Table of Contents
Definition / general | Essential features | Epidemiology | Sites | Clinical and laboratory features | Diagnosis | Prognostic factors | Case reports | Microscopic (histologic) description | Microscopic (histologic) images | Peripheral smear description | Peripheral smear images | Immunophenotype | Electron microscopy images | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Nguyen L, Zhang L. Chronic eosinophilic leukemia. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/myeloproliferativeCEL.html. Accessed April 26th, 2024.
Definition / general
- Chronic eosinophilic leukemia (CEL) is an autonomous, clonal proliferation of eosinophils that leads to a persistent increase in eosinophils in peripheral blood, bone marrow and sometimes tissue
- Absolute eosinophil count is ≥ 1.5 x 109/L on at least 2 occasions for 4 weeks (notable change from 6 months in the 4th edition of WHO)
- Bone marrow shows abnormal morphology (e.g., dysmegakaryopoiesis, eosinophilic infiltrate associated overt fibrosis)
- Clonal
- Clonal myeloid or lymphoid neoplasms and reactive causes of eosinophilia should be excluded
Essential features
- Rare myeloproliferative neoplasm characterized by a gradual increase in circulating eosinophils with cytologic atypia, left shifted granulocytic maturation and often hepatomegaly or splenomegaly
- Organ / tissue infiltration by eosinophils and eosinophilic microabscesses are frequently present, leading to tissue damage and organ dysfunction
- Clinical course is variable from indolent to life threatening; transformation to acute myeloid leukemia can occur and prognosis is generally poor
- Fatal cardiac damage has been documented secondary to the release of cytokines produced by eosinophils
- Diagnosis should be made after exclusion of reactive causes of eosinophilia and myeloid and lymphoid neoplasms associated with PDGFRA, PDGFRB, FGFR1, FLT3 or JAK2 rearrangements with evidence of clonality (see updated full WHO criteria) (Am J Hematol 2017;92:1243)
