Table of Contents
Definition / general | Essential features | Terminology | Diagrams / tables | Quality management principles | Quality management system model | Implementing quality management system | Quality toolbox | Goals and benefits of quality management systems | Disaster preparedness in the laboratory | Videos | Additional references | Practice question #1 | Practice answer #1 | Practice question #2 | Practice answer #2Cite this page: Nguyen HD, Hassell LA. Quality management systems. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/managementlabqualitymanagement.html. Accessed August 6th, 2025.
Definition / general
- A quality management system (QMS) can be defined as coordinated activities to direct and control an organization with regard to quality (WHO: Laboratory Quality Management System - Handbook [Accessed 10 July 2023])
- QMS helps coordinate and direct an organization's activities to meet customer and regulatory requirements and improve its effectiveness and efficiency on a continuous basis
Essential features
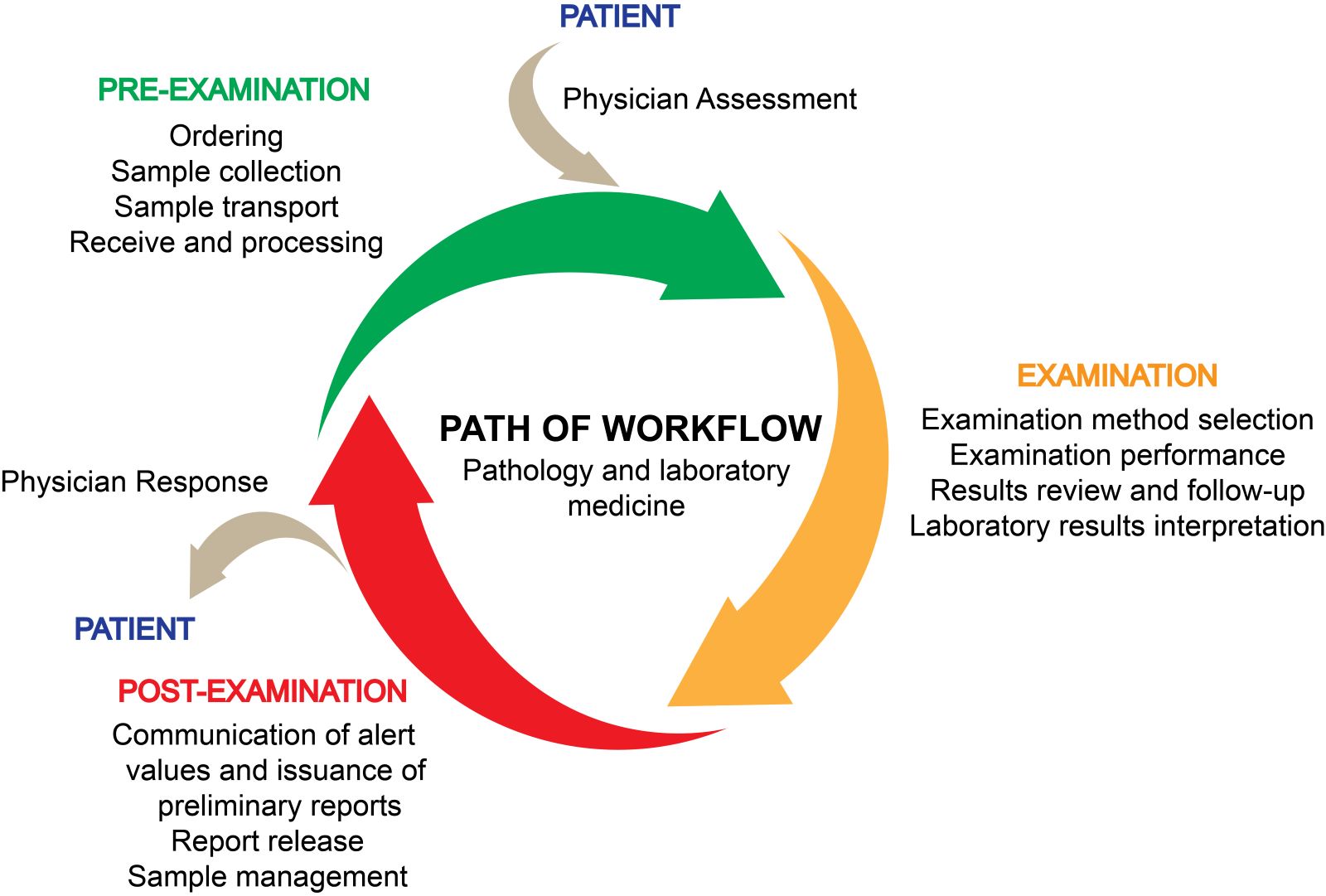
- Concept of the path of workflow is key to the QMS
- 7 quality management principles form the basis for the QMS standards
- 12 quality system essentials (QSEs) represent the most fundamental elements of the QMS model
- Implementing the QMS in all laboratory processes based on
- 12 QSEs
- 10 clauses of the ISO 9001:2015 standard
- Plan, do, check, act (PDCA) cycle
- Several tools, techniques and methods can be used individually or in combination to ensure consistent quality management and continual improvement
- QMS is designed to meet specific goals and offer numerous benefits to the laboratory
- Primary objective of QMS is to improve patient safety, satisfaction and outcome
- Laboratory is always prepared to respond effectively to a disaster
Terminology
- International Organization for Standardization (ISO): a widely recognized and respected international standard setting organization that develops and publishes standards in quality management
- Laboratory QMS is applicable to 2 ISO standards
- ISO 15189: medical laboratories - requirements of quality and compliance (Clin Biochem 2009;42:274)
- ISO 17025: testing and calibration laboratories (J AOAC Int 2003;86:1038)
- Laboratory QMS is applicable to 2 ISO standards
- Quality management (QM): the assembly and management of all activities aimed at the production of quality by organizations of various kinds
- Quality control (QC): the process of monitoring and verifying that a product or service meets the specified quality requirements
