Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Practice question #1 | Practice answer #1 | Practice question #2 | Practice answer #2 | Practice question #3 | Practice answer #3Cite this page: Lérias S, Lerwill M. Usual ductal hyperplasia. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/breastepithelialductalhyperplasia.html. Accessed August 25th, 2025.
Definition / general
- Benign intraductal proliferation of progenitor epithelial cells with varying degrees of solid or fenestrated growth
Essential features
- Component of fibrocystic changes
- Mild cytologic variability
- Streaming growth pattern with fenestrated spaces and lack of cellular polarity
- Immunoreactive for high molecular weight cytokeratins
- Associated with slight increase in subsequent breast cancer risk (1.5 - 2 times)
Terminology
- Also called epithelial hyperplasia, intraductal hyperplasia, hyperplasia of usual type, ductal hyperplasia without atypia, epitheliosis
ICD coding
Epidemiology
- Mean age is 54 (N Engl J Med 2005;353:229)
- Most significant finding in 20% of benign breast biopsies (Cancer 2006;106:732)
Sites
- Terminal duct lobular units
- Occasionally, extralobular ducts
Pathophysiology
- Proliferation of CK5+ progenitor cells that can differentiate along glandular or myoepithelial lineages; glandular progenitor cells appear to predominate and show intermediate levels of differentiation (J Pathol 2002;198:458)
Etiology
- No specific etiologic factors
Clinical features
