Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Sample pathology report | Differential diagnosis | Practice question #1 | Practice answer #1 | Practice question #2 | Practice answer #2Cite this page: Moreno G, Jorns JM. Atypical ductal hyperplasia. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/breastadh.html. Accessed August 14th, 2025.
Definition / general
- Intraductal clonal epithelial cell proliferation with similar histologic features to (but insufficient involvement or volume for the diagnosis of) low grade ductal carcinoma in situ (DCIS)
Essential features
- Intraductal clonal epithelial cell proliferation with cytologic and morphologic features similar to low grade ductal carcinoma in situ
- Differentiated from low grade ductal carcinoma in situ by size (≤ 2 mm) or space occupied by clonal cells (≤ 2 spaces or only portion of involved space)
- Rigid bridges, polarization around lumen and estrogen receptor (ER) (diffuse, strong) positivity
Terminology
- B3 lesion (uncertain malignant potential)
- Atypical intraductal hyperplasia (AIDH)
- Ductal intraepithelial neoplasia (DIN) 1B
- Mammary intraepithelial neoplasia (MIN)
- Atypical hyperplasia is not recommended by WHO unless ductal versus lobular differentiation cannot be discerned
ICD coding
Epidemiology
- F > M (rare incidental finding in gynecomastia)
- Commonly presents in fourth decade
- 5 - 20% of breast biopsies (StatPearls: Atypical Breast Hyperplasia [Accessed 19 November 2019])
- Reduction of postmenopausal hormonal therapy linked to decreased rates (Cancer Epidemiol Biomarkers Prev 2009;18:2822)
- 4 - 5x risk of developing ductal carcinoma in situ within 5 years (Cancer Prev Res (Phila) 2014;7:211)
- Absolute risk of breast cancer is approximately 1% per year for at least 25 years, with a mean latency period of 8 - 12 years after initial diagnosis
- Recent radiological pathological concordance series have shown that biopsy diagnosed atypical ductal hyperplasia (ADH) has an upgrade rate of 10 - 20% to DCIS or invasive carcinoma
Sites
- 2:1 ratio of ipsilateral to contralateral breast (Cancer Prev Res (Phila) 2014;7:211)
Pathophysiology
- Low grade model of progression from normal breast / benign proliferative breast disease to atypical ductal hyperplasia (Histopathology 2010;57:171)