- Quality improvement (QI): the framework used to systematically improve processes and systems
- Quality assurance: a systematic approach to ensuring that a product or service meets specified quality requirements
- Customer satisfaction: the degree to which a product or service meets or exceeds a customer's expectations
- Process improvement: a process of making changes to a process or system to increase its effectiveness, efficiency or quality (Clin Microbiol Rev 2018;31:e00062)
- Plan, do, check, act (PDCA): a cyclical model for continual quality improvement
- Audit: a systematic and independent examination of an organization to determine whether it conforms to specified requirements
- Quality toolbox: a collection of tools and techniques used in quality management
- Root cause analysis (RCA): a set of problem solving tools targeted at identifying the true root cause for a nonconformity
- Failure mode and effects analysis (FMEA): the prospective risk analysis of high risk processes to identify needed improvements that will reduce the chance of an unintended adverse event
- Lean management: a methodology that aims to optimize processes and reduce waste by eliminating nonvalue added activities
- Six Sigma: a quality management approach that focuses on identifying and eliminating defects in a process, resulting in improved quality and efficiency
- Define, measure, analyze, improve, control (DMAIC): Six Sigma model for continual quality improvement
- External quality assessment (EQA) or proficiency testing: a method used to evaluate the performance of a laboratory's testing processes by comparing its results to those of other laboratories
- External quality assessment program: a provider sends a set of blinded samples to participating laboratories, which are then analyzed and the results are reported back to the provider for analysis
- Quality dashboards: a type of health information technology that uses data visualization techniques to support clinicians and managers in viewing and exploring data on processes and outcomes of care
- 5 whys: a simple technique that involves asking why multiple times in order to identify the root cause of a problem
- Fault tree analysis (FTA): a graphical tool used to identify the various events or conditions that can lead to a particular problem
- Brainstorming: a group technique used to generate a large number of ideas and potential causes for a problem
- Design of experiments (DOE): a statistical technique used to determine the relationship between multiple variables in a process and the output of that process