- No specific clinical findings
Diagnosis
- Diagnosis by histologic examination of tissue removed via biopsy or surgical excision
Radiology description
- No specific mammographic findings; occasional examples are associated with microcalcifications
- Can involve an underlying lesion (e.g. radial scar or papilloma) that is identified on imaging
- May show enhancement on magnetic resonance imaging (Arch Pathol Lab Med 2017;141:1513)
Prognostic factors
- Associated with 1.5 - 2 times increased risk for subsequent breast cancer (N Engl J Med 2005;353:229, Cancer 2006;107:1240)
- Risk may be slightly higher for patients with a positive family history of breast cancer (Cancer 2006;107:1240)
- Indicator of general breast cancer risk rather than direct precursor lesion
Case reports
- 30 year old woman with immature-like usual ductal hyperplasia in a fibroadenoma (Breast Dis 2016;36:157)
- 75 year old woman with malignant phyllodes tumor with liposarcomatous differentiation and intraductal hyperplasia (Breast Dis 2015;35:59)
- Usual ductal hyperplasia within gynecomastia-like changes of the female breast (Arch Pathol Lab Med 2001;125:506)
Treatment
- No treatment necessary
Gross description
- No macroscopic findings
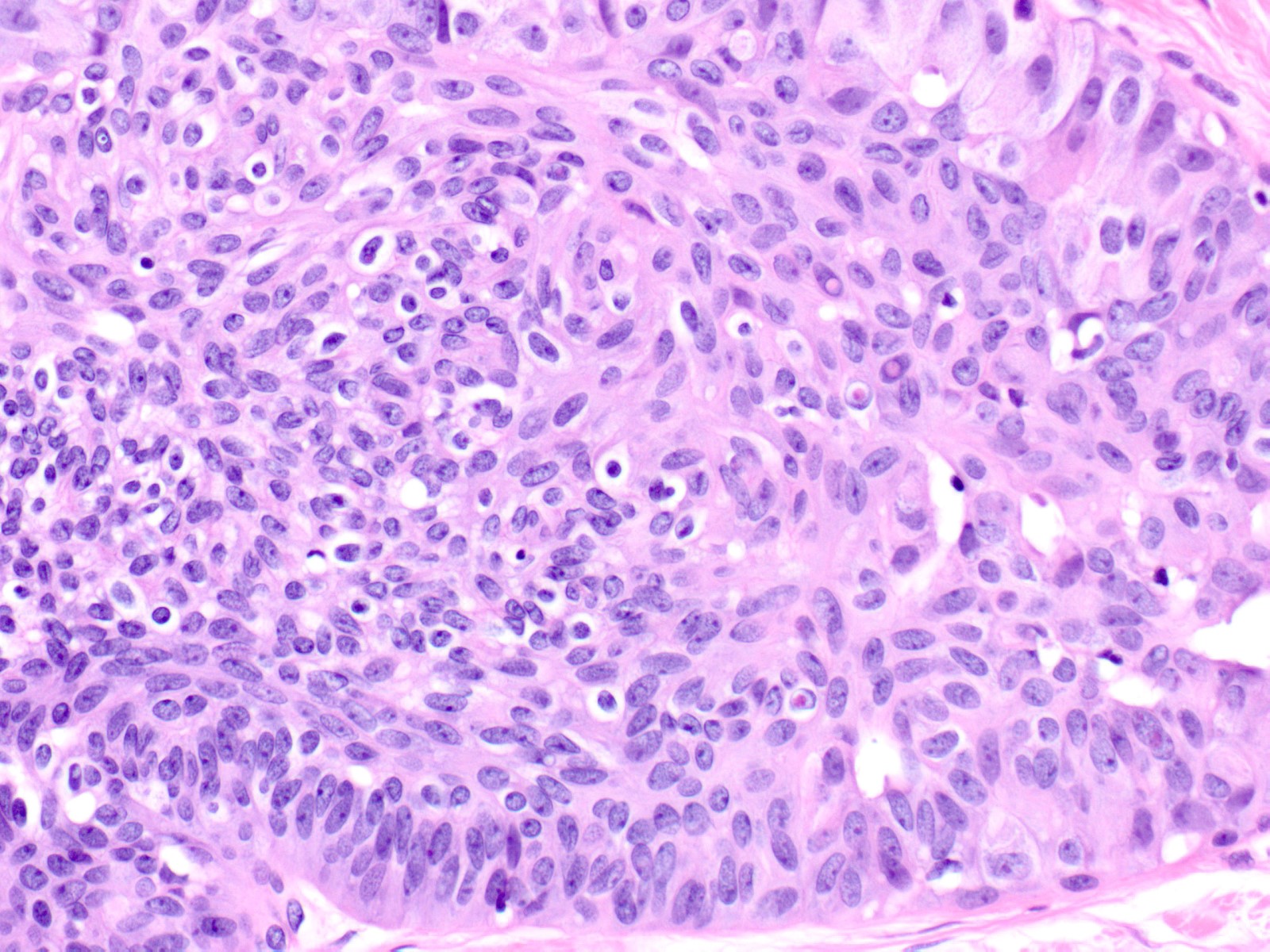
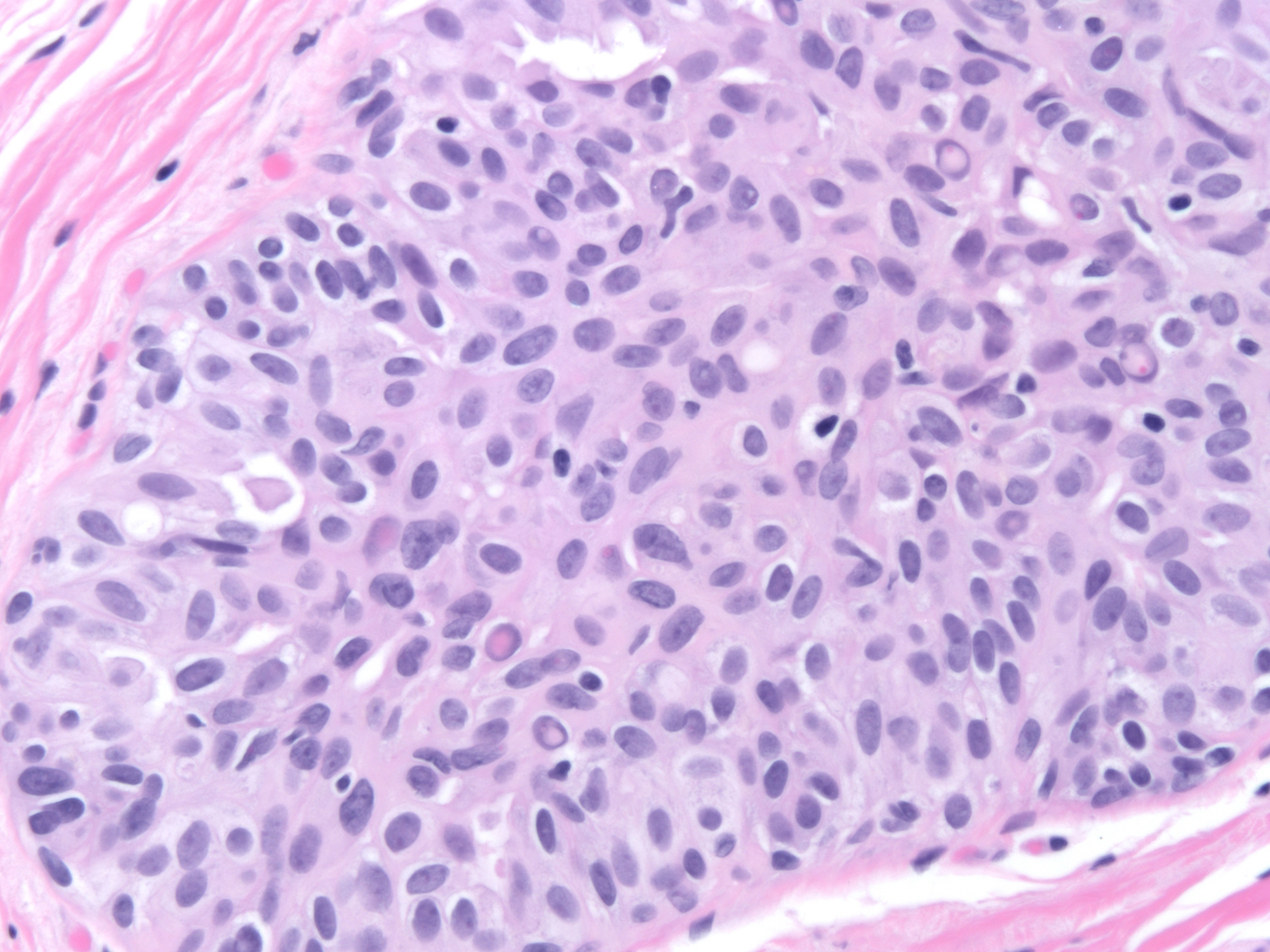
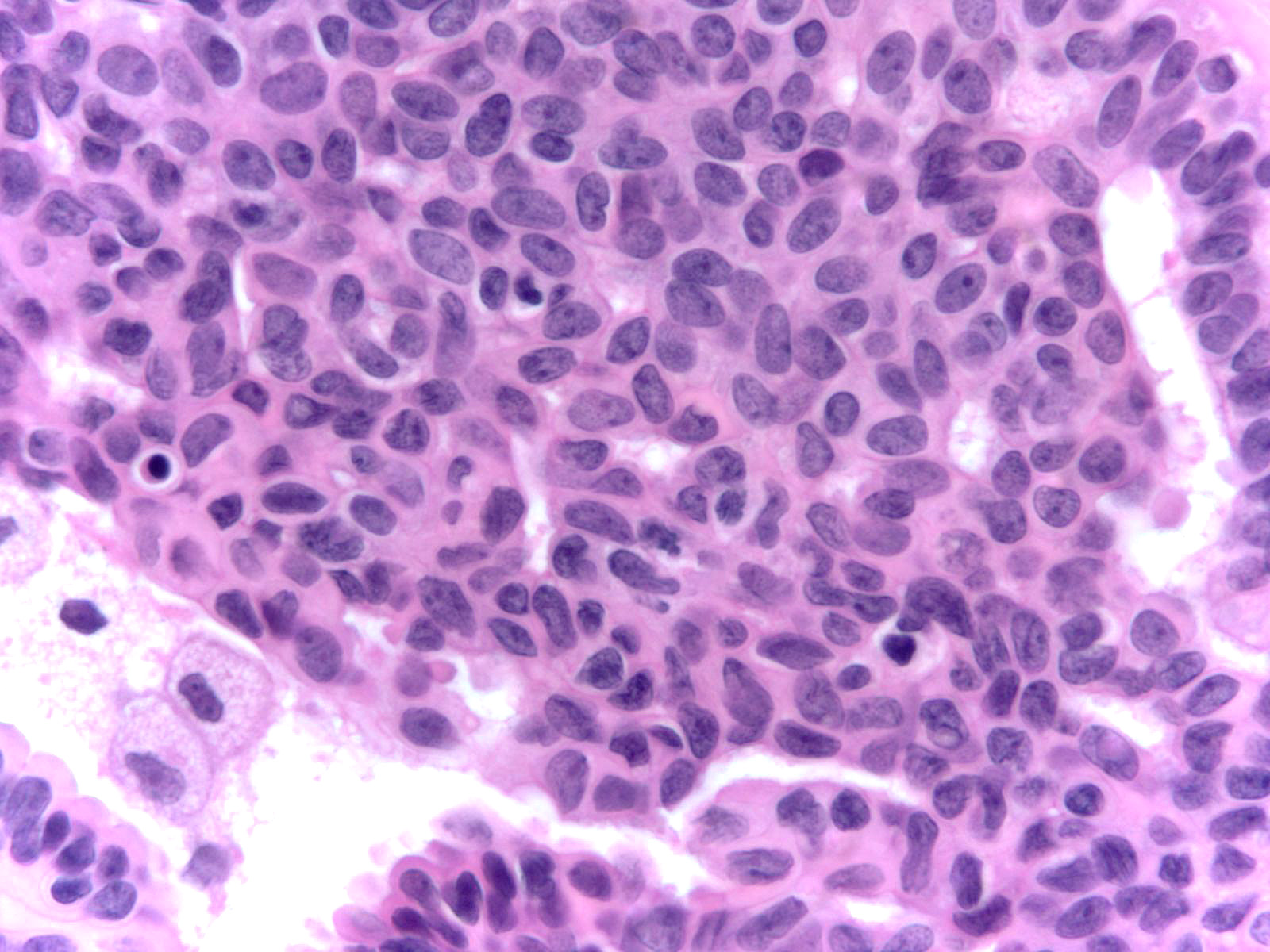
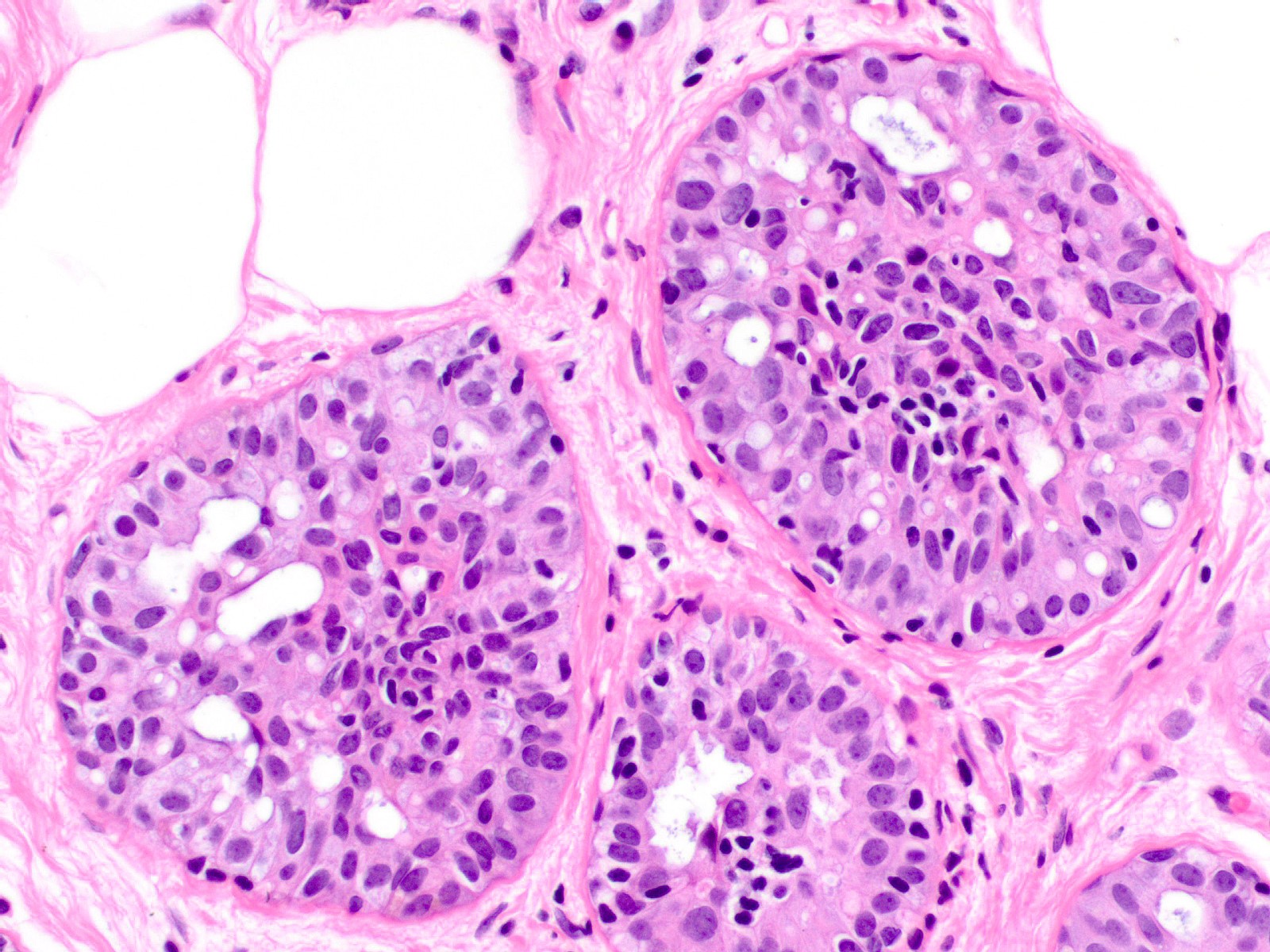
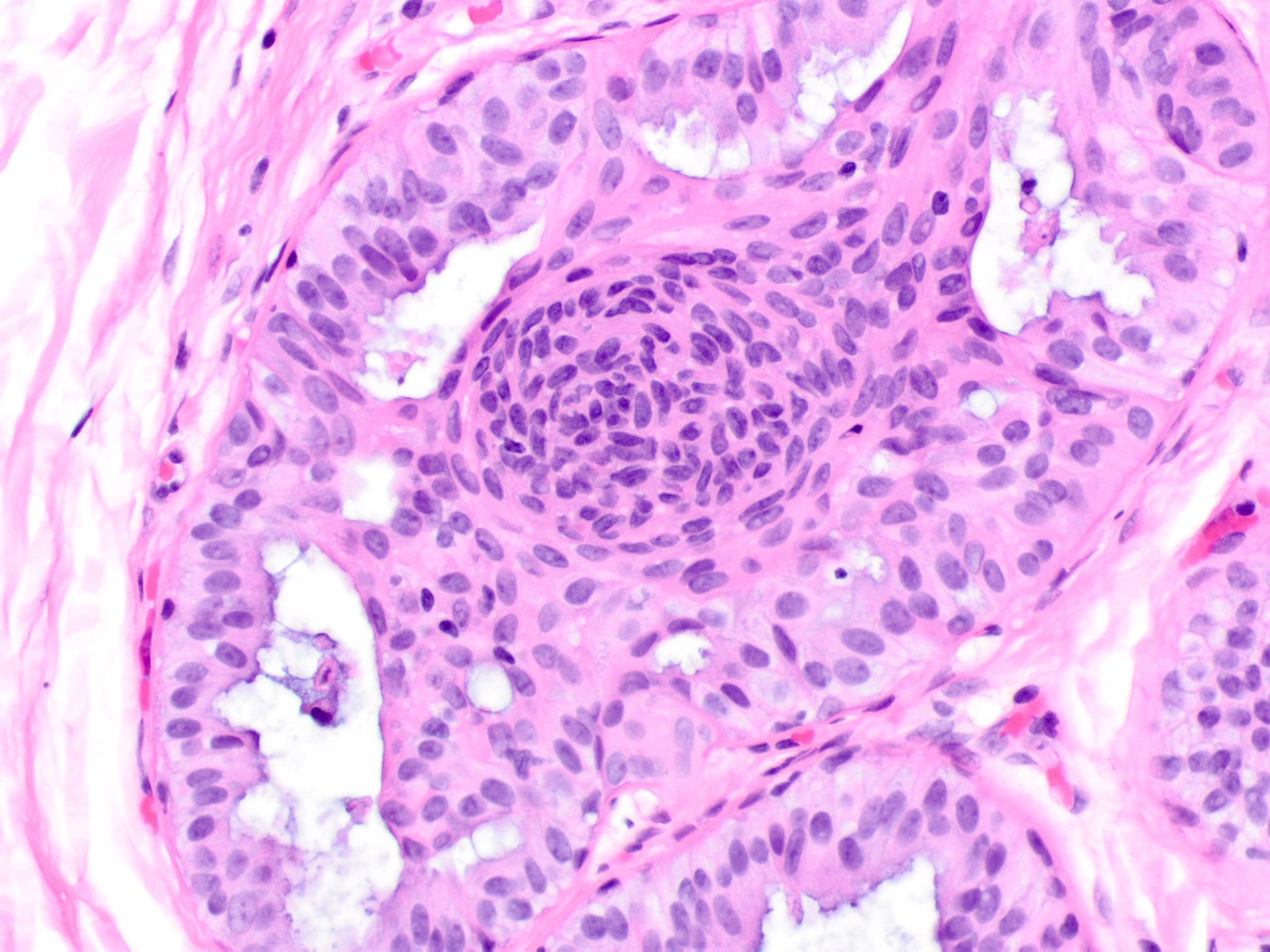
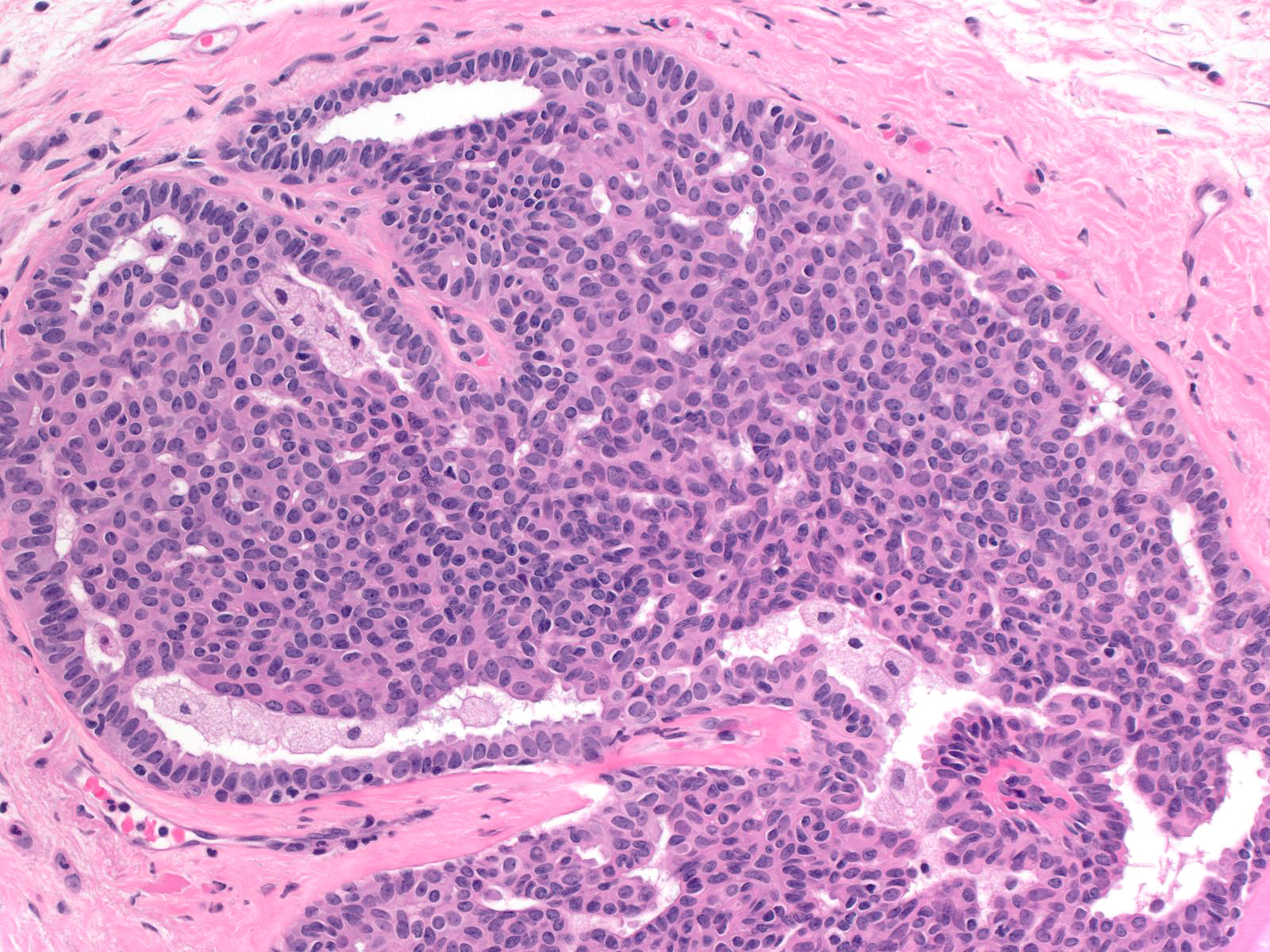
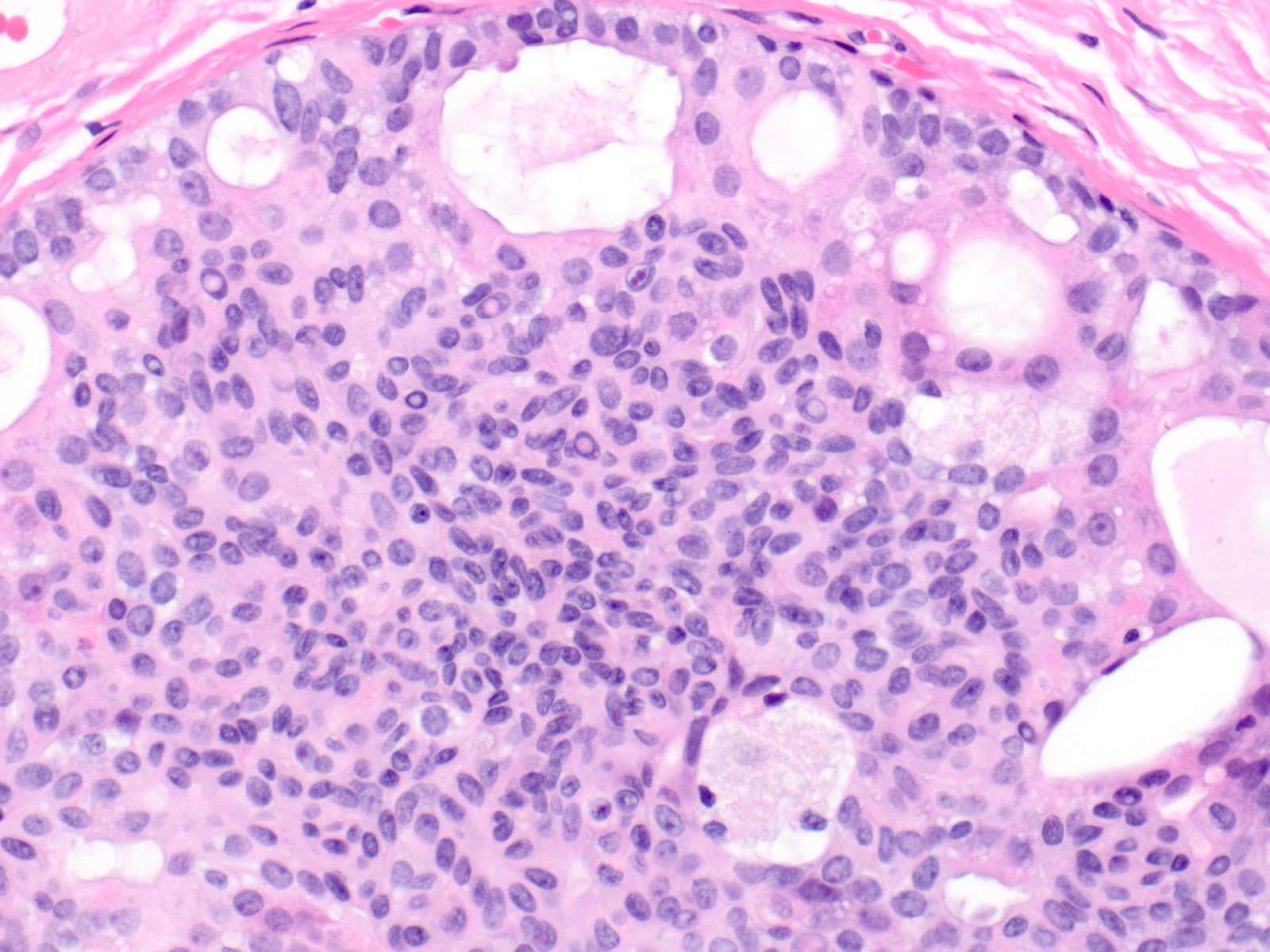