- Cell of origin: pluripotent hematopoietic stem cell
Epidemiology
- Due to difficulty in distinguishing CEL from nonclonal hypereosinophilic syndrome, the true incidence is unknown
- A recent study indicates that only 1.2% of cases with peripheral eosinophilia met criteria for CEL (Am J Hematol 2020;95:E172)
- The Surveillance, Epidemiology and End Results (SEER) database from 2004 - 2015 estimates the incidence of hypereosinophilic syndrome (HES) including CEL, not otherwise specified (NOS) is 0.4 cases per 1,000,000 (Am J Hematol 2022;97:129)
- Some reports show a predilection for men in the seventh decade (Mod Pathol 2016;29:854, Am J Hematol 2012;87:643)
Sites
- Peripheral blood and bone marrow are always involved
- Splenic and hepatic involvement are also common
- Other frequent sites of involvement include the heart, lungs, central nervous system (CNS), skin and gastrointestinal (GI) tract
Clinical and laboratory features
- Natural history varies considerably between individuals
- Patients may be asymptomatic with longstanding eosinophilia (2 episodes over a 4 week interval), without specific etiology (i.e., no allergy, asthma, drug reaction, parasitic infection or connective disease)
- Absolute eosinophil count can vary from 1.5 to 400 x 109/L
- Eosinophils account for 10% of white blood cells (WBCs)
- Can have leukocytosis (20 - 30 x 109/L), anemia, thrombocytopenia or thrombocytosis
- Symptoms: weight loss, night sweats, fever (12%), fatigue (26%), myalgias or angioedema (14%), cough (24%), dyspnea (16%), rhinitis (10%), pruritus and diarrhea
- Skin manifestations are the most common, followed by lung (44%) and GI tract (38%)
- Cardiovascular
- Endomyocarditis, endomyocardial fibrosis followed by restrictive cardiomegaly
- Hypertension, atherosclerosis and heart failure