- Value stream mapping: a lean management technique to identify and eliminate waste in laboratory processes, reduce turnaround times and enhance the quality of test results
- 5S: a lean management technique that involves sorting, simplifying, sweeping, standardizing and sustaining work areas to reduce waste and improve efficiency
- Kaizen: a continuous improvement approach to improve laboratory processes, reduce errors and enhance the quality of test results
- Standardization: the process of establishing a set of guidelines, procedures or best practices to improve efficiency, reduce errors and ensure consistency in processes and outcomes
- Visual management: a lean management technique that uses visual aids, such as signs, symbols, charts and graphs, to communicate information and improve the efficiency of processes
- Clinical and Laboratory Standards Institute (CLSI): a nonprofit organization whose goal is to develop quality assurance standards for the operation of various types of laboratories
- World Health Organization (WHO): a specialized agency of the United Nations responsible for international public health
Diagrams / tables
Quality management principles
- There are 7 QM principles based on ISO 9001:2015 (Cell Tissue Bank 2020;21:563)
- Customer focus
- Leadership
- Engagement of people
- Process approach
- Improvement
- Evidence based decision making
- Relationship management
- These 7 quality management principles form the basis for the QMS standards
(ISO: Quality Management Principles [Accessed 10 July 2023])
- Used by top management in order to lead the organization toward improved performance
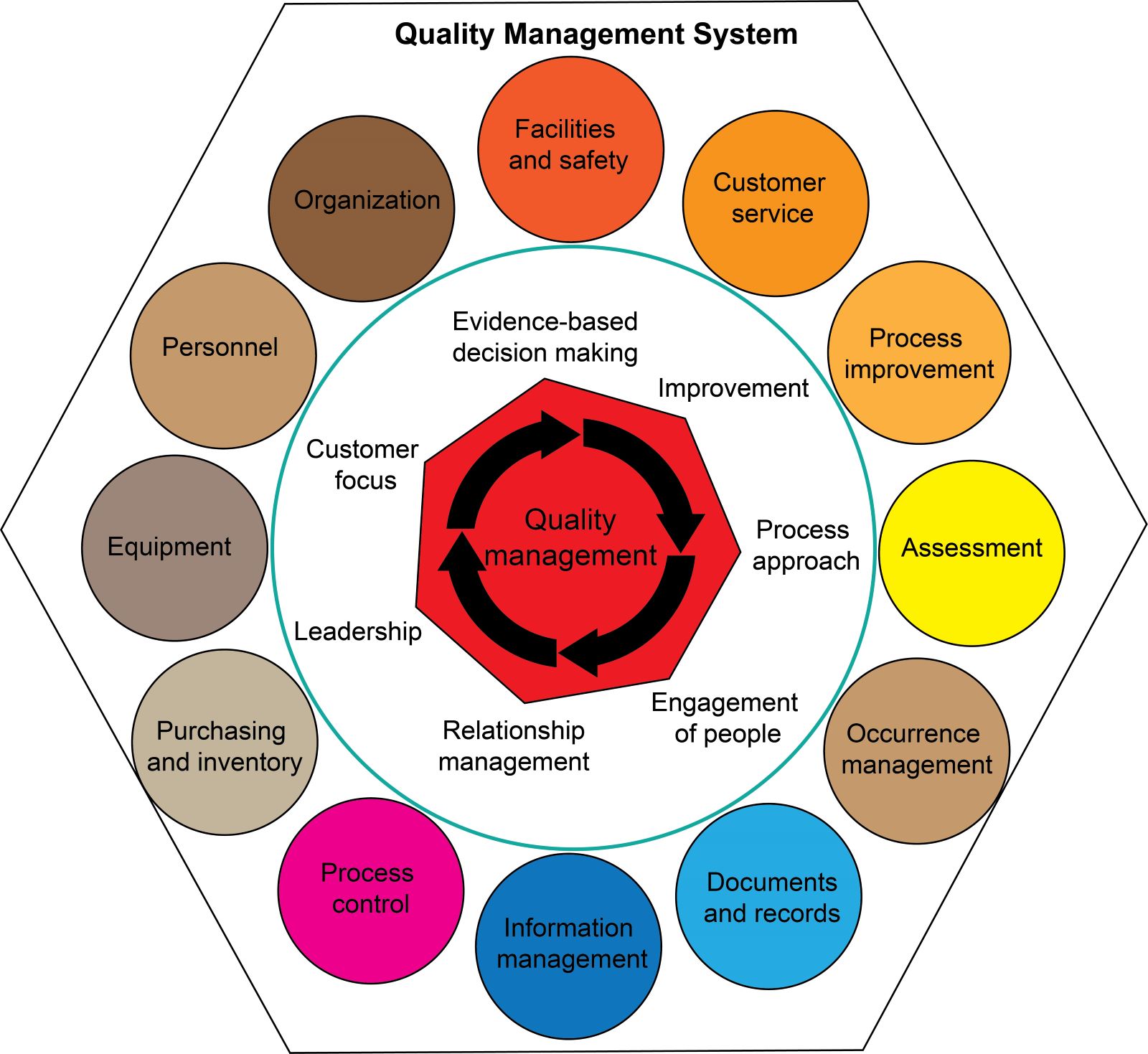
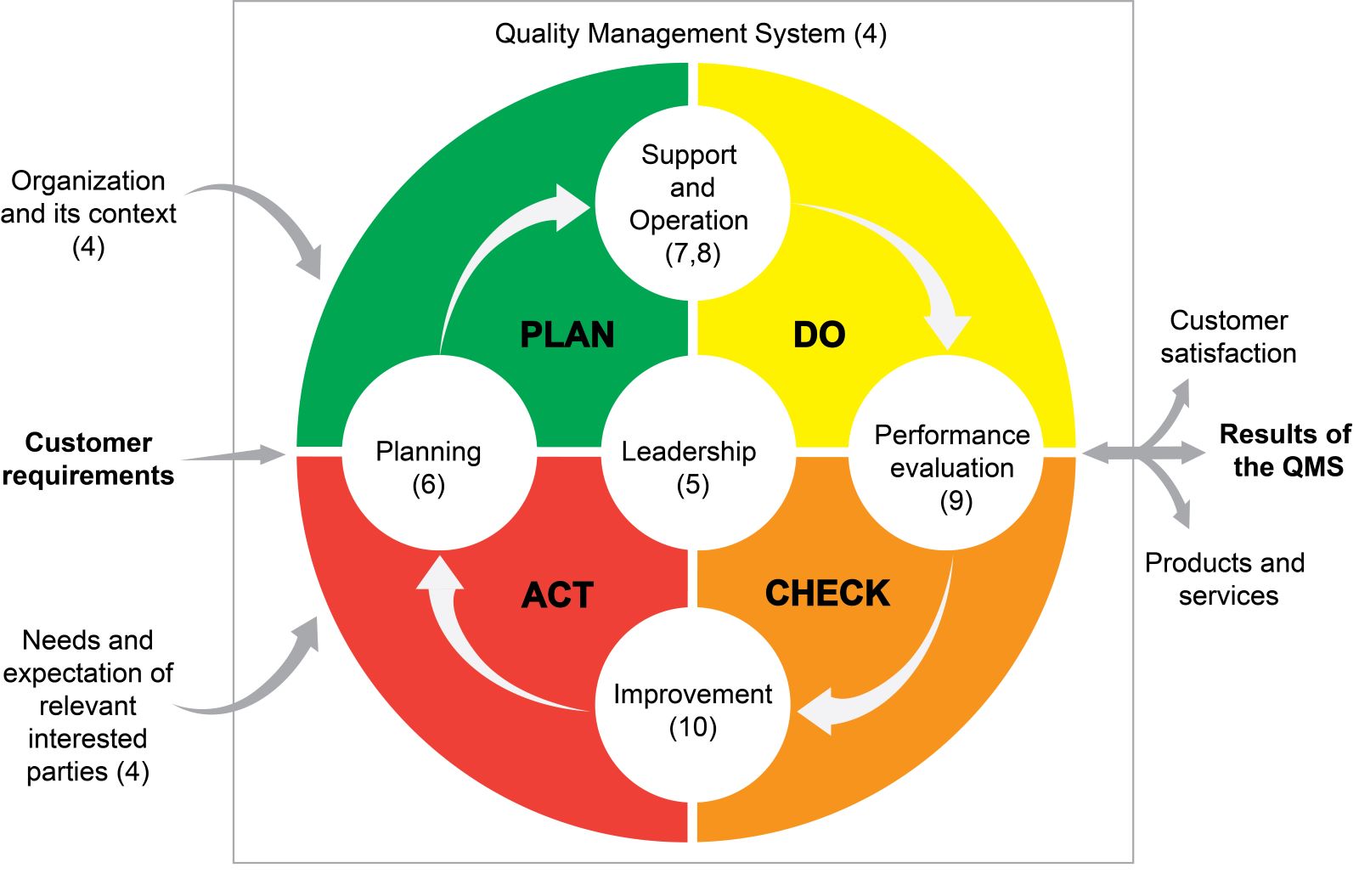
Quality management system model
- The QMS model was developed by CLSI and is fully compatible with ISO standards
- Concept of the path of workflow is key to the QMS; it begins with the patient and ends with reporting and result interpretation (see Diagram 3)
- QMS model used here organizes all of the laboratory activities into 12 QSEs
- Organization
- Personnel
- Equipment
- Purchasing and inventory
- Process control
- Information management
- Documents and records
- Occurrence management
- Assessment
- Process improvement
- Customer service
- Facilities and safety
- 12 QSEs represent the most fundamental elements for maintaining quality, safety and efficiency throughout the laboratory's path of workflow (see Diagram 4)
- Diagram 1 provides a comprehensive framework for the development and implementation of an effective QMS in healthcare organizations
- 12 QSEs provide specific guidance on how to achieve the principles in practice