Microscopic (histologic) description
- Proliferation of cells of luminal and myoepithelial lineages, occasionally with intermixed apocrine cells
- Cytologic features (Semin Diagn Pathol 2004;21:10)
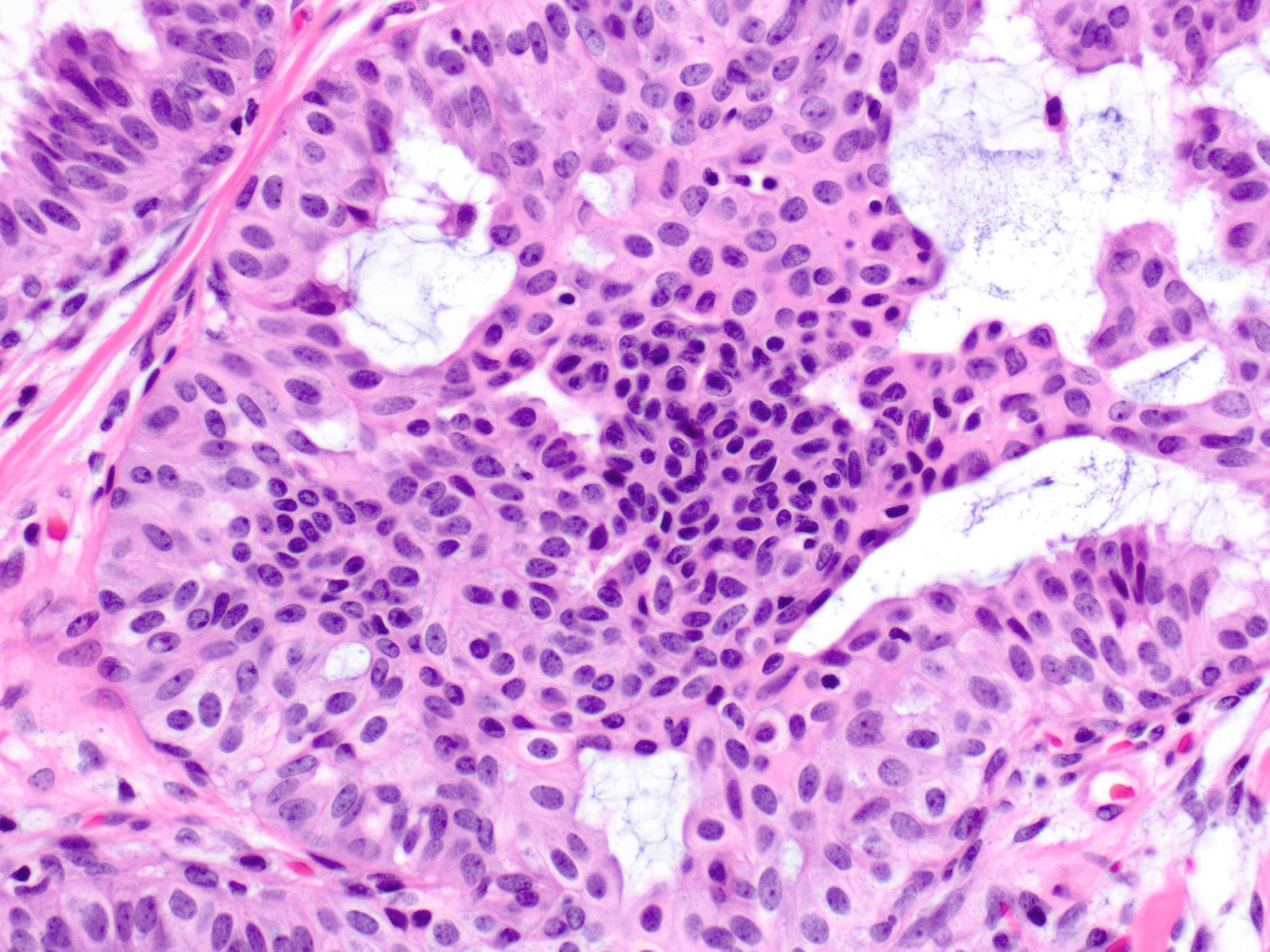
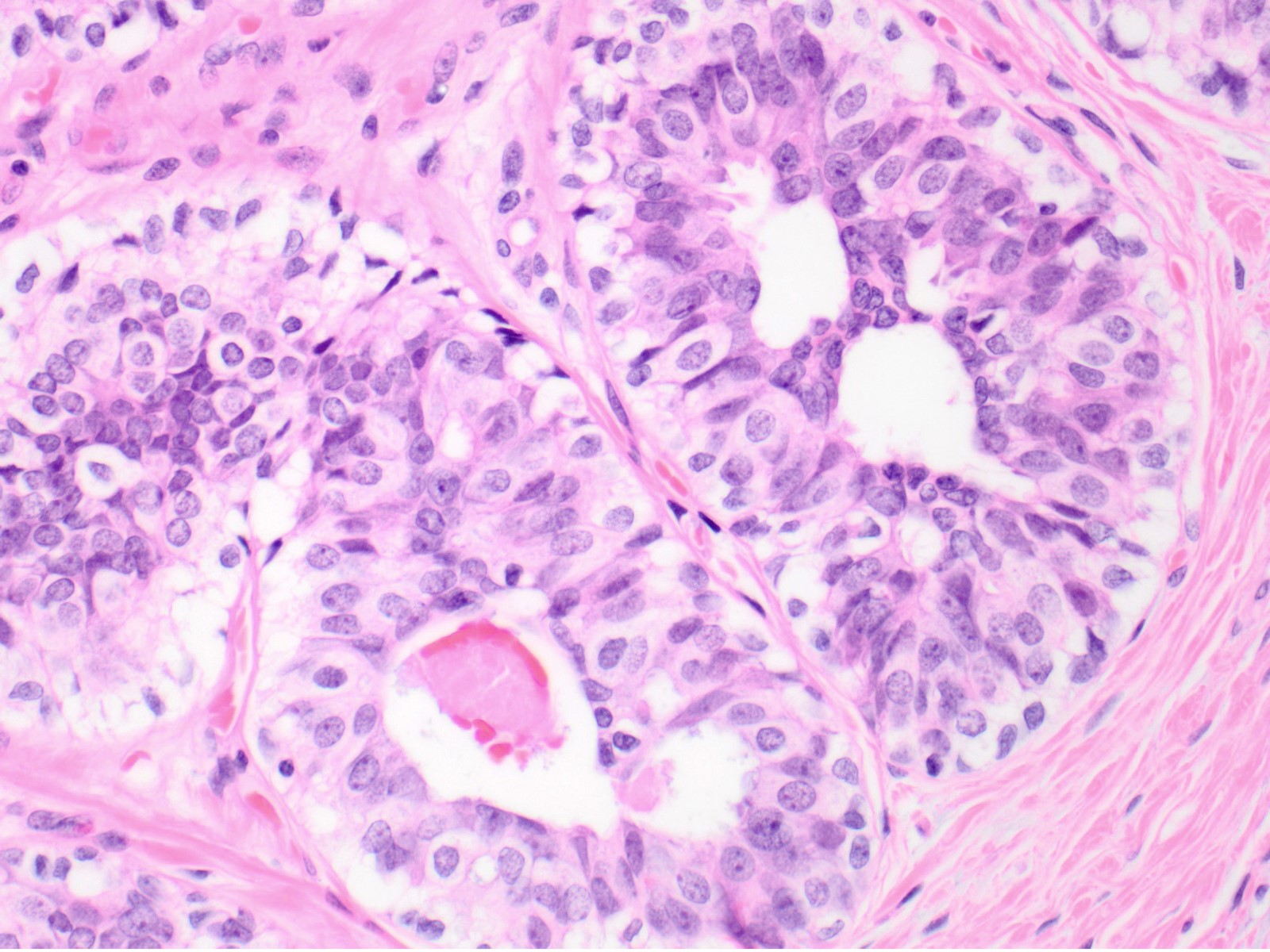
- Mild variation in cellular and nuclear size and shape
- Relatively small ovoid nuclei with frequent elongated or asymmetrically tapered (pear shaped) forms
- Lightly granular euchromatic chromatin and small nucleoli
- Frequent longitudinal nuclear grooves (coffee bean-like) and occasional nuclear pseudoinclusions
- Many examples demonstrate cellular maturation, where the cells shrink as they progress from a basal location to the center of the proliferation, becoming small and nearly pyknotic
- Eosinophilic, nonabundant cytoplasm with indistinct cell borders
- Architectural features (Semin Diagn Pathol 2004;21:10)
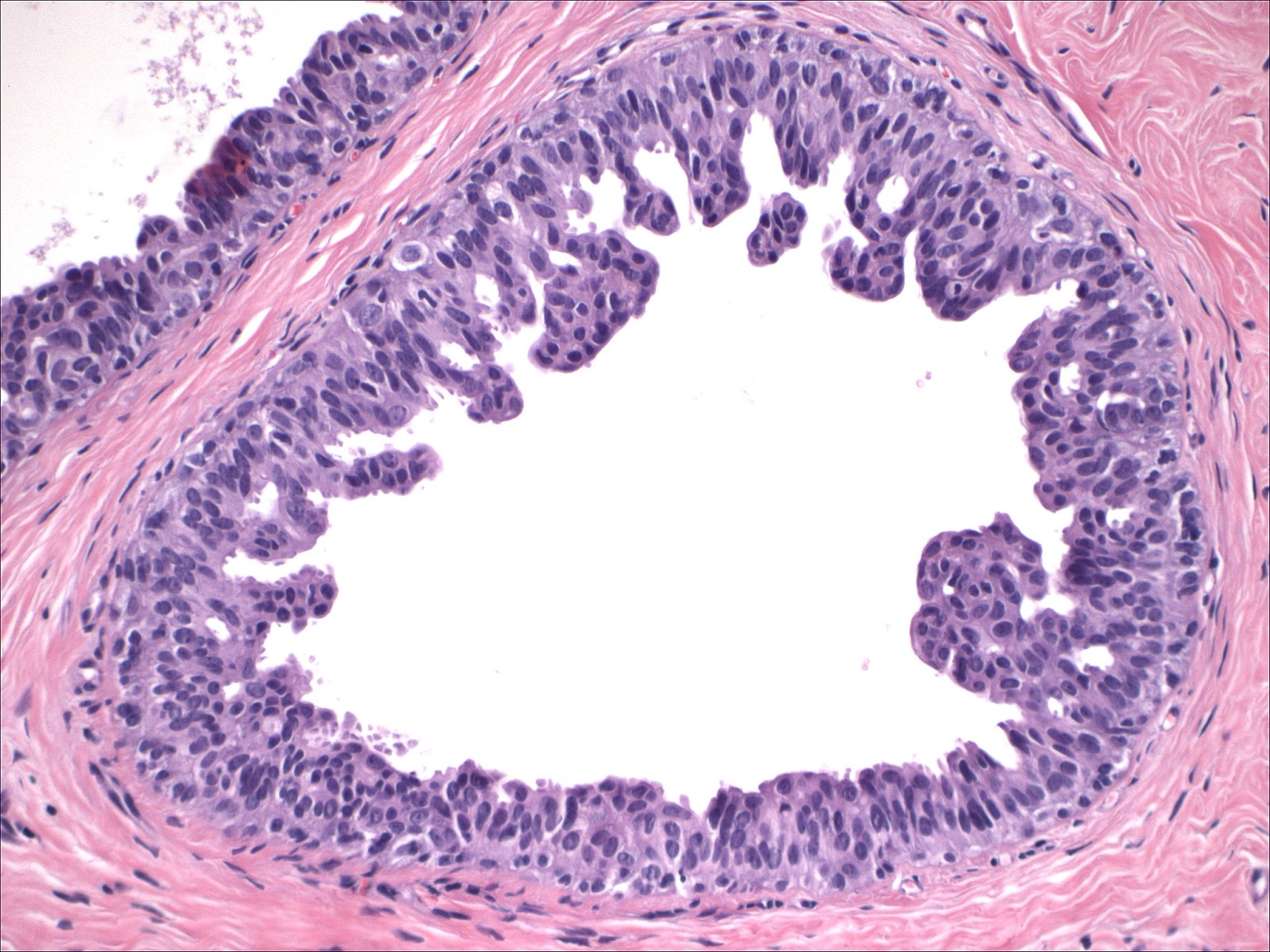
- Cohesive proliferation with haphazard, jumbled cell arrangement or streaming growth pattern
- Fenestrated, solid and occasional micropapillary patterns
- Irregular slit-like fenestrations are common, especially along periphery
- Cells run parallel to the edges of secondary spaces and do not exhibit a polarized orientation (this contrasts with the cells of atypical ductal hyperplasia and ductal carcinoma in situ, which have apical-basal polarity and radially orient their apical poles toward the spaces)
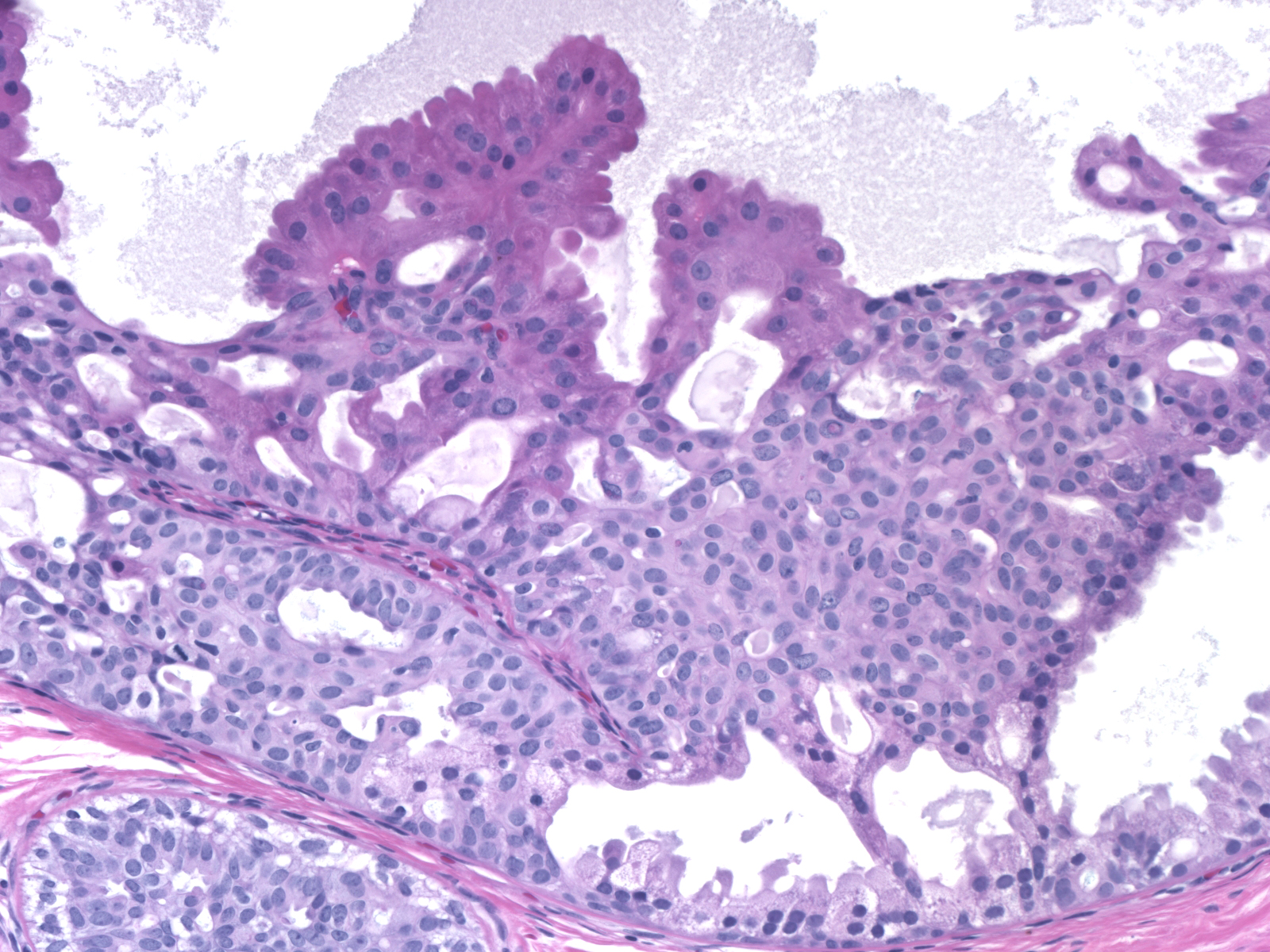