- Scarring of mitral and tricuspid valves leading to regurgitation, thrombi formation and embolization in end organs
- Peripheral neuropathy, CNS dysfunction and rheumatologic findings (Am J Hematol 2012;87:643)
Diagnosis
- World Health Organization (WHO) classification (5th edition)
- Essential
- Persistent eosinophilia (≥ 1.5 x 109/L) on at least 2 occasions over a minimum of 4 weeks in which reactive and genetically defined causes have been excluded
- Evidence of clonality (see note 2)
- Abnormal bone marrow morphology
- Does not meet WHO diagnostic criteria for other myeloid or lymphoid neoplasms (see note 3)
- Blasts of < 20% in peripheral blood and bone marrow cells and does not have the following cytogenetic aberrations
- Tyrosine kinase gene fusions including PDGFRA, PDGFRB or FGFR1 rearrangements
- FLT3 rearrangements (Leukemia 2022;36:1703)
- ETV6::ABL1 fusion (Leukemia 2022;36:1703)
- JAK2 rearrangements: t(8;9)(p22;p24.1) / PCM1::JAK2, t(9;12)(p24.1;p13.2) / ETV6::JAK2 or t(9;22)(p24.1;q11.2) / BCR::JAK2 fusions
- AML with inv(16)(p13.1;q22) or t(16;16)(p13.1;q22) / CBFB::MYH11; t(15;17)(q22;q11-12) / PML::RARA or t(8;21)(q22;q22.1) / RUNX1::RUNX1T1
- CML with t(9;22)(q24;q31) / BCR::ABL1
- Blasts of < 20% in peripheral blood and bone marrow cells and does not have the following cytogenetic aberrations
- Notes
- Note 1
- Note 2: possibility of clonal hematopoiesis of indeterminate potential (CHIP) should be considered
- Certain gene mutations (e.g., DNMT3A, TET2, ASXL1 with variant allele frequency ≥ 2%) can be seen in a subpopulation of elderly patients who do not have hematologic disorders, otherwise known as CHIP
- Diagnostic interpretation should be done with caution (N Engl J Med 2014;371:2477, Nat Med 2014;20:1472)
- Mutations in other genes with variant allele frequency (VAF) > 10% in combination with other mutations is a better indicator of clonality