- 7 QM principles provide a broad framework for the development and implementation of a QMS
- Table below describes brief instructions for all aspects of a QMS; for detailed and specific instructions, please see the laboratory quality management system handbook by WHO (WHO: Laboratory Quality Management System - Handbook [Accessed 10 July 2023])
| 12 QSEs | Brief instructions |
| Documents and records |
|
| Organizational structure |
|
| Personnel |
|
| Equipment |
|
| Purchasing and inventory |
|
| Process control |
|
| Information management |
|
| Occurrence management |
|
| Assessment |
|
| Process improvement |
|
| Customer service |
|
| Facilities and safety |
|
| Reference: WHO: Laboratory Quality Management System - Handbook [Accessed 10 July 2023] | |
Implementing quality management system
- 12 QSEs provide a framework for implementing a laboratory QMS
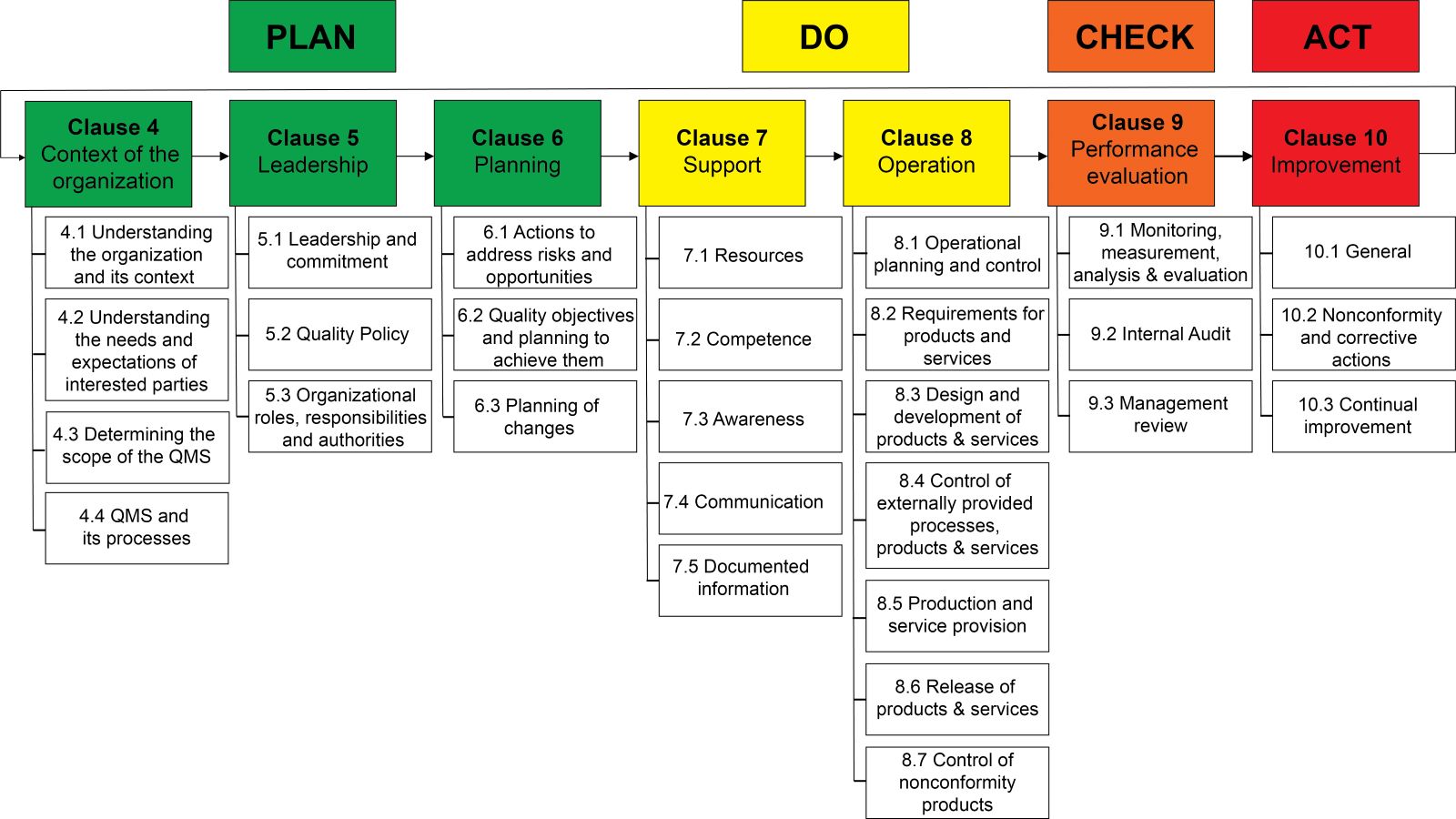
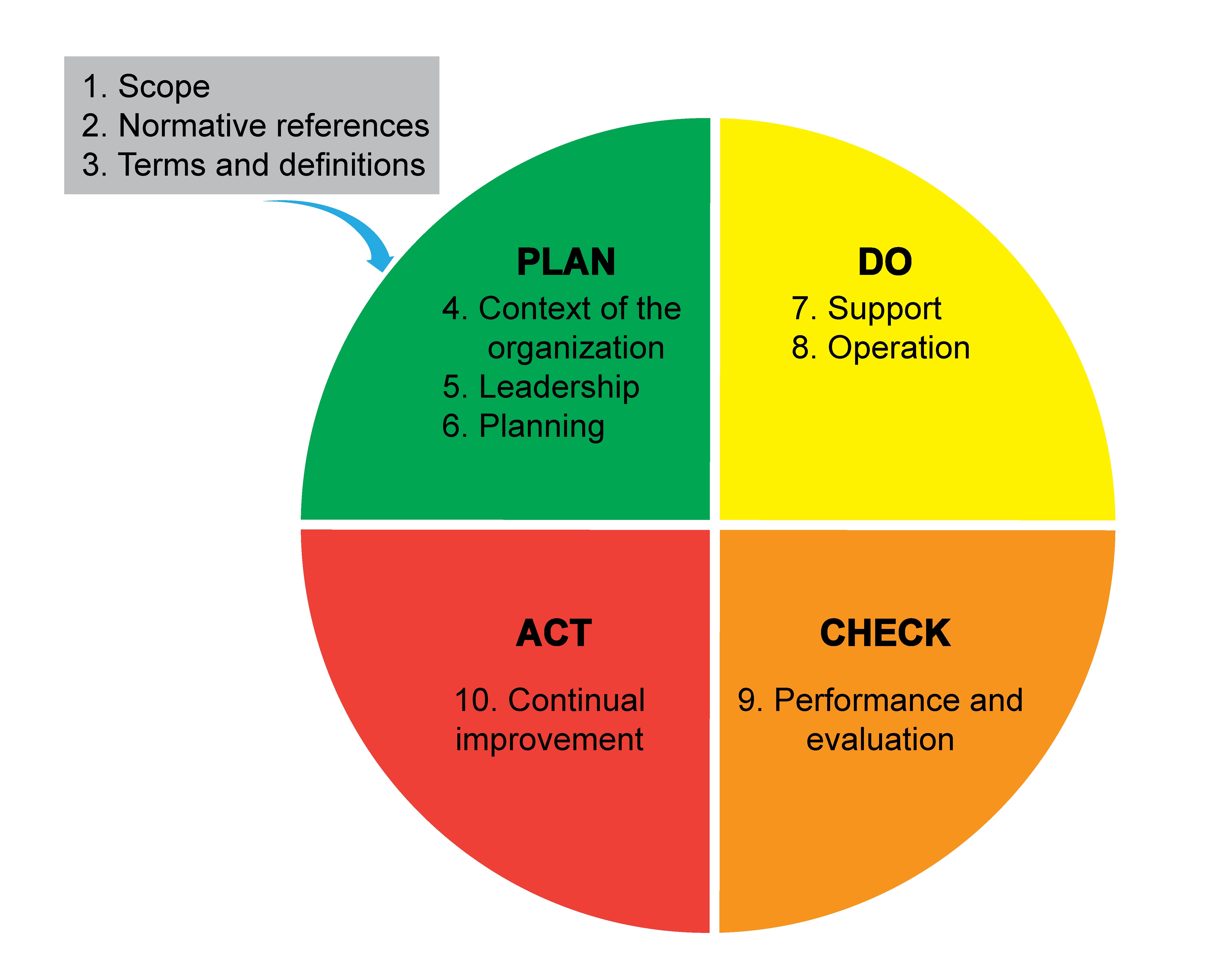
- 10 clauses of the ISO 9001:2015 standard provide a comprehensive framework for QM (Ann Ig 2022;34:627)
- Reflecting on the PDCA cycle and covering all aspects of a QMS
- Diagrams 5 and 6 illustrate the PDCA cycle in the ISO 9001:2015 structure
- Diagram 2 shows the contents of the PDCA division according to the ISO 9001:2015 structure
- Helping organizations implement and maintain effective QMS
- Providing guidance on how to establish, implement, maintain and continually improve a QMS
- Reflecting on the PDCA cycle and covering all aspects of a QMS
- Some key steps to follow (Indian J Med Microbiol 2020;38:243, Afr J Lab Med 2017;6:490)
- Developing a quality policy
- Defining laboratory processes
- Conducting a gap analysis
- Developing a quality manual
- Establishing document control procedures
- Implementing quality control procedures
- Developing a training program
- Conducting competency assessments
- Establishing management review processes
- Seeking accreditation
- PDCA cycle can be briefly described as follows
- Plan: establish the objectives of the system and its processes
- Do: implement the processes defined in the plan stage
- Check: monitor and measure the effectiveness of the implemented processes
- Act: take actions to continually improve the QMS
- Continual improvement: start the cycle again by planning further improvements based on the results achieved
Quality toolbox
- Statistical process control (SPC) chart (Qual Saf Health Care 2007;16:387)
- Graphical tools used to monitor, control and improve processes by displaying process data over time
- There are several types of SPC charts but the most common ones are