Etiology
- EZH2 (enhancer of zeste homolog 2) overexpression has an important role in oncogenesis (Arch Pathol Lab Med 2018;142:1182)
- Lifelong exposure to estrogen causes a continued accumulation of genomic changes that leads to defective growth control (Cancer Prev Res (Phila) 2014;7:211)
- Shared alterations with low grade ductal carcinoma in situ: gains 1q and loss 16q-17p (Histopathology 2010;57:171)
Radiology description
- No specific diagnostic features differentiate atypia from malignant lesions (AJR Am J Roentgenol 2014;202:1389)
- Incidentally discovered on routine screening mammograms (3.3 per 10,000)
- Often identified in breast biopsies performed as a result of calcifications on imaging (Acad Radiol 2019;26:893)
- May be seen as nonmass enhancement on breast MRI
Prognostic factors
- Higher risk with
- Younger age
- BRCA1 / BRCA2 mutations
- Family history
- Higher number of separate foci (overall volume)
- Extent of lobular involution (complete / partial) correlated with age (J Natl Cancer Inst 2017;109:djx035, Cancer Prev Res (Phila) 2014;7:211)
Case reports
- 15 year old girl with atypical ductal hyperplasia and juvenile fibroadenoma (Clin Breast Cancer 2018;18:e751)
- 21 year old man with atypical ductal hyperplasia in gynecomastia (Adv Plast Reconstr Surg 2017;1:1)
- 42 year old man with gynecomastia, atypical ductal hyperplasia and ductal carcinoma in situ associated with invasive breast carcinoma, undergoing antiretroviral therapy (Int J Surg Pathol 2016;24:139)
Treatment
- Most biopsy proven lesions undergo excision with some variation, as directed by
- Imaging findings (e.g., small versus larger extent of calcifications)
- Biopsy type / size: excision rate 60% if diagnosed by vacuum assisted biopsy
- Patient / surgeon preference
- Tamoxifen reduced the risk of subsequent cancer from 21 to 7.5% (Breast Cancer Res 2018;20:39)
Gross description
- Usually difficult to detect grossly
- Associated calcifications may feel gritty
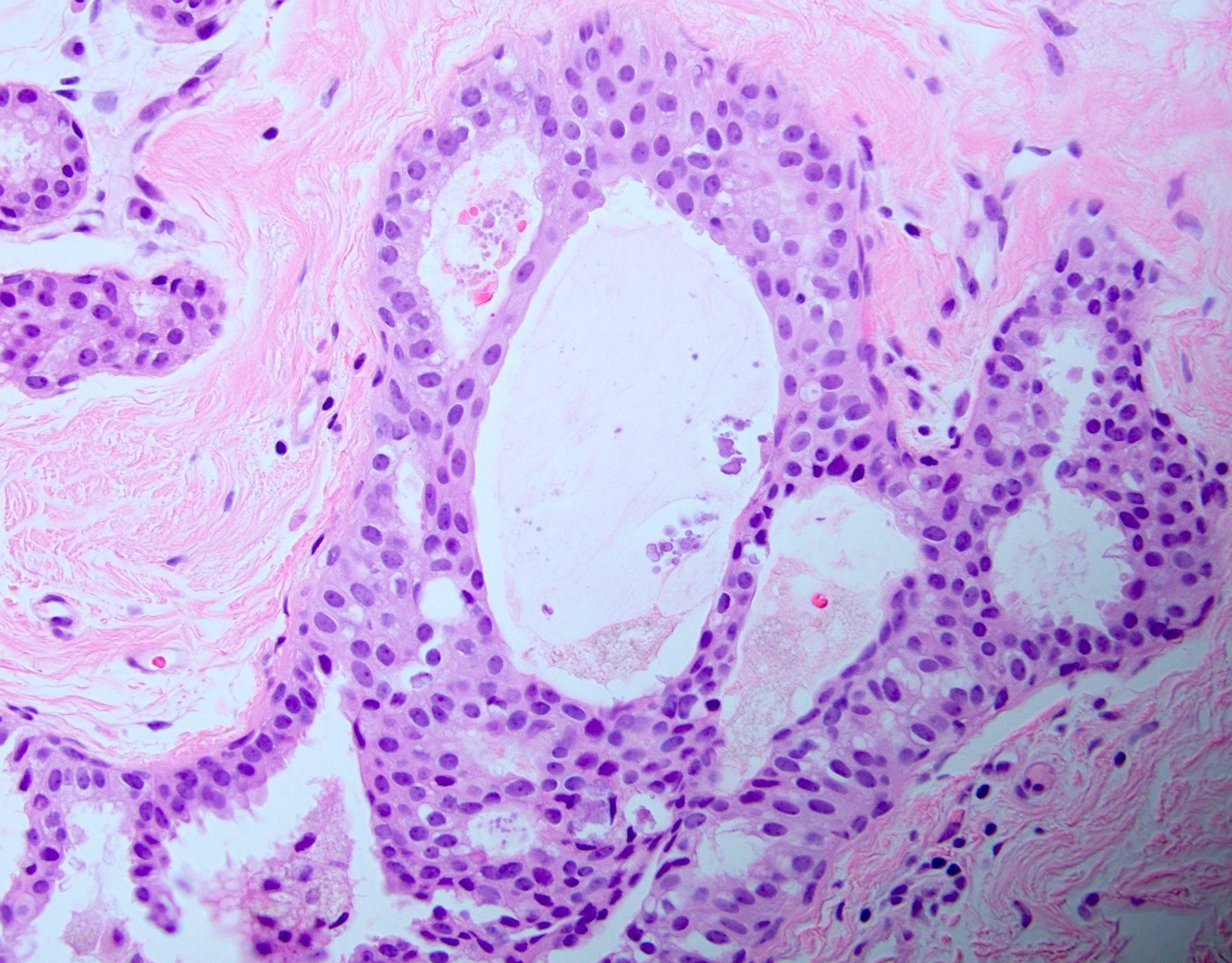
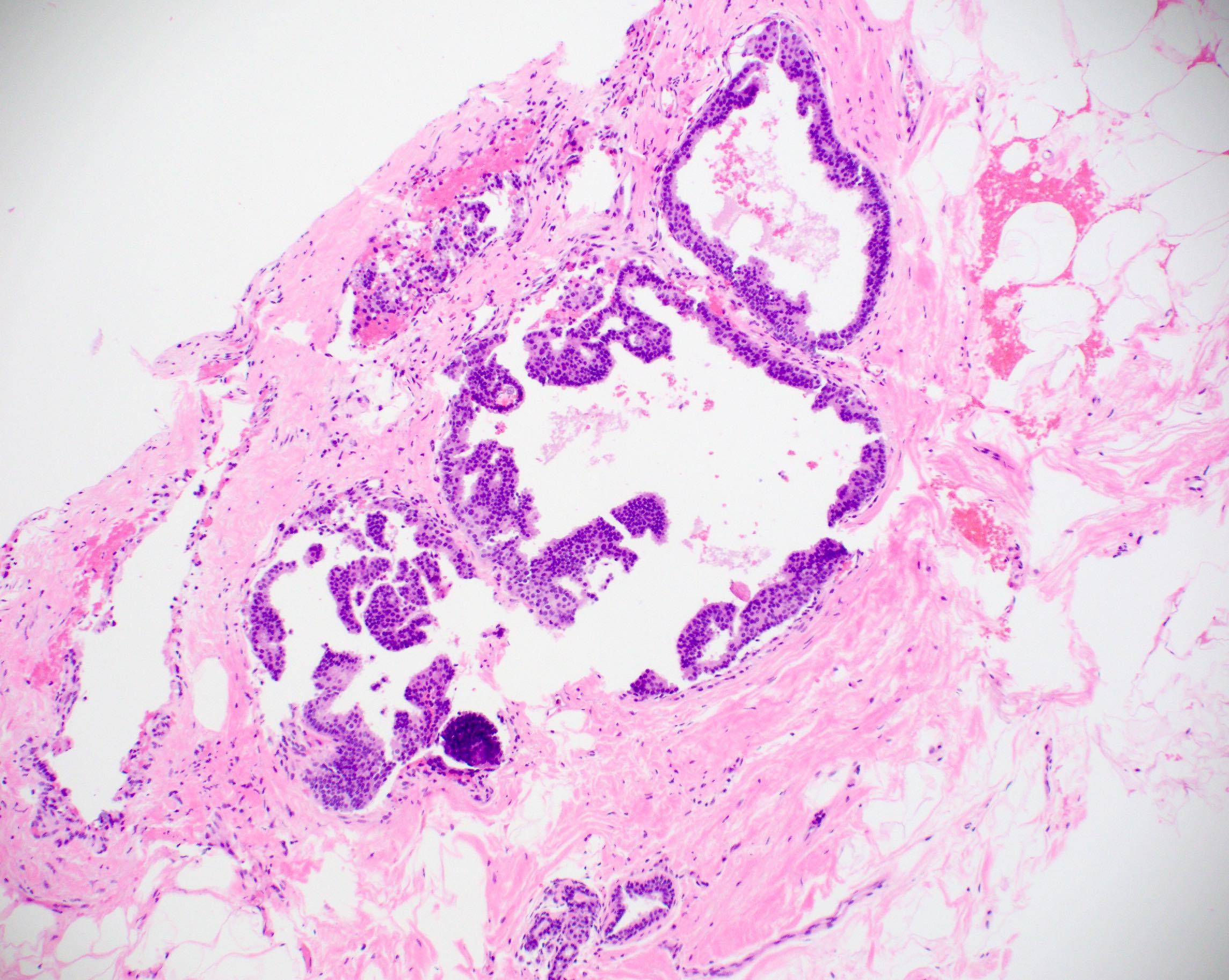
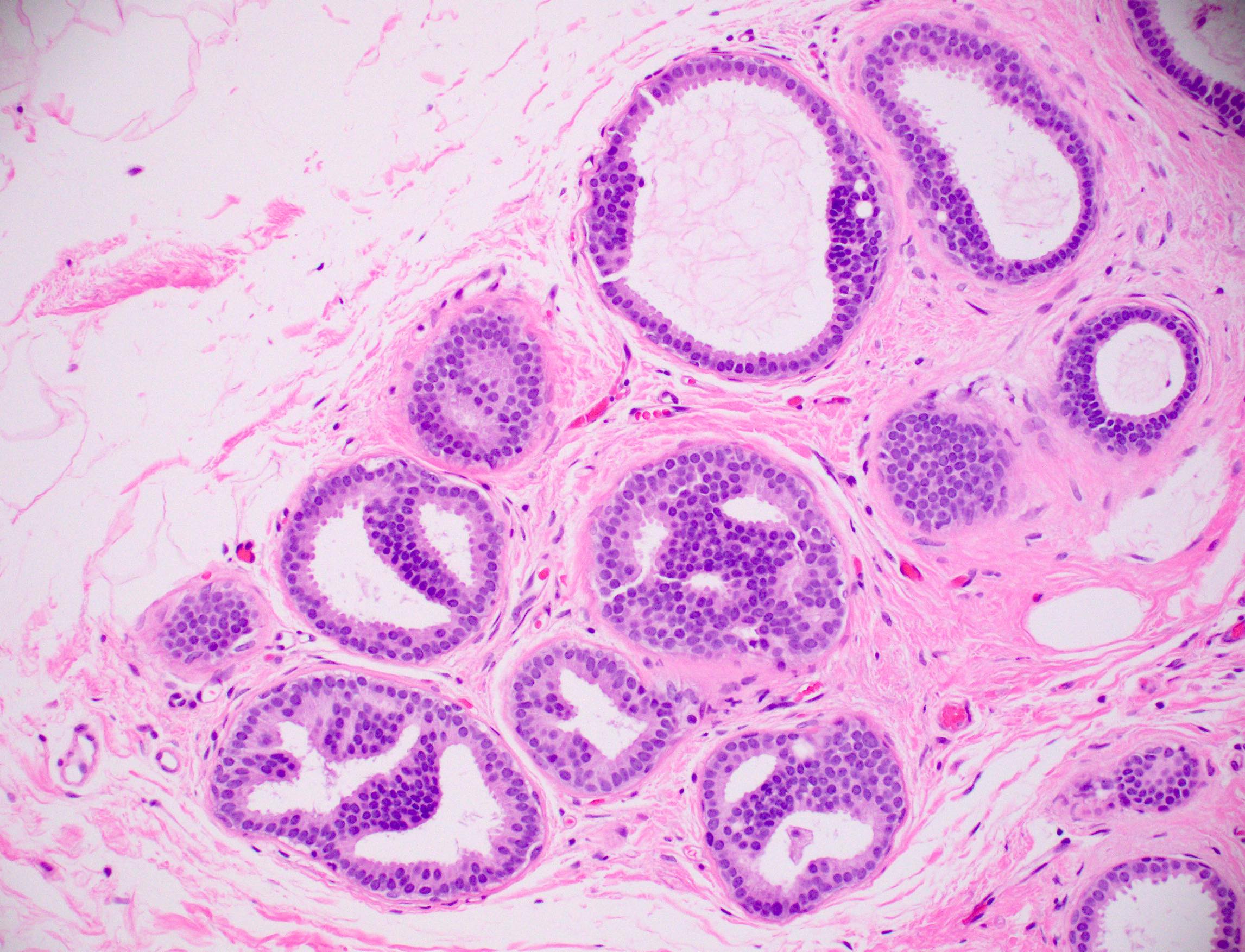
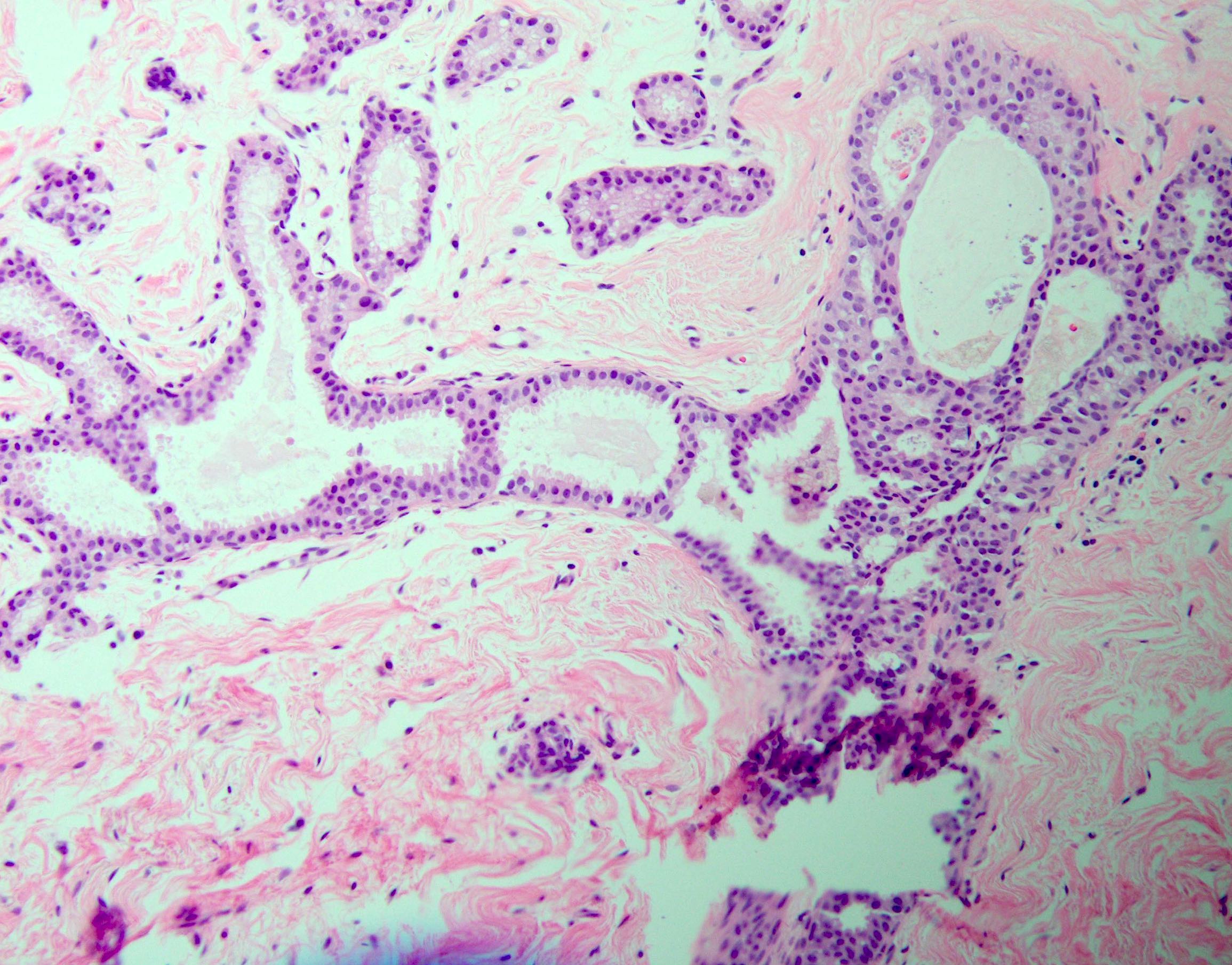
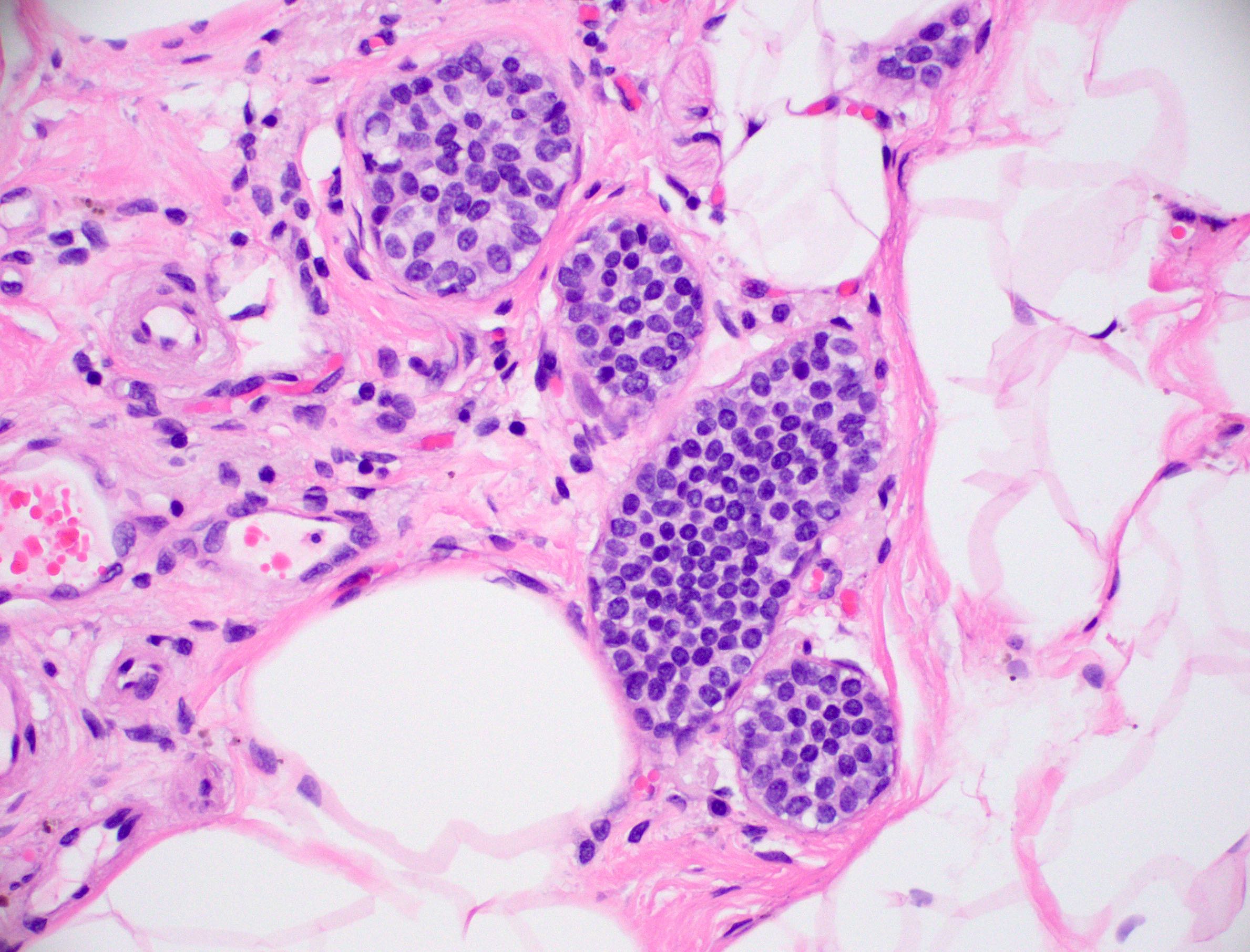
