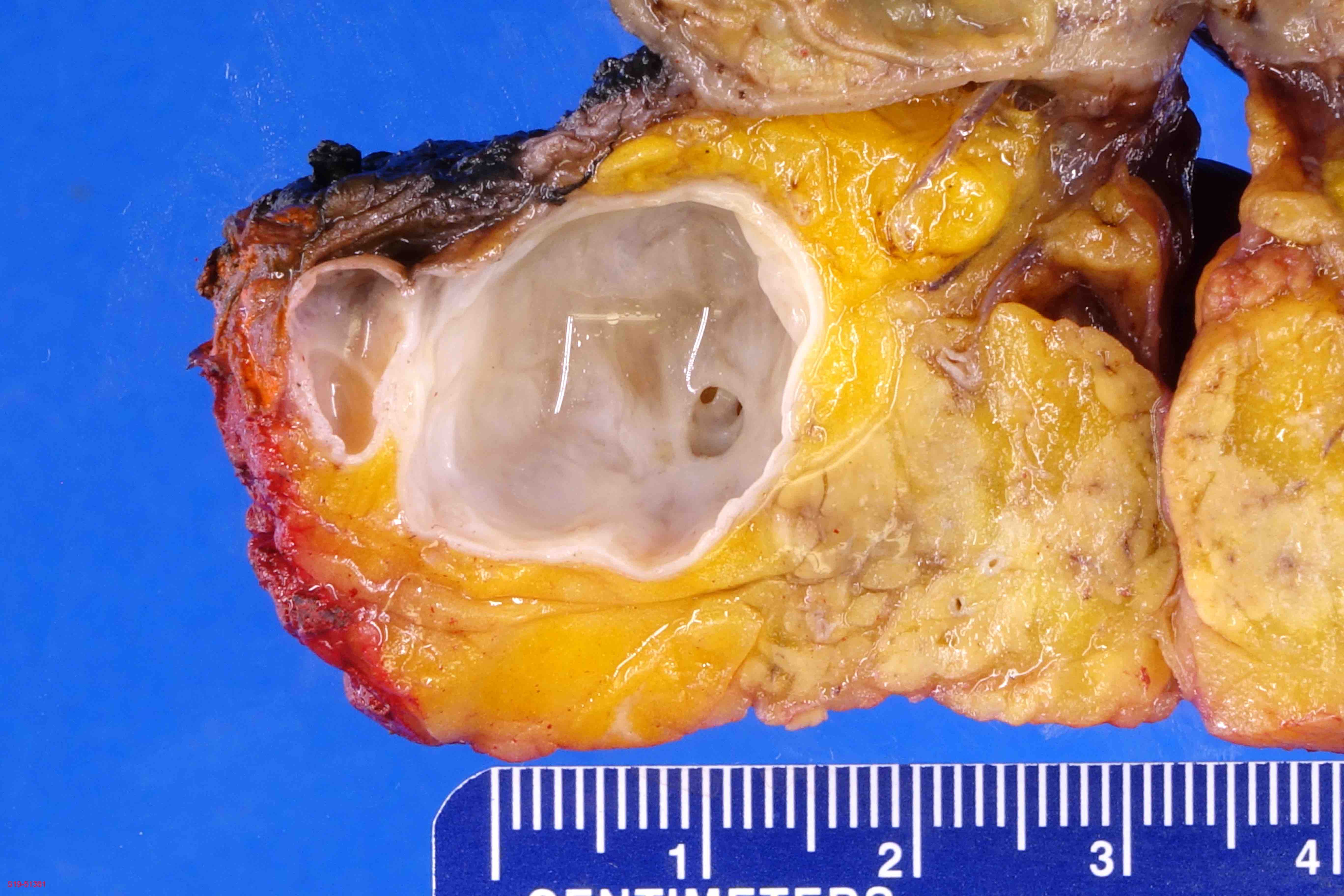
- Branch duct involvement:
- Often in uncinate process
- Forms multicystic, grape-like structures; cystically dilated ducts, filled with tenacious mucin; cyst walls are usually thin with flat or papillary lining
- Cysts separated by normal pancreas, suggesting that cysts are separate on cut sections
- Multicentricity seen in up to 40% of cases (Am J Gastroenterol 2007;102:1759)
- Extensive sampling / complete submission of cyst for microscopic evaluation important to rule out an associated invasive carcinoma (Am J Surg Pathol 2014;38:480, Ann Surg 2016;263:162)
- Greater than 5 mm in diameter
- Incipient IPMN is a term that can be used for lesions 0.5 - 1.0 cm in size
Gross images
Frozen section description