- Control charts for variables: measure continuous variables
- Control charts for attributes: measure categorical or binary data
- Control charts for count data: measure count data
- Identifying a process that is out of control or is trending towards an out of control condition
- Moving average quality control (Biochem Med (Zagreb) 2022;32:010705)
- Detecting changes in the central tendency of the process over time
- 5 steps
- Determining the sample size and time period
- Collecting data
- Calculating the moving average
- Plotting the moving average on a control chart
- Monitoring the control chart
- Identifying shifts or trends that may indicate a need for corrective action
- Flow chart or process flow diagram (BMC Health Serv Res 2021;21:342)
- A useful tool for documenting, analyzing and improving laboratory processes in quality control
- Documenting laboratory procedures
- Identifying potential sources of error
- Standardizing laboratory processes
- Communicating laboratory processes to stakeholders
- Facilitating continuous improvement
- A useful tool for documenting, analyzing and improving laboratory processes in quality control
- Root cause analysis (J Clin Diagn Res 2014;8:FC05, StatPearls: Root Cause Analysis and Medical Error Prevention [Accessed 10 July 2023])
- A problem solving method to investigate and resolve issues that may arise in laboratory processes, procedures or test results
- Some key steps in the root cause analysis process
- Defining the problem
- Identifying possible causes
- Determining the root cause
- Developing corrective actions
- Implementing corrective actions
- Verifying the solution
- Some common tools and techniques used in root cause analysis include fishbone diagrams, Pareto charts, process flow diagrams, 5 whys, fault tree analysis, failure mode and effects analysis, brainstorming and statistical analysis
- Failure mode and effects analysis (J Am Coll Radiol 2014;11:572)
- A systematic approach to identifying and preventing potential failures in laboratory processes
- Typically conducted in 3 stages
- Identification of potential failures
- Assessment of potential failures
- Mitigation of potential failures
- Design of experiments (Eur J Pharm Biopharm 2021;166:144)
- Optimizing laboratory processes, improving the accuracy and precision of laboratory test results and reducing the time required to perform tests
- Involves several steps, including
- Identifying the process to be improved
- Identifying the factors that may affect the process output
- Designing the experiment to test the effects of these factors on the process output