- Note 3: including myeloproliferative neoplasms (MPN), myelodysplastic syndrome (MDS), MDS / MPN, myeloid / lymphoid neoplasms with eosinophilia (MLN-eo), mastocytosis and acute myeloid leukemia (AML)
- Essential
- International Consensus Classification (ICC) criteria; requires all 6 criteria
- Hypereosinophilia (≥ 1.5 x 109/L) with eosinophils comprising ≥ 10% of white blood cells
- Does not meet criteria for AML; blasts < 20% in the peripheral blood and bone marrow, excluding AML with recurrent genetic abnormalities
- AML with recurrent genetic aberrations with < 20% is also excluded
- No tyrosine kinase fusion genes including BCR::ABL1, other ABL, PDGFRA, PDGFRB, FGFR1, JAK2 or FLT3 fusions
- Does not meet criteria for MPN, chronic myelomonocytic leukemia (CMML) or systemic mastocytosis (SM)
- Of note, although eosinophilia can be seen with SM, true CEL, NOS may also occur as SM with an associated myeloid neoplasm (Blood 2022;140:1200)
- Marrow shows increased cellularity with dysplastic megakaryocytes with or without dysplasia in other cell lineages; often with significant myelofibrosis, associated eosinophilic infiltrate or increased blasts ≥ 5% in bone marrow or ≥ 2% in peripheral blood
- Clonal cytogenetic abnormality or somatic mutation identified
- If no clonality / somatic mutation demonstrated or no increased blasts, persistent eosinophilia may suffice provided the bone marrow findings are supportive and other causes of eosinophilia have been ruled out
Prognostic factors
- WHO classification (5th edition)
- Variable survival; median survival is 22.2 months secondary to infection, bleeding and age related comorbidity
- Transformation to acute leukemia can occur
- Response to imatinib therapy is uncommon except for some CEL with PDGFRA mutations (Am J Hematol 2012;87:643, Blood 2011;117:2935)
- STAT5B p. N642H with or without SF3B1 mutations demonstrated better overall survival (Leukemia 2019;33:415)
- 4 cases showed somatic activating KIT M541L mutation that was responsive to low dose imatinib therapy (Oncotarget 2014;5:4665)