- Typically pancreatic resection margin status will be evaluated to exclude IPMN; additional margins will be submitted if invasive carcinoma is present or possibly for the presence of high grade dysplasia (Am J Surg Pathol 2015;39:1730, Pancreatology 2017;17:738)
- If low grade dysplasia is seen, typically no additional resection done; this may represent pancreatic intraepithelial neoplasia (PanIN) versus IPMN (Am J Surg Pathol 2015;39:1730, Pancreatology 2017;17:738)
Frozen section images
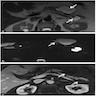
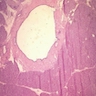
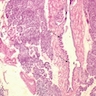
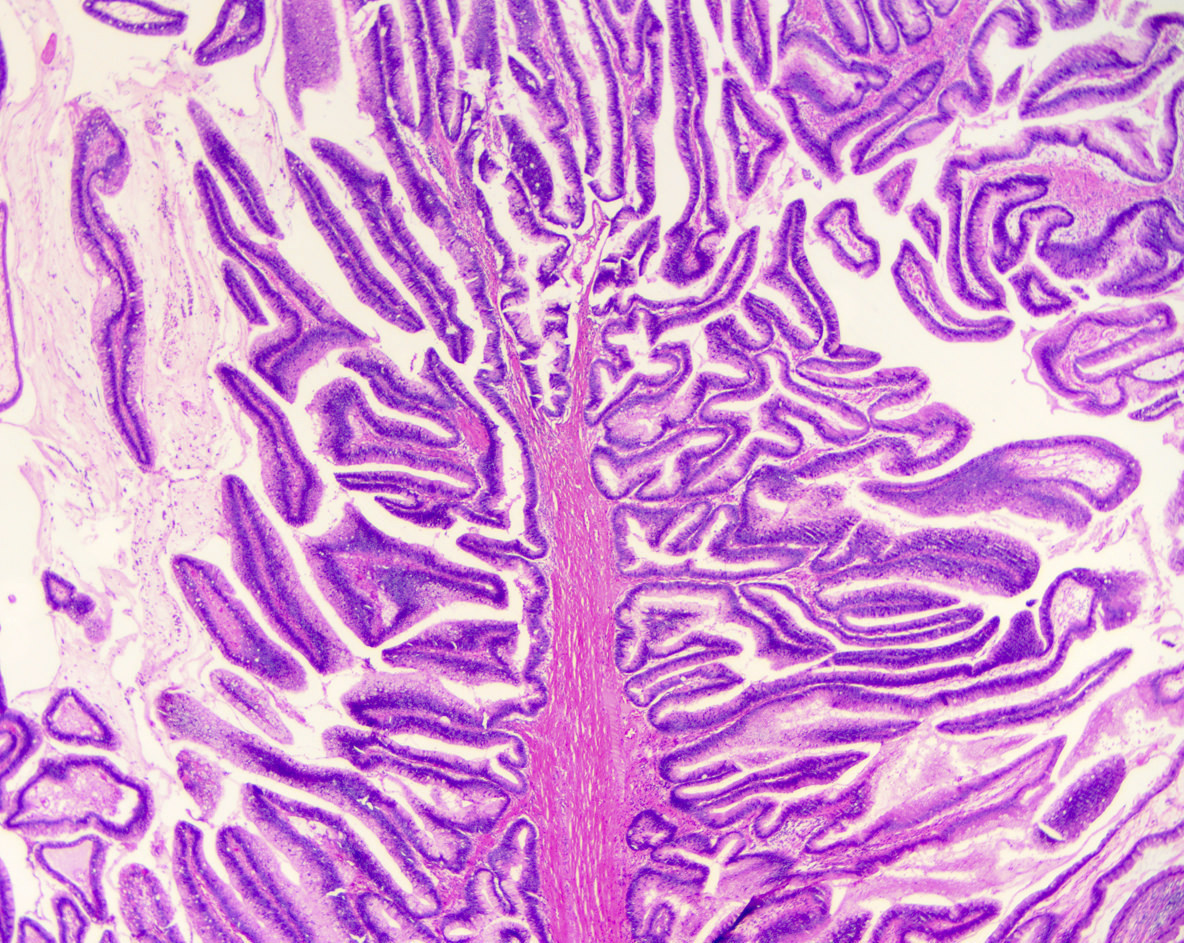
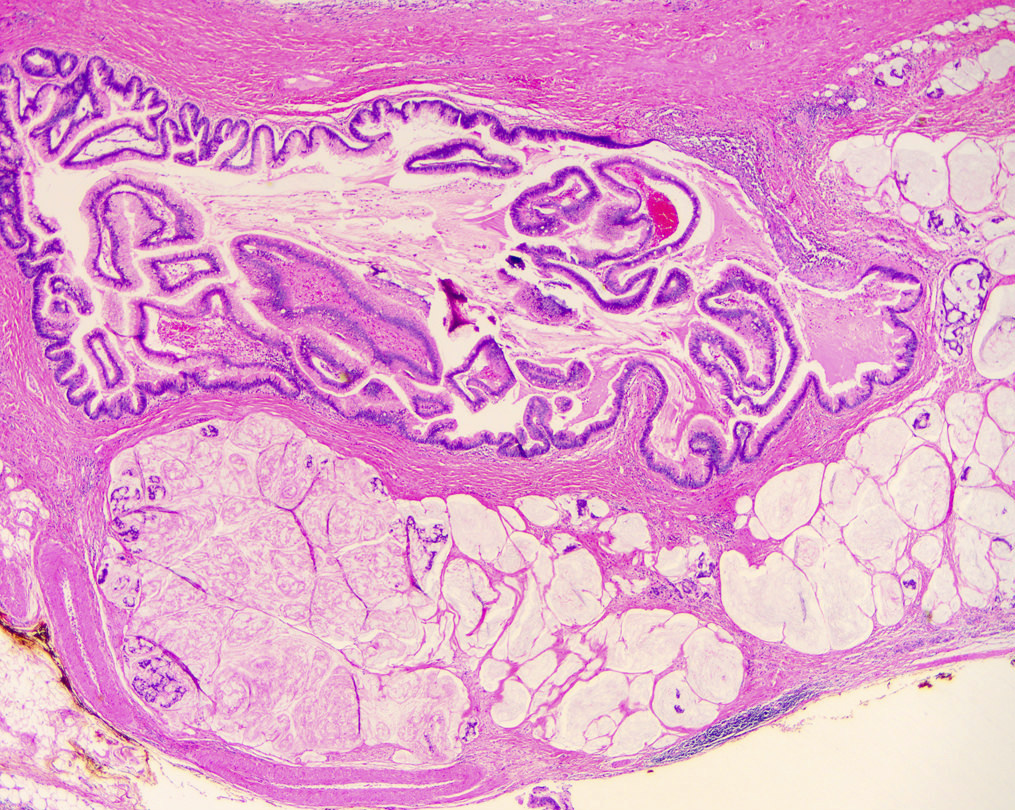
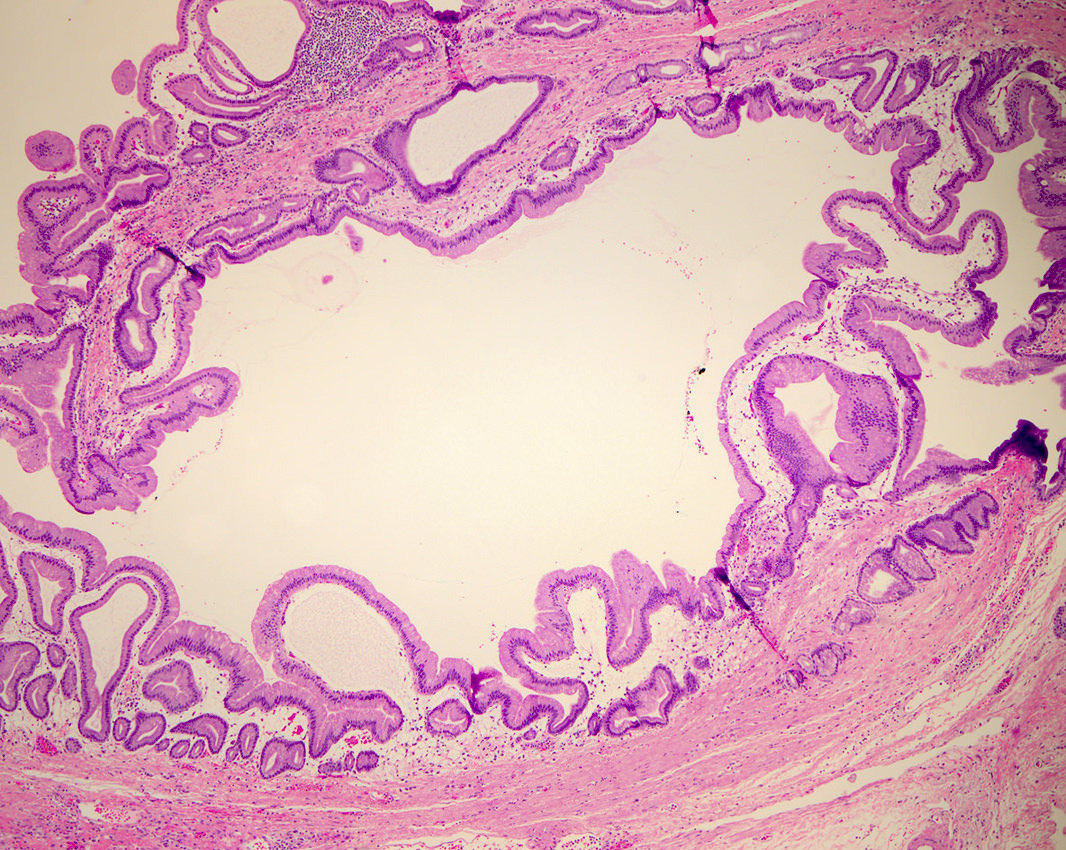
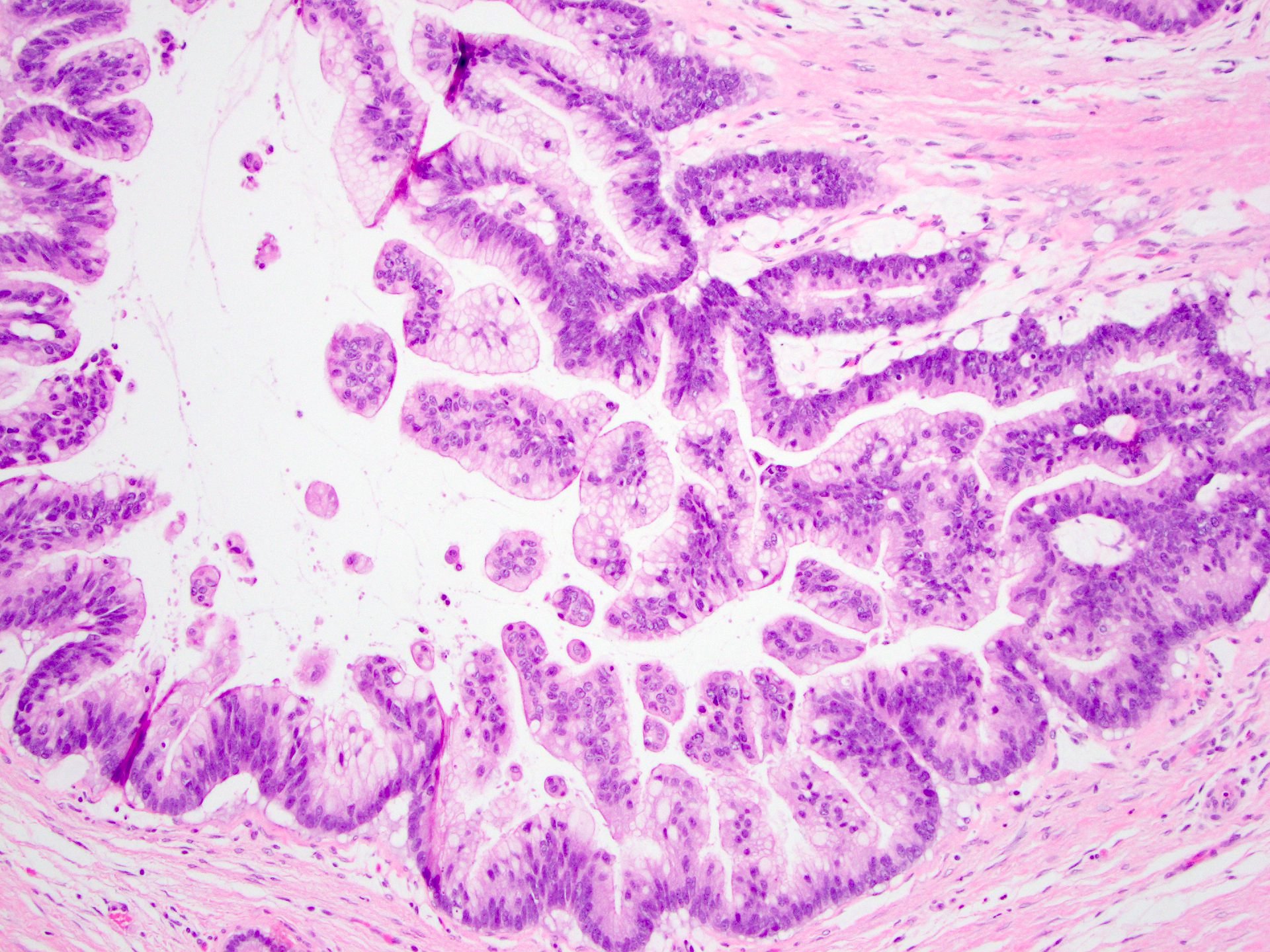
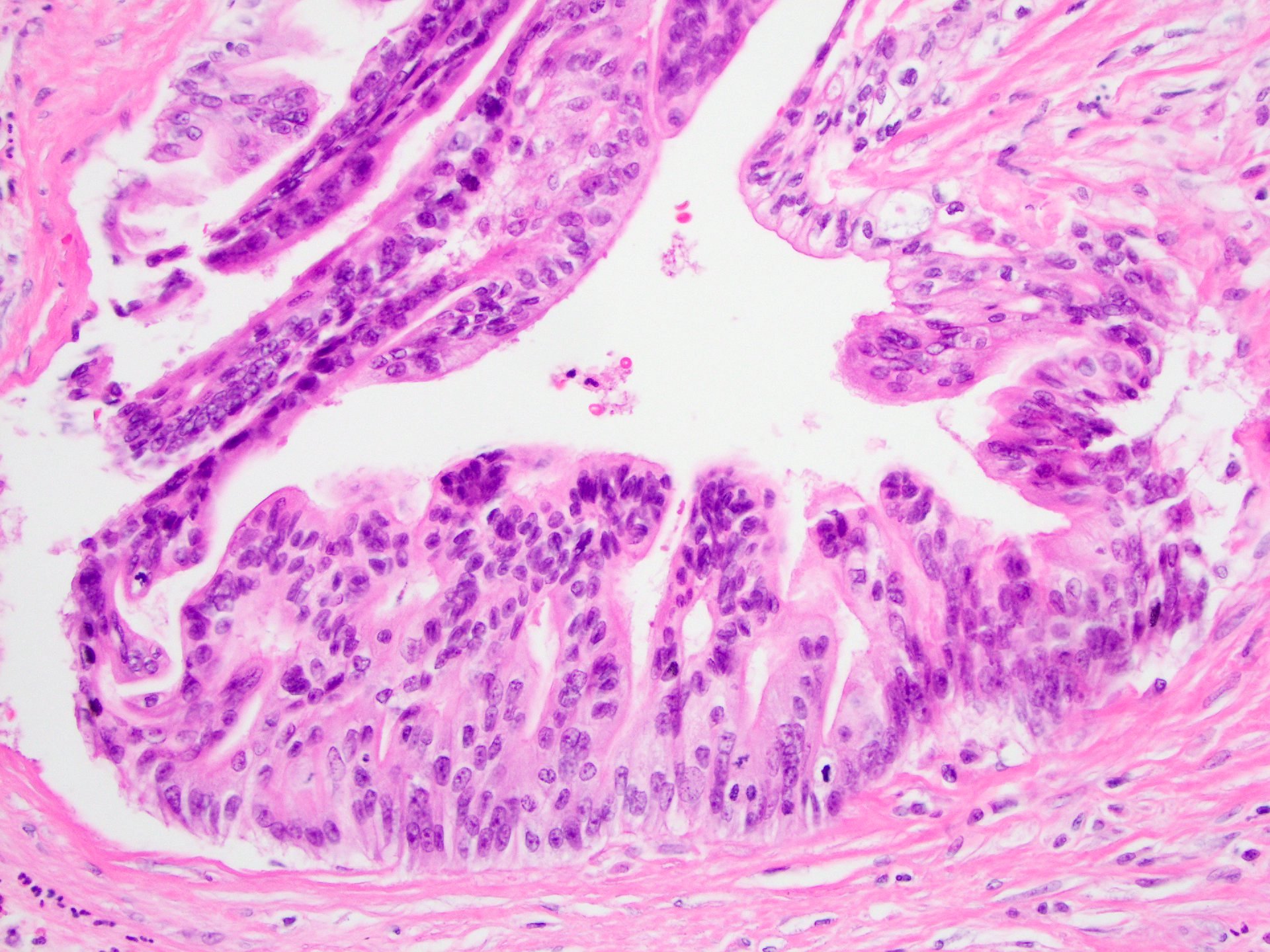
Microscopic (histologic) description
- Mucin producing epithelial cells with varied degrees of dysplasia (Am J Surg Pathol 2015;39:1730)