- Conducting the experiment and collecting data
- Analyzing the data to determine the effects of the factors on the process output
- Optimizing the process by identifying the optimal settings for the factors that have the most significant impact on the process output
- Lean management (J Clin Lab Anal 2018;32:e22180)
- Principles of Lean are applied to laboratory quality management to optimize processes, reduce errors and enhance patient care
- Some of the ways Lean principles are applied to laboratory quality management include
- Value stream mapping
- Kaizen
- Standardization
- 5S
- Visual management
- Six Sigma (J Clin Lab Anal 2018;32:e22180, J Clin Lab Anal 2021;35:e24041, J Clin Lab Anal 2020;34:e23126)
- A data driven approach to quality management that seeks to minimize defects and variability in processes
- A set of tools and techniques for improving process efficiency and effectiveness by identifying and removing causes of defects and minimizing variability
- Some of the tools and techniques used in Six Sigma include
- DMAIC methodology for process improvement
- Statistical process control charts to monitor and control process variation
- Design of experiments to optimize process settings
- Root cause analysis to identify and eliminate causes of defects and errors
- Failure mode and effects analysis to identify and eliminate potential failure modes in processes
- External quality assessment / proficiency testing (Clin Chem Lab Med 2006;44:740, Cancers (Basel) 2022;14:3686, Biochem Med (Zagreb) 2017;27:19, Clin Chem 2011;57:1670)
- Comparing laboratory results to those of other laboratories
- Proficiency testing programs can help identify errors and areas for improvement, ultimately leading to improved patient care and outcomes
- External quality assessment process typically involves the following steps
- Sample preparation
- Sample distribution
- Sample analysis
- Result reports
- Data analysis
- Feedback and improvement
- Quality dashboards (J Pathol Inform 2016;7:24, AMIA Annu Symp Proc 2020;2019:735)
- A visual representation of key performance indicators (KPIs) and metrics
- Some of the key performance indicators and metrics
- Turnaround time
- Error rates
- Quality control results
- Test volumes
- Customer satisfaction
- Allowing laboratory managers to identify areas for improvement and take corrective action
Goals and benefits of quality management systems
- Improving patient safety by ensuring accurate and reliable test results
- Increasing efficiency and productivity in laboratory processes
- Meeting regulatory requirements and accreditation standards