- Unfavorable prognostic findings include marked splenomegaly, bone marrow fibrosis, megakaryocytic atypia and thrombocytopenia (Am J Hematol 2020;95:E172)
Case reports
- 52 year old man with t(5;12)(q31;p13) / ETV6::ACSL6 gene fusion, a novel variant of myeloid proliferative neoplasm with eosinophilia (Hum Pathol (N Y) 2016;5:6)
- 68 year old woman with liver infiltration resembling Budd-Chiari syndrome (Rinsho Ketsueki 2007;48:505)
- 72 year old man with autoimmune hemolytic anemia and erythrophagocytosis by eosinophils (Am J Hematol 2006;81:458)
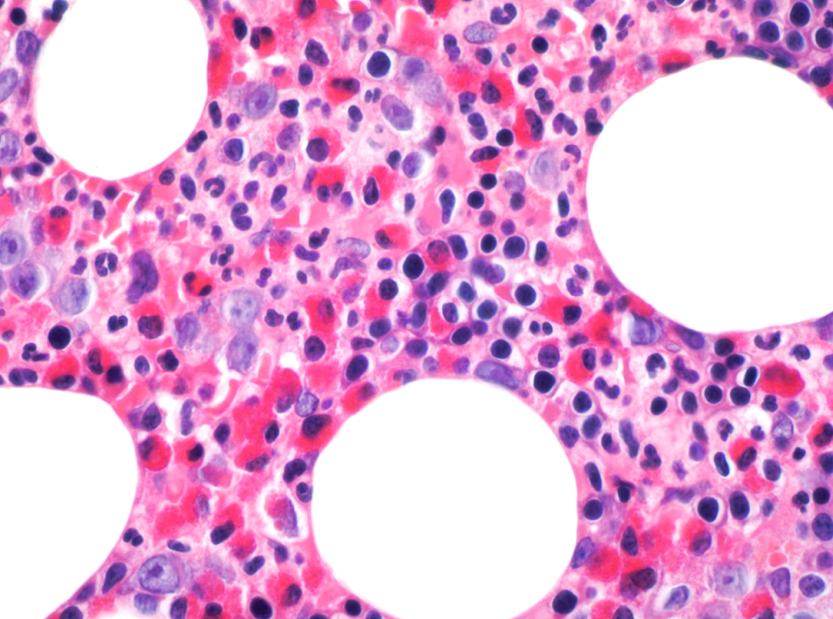
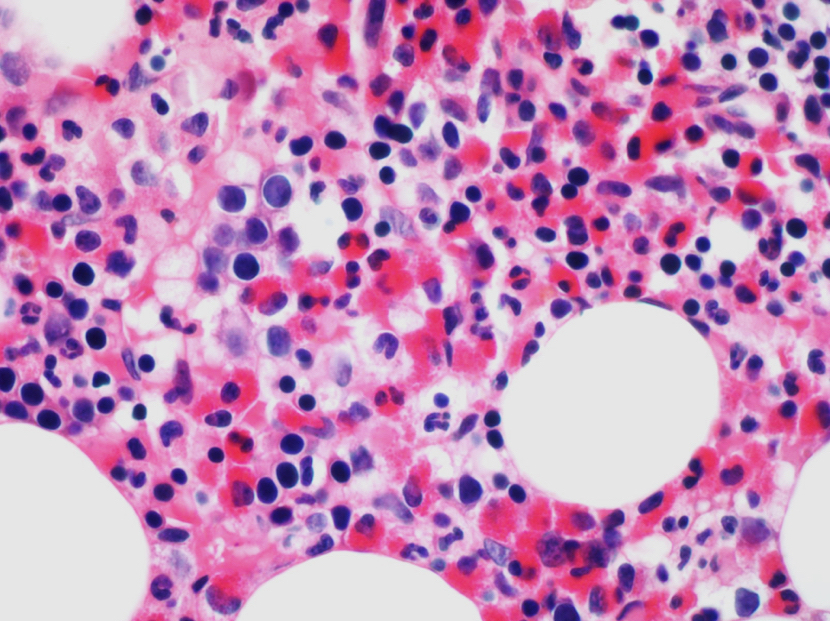
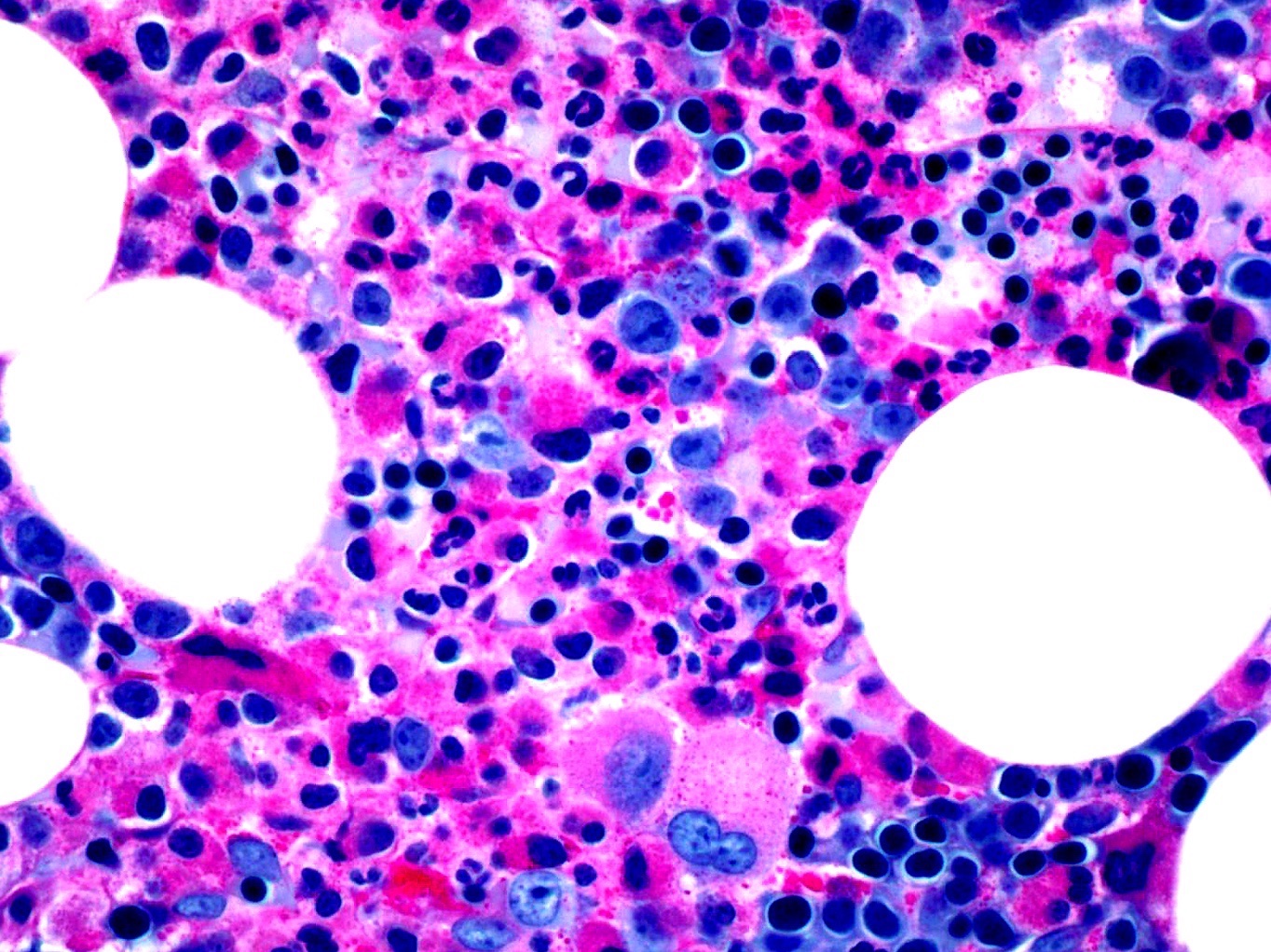
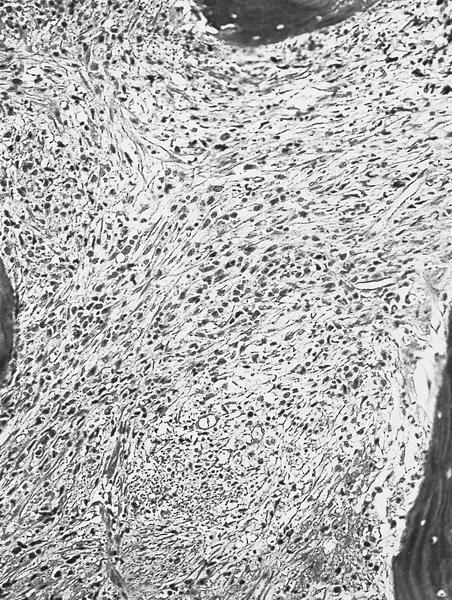
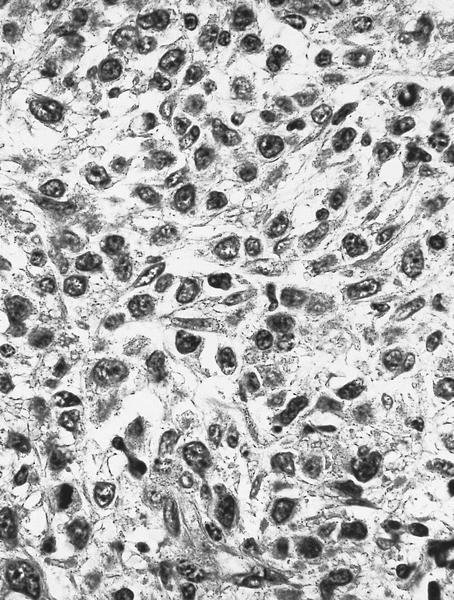
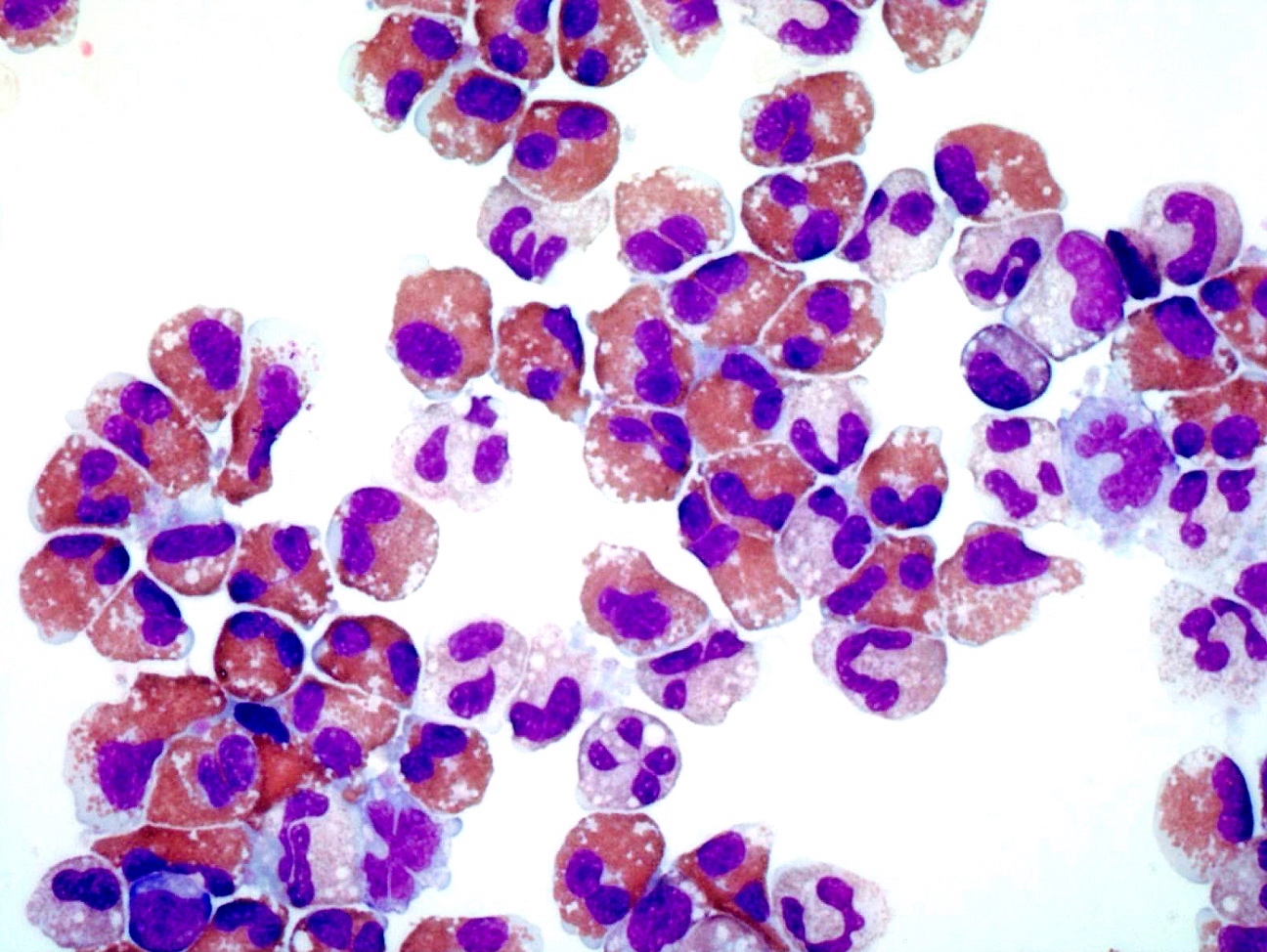
Microscopic (histologic) description
- Bone marrow
- Hypercellular due to eosinophilic proliferation; however, maturation is orderly without disproportionate increase in myeloblasts
- Charcot-Leyden crystals often present
- Usually normal erythropoiesis and megakaryocytopoiesis; however, abnormal dysplastic megakaryocytes can be seen with or without dysplasia in other lineage (Blood 2022;140:1200)
- Subpopulation of patients (27%) show morphologic features resembling BCR::ABL1 negative neoplasms, myelodysplastic syndromes or myelodysplastic / myeloproliferative neoplasms (Mod Pathol 2016;29:854, Haematologica 2017;102:1352)
- Increased myeloblasts (commonly 5 - 19% in bone marrow)
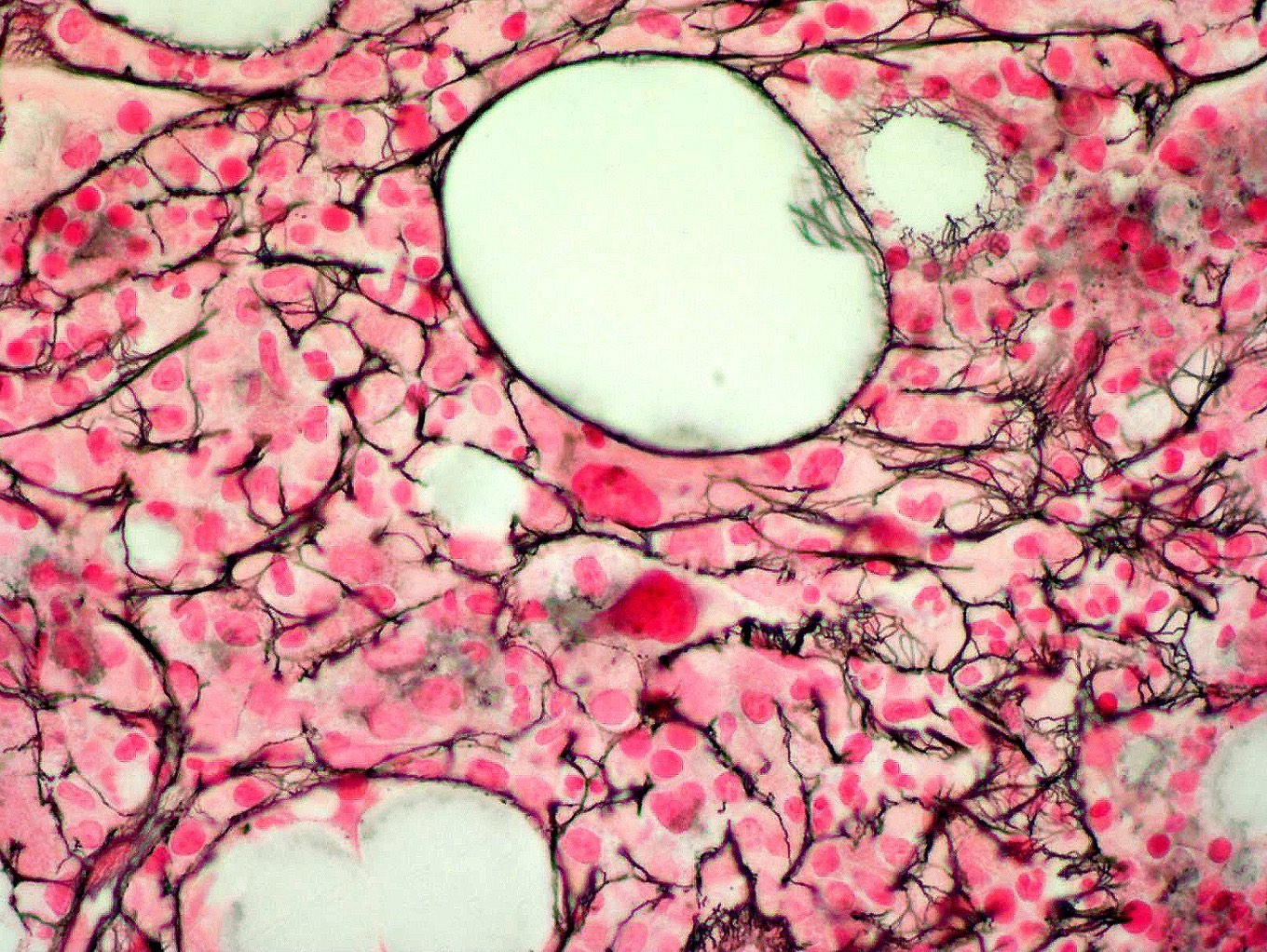
- ~33% of cases show myelofibrosis; often significant fibrosis associated with eosinophilic infiltrate (Blood 2022;140:1200)
- Tissue
- Eosinophilic infiltration or microabscesses
- Charcot-Leyden crystals
- Fibrosis (caused by the degranulation and release of eosinophilic basic and cationic proteins)
Microscopic (histologic) images
