Table of Contents
Definition / general | Essential features | CPT coding | Sites | Case reports | Cytology description | Cytology images | Molecular / cytogenetics description | Sample pathology report | Practice question #1 | Practice answer #1 | Practice question #2 | Practice answer #2Cite this page: Feng G, Choy B. Cytology-nonneoplastic. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/bladdercytologynonneoplastic.html. Accessed September 25th, 2025.
Definition / general
- Benign and reactive cytologic changes based on the Paris System for Reporting Urinary Cytology include nonneoplastic entities such as changes associated with lithiasis, bacterial, fungal and parasitic infections, viral cytopathic effect and posttreatment effect
- Nonneoplastic entities may mimic malignant cells; clinical correlation, as well as the use of ancillary testing when needed, is advised
Essential features
- The Paris System for Reporting Urinary Cytology is the recommended system to report results (Rosenthal: The Paris System for Reporting Urinary Cytology, 1st Edition, 2016)
- Negative for high grade urothelial carcinoma category encompasses nonneoplastic entities that pose no significant risk for the development of high grade urothelial carcinoma, including
- Changes associated with urinary lithiasis
- Bacterial, fungal and parasitic infections
- Viral cytopathic effect
- Posttreatment effect
- Atypical urothelial cells category should be used only if there are cellular alterations (mild to moderate cytologic atypia) that warrant concern but fall short of suspicious for high grade urothelial carcinoma or high grade urothelial carcinoma categories
CPT coding
Sites
- Urinary bladder, upper tracts (renal pelvis, ureters), urethra
- Urinary bladder diversion (ileal conduit, Indiana pouch, neobladder)
Case reports
- 38 year old woman with Schistosoma haematobium in urine cytology (Diagn Cytopathol 2007;35:649)
- 60 year old man with cytologic effects of intravesical mitomycin in urine cytology (Cell J 2014;16:375)
- 67 year old woman with fusariosis in urine cytology (J Clin Pathol 2007;60:422)
- 68 year old woman with adenovirus cytopathic effect in urine cytology of ileal conduit (Diagn Cytopathol 2004;30:284)
- 74 year old woman with herpes simplex viral cytopathic effect in catheterized urine cytology (Diagn Cytopathol 2013;41:61)
Cytology description
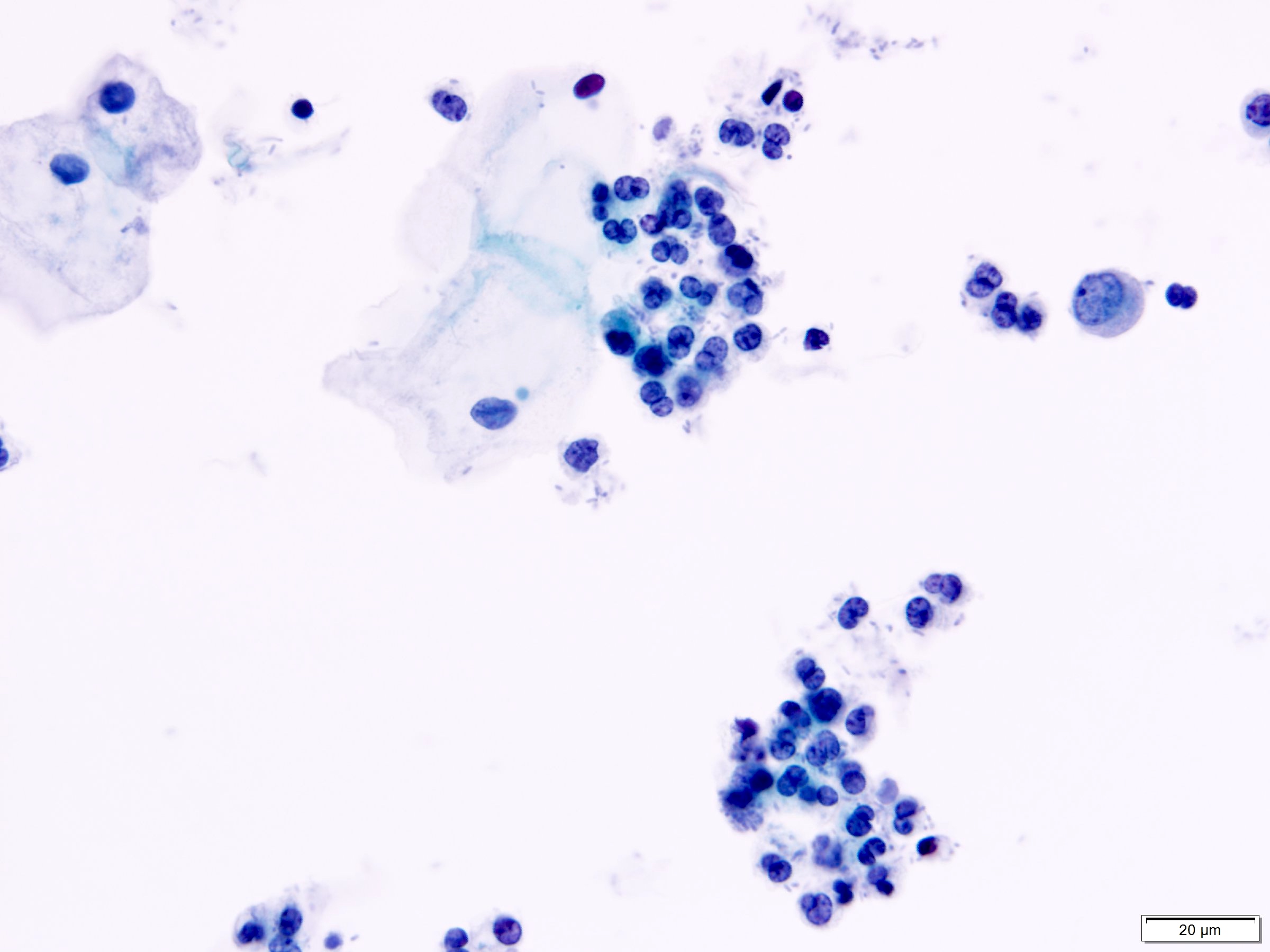
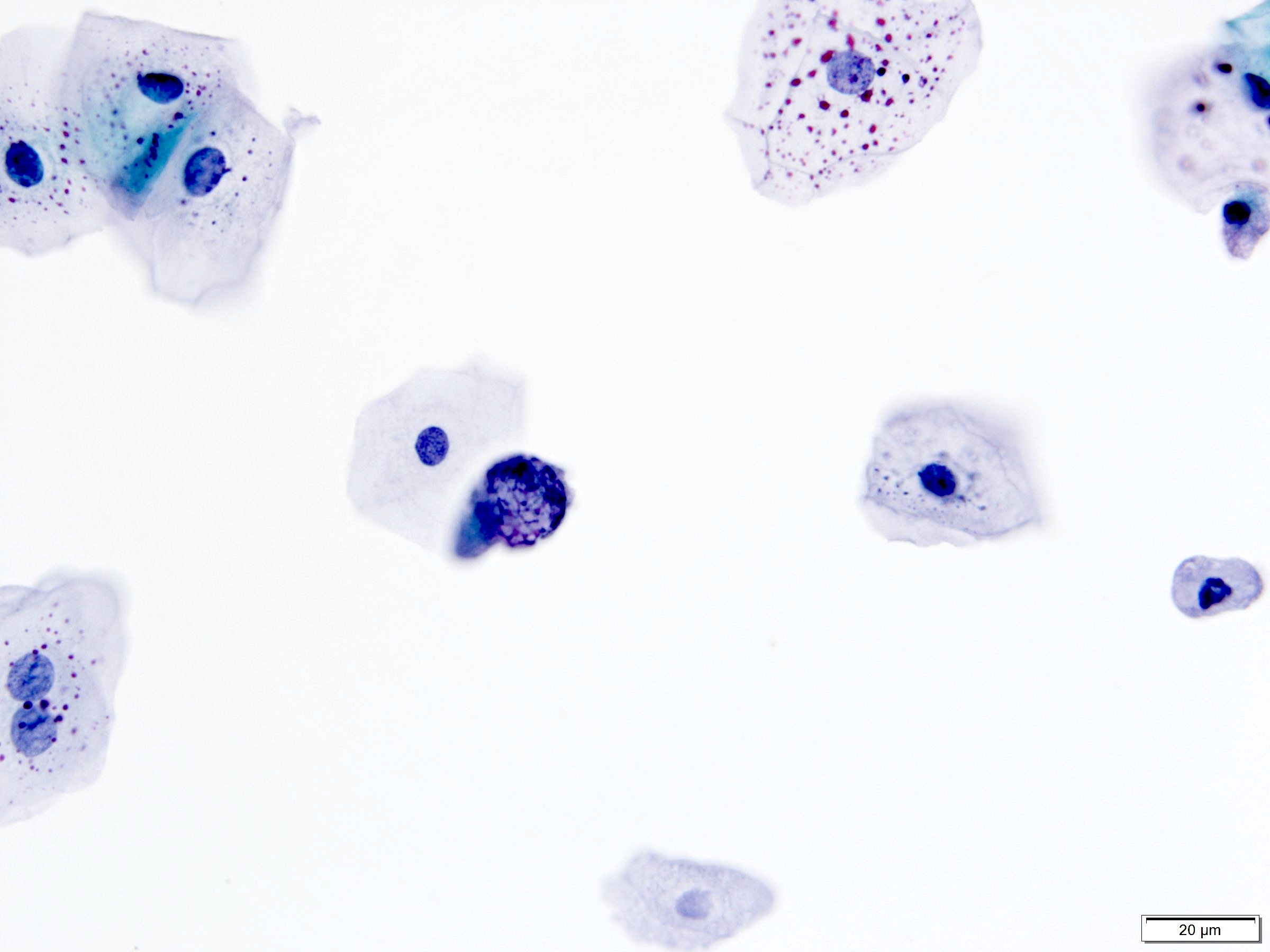
Urothelium with lithiasis (J Am Soc Cytopathol 2015;4:30)
Infections
Treatment effect (J Clin Pathol 2002;55:641)