- Variant patterns and features
- Micropapillary
- Typically focal in a background of conventional pattern usual ductal hyperplasia
- Mild duct dilation
- Short stubby papillae of roughly uniform height
- Cytologic features of usual ductal hyperplasia
- Cellular maturation present, with tips of papillae formed by tight knots of mature cells
- Lack of polarization
- Immature
- Uncommon variant
- Larger immature basal hyperplastic cells predominate or are increased beyond their usual 1 - 2 cell layers and are instead several cell layers thick
- Cellular maturation is still present
- Most often encountered in fibroepithelial lesions with cellular stroma
- Necrosis
- Florid usual ductal hyperplasia can rarely demonstrate central necrosis
- Typically occurs within a radial scar / complex sclerosing lesion, nipple adenoma or juvenile papillomatosis
- Mild nuclear enlargement in radial scars
- Florid usual ductal hyperplasia within radial scars / complex sclerosing lesions can occasionally have more active appearing nuclei with mild nuclear enlargement
- Other cytologic and architectural features of usual ductal hyperplasia remain intact
- Micropapillary
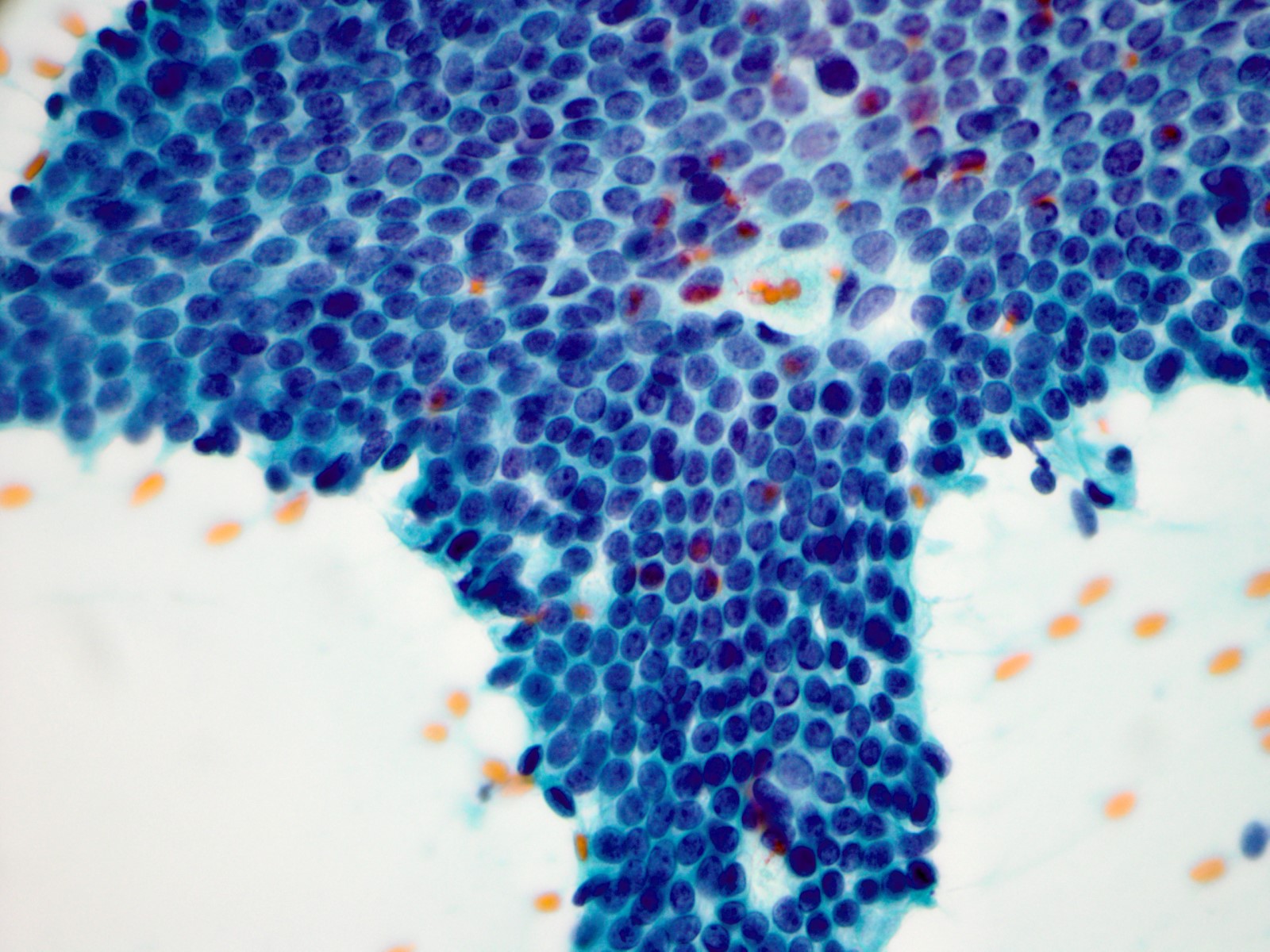
Cytology description
- Sample may be moderately to highly cellular
- Sheets and cohesive clusters of bland ductal cells with regular spacing and associated myoepithelial cells (Am J Clin Pathol 1995;103:438)
- Lack of significant nuclear overlap / crowding