- Minimizing errors and reducing the risk of testing related incidents
- Enhancing customer satisfaction by meeting or exceeding their expectations for quality
- Providing a framework for continuous improvement and optimization of laboratory operations
- Reducing costs associated with rework, repeat testing and equipment downtime
- Enhancing communication and collaboration among laboratory staff and stakeholders
- Ensuring compliance with ethical and legal requirements for laboratory operations
- Improving data management and ensuring the integrity and confidentiality of laboratory records
- Enhancing the reputation and credibility of the laboratory and its services
- Facilitating risk management and mitigation in laboratory operations
- Enhancing staff training and professional development
- Promoting a culture of quality, safety and excellence in laboratory operations
- Supporting evidence based decision making and strategic planning for the laboratory
- References: Comput Med Imaging Graph 2001;25:217, J Oral Biol Craniofac Res 2019;9:180
Disaster preparedness in the laboratory
- Laboratory operations can continue during and after a disaster (Gac Sanit 2021;35:S180, Lab Anim (NY) 2013;42:F18)
- Some steps to prepare for disasters (StatPearls: Disaster Planning [Accessed 10 July 2023])
- Developing a disaster preparedness plan
- Establishing communication protocols
- Identifying and securing critical equipment and supplies
- Training staff on disaster response
- Participating in disaster drills and exercises
- Some of the key elements of a disaster preparedness plan (Int J Environ Res Public Health 2019;16:1046)
- Conducting a risk assessment
- Establishing emergency response procedures
- Creating an evacuation plan
- Ensuring adequate emergency response equipment and supplies
- Reviewing and updating the plan
- Disaster or emergency drills and exercises (Am J Pharm Educ 2016;80:50)
- Testing the effectiveness of emergency response procedures and identifying areas for improvement
- Identifying strengths and weaknesses
- Testing equipment and supplies
- Improving teamwork and communication
- Increasing preparedness
- Laboratory personnel are prepared to respond effectively to emergencies
- Testing the effectiveness of emergency response procedures and identifying areas for improvement
- Evacuation plans (Heliyon 2023;9:e14277)
- Ensuring the safety of pathologists, staff members and patients during a disaster or emergency
- Some steps to developing an effective evacuation plan
- Identifying evacuation routes and procedures