- 3 dimensional pseudopapillary clusters of urothelial cells
- Most clusters with smooth borders
- Rim of cytoplasm (collarette) around clusters
- Urothelial cells with uniform round nuclei, finely granular chromatin and inconspicuous nucleoli; however, reactive atypia (pleomorphism, coarsely granular chromatin, hyperchromasia, occasional mitotic figures) can be seen
- Calcific concretions
- Background of blood or inflammatory cells
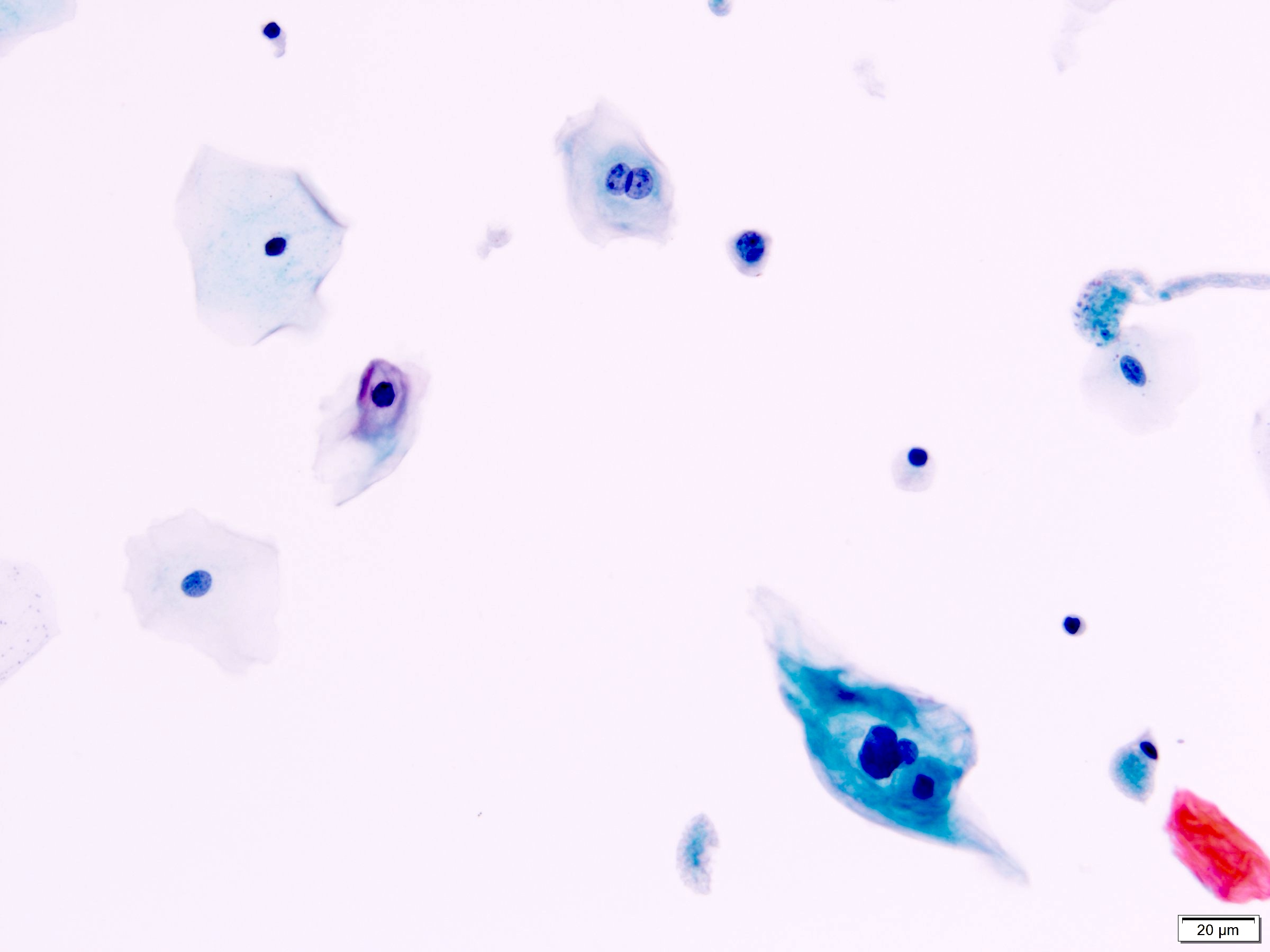
Infections
- Bacterial infections
- Acute bacterial infection
- Numerous neutrophils
- Urothelial cells with reactive changes: slight nuclear enlargement, conspicuous nucleoli but chromatin is fine and uniformly distributed and nuclei remain round
- Bacteria seen in the background
- Note: presence of bacteria without neutrophils is a nonspecific finding
- Malakoplakia
- Histolytic inflammatory condition, often resulting from bacterial infection
- Histiocytes with intracytoplasmic:
- Bacteria
- Basophilic, concentric, laminated structures (Michealis-Gutmann bodies)
- Acute bacterial infection
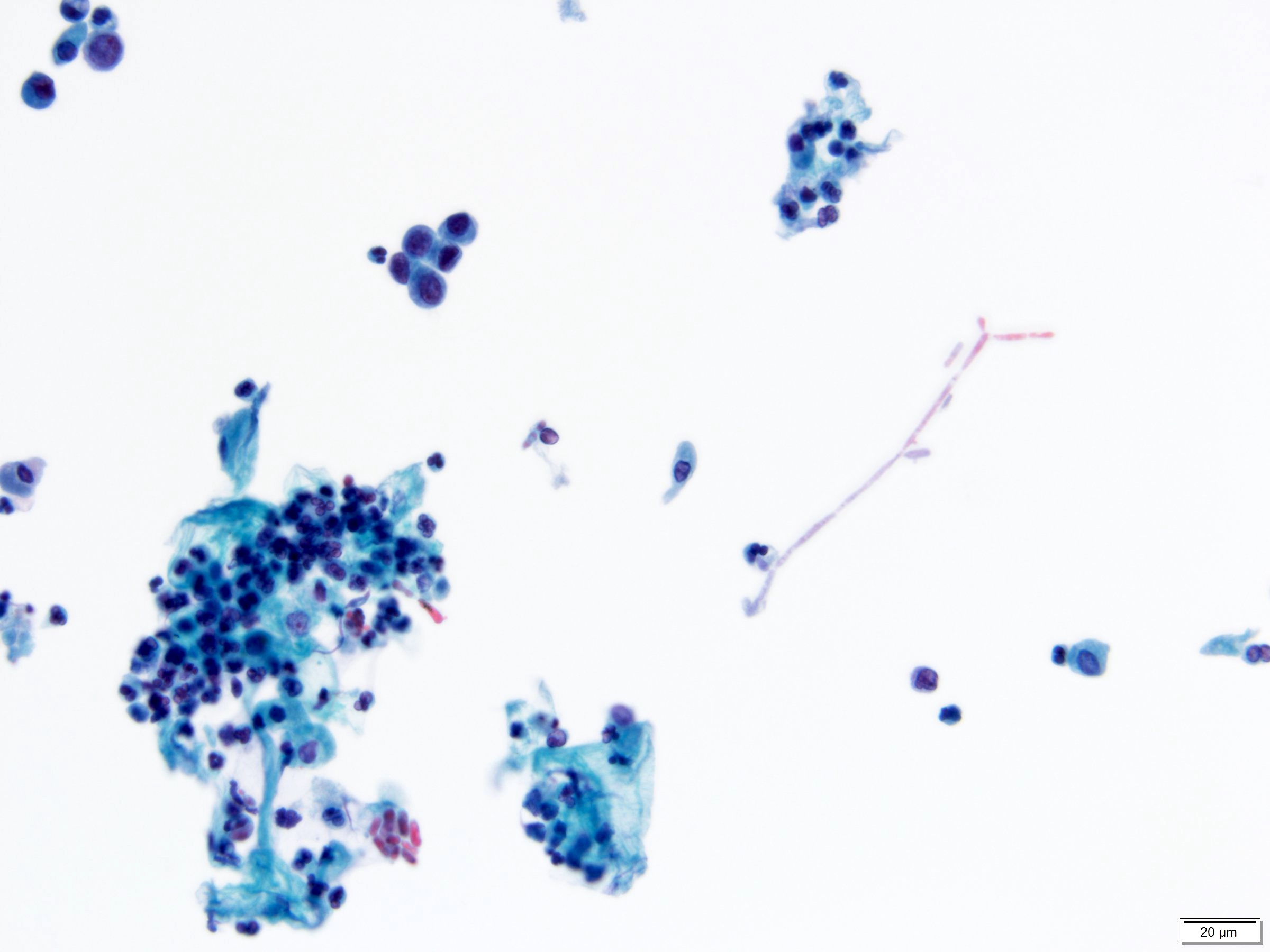
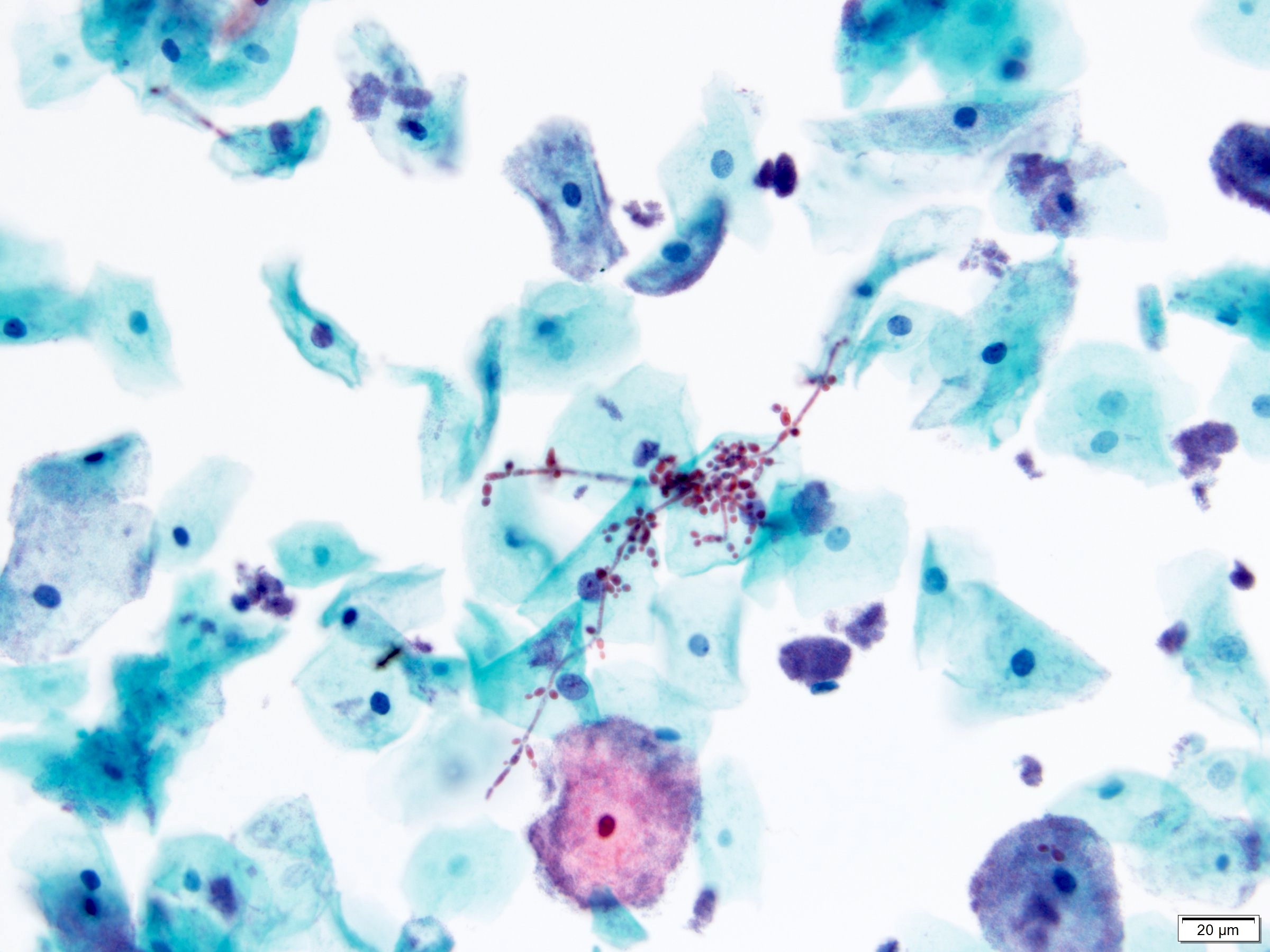
- Fungal infections
- Candida species
- Most common cause of fungal urinary tract infections