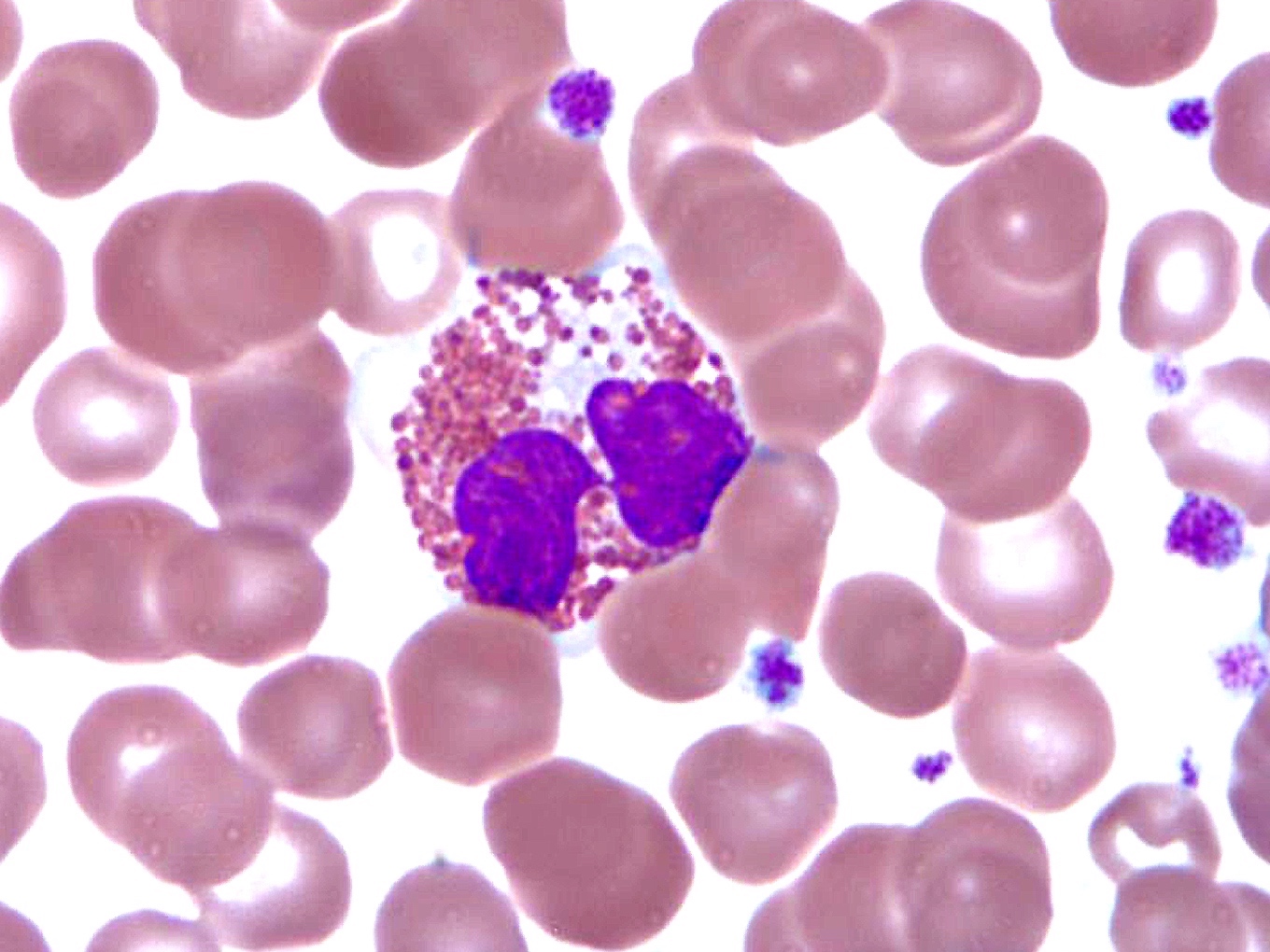
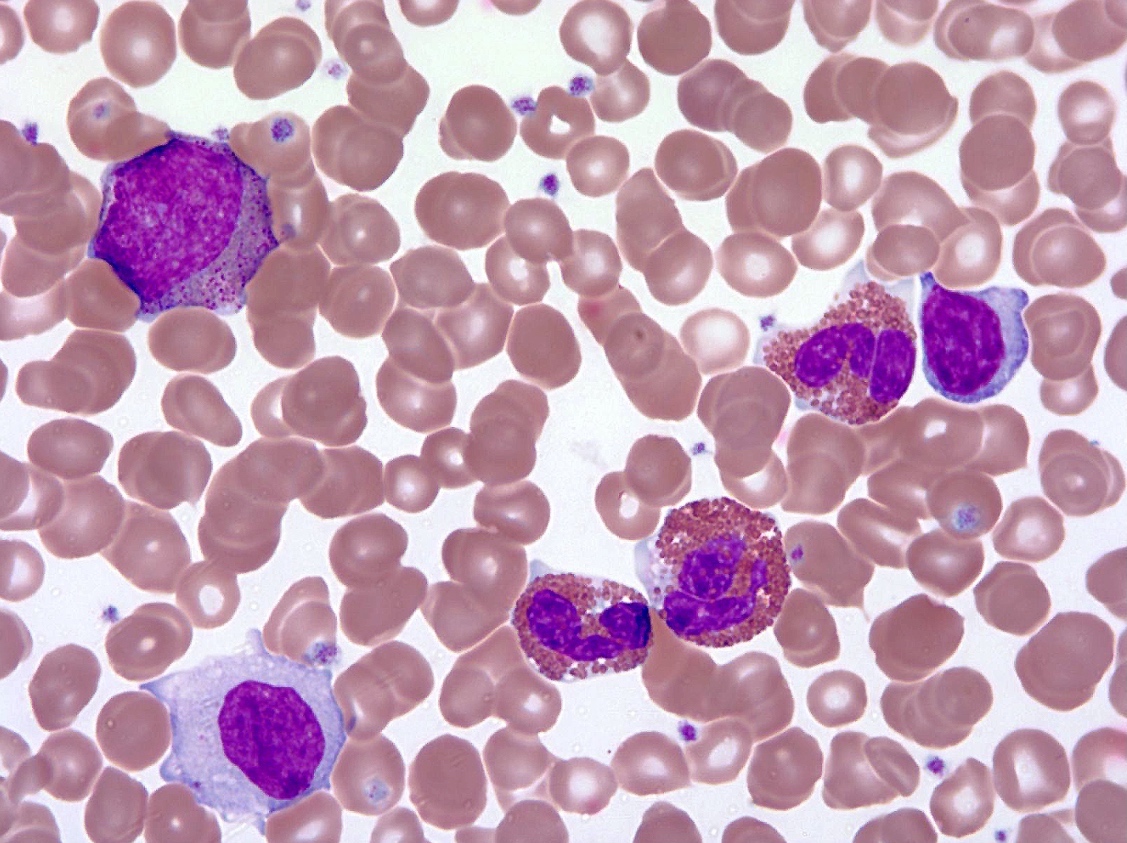
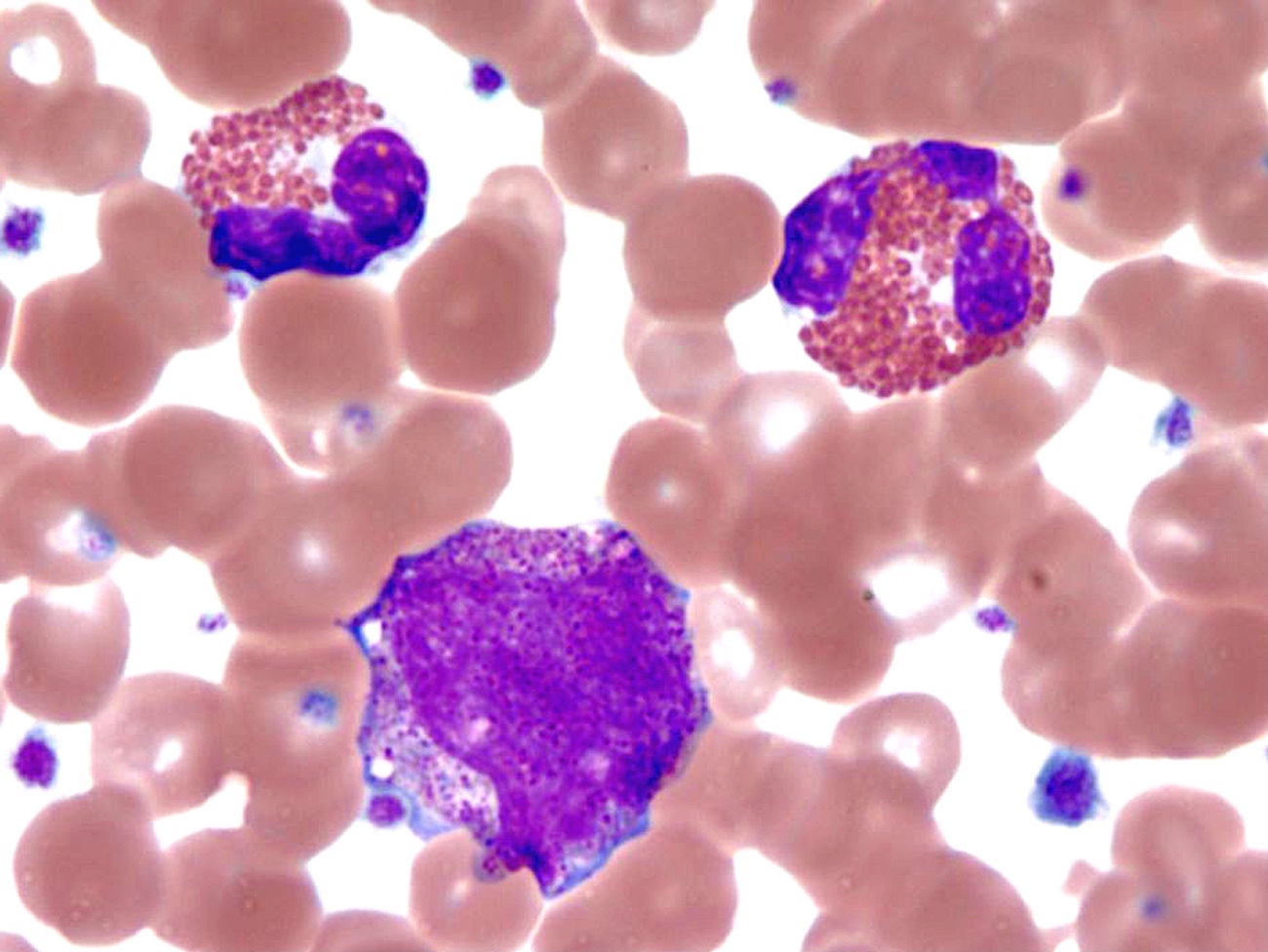
Peripheral smear description
- Peripheral blood
- Striking eosinophilia (≥ 1.5 x 109/L) mainly composed of mature eosinophils and occasional immature eosinophilic precursors
- Circulating blasts can be seen but comprise < 20%
- Spectrum of nonspecific eosinophil abnormalities: sparse granulation, cytoplasmic vacuolation, nuclear hyper / hyposegmentation or increased size
- Often accompanied by neutrophilia, some with mild monocytosis (> 1 x 109/L) and a few with mild basophilia
- Striking eosinophilia (≥ 1.5 x 109/L) mainly composed of mature eosinophils and occasional immature eosinophilic precursors
Peripheral smear images
Immunophenotype
- No specific immunophenotype has been associated with CEL, NOS; however, immunophenotyping is important to exclude T lymphocyte driven eosinophilia or acute leukemia
Molecular / cytogenetics description
- No specific cytogenetic or molecular genetic abnormality identified
- Must exclude neoplasms with rearrangements of BCR::ABL1, PDGFRA, PDGFRB, FGFR1, FLT3 or PCM1::JAK2, ETV6::JAK2 or BCR::JAK2 fusions
- Presence of recurrent translocations and karyotypic abnormalities that are seen in myeloid disorders [-8, -7, i(17q)] also support the diagnosis (Br J Haematol 1996;95:2, Br J Haematol 1986;62:659)
- Next generation sequencing (NGS) is helpful in establishing clonality
- Common mutations in ASXL1, TET2 and EZH2; occasional JAK2 mutation (Blood 2005;106:2162, Mod Pathol 2016;29:854)
- Mutations in TET2, ASXL1 and DNMT3A can be detected in the elderly population without neoplasms (clonal hematopoiesis of indeterminate potential) (N Engl J Med 2014;371:2477, Nat Med 2014;20:1472)
Sample pathology report
- Peripheral blood, peripheral blood smear:
- Mild normocytic anemia
- Leukocytosis with marked eosinophilia
- No circulating blasts identified