- Low grade dysplasia is characterized by a flat epithelial lining with basal located nuclei without significant pleomorphism, while intermediate dysplasia has features between those of low and high grade dysplasia (note: low and intermediate dysplasia are now both grouped as low grade dysplasia) (Am J Surg Pathol 2015;39:1730)
- High grade dysplasia is characterized by complex architectural features (i.e. irregular branching, cribiforming) with loss of nuclear polarity along with increased nuclear hyperchromasia and nuclear irregularities (Am J Surg Pathol 2015;39:1730)
- Epithelial cells show variable differentiation and can be subclassified into: intestinal, gastric and pancreaticobiliary subtypes (oncocytic lining suggests intraductal oncocytic papillary neoplasm)
- Associated with pancreatic intraepithelial neoplasia (PanIN), chronic pancreatitis (Am J Surg Pathol 2004;28:1184)
- No ovarian type stroma
- Assess presence or absence of invasive carcinoma (most important prognostic factor) (Hum Pathol 2012;43:1)
- Type of invasion is associated with MUC1 / MUC2 pattern; see below (Mod Pathol 2002;15:1087)
- Gastric type IPMN:
- Cells resemble gastric foveolae
- Intestinal metaplasia may be seen, usually with low grade dysplasia and branch duct IPMN
- If associated invasive carcinoma present, typically ductal (tubular) adenocarcinoma (Ann Surg 2016;263:162, Virchows Arch 2005;447:794)
- Intestinal type IPMN:
- Cells with tall columnar epithelium (resembling intestinal villous adenomas)
- Usually low or high grade dysplasia and main duct IPMN
- If associated invasive carcinoma present, typically mucinous (colloid) carcinoma
- Pancreaticobiliary type IPMN:
- Complex, thin branching papillae resembling cholangiopapillary neoplasms
- Cuboidal cells with prominent nucleoli