- Ductal cell nuclei with finely granular chromatin and inconspicuous small nucleoli
- Lack of ductal cell discohesion
- Naked myoepithelial cell nuclei in the background may be present
Positive stains
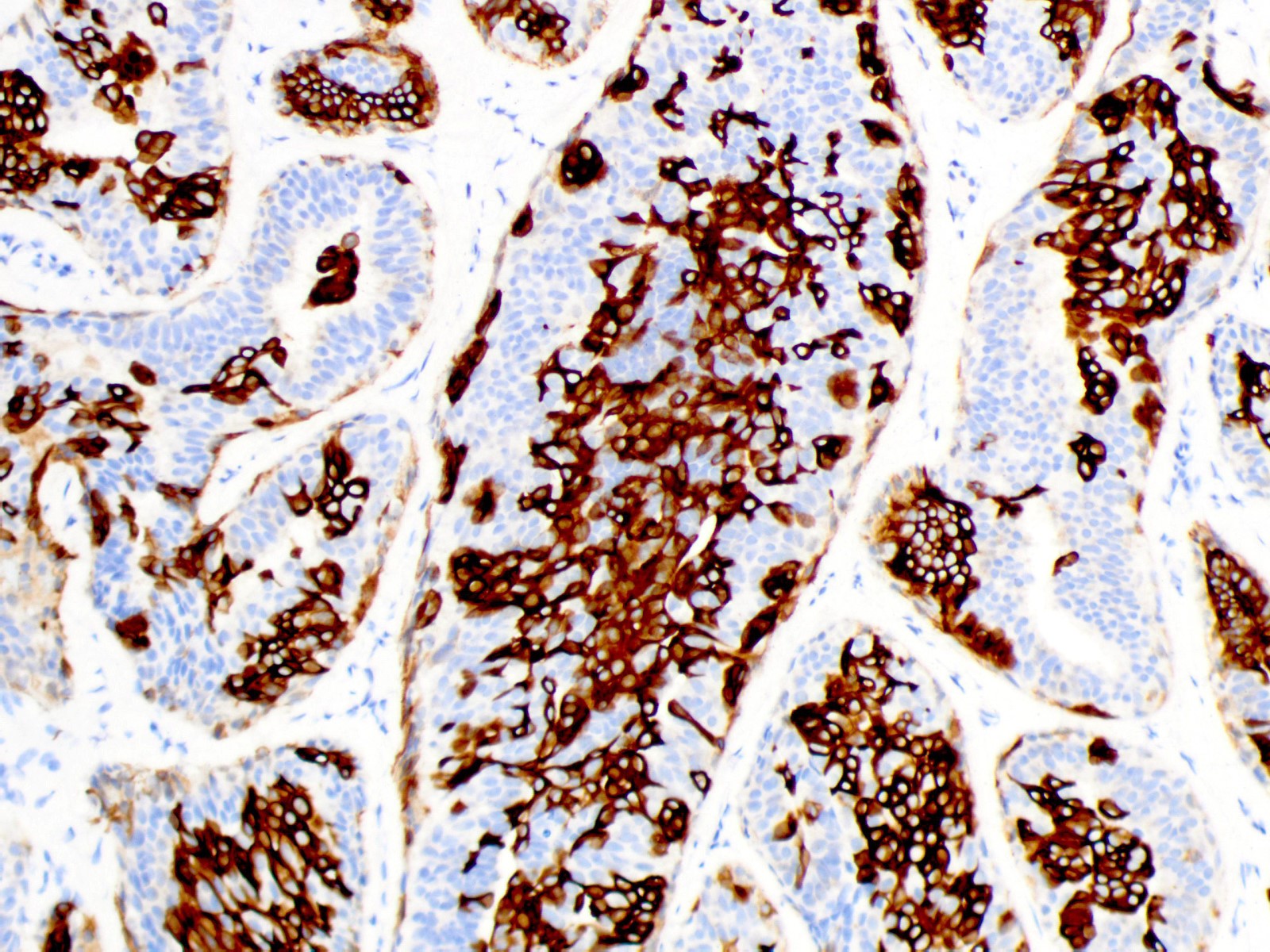
- High molecular weight cytokeratins (e.g. CK5, CK14, 34 beta E12): mosaic to occasionally diffuse pattern of positivity (Histopathology 2000;37:232)
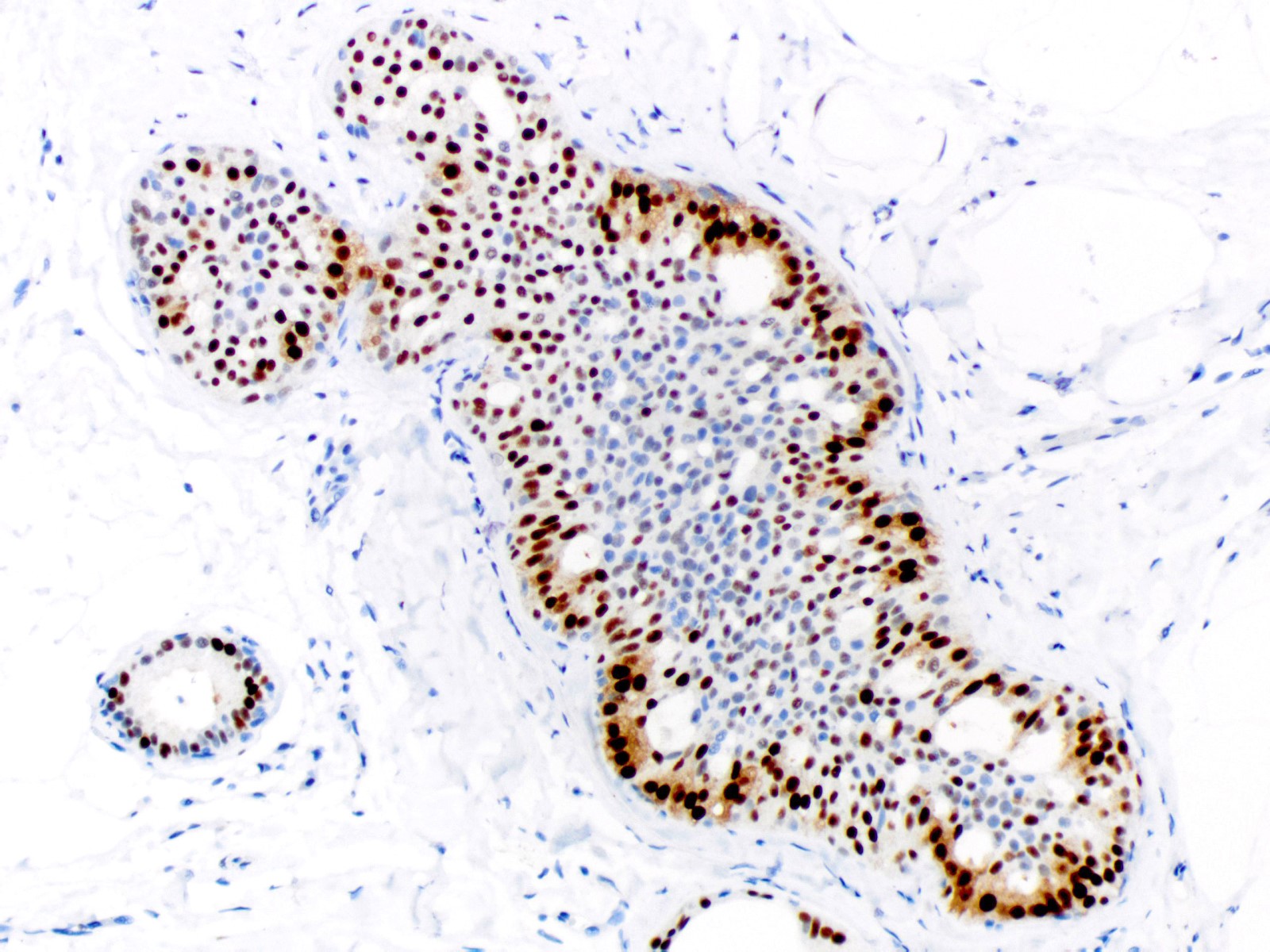
- Estrogen receptor: heterogeneous positivity with variation in staining intensity
- E-cadherin
- Low molecular weight cytokeratins (e.g. CK7, CK8, CK18)
Molecular / cytogenetics description
- Activating mutations in the PI3K / AKT / mTOR pathway may play a role in pathogenesis (Am J Pathol 2016;186:15)
Videos
High yield breast pathology cases
Sample pathology report
- Left breast, needle core biopsy:
- Usual ductal hyperplasia
Differential diagnosis
- Atypical ductal hyperplasia / low grade ductal carcinoma in situ:
- Monomorphic cell population
- Round to oval nuclei with homogeneous, fine and hyperchromatic chromatin; inconspicuous nucleoli; and smooth nuclear contours
- Increased amounts of pale eosinophilic to amphophilic cytoplasm with conspicuous cell borders
- Cellular maturation absent
- Cellular polarization around luminal and secondary spaces
- Atypical architectural patterns formed by polarized growth (cribriform spaces, Roman arches, trabecular bars, micropapillae)
- High molecular weight cytokeratin / CK5 negative and estrogen receptor diffusely positive (Arch Pathol Lab Med 2016;140:686)