- Bone marrow, aspirate, clot section and biopsy:
- Markedly hypercellular marrow with myeloid preponderance, marked eosinophilic hyperplasia and scattered blasts (2%) (see comment)
- Moderate myelofibrosis (MF2)
- Comment: Examination of the peripheral blood and bone marrow aspirate smears show a marked increase in eosinophils with abnormal morphology including sparse granulation and nuclear hyper / hyposegmentation. There is no peripheral monocytosis or basophilia. Manual differential counts performed on the submitted bone marrow aspirate demonstrate approximately 2% myeloblasts. Megakaryocytes are present and slightly increased in number. Erythroid precursors show essentially normoblastic maturation. The M:E ratio is increased, estimated 5:1. The bone marrow core biopsy demonstrates normal bony trabeculae surrounded marrow space with overt myeloid predominance, increased eosinophils and their precursors. Occasional blasts are identified. Megakaryocytes are evenly distributed without syncytial clustering. Erythroid precursors are present and relatively low in proportion. Occasional small loose lymphoid aggregates composed of small mature forms are focally noted. No granulomata are evident. Flow cytometry performed on the bone marrow aspirate with adequate cells showed no immunophenotypic evidence of clonal B cell or aberrant T cell population. Rare CD34 positive blasts (1 - 2%) are identified.
- Karyotyping showed normal karyotype, 46,XX[20]. Fluorescence in situ hybridization is negative for BCR::ABL1, PDGFRA, PDGFRB or FGFR1 rearrangements. Next generation sequencing identified multiple mutations involving ASXL1, TET2, EZH2, SETBP1, CBL and NOTCH genes. According to WHO classification, the patient showed typical clinical presentation, along with morphologic and molecular findings supporting a diagnosis of chronic eosinophilic leukemia. Clinically, all other neoplastic and reactive causes of eosinophilia have been excluded.