- Usually high grade dysplasia and main duct IPMN
- If associated invasive carcinoma present, typically ductal (tubular) adenocarcinoma
Microscopic (histologic) images
Cytology description
- Cannot distinguish IPMN from mucinous cystic neoplasm on cytology (neoplastic mucinous cyst)
- Disordered mucinous epithelial clusters with nuclear overlap and variable cytologic atypia
- Distinguishing a low grade mucinous neoplasm from gastrointestinal tract contaminant (particularly gastric mucosa) can be challenging
Cytology images
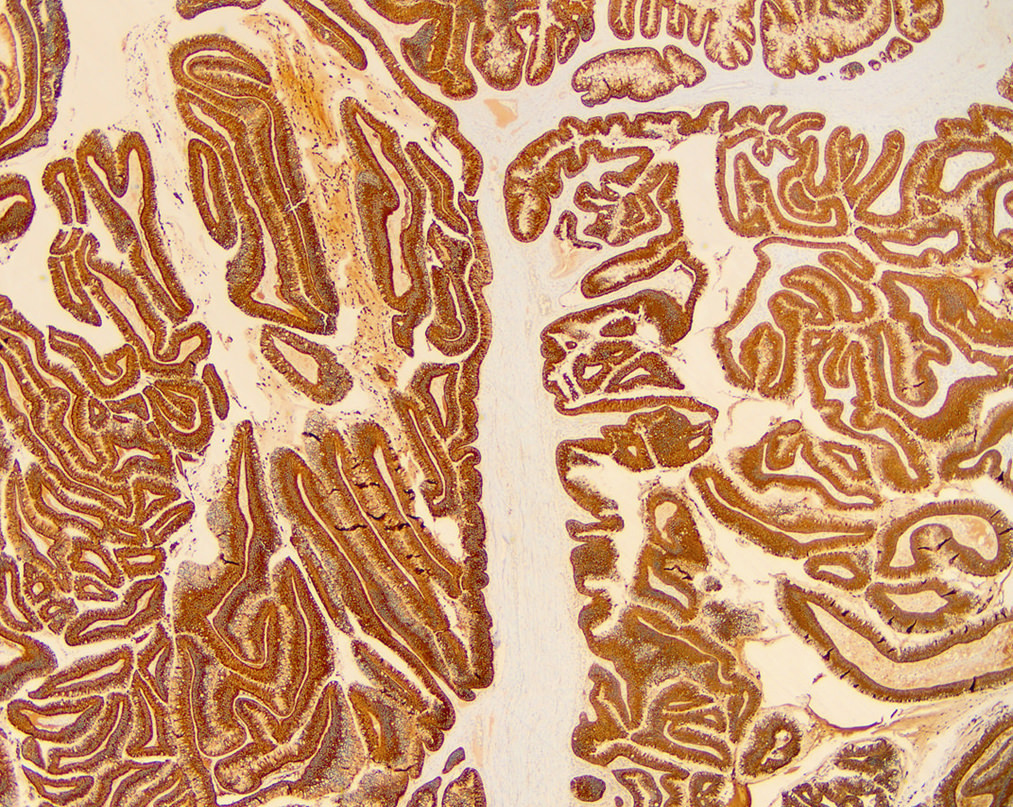
Positive stains
- General ductal markers are positive, including CK7, CK19, CA 19-9, B72.3 and CEA
- Subtypes show various mucin glycoprotein (MUC) expression (as below):
- Gastric type IPMN: MUC5AC+, MUC6 variable (Am J Surg Pathol 2006;30:1561, Mod Pathol 2016;29:977)