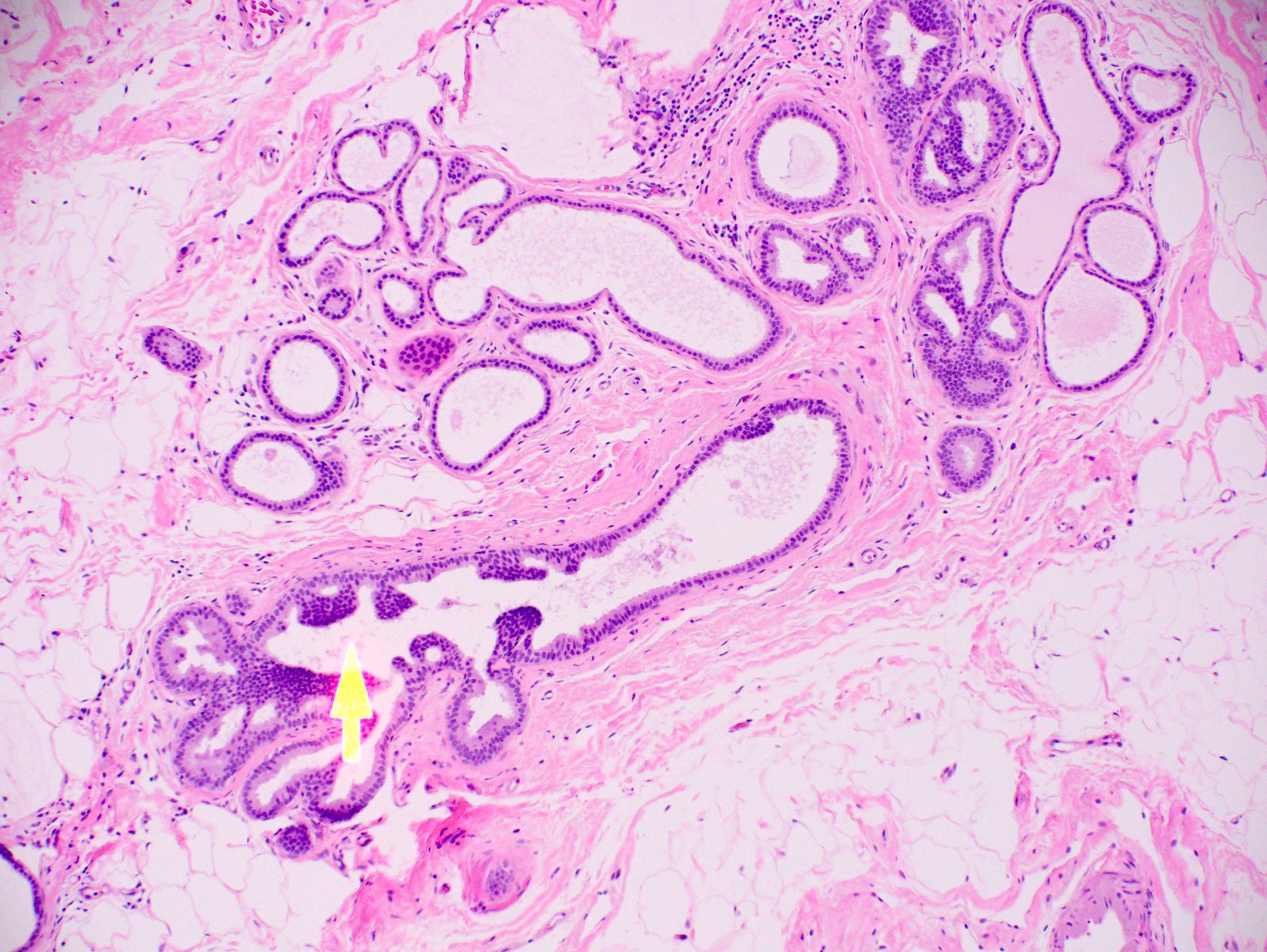
Microscopic (histologic) description
- Histologic sections show well defined, monomorphic cells with evenly spaced, small rounded nuclei
- Variable patterns:
- Solid
- Cribriform (polarization around lumina)
- Micropapillary (club shaped)
- Unicentric or multicentric
- Uniform thickness of rigid bridges or arcades (differential feature from usual ductal hyperplasia)
- Intraluminal microcalcifications are often seen
- Rarely can be mixed with usual ductal hyperplasia or other proliferations within the same lobule
- Differentiation from low grade ductal carcinoma in situ is controversial and shows interobserver variability with low agreement rate on biopsy (40 - 60%), especially because the majority of the distinction is based on the following (generally accepted) quantitative features:
- Size: ≤ 2 mm (Cancer 1990;65:518)
- Space involvement: ≤ 2 spaces or only portion of involved space (Cancer 1985;55:2698)
- Conservative approach is suggested on biopsy; definitive diagnosis should be upon surgical excision if the lesion is borderline between atypical ductal hyperplasia and low grade ductal carcinoma in situ