- Markedly hypercellular marrow with myeloid preponderance, marked eosinophilic hyperplasia and scattered blasts (2%) (see comment)
Differential diagnosis
- Acute myeloid leukemia with inv(16)(p13.1;q22) or t(16;16)(p13.1;q22); CBFB::MYH11:
- Presence of inv(16)(p13.1;q22) or t(16;16)(p13.1;q22)
- Most cases show myelomonocytic differentiation and presence of abnormal eosinophils with immature basoeosinophilic granules in addition to increased myeloblasts
- Atypical chronic myeloid leukemia (aCML); BCR::ABL1 negative:
- Myelodysplastic / myeloproliferative neoplasm with neutrophilia according to the 5th edition of WHO
- Besides features of myeloproliferative neoplasms and eosinophilia, dysplasia is present in > 10% of neutrophilic precursors but there is no monocytosis
- Myeloproliferative chronic myelomonocytic leukemia (MP-CMML) (5th edition of WHO):
- Sustained monocytosis ≥ 1 x 109/L for > 6 months with bone marrow morphologic findings compatible with CMML
- Nonclonal hypereosinophilic syndrome:
- Diagnosis of exclusion given when
- Eosinophil count ≥ 1.5 x 109/L for ≥ 6 months
- Exclude reactive causes of eosinophilia
- Exclude acute myeloid leukemia, myeloproliferative neoplasms, myelodysplastic syndromes, myelodysplastic / myeloproliferative neoplasms and systemic mastocytosis and familial causes of eosinophilia
- Exclude cytokine producing, immunophenotypically aberrant T cell population
- Tissue damage present due to hypereosinophilia
- Diagnosis of exclusion given when
- Idiopathic hypereosinophilia (hypereosinophilia of uncertain significance):
- When criteria 1 - 4 listed above for idiopathic hypereosinophilic syndrome are met but there is no end organ damage
- Presence of clonal genetic aberration favors chronic eosinophilic leukemia, not otherwise specified
- Lymphocytic variant of hypereosinophilic syndrome:
- Abnormal cytokine release by immunophenotypically abnormal or clonal T cells
- Aberrant CD3- / CD4+ T cell population which can be detected by flow cytometry can be used to differentiate; absolute CD3- / CD4+ T cell count of 0.01 - 28.3 k/L with median of 0.35 k/L
- TCRγδ rearrangements were frequently identified (76%) (Medicine (Baltimore) 2014;93:255)
- Other phenotypic changes (e.g., CD3+ / CD4+ / CD7- and CD3+ / CD4- / CD8- TCRαβ+) have been reported (Immunol Allergy Clin North Am 2007;27:389, N Engl J Med 1999;341:1112)
- Medication related:
- Administration of cytokines IL3, IL5 or GMCSF
- Myeloid neoplasms with rearrangement of PDGFRA, PDGFRB, FGFR1 or FLT3 or PCM1::JAK2, ETV6::JAK2 or BCR::JAK2 fusion (Blood 2017;129:704):
- Presence of these genetic abnormalities show morphologic findings similar to other myeloid and lymphoid neoplasms
- PDGFRA rearrangement: can present as CEL, AML or T ALL with prominent eosinophilia
- PDGFRB rearrangement: most often presents as CMML but have been characterized as atypical CML, BCR::ABL negative, CEL or MPN with eosinophilia; atypical CML is now called myelodysplastic / myeloproliferative neoplasm with neutrophilia
- FGFR1 rearrangement: presents as a AML, T or B ALL or mixed phenotype acute leukemia with prominent eosinophilia
- PCM1::JAK2: CEL, PMF or atypical CML, BCR::ABL negative
- ETV6::JAK2: B and T ALL but can also present as MDS
- BCR::JAK2: most commonly as atypical CML, BCR::ABL negative
- Paraneoplastic eosinophilia:
- T cell lymphoma, Hodgkin lymphoma, systemic mastocytosis, acute lymphoblastic leukemia t(5;14) / IgH::IL3, myeloproliferative neoplasms or certain solid tumors (e.g., lung cancer, metastatic renal cell carcinoma, etc.) associated with abnormal release of IL2, IL3, IL5 or GMCSF (Arch Pathol Lab Med 2013;137:259, Rinsho Ketsueki 1991;32:874, Acta Clin Belg 2011;66:293, Am J Hematol 2007;82:234)
- Reactive eosinophilia:
- Parasitic or fungal infections, allergies, pulmonary disease (Löffler syndrome), cyclical eosinophilia, skin diseases (angiolymphoid hyperplasia), collagen vascular diseases such as Churg-Strauss syndrome and Kimura disease
- Systemic mastocytosis:
- Clonal proliferation of atypical mast cells that meet WHO diagnostic criteria
- Eosinophils are present or increased, which could be a secondary reaction or derived from a neoplastic clone
Additional references
Board review style question #1
Which of the following is useful in definitively diagnosing the lymphocytic variant of hypereosinophilic syndrome?
- Abnormal T cell population (e.g., CD3- / CD4+)
- Elevated IL5 level
- Eosinophilia ≥ 1.5 k/L
- Eosinophilic tissue infiltrate
- Skin rush and pruritus
Board review style answer #1
A. Abnormal T cell population (e.g., CD3- / CD4+). Lymphocytic variant of hypereosinophilic syndrome (L-HES) is characterized by abnormal cytokine release by clonal T cells. CD3- / CD4+ T cells are often found in circulating blood but CD3+ / CD4+ / CD7- and CD3+ / CD4- / CD8- T cells have also been described. TCRγδ rearrangements are frequently identified in these patients; however, TCRαβ+ L-HES has also been reported. Answers B - D are incorrect because elevated IL5 levels, eosinophilia and eosinophilic infiltration can be seen in a number of reactive, neoplastic and iatrogenic conditions and are not diagnostic or specific for L-HES. Answer E is incorrect because pruritus and skin rash can occur as a result of eosinophilia but this finding is not specific.
Comment Here
Reference: Chronic eosinophilic leukemia
Comment Here
Reference: Chronic eosinophilic leukemia
Board review style question #2
Which of following morphologic or molecular genetic findings is frequently identified in chronic eosinophilic leukemia, not otherwise specified?
- Dysplastic noneosinophilic granulocytes
- ETV6::JAK2 fusion
- Hypercellular marrow with markedly increased eosinophils and left shifted myelopoiesis
- KIT D816V gene mutation
- Marked monocytosis
Board review style answer #2
C. Hypercellular marrow with markedly increased eosinophils and left shifted myelopoiesis. Abnormal bone marrow findings are 1 of 3 key diagnostic features of CEL (see above). Hypercellular marrow along with eosinophilic proliferation and left shifted myelopoiesis, though nonspecific, are the most common features of CEL. Answers A and E are incorrect. Dysplastic megakaryocytes with or without dysplasia in other cell lineages can be seen in CEL. Spectrum of nonspecific eosinophil abnormalities can be seen (sparse granulation, cytoplasmic vacuolation, nuclear hyper / hyposegmentation or increased size). CEL often shows neutrophilia, some with mild monocytosis (> 1 x 109/L) and a few with mild basophilia. Answers B and D are incorrect. ETV6::JAK2 fusion can seen in myeloid / lymphoid neoplasms with eosinophilia (MLN-eo) and tyrosine kinase (TK) gene fusions while KIT D816V gene mutation is frequently seen in systemic mastocytosis.
Comment Here
Reference: Chronic eosinophilic leukemia
Comment Here
Reference: Chronic eosinophilic leukemia