- Intestinal type IPMN: MUC2+, CDX2+, MUC5AC+; similar staining pattern for associated invasive colloid carcinoma (Mod Pathol 2016;29:977)
- Pancreaticobiliary type IPMN: MUC1+, MUC6+, MUC5AC+; similar staining pattern for associated invasive ductal adenocarcinoma (Am J Surg Pathol 2006;30:1561, Mod Pathol 2016;29:977)
Negative stains
- Gastric type IPMN: MUC1-, MUC2- (Am J Surg Pathol 2006;30:1561, Mod Pathol 2016;29:977)
- Intestinal type IPMN: MUC1-; similar staining pattern for associated invasive colloid carcinoma (Mod Pathol 2016;29:977)
- Pancreaticobiliary type IPMN: MUC2-; similar staining pattern for associated invasive ductal adenocarcinoma (Am J Surg Pathol 2010;34:364, Mod Pathol 2016;29:977)
Molecular / cytogenetics description
- KRAS, GNAS and RNF43 mutations seen in decreasing frequency in noninvasive IPMNs and IPMNs with an associated invasive carcinoma (J Am Coll Surg 2015;220:845)
- GNAS mutations more commonly associated with IPMNs, which harbor an invasive colloid carcinoma
- KRAS mutations more commonly associated with IPMNs, which harbor an invasive tubular (ductal) adenocarcinoma
- With increasing grades of dysplasia, increased mutations in KRAS, TP53, CDKN2A (p16); also hypermethylation and reduced BRG1 protein (Hum Pathol 2012;43:585)
- Loss of programmed cell death 4 (PDCD4) and CD24 expression associated with tumor progression and proliferation (Hum Pathol 2010;41:1507, Hum Pathol 2010;41:1466)
- Cyst fluid shows mutations in KRAS2 and GNAS
Sample pathology report
- Pancreas, small bowel and stomach, pancreaticoduodenectomy (Whipple resection):
- Intraductal papillary mucinous neoplasm (IPMN), low grade, gastric phenotype, branch duct type, 3.0 cm (see comment)
- Negative for high grade dysplasia or malignancy.