- Micropapillary ductal carcinoma in situ:
- Often extensive (Mod Pathol 2010;23:260)
- Moderate to marked duct dilation
- Variation in papillae size and shape
- Elongated, bulbous or complex papillae
- Cellular polarization
- Cytologic atypia
- Lack of maturation
- Frequent luminal cellular debris
- High molecular weight cytokeratin / CK5 negative and estrogen receptor diffusely positive in low and intermediate grade cases
- Intermediate grade ductal carcinoma in situ:
- Populational uniformity (one cell type)
- Moderate nuclear enlargement throughout the proliferation
- Abnormal chromatin, which may be hyperchromatic, cleared and clumped or coarsely granular
- May show marked nuclear irregularity
- Can have red nucleoli
- Polarized architectural atypia
- Necrosis may be present
- Usually high molecular weight cytokeratin / CK5 negative
- Estrogen receptor often positive but more variability in degree of staining than in low grade ductal carcinoma in situ
- Uncommon basal-like examples may have a high molecular weight cytokeratin / CK5 positive and estrogen receptor negative phenotype (Breast Cancer Res 2008;10:R67)
- Solid papillary carcinoma:
- Solid epithelial proliferation showing marked expansion of multiple circumscribed duct spaces (Arch Pathol Lab Med 2012;136:1308)
- Thin fibrovascular cores punctuate the proliferation, with cellular palisading around the cores
- Myoepithelial cells often sparse or absent along fibrovascular cores