- Assigning responsibilities
- Establishing a communication plan
- Considering special needs
- Practicing drills
- Well designed disaster preparedness plan can help to minimize the impact of a disaster on laboratory operations, staff and patients (J Emerg Manag 2022;20:351)
Videos
WHO: LQSI series - implementing a laboratory quality management system
Laboratory quality management and standards
CAP: quality management
Additional references
Practice question #1
What is the primary objective of a laboratory quality management system (QMS)?
- Expand laboratory services
- Improve patient outcomes
- Increase laboratory efficiency
- Reduce laboratory costs
- Support evidence based decision making
Practice answer #1
B. Improve patient outcomes. A laboratory QMS is a comprehensive approach to ensuring that a laboratory's services are accurate, reliable and timely and that they meet the needs of patients and healthcare providers. The primary objective of a laboratory QMS is to improve patient outcomes by ensuring that laboratory testing is conducted according to established protocols, that results are accurate and reliable and that they are reported in a timely and efficient manner. Answers A, C, D and E are incorrect because while these may be important secondary objectives of a laboratory QMS, the ultimate goal is to improve patient care and outcomes.
Comment Here
Reference: Quality management systems
Comment Here
Reference: Quality management systems
Practice question #2
Which of the following quality improvement tools is best suited for identifying the underlying causes of a recurring problem in a laboratory process?
- Failure mode and effects analysis
- Flow chart
- Pareto chart
- Root cause analysis
- Statistical process control chart
Practice answer #2
D. Root cause analysis. Root cause analysis is a quality improvement tool that is widely used in the laboratory to identify the underlying causes of a recurring problem or issue. Root cause analysis involves a systematic process of investigation and problem solving, which helps to identify the fundamental reasons why a problem is occurring, rather than simply addressing the symptoms or immediate causes. Answers A, B, C and E are incorrect because while a statistical process control chart, failure mode and effects analysis, Pareto charts and flow charts are all useful quality improvement tools, root cause analysis is specifically designed to help identify and address the underlying causes of recurring problems in a laboratory process.
Comment Here
Reference: Quality management systems
Comment Here
Reference: Quality management systems