- Margins are negative for IPMN.
- 23 lymph nodes with no significant histologic abnormality.
- Comment: The entire cyst is submitted for histologic examination.
Differential diagnosis
- Intraductal oncocytic papillary neoplasm:
- Lined by oncocytic cells with complex growth patterns and arborizing papillae
- Distinct molecular pathway with ARHGAP26, ASXL1, EPHA8 and ERBB4 genetic alterations (Mod Pathol 2016;29:1058)
- DNAJB1-PRKACA fusions (Mod Pathol 2020;33:648)
- MUC1+, MUC6+
- Intraductal tubulopapillary neoplasm:
- Recently recognized subtype with potential origin from peribiliary cysts (Am J Surg Pathol 2009;33:1164)
- Confluent epithelial cells with tubular and papillary architectural patterns and usually high grade dysplasia
- Necrotic foci, more solid growth without visible mucin or scanty cytoplasmic mucin, no KRAS2 gene mutations
- MUC1+, MUC2-, MUC6+
- Mucinous cystic neoplasm:
- Epithelial mucinous lining with ovarian type stroma
- No communication to pancreatic ductal system
- Pancreatic intraepithelial neoplasia (PanIN):
- Only detectable microscopically
- No mass lesion, unlike IPMN
- Almost always has gastric foveolar differentiation
- Simple mucinous cyst:
- Cyst > 1 cm with flat (not papillary) gastric mucinous lining and no significant atypia (Am J Surg Pathol 2015;39:1730, Am J Surg Pathol 2017;41:121)
- Retention cyst:
- Cyst > 1 cm with flat (not papillary) ductal lining and no significant atypia in a background of obstruction (Am J Surg Pathol 2015;39:1730)
Additional references
Practice question #1
Practice answer #1
A. Gastric. The histologic features are typical of the gastric type of IPMN, no features of intestinal or pancreatobiliary differentiation are seen (choices B and E); choice D is not a morphologic type of IPMN; rather, intraductal oncocytic papillary neoplams are a distinct separate entity.
Comment Here
Reference: Intraductal papillary mucinous neoplasm (IPMN)
Comment Here
Reference: Intraductal papillary mucinous neoplasm (IPMN)
Practice question #2
Which of the following clinical scenarios would most likely represent an intraductal papillary mucinous neoplasm (IPMN)?
- 29 year old woman with 8 cm solid and cystic pancreatic tail mass
- 45 year old woman with 3 cm cystic pancreatic tail mass
- 58 year old man with 3 cm solid pancreatic head mass
- 66 year old man with 2 cm cystic pancreatic head mass
Practice answer #2
D. 66 year old man with 2 cm cystic pancreatic head mass. IPMNs are more often seen in older men and are cystic; thus choices A - C are incorrect (choice A would be more typical of a solid pseudopapillary neoplasm; choice B would more typical of a mucinous cystic neoplasm; choice C would be more typical of pancreatic ductal adenocarcinoma or a pancreatic neuroendocrine tumor).
Comment Here
Reference: Intraductal papillary mucinous neoplasm (IPMN)
Comment Here
Reference: Intraductal papillary mucinous neoplasm (IPMN)