- Nuclei may superficially resemble those in usual ductal hyperplasia but demonstrate greater populational uniformity, are slightly larger and have abnormal chromatin
- Granular cytoplasm may be present
- Intra or extracellular mucin common
- High molecular weight cytokeratin / CK5 negative and estrogen receptor diffusely positive
- Often positive for neuroendocrine markers (synaptophysin or chromogranin)
Practice question #1
Practice answer #1
A. 1.5 - 2 times increased risk
This is usual ductal hyperplasia. Usual ductal hyperplasia is associated with a slight increase in risk (1.5 - 2 times) for subsequent breast cancer. Risk appears to be slightly higher in those patients with a positive family history of breast cancer.
Comment Here
Reference: Usual ductal hyperplasia
This is usual ductal hyperplasia. Usual ductal hyperplasia is associated with a slight increase in risk (1.5 - 2 times) for subsequent breast cancer. Risk appears to be slightly higher in those patients with a positive family history of breast cancer.
Comment Here
Reference: Usual ductal hyperplasia
Practice question #2
- What is the typical high molecular weight cytokeratin / estrogen receptor (HWMCK / ER) immunoprofile for usual ductal hyperplasia of the breast?
- HMWCK negative / ER diffusely positive
- HMWCK negative / ER negative
- HMWCK mosaic positive / ER diffusely positive
- HMWCK mosaic positive / ER heterogeneously positive
- HMWCK mosaic positive / ER negative
Practice answer #2
