Table of Contents
Definition / general | Essential features | Diagrams / tables | Clinical features | Types of specimen | Processing / preservation of specimen | Format of diagnostic report | Specimen adequacy | Negative for High Grade Urothelial Carcinoma (NHGUC) | Atypical Urothelial Cells (AUC) | Suspicious for High Grade Urothelial Carcinoma (SHGUC) | High Grade Urothelial Carcinoma (HGUC) | Low Grade Urothelial Neoplasia (LGUN) | Squamous Cell Carcinoma (SCC) | Adenocarcinoma | Small cell carcinoma | Secondary neoplasmsCite this page: Pansare V., Parakh R. Paris system for urothelial neoplasia. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/bladdercytologyparissystemnew.html. Accessed August 15th, 2025.
Definition / general
- Urine Cytology represents a significant portion on non-gynecologic cytology specimens in daily practice, primarily because of the simplicity and ease of specimen procurement and its significant impact on management
- However there was no consensus on the various categories and their cytomorphologic features used for reporting
- Paris System for reporting urinary cytology was an effort to standardize terminology with standardized cytomorphologic criteria for reporting urine cytology (2016)
- Paris System is based on the principle that the ultimate goal of urine cytology is detection of high grade urothelial carcinoma
Essential features
- Identification of high grade urothelial carcinoma (HGUC) is the ultimate goal of urinary cytology and should always be kept in mind while reporting urine cytology; these patients will receive active investigation to find the lesion
- Suspicious for High Grade Urothelial Carcinoma (SHGUC) has similar management due to its strong association with HGUC
- Carcinoma in Situ (CIS) cannot be distinguished from HGUC in urine cytology
- Low grade urothelial neoplasia (LGUN) can only be definitively diagnosed in the presence of 3 dimensional cellular papillary clusters with fibrovascular cores; if not present, report as negative for high grade urothelial carcinoma (NHGUC) with a possible comment for consideration of LGUN
- Atypical urothelial cells (AUC) should be reported using strict morphologic criteria with 1 major and 1 minor criterion (see below)
Clinical features
- Cytology is useful to detect carcinoma in situ or marked chronic inflammation (i.e. when there is no specific lesion to biopsy), carcinoma hidden in diverticula or for detecting residual tumor from urine specimens
- Cystoscopic biopsy of visible lesions is more sensitive than cytology in most cases
- Bladder irrigation is superior to collecting voided urine
- Most sensitive and highly specific for high grade tumors (diagnosis or follow-up) whether flat (carcinoma in situ), papillary or mixed
- Low sensitivity (difficult to diagnose) for papilloma and low malignant potential lesions because they have normal histology (Mod Pathol 1995;8:394)
- Follow up examination of urine with FISH may improve sensitivity and specificity of cytology (Am J Clin Pathol 2001;116:79)
Types of specimen
Voided urine:
Instrumented urine:
Ileal conduit urine:
- Non invasive, easiest to obtain
- Obtaining 3 second morning voided midstream urine samples collected over 3 consecutive days appears to optimize the detection of urothelial malignancies
Instrumented urine:
- Catheterization of the bladder or irrigation of bladder
Ileal conduit urine:
- Ileal conduit and neobladder are the most common urine diversion techniques used in patients who have undergone cystectomy
- A portion of the ileum is anastomosed with the ureters to the skin or to the urethra
Processing / preservation of specimen
- Immediate processing is recommended or refrigerate if immediate processing cannot be done
- If fixation if needed, use equal volumes of 50% ethanol or a methanol based fixative (Cytolyt® or similar)
Format of diagnostic report
Recommendations for Diagnostic Format and Categories for Urinary Cytology Specimens
Papanicolaou Society of Cytopathology Practice Guidelines Task Force
I. Adequacy Statement (Optional)
II. General Categorization
III. Descriptive Diagnosis
IV. Other
I. Adequacy Statement (Optional)
- Satisfactory for evaluation
- List any quality factors affecting specimen
- Unsatisfactory for evaluation (give reason)
II. General Categorization
- Negative for epithelial cell abnormality (see Descriptive Diagnoses)
- Epithelial cell abnormality present (see Descriptive diagnosis)
III. Descriptive Diagnosis
- Negative for epithelial cell abnormality
- Infectious agents
- Bacterial organisms
- Fungal organisms
- Viral changes (CMV, herpes, adenovirus, polyomavirus)
- Nonspecific inflammatory changes
- Acute inflammation
- Chronic inflammation
- Changes consistent with xanthogranulomatous pyelonephritis
- Cellular changes associated with:
Chemotherapeutic agents
Radiation - Epithelial Cell Abnormalities
Atypical urothelial cells (*see comment)
Low-grade urothelial carcinoma
High-grade urothelial carcinoma (invasive carcinoma vs. carcinoma in situ)
Squamous cell carcinoma
Adenocarcinoma
Other malignant neoplasms (specify type)
IV. Other
- An optional comment section should be used to list additional findings or further clarification of findings
- Diagn Cytopathol 2004;30:24
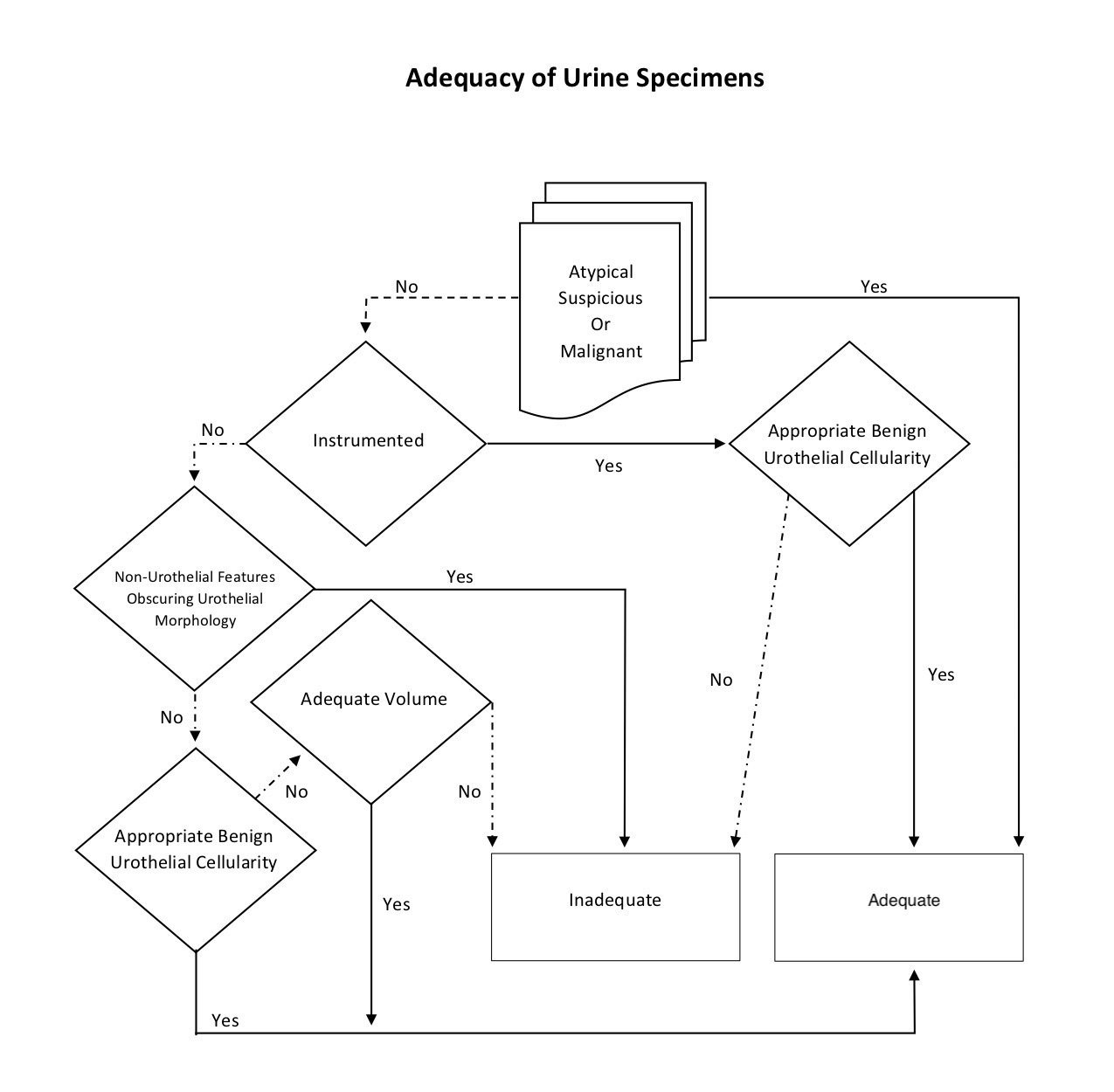
Specimen adequacy
- Adequacy refers to the usefulness of the specimen to diagnose or raise suspicion of urothelial carcinoma
- Adequacy is determined by the interplay of 4 specimen characteristics: collection type, cellularity, volume and cytomorphologic findings
- Of these, the cytomorphologic findings must be considered first because any atypical, suspicious or malignant cells make the specimen intrinsically adequate regardless of the volume, cellularity or collection type
- Similar to Pap smear adequacy, any atypical, suspicious or malignant cells on cytology automatically becomes an adequate specimen
- Adequate number of benign urothelial cells also supercedes any volume requirements for adequacy
- Volume is only considered for voided specimens; low volume samples are less likely to have adequate urothelial cells, but an optimal volume has not been established
- The Paris system indicates that for SurePath®, 30 ml is an optimal volume for voided urines but specimens should not be rejected based on low volume
- Benign urothelial cellularity cut offs should be validated for instrumented and voided urines
Negative for High Grade Urothelial Carcinoma (NHGUC)
- To minimize lumping everything into the "atypical" category, the terminology Negative for High Grade Urothelial Carcinoma includes all entities that pose no significant risk to the patient for developing HGUC based upon available studies
- This term also clarifies the goal of the Paris System - to highlight those cases at risk for HGUC
- For example, radiation associated atypia is classified as Negative for High Grade Urothelial Carcinoma and not atypical
- A urine sample (voided or instrumented) is considered Negative for High Grade Urothelial Carcinoma if any of the following components are present:
- Benign urothelial, squamous and glandular cells
- Benign urothelial tissue fragments
- Changes associated with stones
- Viral cytopathic effect due to polyoma virus
- Post therapy effect, including epithelial cells from urinary diversions
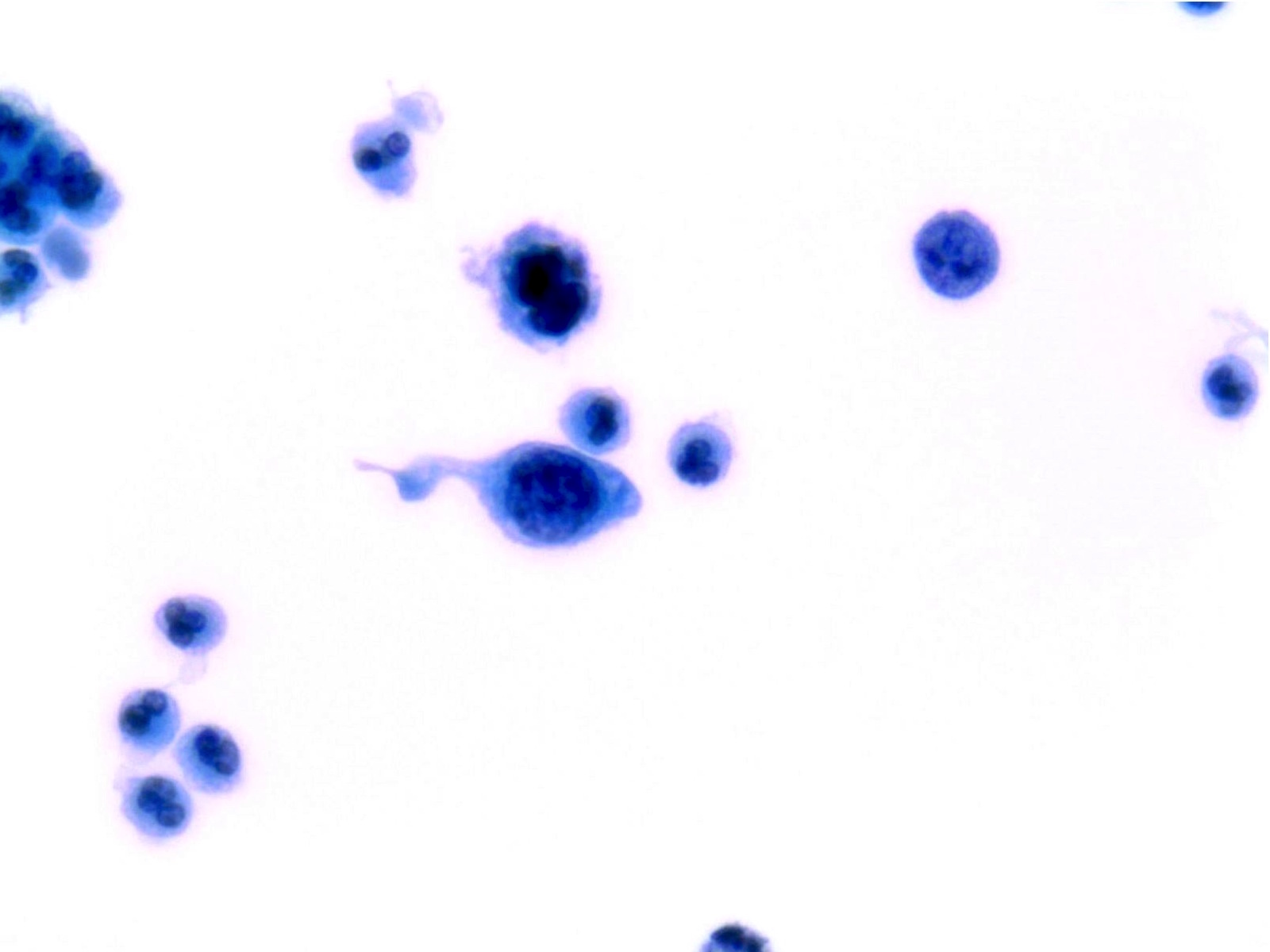
Atypical Urothelial Cells (AUC)
- Defined as cellular changes that fulfill the major (required) criterion and only 1 minor criterion
- Note: the presence of 2 or more minor criterion including nuclear hyperchromasia is diagnostic of Suspicious for HGUC (see below)
- Major criterion (required):
- Non superficial and non degenerated urothelial cells with an increased N/C ratio (> 0.5)
- Minor criteria (one required):
- Nuclear hyperchromasia
- Irregular nuclear membranes
- Irregular, coarse and clumped chromatin
- Diagnosis of AUC is appropriate when cells are more abnormal than NHGUC
- AUC is appropriate when there is suspicion of HGUC but also extensive degeneration
- Normal intermediate and basal urothelial cells, typically seen in instrumented urine, have high N/C ratio and frequently occur in groups; should be regarded as NHGUC
Suspicious for High Grade Urothelial Carcinoma (SHGUC)
- Reflects the presence of urothelial cells with severe atypia that falls short for a diagnosis of high grade urothelial carcinoma but beyond atypical urothelial cells
- A diagnosis of SHGUC is defined as non superficial and non degenerated urothelial cells showing:
- Increased N/C ratio, at least 0.5 - 0.7 (required criterion)
- Moderate to severe nuclear hyperchromasia (required criterion) and at least one of the following:
- Irregular clumpy chromatin
- Marked irregular nuclear membranes
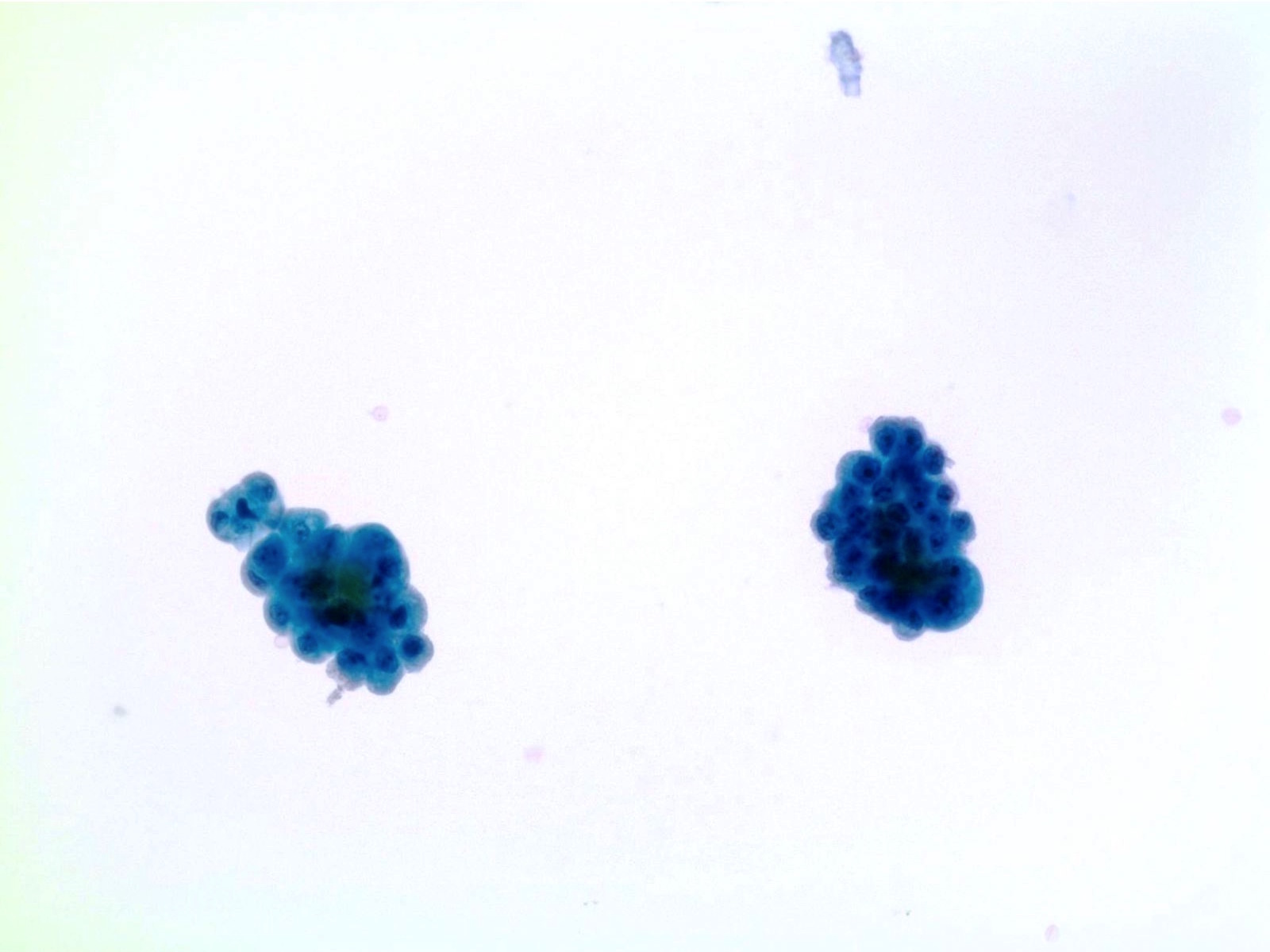
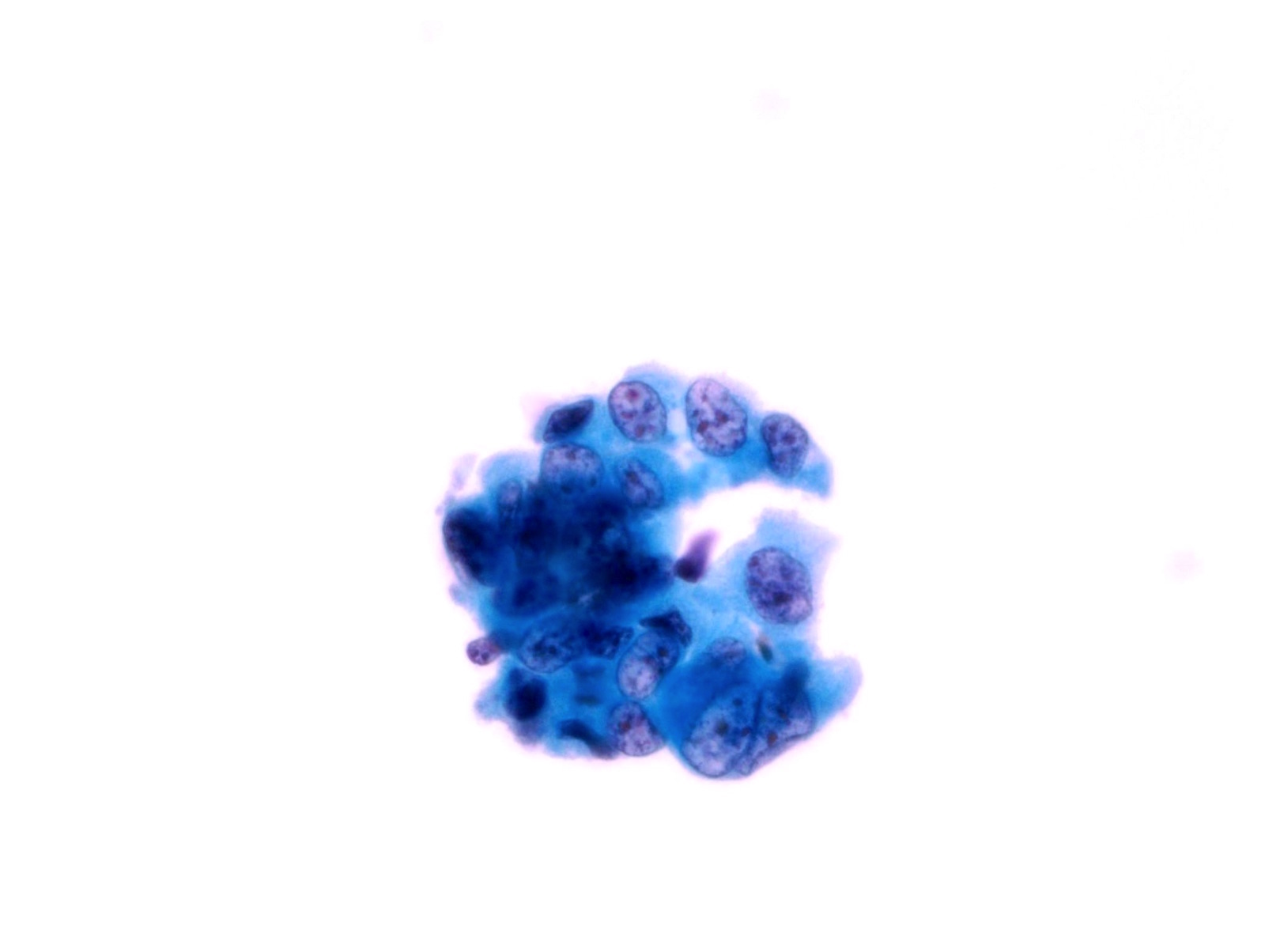
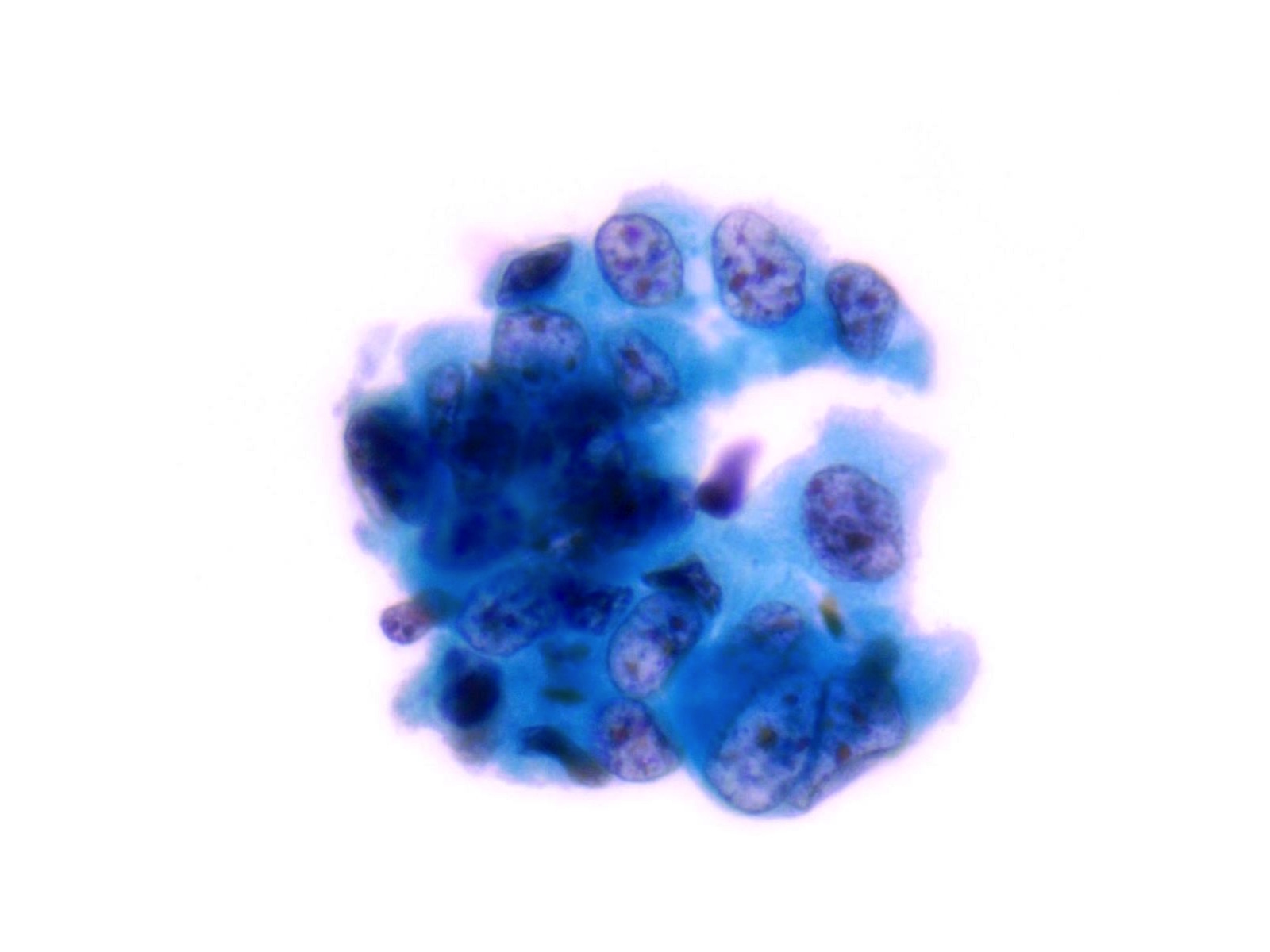
High Grade Urothelial Carcinoma (HGUC)
- Sensitivity of urine cytology for HGUC is 50 - 85%
- Positive urine cytology is clinically meaningful, is significantly associated with tumor recurrence and is independent of other clinicopathologic variables
- Hence, positive urine cytology in primary upper urinary tract urothelial carcinoma is valuable to predict prognosis and preoperative positive urine cytology may be associated with higher prevalence of tumor recurrence
- It can be useful to predict tumor progression
-
Notes:
- Urine cytology cannot distinguish invasive HGUC and carcinoma in Situ (CIS)
- Squamous or glandular differentiation of urothelial carcinoma may be seen in urine cytology but a diagnosis of squamous cell carcinoma or adenocarcinoma of the urinary tract can only be made after examination of biopsy or cystectomy specimens
- HGUC is diagnosed on the basis of this criteria according to the Paris System consensus:
- Cellularity; at least 5 - 10 abnormal cells
- N:C ratio: 0.7 or greater
- Nucleus: moderate to severe hyperchromasia
- Nuclear membrane: markedly irregular
- Chromatin: coarse / clumped
- Other notable cytomorphologic features of HGUC are
- Cellular pleomorphism
- Marked variation in cellular size and shapes. i.e., oval, rounded, elongated or plasmacytoid (comet cells)
- Scant, pale or dense cytoplasm
- Prominent nucleoli
- Mitoses
- Necrotic debris
- Inflammation
Low Grade Urothelial Neoplasia (LGUN)
- LGUN is a combined cytologic term for low grade papillary urothelial neoplasms, which includes urothelial papilloma, papillary urothelial neoplasm of uncertain malignant potential (PUNLMP) and low grade papillary urothelial carcinoma (LGPUC)
- Definitive diagnosis of LGUN is possible only in the presence of this cytologic criteria (regardless of voided urine or instrumented urine):
- 3 dimensional cellular papillary clusters with fibrovascular cores including capillaries
- Cellular papillary clusters are defined as clusters of cells with nuclear overlapping forming papillae
- The following cytologic features should be categorized as NHGUC:
- 3 dimensional cellular clusters without fibrovascular cores
- Increased numbers of single monotonous (non umbrella) cells
- Cytoplasmic homogeneity
- Nuclear border irregularity
- Increased N/C ratio
- A comment may be added to suggest LGUN in these cases without definitive cytomorphologic features
- Rate of progression is 0% for papillomas, 3.6% for PUNLMP and 5 - 25% for LGPUC (WHO / ISUP classification (2004))
Squamous Cell Carcinoma (SCC)
- Accounts for 2 - 5% of all bladder cancer in West
- In North Africa and Middle East where Schistosoma hematobium infestation is endemic, it accounts for 25 - 30% of bladder malignancies
- Cases not associated with Schistosoma (non bilharzial) are usually associated with conditions causing urinary stasis with epithelial injury, such as spinal cord injury or paraplegia
- Cytologic features of bladder SCC are similar to SCC elsewhere
- Diagnostic criteria for SCC:
- Cellular specimen with numerous individual and nests of squamous cells
- Tumor cells are large, polygonal with keratinized cytoplasm, sharp borders and mildly to markedly atypical hyperchromatic nuclei
- Fiber and tadpole cells, squamous pearls and "cell in cell" arrangement may be present
- Background may show fragments of anucleated squamous cells, small atypical parakeratotic cells, necrosis, RBCs and neutrophils
- Nonkeratinizing malignant cell groups with metaplastic appearance may be present
- Liquid based preparations show similar morphology but the background is cleaner so cell details are better preserved
Adenocarcinoma
- Accounts for 0.5 - 2.5% of all primary bladder malignancies and includes vesical and urachal subtypes
- Urachal adenocarcinoma develops within urachal remnants located in bladder dome
- Primary urinary tract adenocarcinoma is less common than secondary involvement from adjacent organs
- Risk factors include cystitis glandularis of intestinal type and bladder exstrophy
- Diagnostic criteria:
- Variable cellularity
- Enteric / colonic type columnar cell clusters and single degenerated cells in a background of necrosis and mucin
- Nuclei are large vesicular or hyperchromatic, with irregular shapes and prominent nucleoli
- Cytoplasm may be vacuolated
- Mucinous / colloid type has rounded 3-D clusters of crowded, bland cells with small to moderate amounts of lacy cytoplasm with occasional mucin vacuoles and medium sized nuclei with visible nucleoli in mucinous background
- Signet ring cell adenocarcinoma: cells with large cytoplasmic mucin filled vacuole that appear optically clear or finely vacuolated, pushing the nucleus to the periphery
- Clear cell carcinoma: cells with abundant vacuolated cytoplasm and centrally located nuclei that may be present in clusters with hobnail configuration
Small cell carcinoma
- Accounts for less than 1% of all bladder malignancies
- Diagnostic criteria:
- Moderate to high cellularity
- Hemorrhagic and necrotic background with apoptosis, isolated or small groups of small, undifferentiated malignant cells, mitoses and numerous neutrophils
- Cells are arranged singly, in linear pattern with rosettes, loosely or tightly cohesive clusters
- Tumor cells are round to oval or irregular and small to medium in size (2 - 3 x lymphocytes)
- Nuclei are small to oval, hyperchromatic with finely granular evenly distributed or smudged chromatin, ill defined membranes, prominent molding and display crush artifact
- Nucleoli are inconspicuous
- Scanty cytoplasm
- High N:C ratio
Secondary neoplasms
- Renal cell carcinoma:
- May be seen in cases of renal pelvis invasion by renal cell carcinoma
- Degenerated cells
- Cells maintain the same morphology as the tumors in the kidney
- Prostatic carcinoma:
- Bladder neck involvement by prostatic carcinoma
- Large cells in clusters with ill defined cell borders, vacuolated cytoplasm, round nuclei and prominent nucleoli
- Immunohistochemistry may be helpful
- Colonic carcinoma:
- Direct extension to bladder with possible fistulae formation
- Cells arranged in acinar configuration
- Coarse chromatin pattern and prominent nucleoli
- Necrosis and abundant red blood cells
- Fecal material present