- Seen in yeast forms and pseudohyphae
- Reactive urothelial cells and mixed inflammatory background
- Note: presence of Candida may be due to vaginal contamination, often seen with numerous squamous cells but few neutrophils
- Other less common fungal organisms reported in urine cytology
- Aspergillus (Diagn Cytopathol 2015;43:403)
- Histoplasma (Diagn Cytopathol 2002;26:243)
- Fusarium species (J Clin Pathol 2007;60:422)
- Candida species
- Viral cytopathic effects
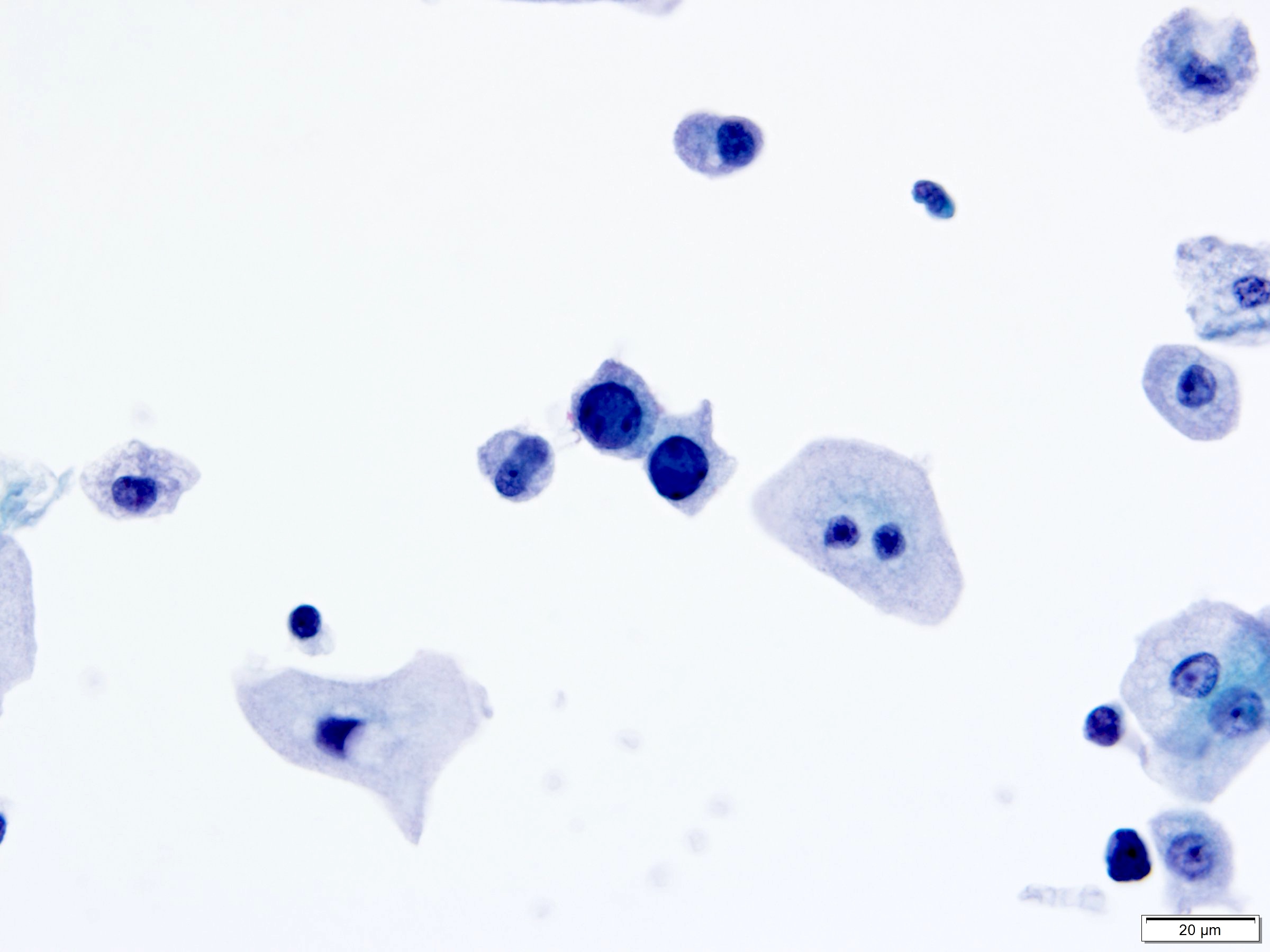
- Polyomavirus (BK virus > JC virus) (Madame Curie Bioscience Database: Urine Cytology Findings of Polyomavirus Infections [Accessed 3 November 2020], Arch Pathol Lab Med 1998;122:333)
- Classic appearance: infected cells with single, large, homogenous, basophilic, glassy nuclear inclusions and small condensed rim of chromatin
- Other appearances:
- Central nuclear inclusion surrounded by irregular and incomplete clear halo (cytomegalovirus-like)
- Granular chromatin, sometimes multinucleated
- Degenerated, vesicular nuclei with coarsely granular and clumped (spider web) chromatin, nucleoli can be seen
- Round or oval, smooth but thickened nuclear membrane
- Many with eccentric comet-like cytoplasm
- Note: infected cells are descriptively known as decoy cells because they mimic malignant cells (Koss: Koss' Diagnostic Cytology and Its Histopathologic Bases, 5th Edition, 2005)
- Adenovirus (Madame Curie Bioscience Database: Urine Cytology Findings of Polyomavirus Infections [Accessed 3 November 2020], Diagn Cytopathol 2004;30:284)
- Similar to cells with polyomavirus cytopathic changes
- Ground glass inclusions with margination of chromatin
- Cytomegalovirus (CMV)
- Infected cells are markedly enlarged and have both nuclear and cytoplasmic inclusions
- Nuclear inclusions: single, large, basophilic, surrounded by halo (owl's eye appearance)
- Cytoplasmic inclusions: multiple, smaller, basophilic, finely or coarsely granular
- Herpes simplex virus (HSV) (Diagn Cytopathol 2013;41:61)
- Infected urothelial cells show multinucleation, nuclear molding, homogenous, ground glass nuclei with margination of chromatin
- Human papillomavirus (HPV)
- Infected cells show koilocytosis, dyskeratocytosis, multinucleation
- Polyomavirus (BK virus > JC virus) (Madame Curie Bioscience Database: Urine Cytology Findings of Polyomavirus Infections [Accessed 3 November 2020], Arch Pathol Lab Med 1998;122:333)
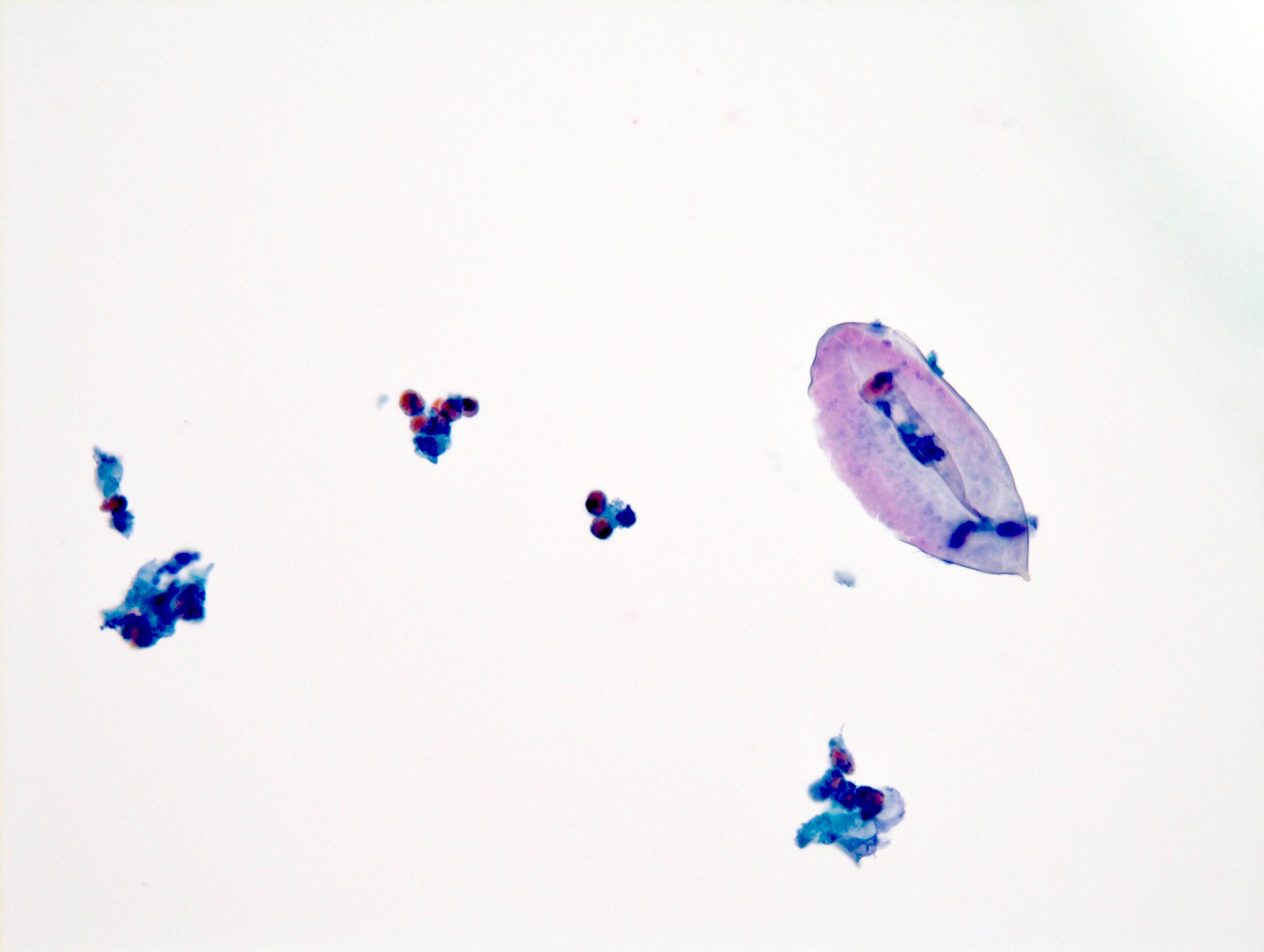
- Parasitic infections
- Schistosoma haematobium
- Oval shaped eggs with terminal spine
- Hatched eggs with empty shells
- Unhatched eggs contain miracidia
- Eosinophils in the background
- Oval shaped eggs with terminal spine
- Trichomonas vaginalis (Acta Cytol 2000;44:484)
- Pear shaped parasites with small and oval nucleus and red cytoplasmic granules
- Note: presence of Trichomonas may be due to contamination from vaginal infection, often seen with numerous squamous cells and vaginal flora
- Schistosoma haematobium
Treatment effect (J Clin Pathol 2002;55:641)
- Radiation
- Urothelial cells show significant cytomegaly and nucleomegaly but maintain N/C ratio (not increased)
- Multinucleation and nuclear and cytoplasmic vacuoles may be seen
- Nucleus and cytoplasm often have degenerative changes
- Finely granular chromatin and smooth nuclear membrane
- Cytologic changes can be seen for weeks to years
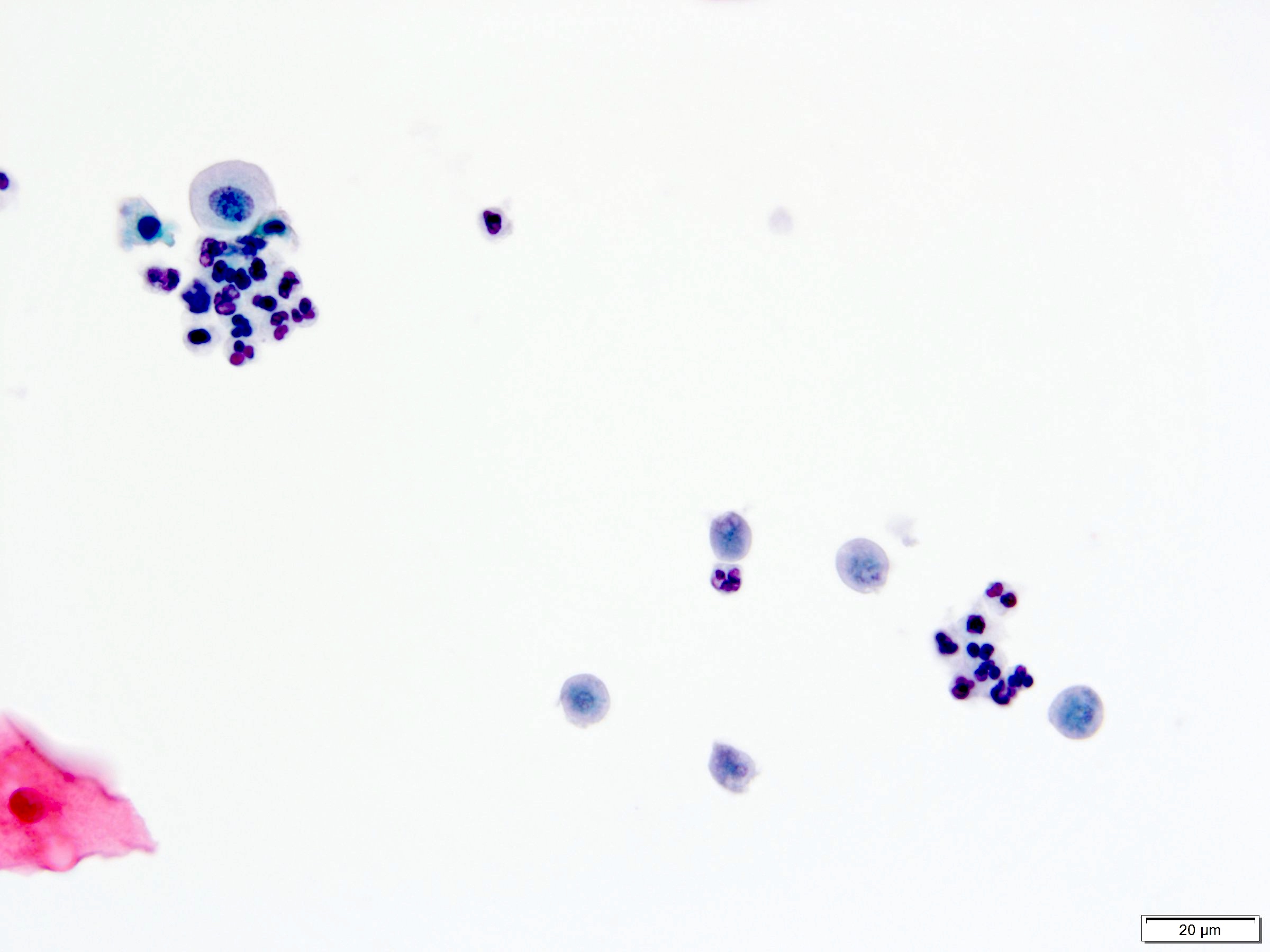
- Immunotherapy
- Intravesical Bacillus Calmette-Guérin (BCG) immunotherapy can cause granulomatous inflammation
- Granulomas composed of epithelioid histiocytes with carrot shaped nuclei and lymphocytes
- Langhans type multinucleated giant cells from fused macrophages have small, hyperchromatic nuclei clustered at one cytoplasmic pole
- Chemotherapy
- Mitomycin and thiotepa
- Intravesical treatment usually affects superficial cells
- Nuclear enlargement, multinucleation and hyperchromasia
- Background eosinophils for mitomycin
- Cyclophosphamide
- Systemic treatment may cause similar cytologic changes as intravesical mitomycin and thiotepa
- Hyperchromasia, degeneration, large nuclei and increased N/C ratio
- Mitomycin and thiotepa
Cytology images
Molecular / cytogenetics description
Sample pathology report
- Bladder, voided urine:
- Specimen adequacy:
- Satisfactory for evaluation
- Interpretation:
- Negative for high grade urothelial carcinoma
- Diagnosis:
- Urothelial cells with viral cytopathic effects consistent with polyomavirus
- Specimen adequacy:
Practice question #1
A bladder washing from a 65 year old man shows urothelial cells with large basophilic, glassy nuclear inclusions. Which of the following features differentiated polyomavirus infected urothelial cells from high grade urothelial carcinoma cells?
- Comet shaped cells
- Homogenous chromatin with ground glass appearance
- Increased N/C ratio
- Irregular nuclear membrane
- Prominent nuclear membrane
Practice answer #1
D. Irregular nuclear membrane. Both polyomavirus infected cells and high grade urothelial carcinoma cells have increased N/C ratio. When high grade urothelial carcinoma cells are infected by polyomavirus, they also can show ground glass chromatin, prominent nuclear membrane and comet shaped cells. However, only high grade urothelial carcinoma cells have irregular nuclear membranes.
Comment Here
Reference: Cytology-nonneoplastic
Comment Here
Reference: Cytology-nonneoplastic
Practice question #2
A voided urine sample from a 50 year old woman shows urothelial cells admixed with histiocytes with round, laminated and basophilic cytoplasmic inclusions. What is the most likely etiology?
- Candida infection
- Cytomegalovirus infection
- Malakoplakia
- Melamed-Wolinska bodies
- Polyomavirus infection
Practice answer #2
C. Malakoplakia. The described structures are Michealis-Gutmann bodies, which are seen in malakoplakia. Fungal yeast forms and pseudohyphae with a mixed inflammatory background are seen in Candida infection. Cytomegalovirus infected cells show nuclear inclusions with an owl's eye appearance as well as cytoplasmic inclusions. Melamed-Wolinska bodies are intracytoplasmic eosinophilic inclusions seen in degenerated urothelial cells. The classic appearance of cells infected by polyomavirus shows a single, large, basophilic, glassy nuclear inclusion.
Comment Here
Reference: Cytology-nonneoplastic
Comment Here
Reference: Cytology-nonneoplastic