D. HMWCK mosaic positive / ER heterogeneously positive
Usual ductal hyperplasia is positive for HMWCK in a mosaic to occasionally diffuse pattern and demonstrates heterogeneous positivity for ER.
Comment Here
Reference: Usual ductal hyperplasia
Usual ductal hyperplasia is positive for HMWCK in a mosaic to occasionally diffuse pattern and demonstrates heterogeneous positivity for ER.
Comment Here
Reference: Usual ductal hyperplasia
Practice question #3
- Which of the following is a feature of usual ductal hyperplasia that aids in distinguishing it from low grade ductal carcinoma in situ?
- Cellular maturation
- Monomorphic hyperchromatic nuclei
- Palisading around fibrovascular cores
- Polarization around secondary spaces
- Red macronucleoli
Practice answer #3
A. Cellular maturation
Many examples of usual ductal hyperplasia demonstrate cellular maturation, where the cells shrink as they progress from a basal location to the center of the proliferation, becoming small and nearly pyknotic. Cellular maturation is not a feature of low grade ductal carcinoma in situ or atypical ductal hyperplasia.
Comment Here
Reference: Usual ductal hyperplasia
Many examples of usual ductal hyperplasia demonstrate cellular maturation, where the cells shrink as they progress from a basal location to the center of the proliferation, becoming small and nearly pyknotic. Cellular maturation is not a feature of low grade ductal carcinoma in situ or atypical ductal hyperplasia.
Comment Here
Reference: Usual ductal hyperplasia