Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Rytych J, Escobar D. Rejection. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/smallbowelrejection.html. Accessed April 25th, 2024.
Definition / general
- Small bowel transplantation is a surgical option for patients who have intestinal failure and have failed parenteral nutrition
- Rejection of the transplanted bowel occurs through cellular or antibody mediated mechanisms and is responsible for a significant portion of graft failures
Essential features
- Rejection is a major complication of small bowel transplantation, with 30 - 40% of patients developing an episode of acute cellular rejection (ACR) within the first year
- ACR is characterized by increased crypt apoptotic figures, increased lamina propria mixed inflammation and epithelial injury
- Repeated episodes of ACR can lead to chronic rejection and graft dysfunction, which shows nonspecific histologic findings, including ischemic changes and lamina propria fibrosis
Terminology
- Acute cellular rejection (ACR)
- Antibody mediated rejection (AMR)
- Chronic rejection
ICD coding
Epidemiology
- Small bowel transplants are uncommon; < 100 performed in the U.S. per year since 2019 (Am J Transplant 2023;23:S264)
- 30 - 40% of adult transplants develop acute cellular rejection within the first year posttransplant (Am J Transplant 2021;21:1705)
- Chronic rejection was documented in 15% of transplants in 1 series (Ann Surg 2009;250:567)
- Rejection is the primary cause of graft loss in ~13% of cases (sepsis causes ~50% and cardiac complications account for ~8%) (Am J Transplant 2015;15:210)
Sites
- Transplants performed as small intestine only, with liver or multivisceral (with liver, stomach, pancreas, colon, spleen or kidney)
Pathophysiology
- Direct pathway
- Donor antigen presenting cells using donor major histocompatibility complex (MHC) class II and class I molecules present allogenic molecules from the graft to recipient CD4+ and CD8+ lymphocytes
- Activated CD4+ and CD8+ lymphocytes are predominantly responsible for early acute cellular rejection occurring within the first several months posttransplant
- Semidirect pathway
- Donor membrane bound MHC molecules are transferred to recipient antigen presenting cells and presented to CD4+ and CD8+ lymphocytes without processing
- Responsible for delayed acute cellular rejection occurring years after transplant
- Indirect pathway
- Donor MHC antigens are internalized and processed by recipient antigen presenting cells and presented to recipient CD4+ lymphocytes
- Responsible for antibody mediated rejection and delayed acute cellular rejection
- Inverted direct pathway
- Donor CD4+ T cells are activated by recipient B cells presenting recipient MHC class II molecules
- Responsible for early antibody mediated rejection
- Reference: Int J Mol Sci 2023;24:4541
Etiology
- Rejection of allograft through cellular mediated response or antibody mediated response involving activation of CD4+ and CD8+ lymphocytes (Int J Mol Sci 2023;24:4541)
Clinical features
- Symptoms are typically nonspecific: fever, diarrhea / increased ostomy output, nausea and abdominal pain
- Acute cellular rejection
- Onset is typically between 1 and 9 weeks posttransplant
- Antibody mediated rejection
- Typically occurs during the first 2 weeks posttransplant
- Detection of lymphocytotoxic antibodies by serologic testing
- Chronic rejection
- Typically occurs after repeated episodes of acute cellular rejection
- Also associated with nutritional deficiency and generalized edema (associated with protein losing enteropathy)
- Reference: Arch Pathol Lab Med 2012;136:761
Diagnosis
- Mucosal biopsies of the graft are the only current reliable method (Scand J Gastroenterol 2021;56:13)
Laboratory
- No specific laboratory markers are routinely used to diagnose or monitor rejection
- Biomarkers such as decreased serum citrulline and increased fecal calprotectin are seen in moderate to severe rejection but are nonspecific (Scand J Gastroenterol 2021;56:13)
- Detection of lymphocytotoxic antibodies may support antibody mediated rejection
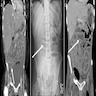
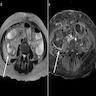
Radiology description
- Imaging findings are nonspecific
- Noncontrast computed tomography (CT): diffuse wall thickening, mucosal hyperenhancement or hypoenhancement and moderate ascites
- Magnetic resonance imaging (MRI): wall thickening and mucosal hyperenhancement with contrast on T1 sequence
- Fluoroscopy: wall thickening, loss of mucosal fold patterns and hypomotility
- Reference: Br J Radiol 2018;91:20180173
Radiology images
Prognostic factors
- Increased number of episodes of ACR, higher grade of ACR and earlier onset of ACR are associated with an increased risk of chronic rejection (Pediatr Dev Pathol 2003;6:240)
- 5 year graft survival for patients < 18 years old is better than adults (62% versus 43%) (Am J Transplant 2023;23:S264)
Case reports
- 18 year old man with small bowel transplant and acute cellular rejection (World J Gastroenterol 2005;11:5332)
- 19 year old woman with small bowel transplant and severe acute rejection (Dig Dis Sci 2010;55:3336)
- 20 year old woman with a history of small bowel transplant and severe late onset acute cellular rejection (Transplant Proc 2019;51:3181)
- 20 year old man with small bowel transplant and acute cellular rejection associated with an elevation of donor specific HLA antibodies (Transplant Proc 2016;48:525)
- 31 year old woman with small bowel transplant, acute cellular rejection with white exudates on endoscopy (Transplant Proc 2017;49:2419)
Treatment
- Induction immunosuppression with antithymocyte globulin or interleukin 2 receptor blocking antibody
- Maintenance immune suppression with tacrolimus and mycophenolate mofetil or corticosteroids
- Reference: Am J Transplant 2023;23:S264
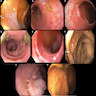
Gross description
- Endoscopically, acute cellular rejection shows an erythematous and friable mucosa with or without erosion or ulceration
- No endoscopic / macroscopic findings have been identified that are specific for chronic ACR or AMR
- Reference: Am J Transplant 2021;21:1705
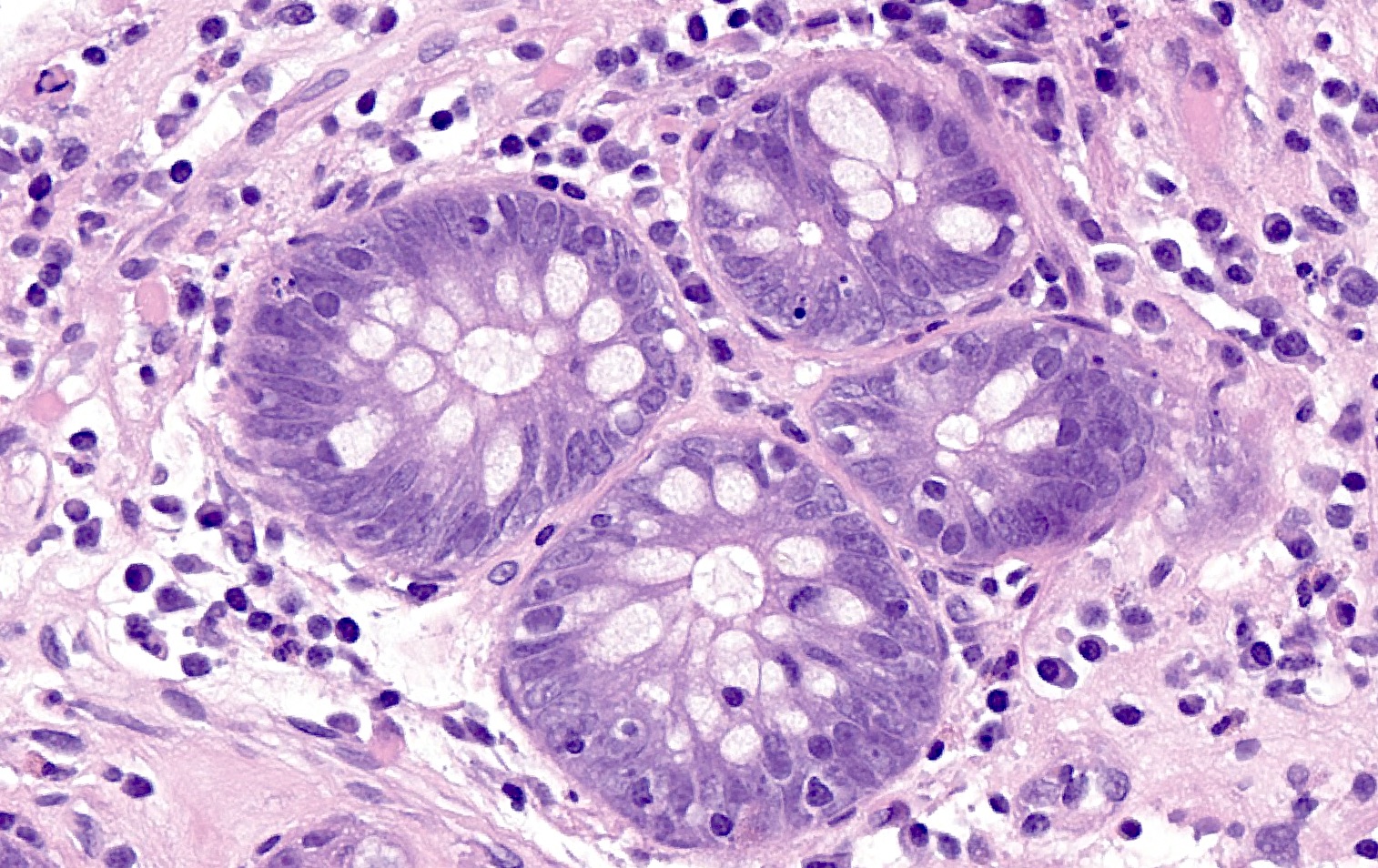
Microscopic (histologic) description
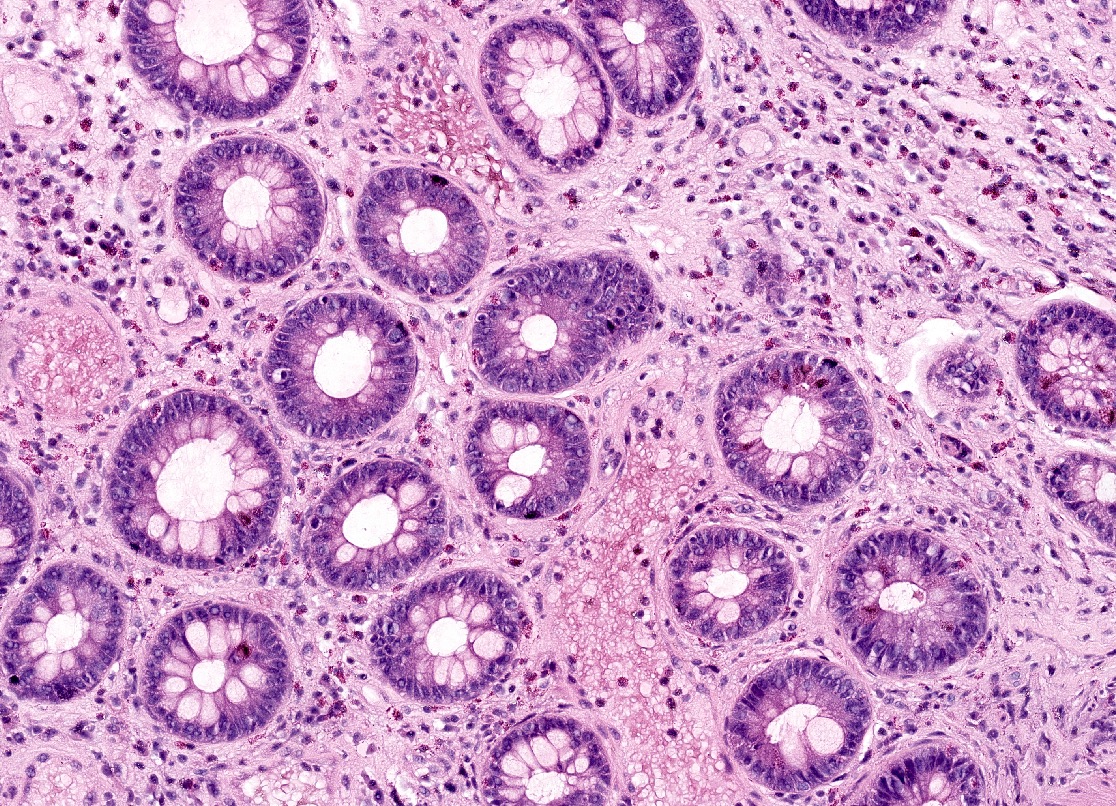
- Standardized histologic criteria were developed at the pathology workshop of the VIII International Small Bowel Transplant Symposium (Transplant Proc 2004;36:335)
- Acute cellular rejection
- Grading based on apoptotic bodies per 10 consecutive crypts, amount of lamina propria mixed inflammation (lymphocytes, eosinophils and neutrophils) and amount of epithelial injury (crypt dropout, erosions or ulceration) (see table below)
- Other features include edema, villous blunting, reactive epithelial changes and vascular congestion
- Antibody mediated rejection
- Capillaritis, lamina propria inflammation, mucosal erosion / ulceration, fibrin thrombi within lamina propria vasculature and edema (Am J Transplant 2018;18:2250)
- Chronic rejection
- Nonspecific findings: mild ischemic changes, apoptotic bodies (< 6/10 consecutive crypts) and mild fibrosis of the lamina propria
Grading of acute cellular rejection (adapted from Arch Pathol Lab Med 2012;136:761, Transplant Proc 2004;36:335)
| Grade | Apoptotic bodies / 10 consecutive crypts | Crypt dropout | Amount of lamina propria inflammation | Surface epithelial damage | Additional findings |
| 0 (no ACR) | < 2 | None | Not increased | None | Unremarkable mucosa |
| Indeterminate (grade ind) | < 6 | None | Focal minimal | None | Focal mild villous blunting |
| Mild (grade 1) | ≥ 6 | None | Mild | Focal damage / erosion | Mucin depletion, nuclear enlargement and hyperchromasia |
| Moderate (grade 2) | ≥ 6; possibly confluent | Focal | Moderate to severe | Erosion | Moderate to marked architectural distortion and villous blunting, edema and congestion |
| Severe (grade 3) | Variable | Extensive | Moderate to severe | Extensive erosion / ulceration | Moderate to marked architectural distortion, granulation tissue formation |
Microscopic (histologic) images
Positive stains
- Complement 4d (C4d) is usually positive in antibody mediated rejection (not routinely performed) (Am J Transplant 2018;18:2250)
Negative stains
- Cytomegalovirus, adenovirus and Epstein-Barr virus stains
Sample pathology report
- Small intestine, transplant, biopsy:
- Moderate mixed lamina propria inflammatory cells, increased crypt apoptotic figures and foci of crypt distortion, crypt loss and ulceration, consistent with moderate, acute cellular rejection (see comment)
- Comment: Up to 8 crypt apoptotic figures per 10 consecutive crypts are identified. This finding, in combination with lamina propria inflammatory infiltrate and focal epithelial injury, is compatible with moderate acute cellular rejection.
Differential diagnosis
- Infectious enteritis (Arch Pathol Lab Med 2012;136:761):
- Most commonly caused by adenovirus and cytomegalovirus (CMV); other etiologies including bacteria, other viruses, fungi and protozoa are possible
- Apoptotic figures are generally less prominent, viral inclusions may be seen
- Positive immunohistochemistry for adenovirus or CMV useful for confirmation
- Correlation with cultures and serologic studies may be helpful
- Medication effect (commonly mycophenolate mofetil) (Transplant Proc 2010;42:82):
- Can display increased crypt apoptotic figures, increased lamina propria inflammation and epithelial erosion / ulceration
- Will affect both transplanted and native bowel
- Correlation with medication history and supportive endoscopic findings is helpful
- Graft versus host disease (Nat Rev Gastroenterol Hepatol 2017;14:711):
- Commonly affects multiple sites: skin, liver, gastrointestinal tract
- Small bowel involvement characterized by increased crypt apoptotic figures and crypt dropout
- Only affects the native tissues (bowel)
Additional references
Board review style question #1
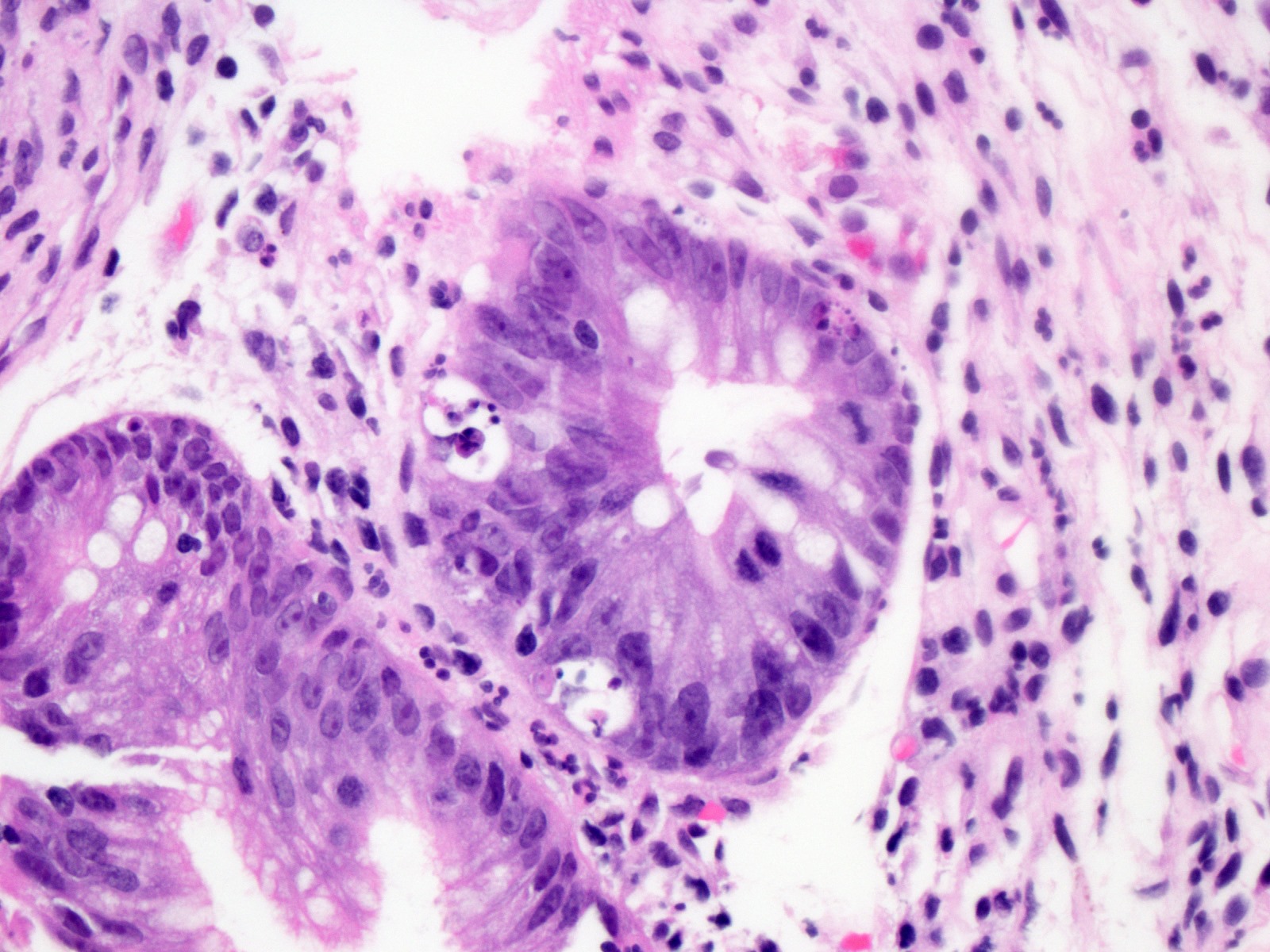
A 35 year old patient with a history of small bowel transplantation presents with diarrhea, abdominal pain and weight loss. Out of concern for acute cellular rejection (ACR), endoscopic biopsies of both the transplanted small bowel and segment of native small bowel are obtained. Histologic examination of the transplanted bowel shows the findings displayed in the representative photomicrograph. What additional findings, if present, would be most supportive of the diagnosis of acute cellular rejection of the transplant bowel in this patient?
- Identification of eosinophilic nuclear inclusions
- Increased mitotic rate
- Occasional crypt dropout and apoptotic figures in the native small bowel
- Occasional crypt dropout and erosion of the surface epithelium in the transplanted small bowel
Board review style answer #1
D. Occasional crypt dropout and erosion of the surface epithelium in the transplanted small bowel. The histological findings described, including ≥ 6 apoptotic bodies per 10 consecutive crypts, when combined with the additional findings of occasional crypt dropout and focal erosion, are indicative of moderate acute cellular rejection (grade 2) in transplanted small bowel. Grade 2 rejection is characterized by ≥ 6 apoptotic bodies per 10 consecutive crypts, moderate to extensive inflammation in the lamina propria, focal crypt dropout and focal erosion of the epithelium. Answer B is incorrect because an increased mitotic rate is not specifically associated with acute cellular rejection. Answer A is incorrect because identification of viral mediated cellular changes, such as Cowdry-like nuclear inclusions, would be more suggestive of infectious etiologies affecting the transplanted bowel. Answer C is incorrect because identification of crypt apoptosis, crypt injury and epithelial injury in the native small bowel may suggest medication induced injury (such as mycophenolate), infection or graft versus host disease.
Comment Here
Reference: Rejection
Comment Here
Reference: Rejection
Board review style question #2
Which of the following factors is a known risk factor for the development of chronic rejection in small bowel transplant recipients?
- Frequent surveillance biopsies
- History of multiple acute cellular rejection episodes
- Long term immunosuppressive therapy
- Short duration of cold ischemia time during transplant surgery
- Younger age at transplant
Board review style answer #2
B. History of multiple acute cellular rejection episodes. The development of chronic rejection is typically preceded by episodes of acute cellular rejection (ACR). Increased number of episodes of ACR, higher grade of ACR and earlier onset of ACR are all associated with an increased risk of chronic rejection. Answers A and C are incorrect because frequent surveillance biopsies and long term immunosuppressive therapy are necessary for the maintenance and surveillance of the health of the graft and are not associated with the development of chronic rejection. