Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Etiology | Clinical features | Diagnosis | Prognostic factors | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Cytology images | Peripheral smear description | Peripheral smear images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Odetola O, Mirza KM. AML with mutated RUNX1. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/bonemarrowneoplasticAMLmutatedRUNX1.html. Accessed April 28th, 2024.
Definition / general
- Due to the new classifications, this topic is now covered here
- Prior to WHO 2022 and ICC 2022:
- Provisional entity in the 2016 WHO classification
- De novo acute leukemia diagnosed with ≥ 20% blasts in blood or bone marrow
- All morphological subtypes of AML, NOS may harbor RUNX1 mutation, with the highest frequency being AML with minimal differentiation
- Diagnosis of AML with mutated RUNX1 excludes cases that fulfill criteria for other specific AML with recurrent genetic abnormalities, therapy related myeloid neoplasms or AML with myelodysplasia related changes (Blood 2016;128:462, Leukemia 2016;30:2282)
- Unfavorable prognostic implication, with mutated RUNX1 being associated with inferior survival than wildtype (Leukemia 2016;30:2282, J Clin Oncol 2012;30:3109)
Essential features
- Provisional WHO entity describing a de novo acute myeloid leukemia
- Cases possess monoallelic RUNX1 mutations (typically frameshift or missense)
- Blast morphology most compatible with M0
- Cases do not meet diagnostic criteria for other AML with genetic abnormality
- Highest incidence in men and patients over 60 years of age
- Associated gene mutations include those of SRSF2 (39%), ASXL1 (36%), DNMT3A (19%), IDH2 (17%) and SF3B1 (17%) (Leukemia 2018;32:295)
- Other coexisting gene mutations include those of FLT3-ITD, TET2, RAS, PTPN11, p53, JAK2 and EZH2 genes (Leukemia 2018;32:295)
ICD coding
- ICD-O: 9861/3 - Acute myeloid leukemia, NOS (NIH: Acute Myeloid Leukemia, NOS [Accessed 16 July 2020])
Epidemiology
- Reportedly accounts for 4 - 16% of cases of AML (Blood 2016;128:462)
- Higher incidence in men (60%) (Blood 2016;128:462, Leukemia 2016;30:2282, Int J Mol Sci 2017;18:1618, J Clin Oncol 2017;35:934)
- Higher incidence in AML in patients older than 60 years (10 - 20%) (Int J Mol Sci 2017;18:1618, J Clin Oncol 2017;35:934)
- RUNX1 mutations may be seen at increased frequency in patients with AML and a history of radiation exposure or inherited disorders such as Fanconi anemia and congenital neutropenia (Leukemia 2016;30:2282)
- However, these AML may be better classified under alternative categories of therapy related myeloid neoplasm or myeloid neoplasm with germline predisposition
Sites
- Peripheral blood
- Bone marrow
Etiology
- Runt related transcription factor 1 (RUNX1) is known to play a key role in hematopoiesis as a key regulator of genes involved in the differentiation of myeloid cells (Int J Mol Sci 2017;18:1618)
- RUNX1 mutation leads to upregulation of those genes normally expressed in primitive hematopoietic cells and B cell progenitors and downregulation of promoters of myelopoiesis (J Clin Oncol 2012;30:3109)
- Mutation of RUNX1 therefore predisposes to the development of AML with more immature / undifferentiated morphology (M0 in the French-American-British [FAB] classification) (Leukemia 2016;30:2282, Int J Mol Sci 2017;18:1618)
- Associated gene mutations include those of SRSF2 (39%), ASXL1 (36%), DNMT3A (19%), IDH2 (17%) and SF3B1 (17%) (Leukemia 2018;32:295)
- Other coexisting gene mutations include those of FLT3-ITD, TET2, RAS, PTPN11, p53, JAK2 and EZH2 genes (Leukemia 2018;32:295)
Clinical features
- Anemia
- Leukopenia
- Thrombocytopenia
- Low LDH
Diagnosis
- Bone marrow aspiration / biopsy to enumerate ≥ 20% blasts
- Flow cytometric evaluation of peripheral blood and bone marrow aspirate to confirm blast phenotype
- Next generation sequencing (NGS) or mutational analysis to evaluate for any RUNX1 alterations
Prognostic factors
- Presence of mutated RUNX1 gene is by itself a poor prognostic factor in this provisional subtype of AML (Blood 2016;128:462, Leukemia 2016;30:2282, N Engl J Med 2016;374:2209)
- Presence of > 1 RUNX1 mutation correlates with even worse prognosis (Leukemia 2018;32:295)
- AML with mutated RUNX1 is characterized by lower rates of response to induction chemotherapy, higher relapse rates and a poor long term clinical outlook (J Clin Oncol 2017;35:934, N Engl J Med 2016;374:2209, Blood 2017;129:424)
- It has a worse prognosis than AML without mutation of RUNX1
- In contrast, AML with RUNX1 / RUNX1T1 fusion has a more favorable outcome (Sci Rep 2018;8:11293)
- Age 60 years and above indicates poor prognosis (Blood 2011;117:2348)
- Higher WBC count is a marker of poor prognosis (Blood 2011;117:2348)
- Associated comutations including those involving FLT3-ITD, ASXL1, SRSF2, DNMT3A and PHF6 confer a worse prognosis (Leukemia 2016;30:2282, J Clin Oncol 2017;35:934, Blood 2011;117:2348, Leuk Lymphoma 2020;61:1395)
- Associated ASXL1 and ≥ 2 additional mutations correlated with shorter overall survival (Leukemia 2018;32:295)
- Comutation of IDH2 confers a favorable prognosis (Leukemia 2016;30:2282)
Case reports
- 23 year old woman with isolated bone marrow non-Langerhans cell histiocytosis preceding AML with mutated RUNX1 (Am J Clin Pathol 2019;151:638)
Treatment
- AML with mutated RUNX1 is currently treated with standard induction chemotherapy, including regimens consisting of cytarabine and an anthracycline
- However, overall response rate, progression free survival and overall survival are known to be poor in the AML subtype (Leukemia 2016;30:2282, Blood 2011;117:2348)
- Use of hypomethylating agent may negate the effect of RUNX1 in elderly patients (Int J Mol Sci 2017;18:1618)
- Stem cell transplantation is a therapeutic option for suitable patients
- However, it may not significantly improve survival (Blood 2011;117:2348)
- Use of BET protein inhibitors in combination with agents such as venetoclax, decitabine or cytarabine, has shown promise in vitro (Leukemia 2018;32:295)
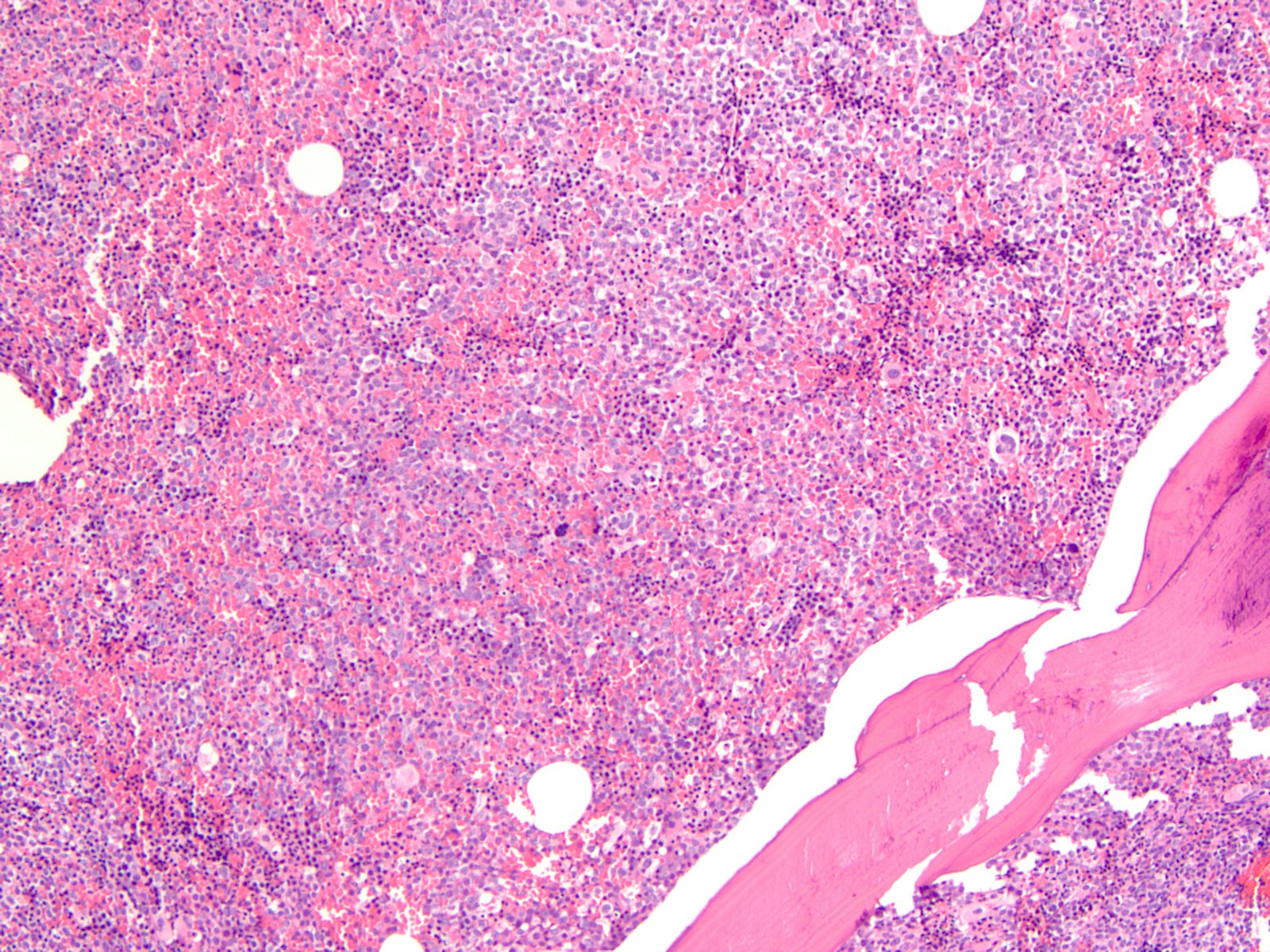
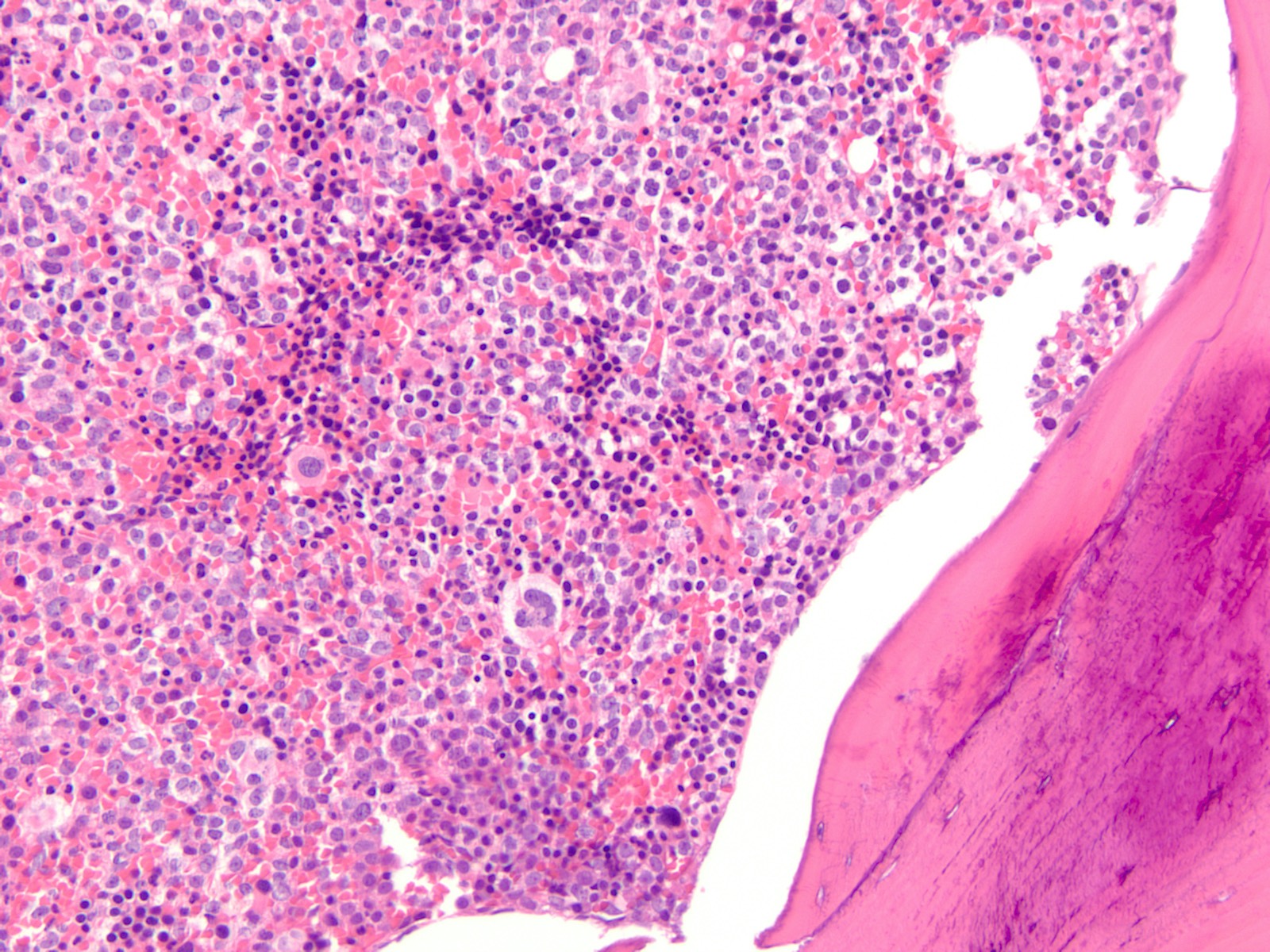
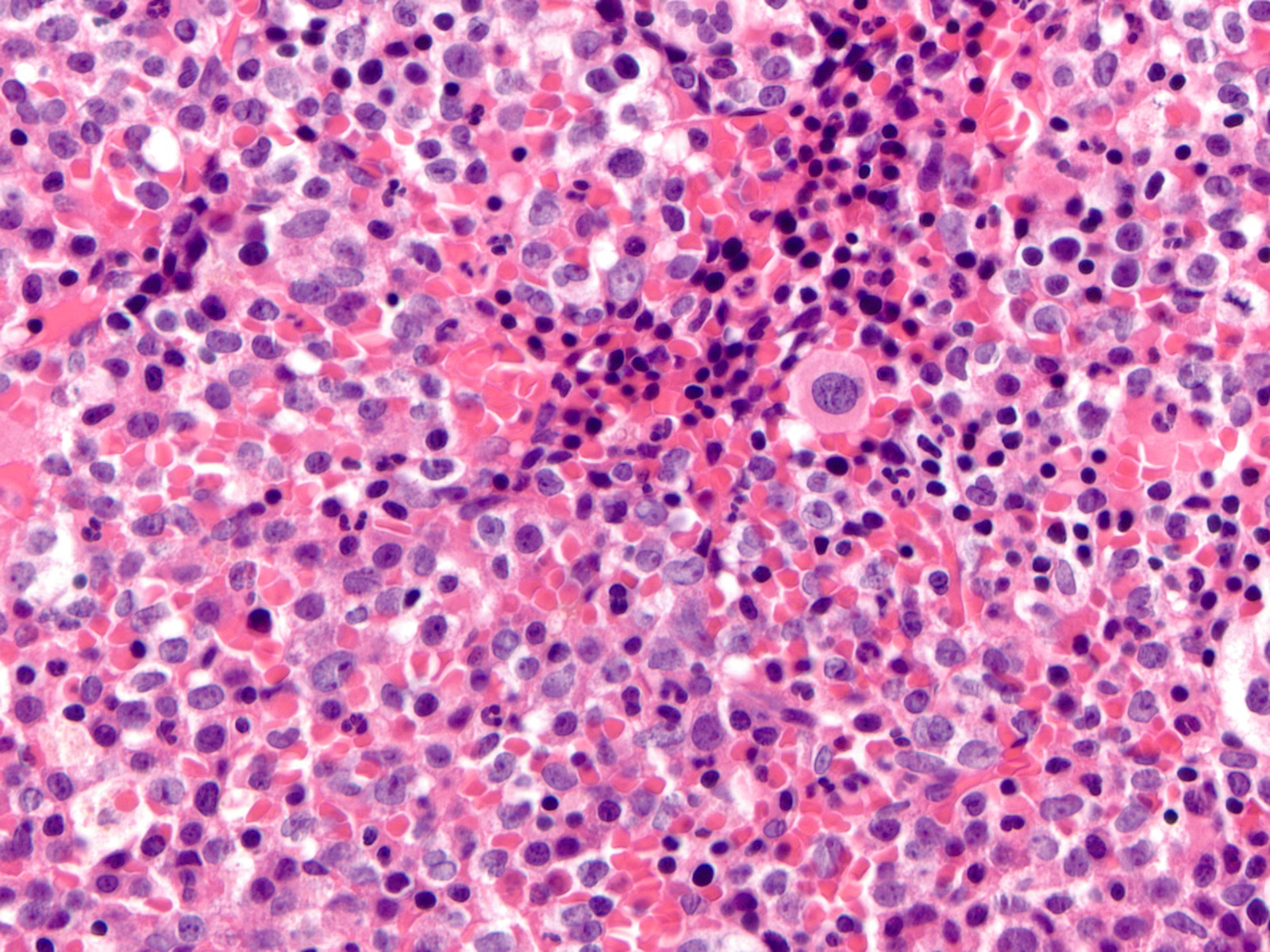
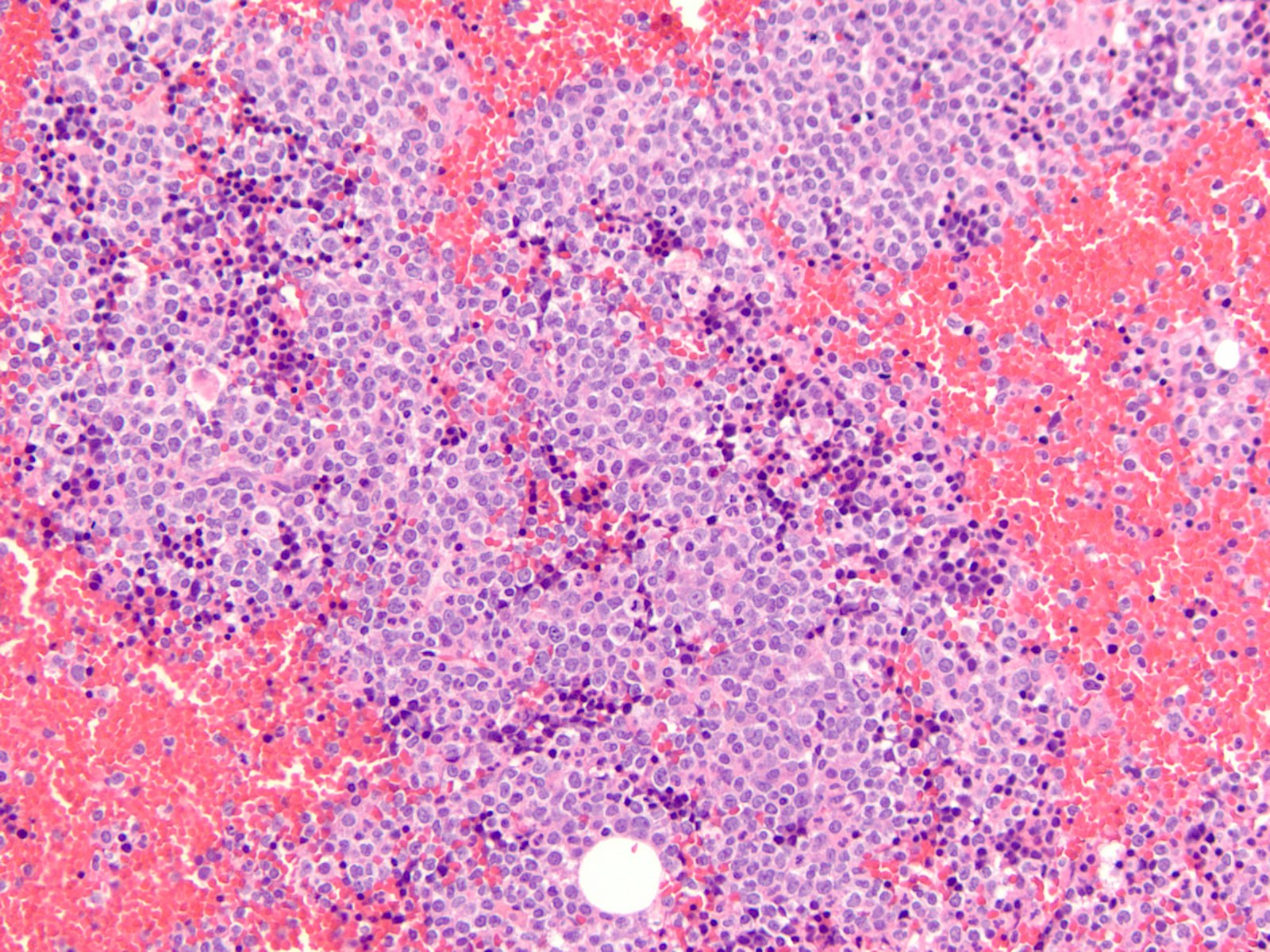
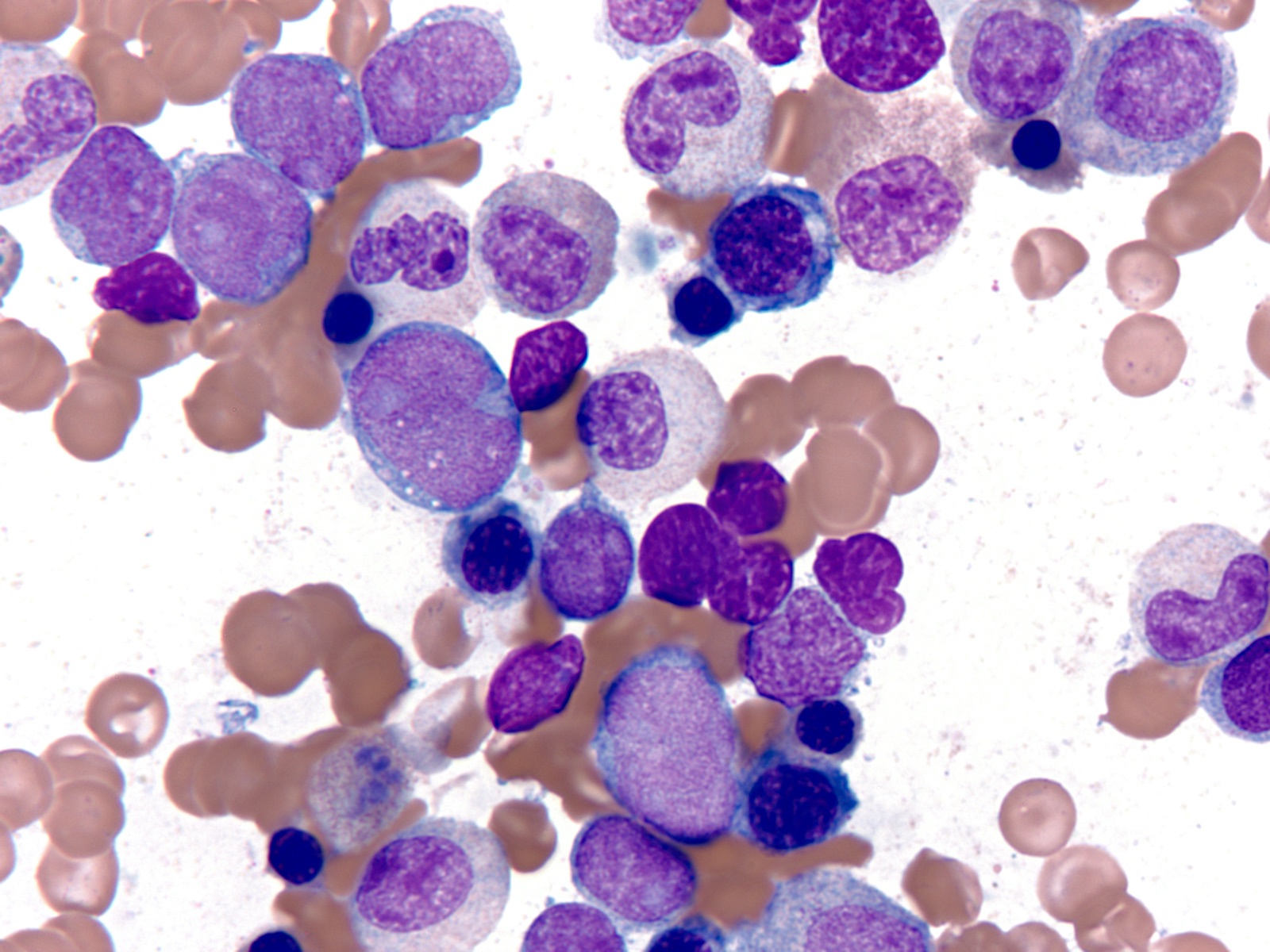
Microscopic (histologic) description
- Majority of cases (~60%) show the M0 (FAB classification) morphology, with very immature / undifferentiated cells (Int J Mol Sci 2017;18:1618, Blood 2011;117:2348)
- Blasts have variable size, high nucleus to cytoplasmic ratio and variably prominent nucleoli with no evidence of differentiation
- M2 and M1 morphology are the next most common (Blood 2011;117:2348)
- RUNX1 mutated AMLs typically demonstrate high bone marrow blast counts (Blood 2011;117:2348)
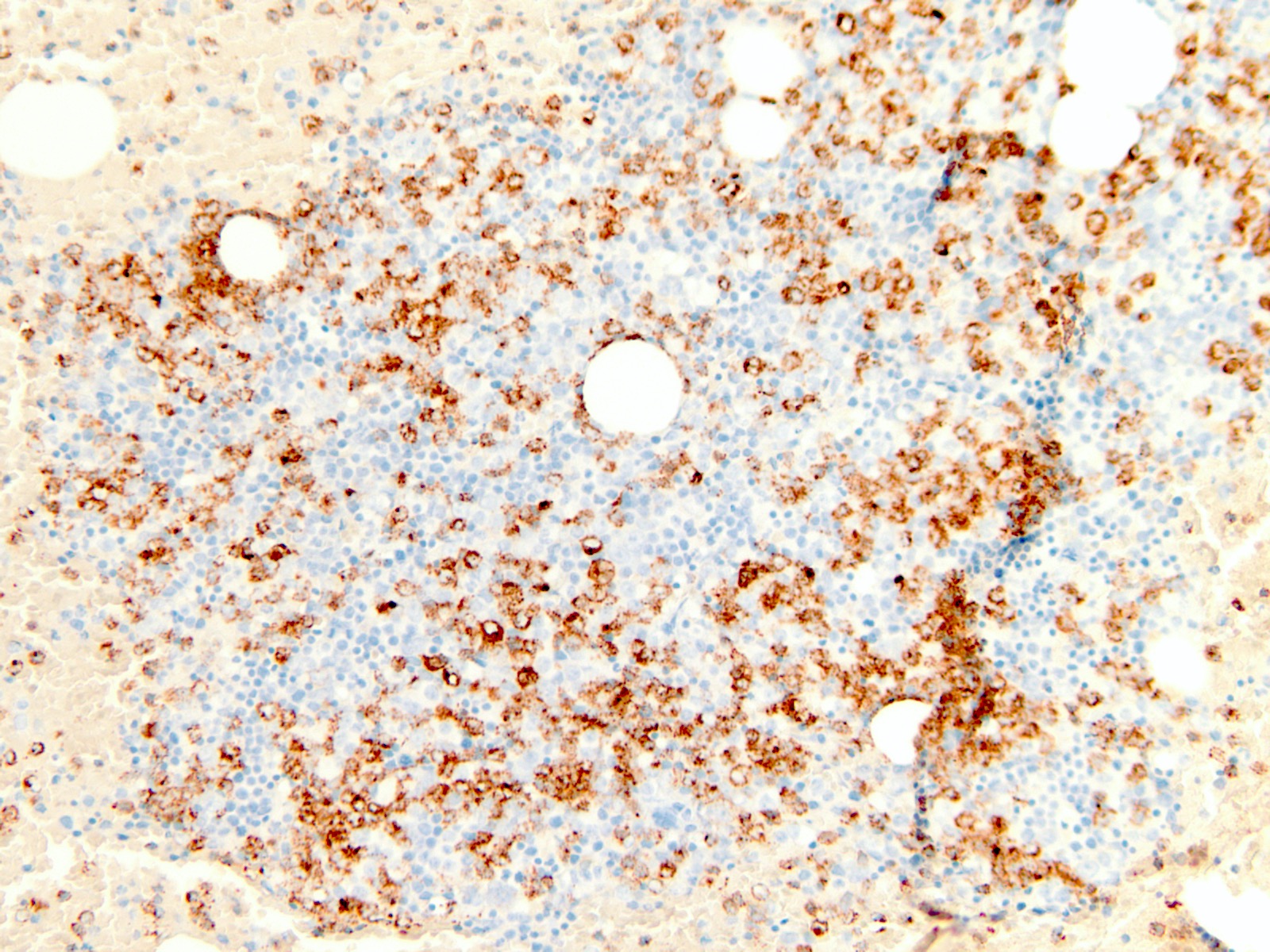
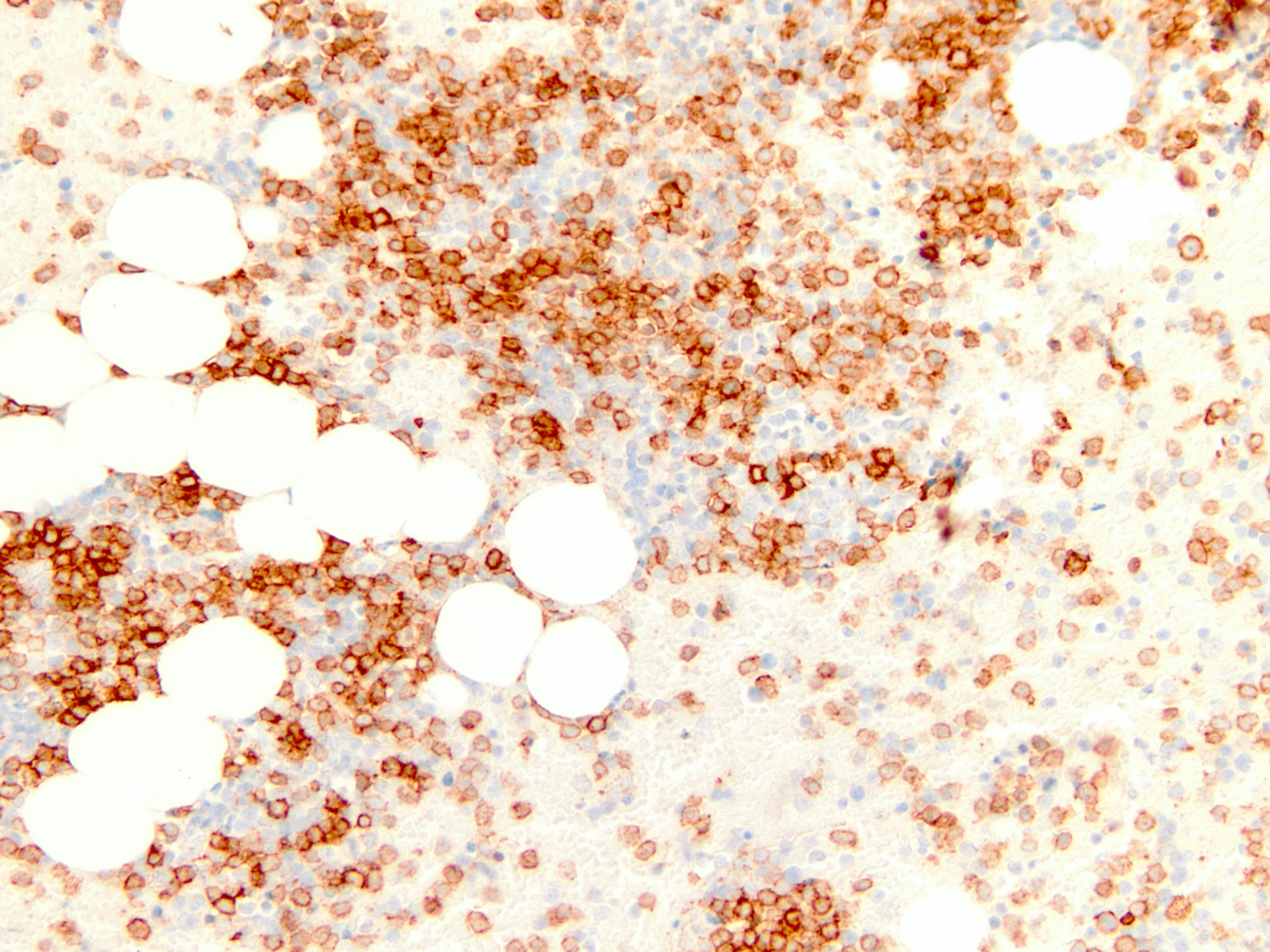
Microscopic (histologic) images
Cytology images
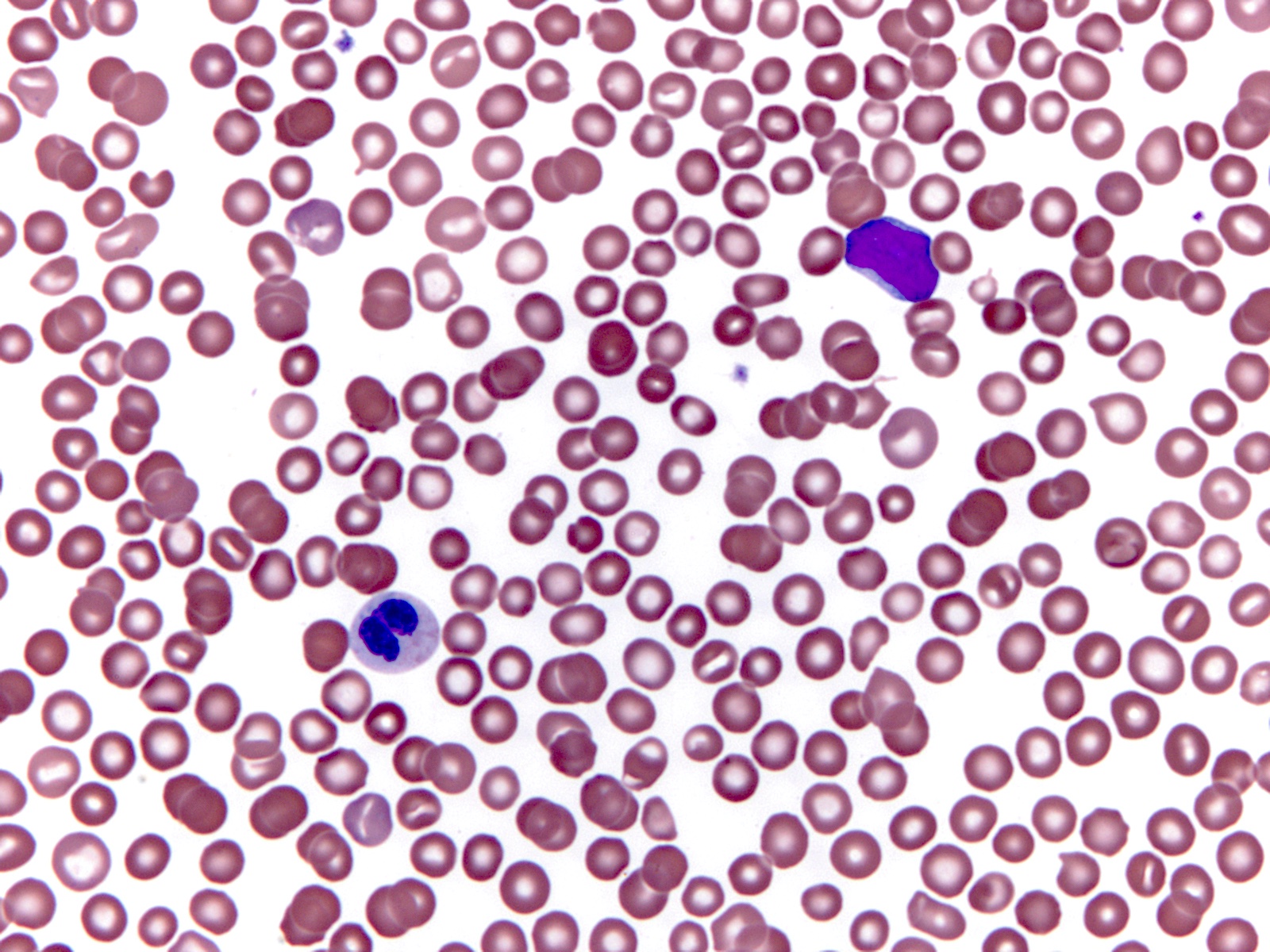
Peripheral smear description
- Lower hemoglobin, white blood cell and blast counts compared with wild type RUNX1
- Thrombocytopenia
Peripheral smear images
Positive stains
- Blasts typically express CD13, CD34 and HLA-DR (Blood 2016;128:462)
- There is variable expression of CD33, monocytic markers and myeloperoxidase (Blood 2016;128:462)
- CD34 higher in RUNX1 mutated blasts compared with RUNX1 wild type (Blood 2011;117:2348)
Negative stains
- Stains for B and T cell differentiation are negative
Molecular / cytogenetics description
- Mutation of RUNX1 (located on chromosome 21q22.12) is disease defining (Blood 2016;128:462)
- Most RUNX1 mutations are monoallelic, within the runt homology domain (RHO) and the transactivation domain (TAD)
- RUNX1 mutations are typically frameshift or missense
- Known associated gene mutations include those of SRSF2, ASXL1, DNMT3A, IDH2, SF3B1, FLT3-ITD, TET2, RAS, PTPN11, p53, JAK2 and EZH2 genes (Leukemia 2018;32:295)
Sample pathology report
- Right posterior iliac crest, bone core biopsy and aspirate smear:
- Acute myeloid leukemia with mutated RUNX1 (see comment)
- Comment: The peripheral blood smear reveals pancytopenia with circulating blasts. The blasts are intermediate in size with high N:C ratio and variably prominent nucleoli. The marrow core biopsy and aspirate smear are packed with sheets of blasts which demonstrate minimal to no myeloid differentiation. Flow cytometry analysis of the marrow aspirate reveals the blast phenotype to be dim CD45, with expression of CD34 and CD13 and absence of B and T lineage markers. Next generation sequencing demonstrates the presence of a missense pathogenic RUNX1 mutation with a variant allele frequency of 65%. FISH panel and cytogenetic analysis are normal.
Differential diagnosis
- AML, NOS, M0:
- Absence of RUNX1 as the defining molecular abnormality
- AML with t(8;21)(q22;q22.1); RUNX1-RUNX1T1:
- Characterized by the RUNX1-RUNX1T1 translocation / fusion product rather than the RUNX1 mutation seen in AML with mutated RUNX1
- Common loss of a sex chromosome or deletion 9q
- Good prognosis compared to AML with mutated RUNX1
- Therapy related AML
- AML with myelodysplasia related changes:
- In the presence of a RUNX1 mutation, this diagnosis takes precedence if the following 3 criteria are met (Swerdlow: WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues, 2017, 4th Edition):
- ≥ 20% bone marrow blasts
- History of MDS or MDS / MPN or MDS related cytogenetic abnormality or multilineage dysplasia (> 50% of cells in at least 2 lineages but not meeting criteria for AML with mutated NPM1 or biallelic mutation of CEBPA)
- Absence of prior cytotoxic therapy or radiation, absence of cytogenetic abnormality defining of another AML category
- In the presence of a RUNX1 mutation, this diagnosis takes precedence if the following 3 criteria are met (Swerdlow: WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues, 2017, 4th Edition):
- Myeloid neoplasm with germline predisposition:
- Patients with a germline mutation in RUNX1 show an autosomal dominant familial platelet disorder and have a predisposition to developing MDS / AML; typically younger than sporadic AML patients (Ther Adv Hematol 2013;4:254, Swerdlow: WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues, 2017, 4th Edition)
Board review style question #1
Which of the following is the most common morphologic correlation (FAB) seen in AML with mutated RUNX1?
- M0 (AML with minimal maturation)
- M1 (AML without maturation)
- M3 (AML with PML-RARA)
- M4 (acute myelomonocytic leukemia)
- M7 (acute megakaryocytic leukemia)
Board review style answer #1
Board review style question #2
Which of the following statements is true about AML with mutated RUNX1?
- ASXL1 and SRSF2 are the most commonly comutated genes
- Comutation with FLT3-ITD negates the poor prognostic impact of RUNX1
- Incidence is higher in people younger than 50 years
- It is associated with a better prognosis than AML with RUNX1-RUNX1T1 fusion
- It has lower frequency of CD34 positivity in blast cells than other subtypes of AML
Board review style answer #2
A. ASXL1 and SRSF2 are the most commonly comutated genes
Comment Here
Reference: AML with mutated RUNX1
Comment Here
Reference: AML with mutated RUNX1