- If nuclear atypia warrants a diagnosis of intermediate or high grade ductal carcinoma in situ, atypical ductal hyperplasia should not be diagnosed based on size
- Intermediate or high grade ductal carcinoma in situ should be diagnosed regardless of size or extent
Microscopic (histologic) images
Positive stains
- Low molecular weight cytokeratins: CK8, CK18, CK19 (Schnitt: Biopsy Interpretation of the Breast, 3rd Edition, 2018)
- Strong and uniform estrogen receptor / ER expression
Negative stains
Sample pathology report
- Left breast, 1 o'clock, stereotactic core biopsy:
- Focal (0.1 cm) atypical ductal hyperplasia (ADH) with microcalcifications
Differential diagnosis
- Usual ductal hyperplasia:
- Nonclonal cell proliferation
- Cells are not uniform; they show different sizes and shapes with overlapping / streaming architecture
- Cells not oriented around lumen
- Bridges stretch with central attenuation and lumina are flattened / slit-like
- Positive mosaic or patchwork pattern of CK5/6 staining
- Low grade ductal carcinoma in situ:
- Same cytologic and morphologic characteristics of atypical ductal hyperplasia but ≥ 2 mm in size, involving the entire lobular space and extending to > 2 lobules
Practice question #1
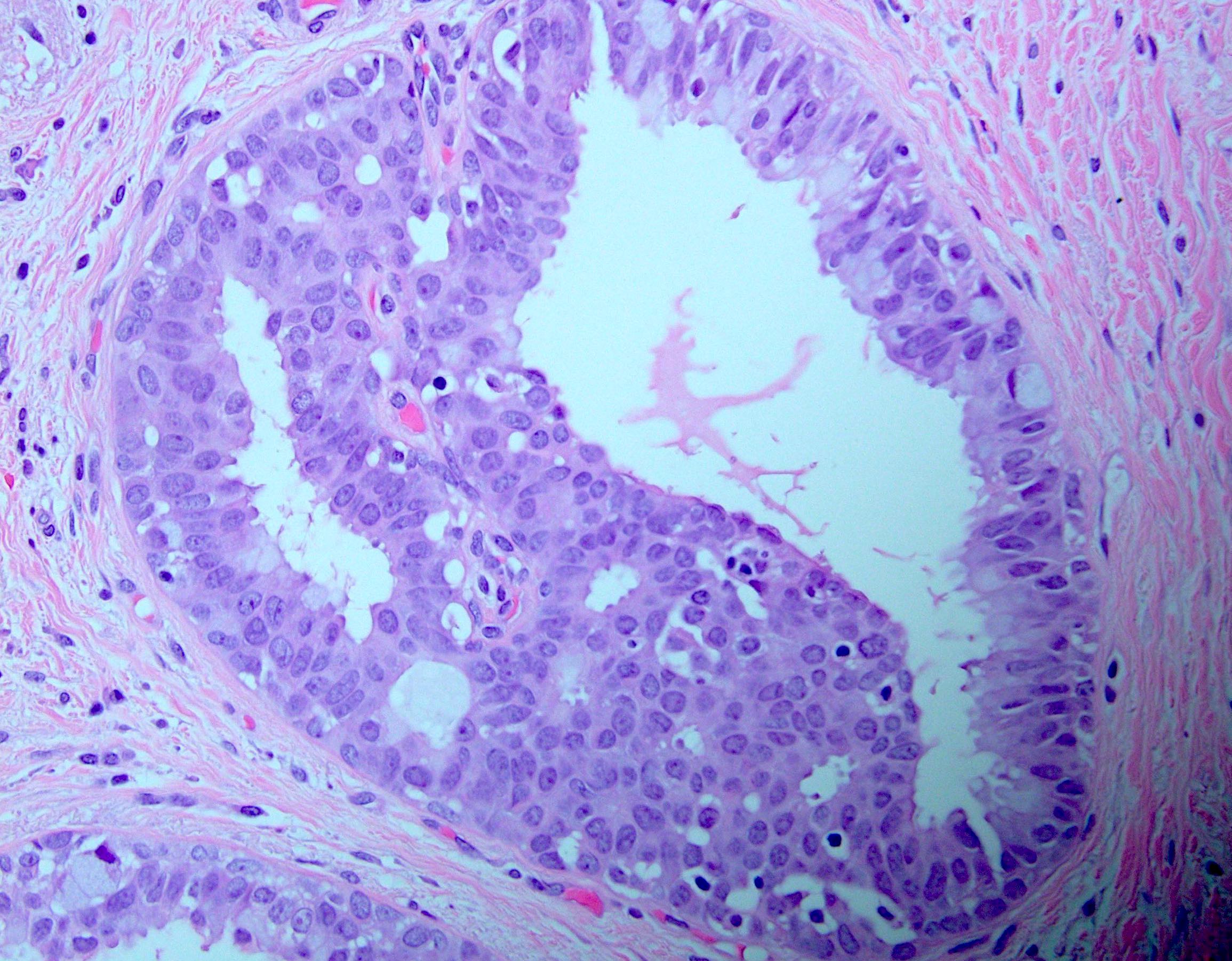
Practice answer #1
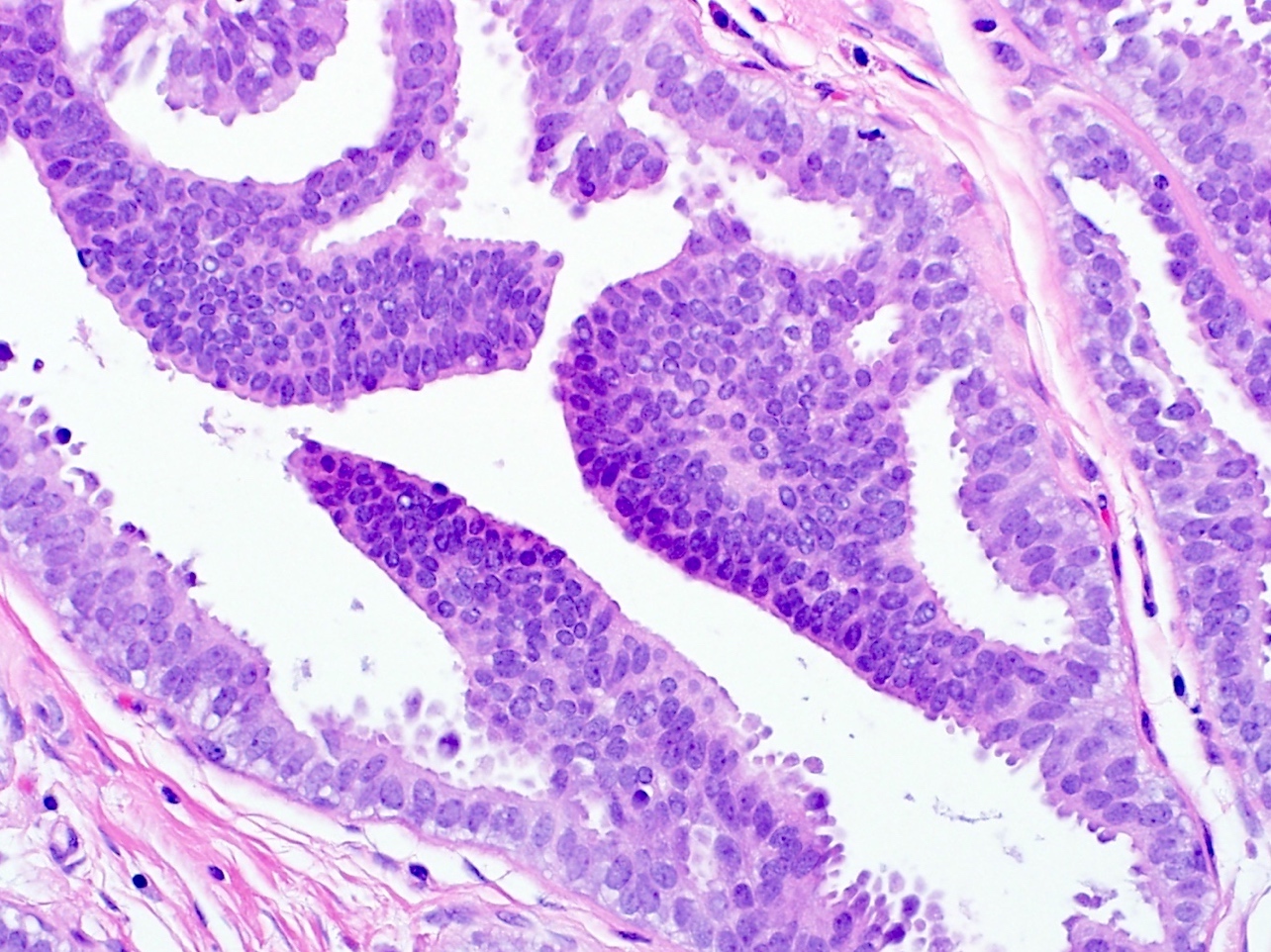
C. Micropapillary
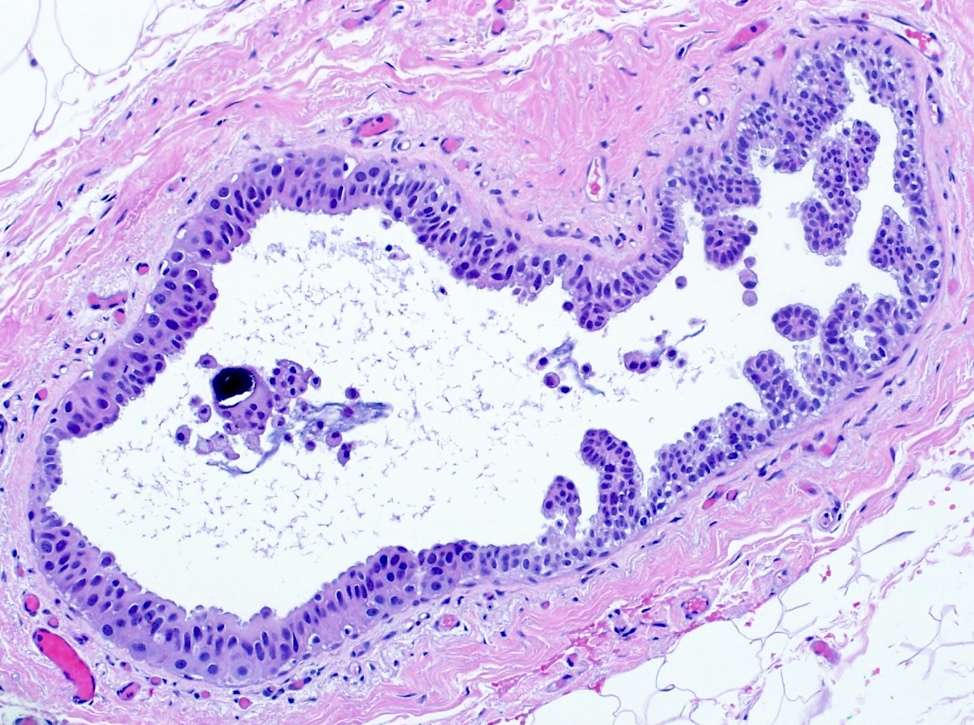
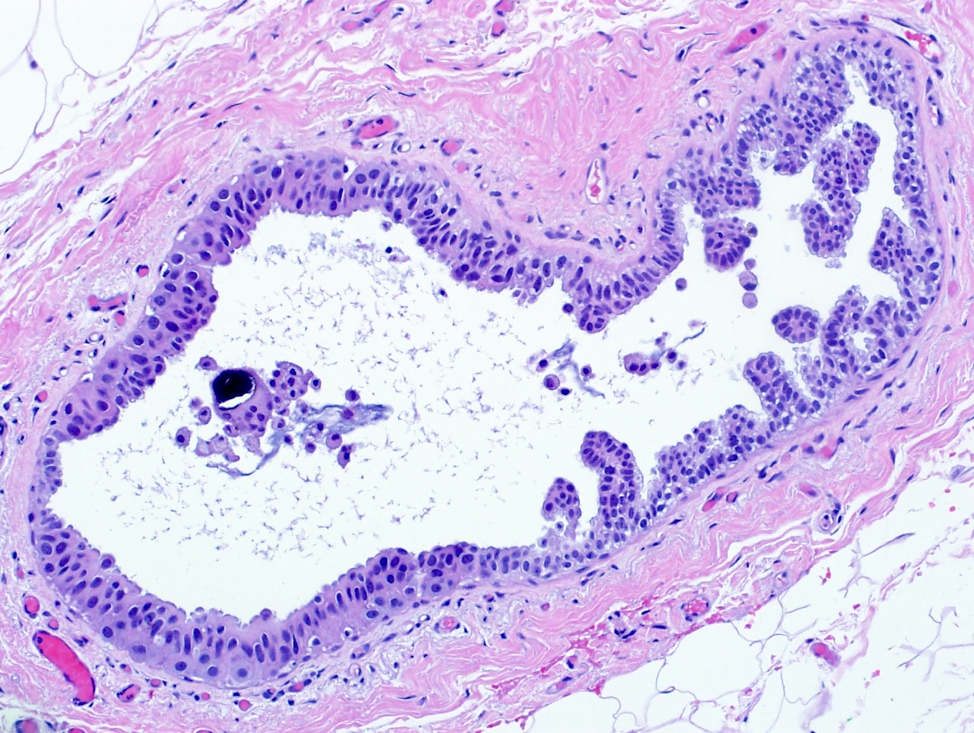
While specifying the pattern of atypical ductal hyperplasia is not clinically important or necessary in the pathology report, recognizing key features and patterns is important in making the diagnosis. Atypical ductal hyperplasia is defined by having some but not all features of low grade ductal carcinoma in situ, with low volume or size (≤ 2 spaces or including only a portion of the involved space or ≤ 2 mm in overall size). Common patterns of atypical ductal hyperplasia include cribriform, micropapillary and solid. Flat patterns are more in keeping with the diagnosis of flat epithelial atypia. The example above has short, club shaped micropapillae, characteristic of the micropapillary pattern of atypical ductal hyperplasia.
Comment Here
Reference: Atypical ductal hyperplasia
While specifying the pattern of atypical ductal hyperplasia is not clinically important or necessary in the pathology report, recognizing key features and patterns is important in making the diagnosis. Atypical ductal hyperplasia is defined by having some but not all features of low grade ductal carcinoma in situ, with low volume or size (≤ 2 spaces or including only a portion of the involved space or ≤ 2 mm in overall size). Common patterns of atypical ductal hyperplasia include cribriform, micropapillary and solid. Flat patterns are more in keeping with the diagnosis of flat epithelial atypia. The example above has short, club shaped micropapillae, characteristic of the micropapillary pattern of atypical ductal hyperplasia.
Comment Here
Reference: Atypical ductal hyperplasia
Practice question #2
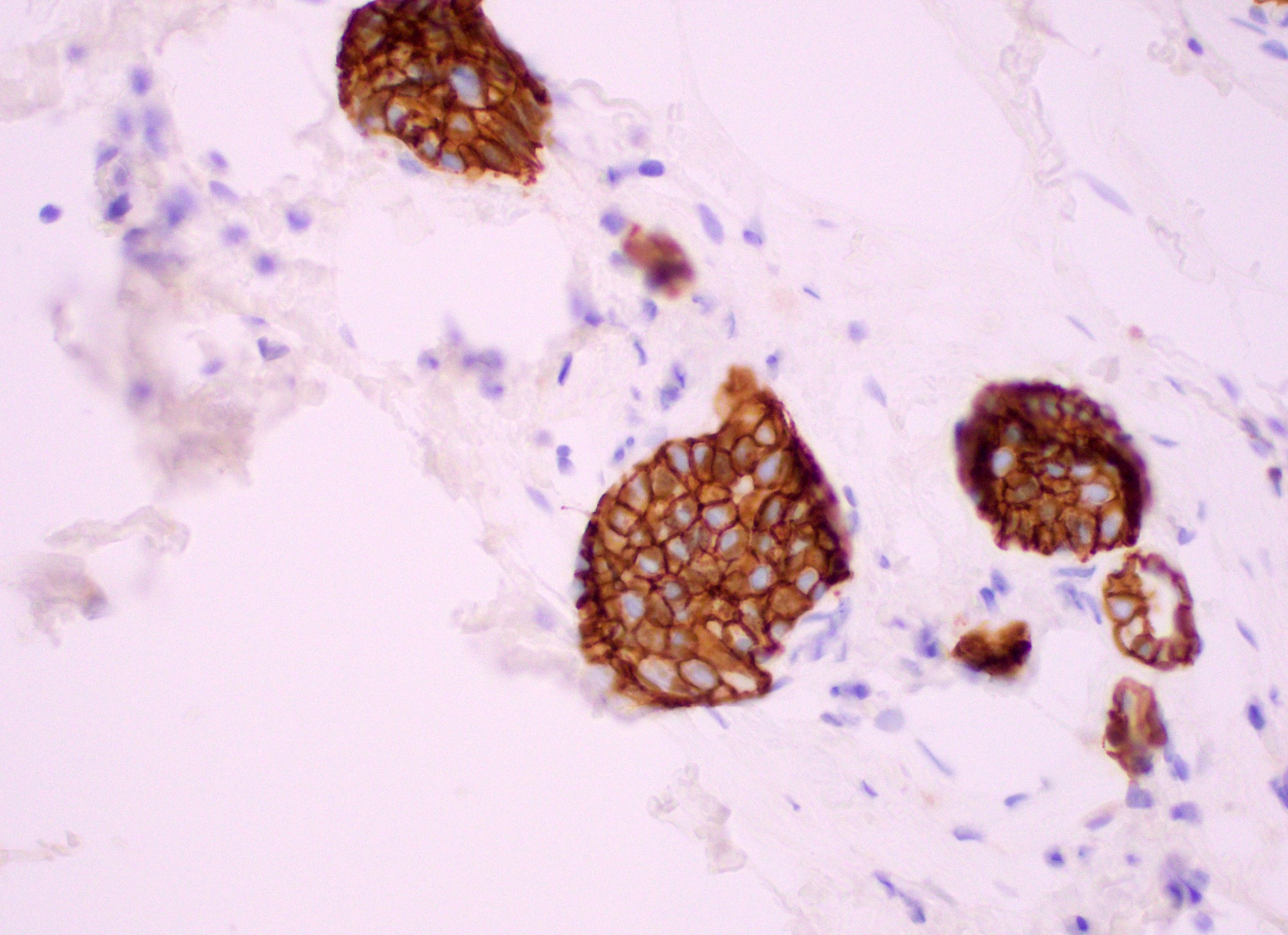
Practice answer #2
B. CK5/6
Usual ductal hyperplasia and atypical ductal hyperplasia of the breast may sometimes be difficult to distinguish morphologically. Thus, use of CK5/6 immunohistochemical staining may be helpful in the differential diagnosis, as usual ductal hyperplasia is positive (in a mosaic or patchwork pattern) and atypical ductal hyperplasia is negative for CK5/6. Both usual ductal hyperplasia and atypical ductal hyperplasia are typically positive for CK7 and negative for CK20 (breast ductal epithelium immunophenotype). Myoepithelial markers p63 and calponin show positive myoepithelial staining in both usual ductal hyperplasia and atypical ductal hyperplasia.
Comment Here
Reference: Atypical ductal hyperplasia
Usual ductal hyperplasia and atypical ductal hyperplasia of the breast may sometimes be difficult to distinguish morphologically. Thus, use of CK5/6 immunohistochemical staining may be helpful in the differential diagnosis, as usual ductal hyperplasia is positive (in a mosaic or patchwork pattern) and atypical ductal hyperplasia is negative for CK5/6. Both usual ductal hyperplasia and atypical ductal hyperplasia are typically positive for CK7 and negative for CK20 (breast ductal epithelium immunophenotype). Myoepithelial markers p63 and calponin show positive myoepithelial staining in both usual ductal hyperplasia and atypical ductal hyperplasia.
Comment Here
Reference: Atypical ductal hyperplasia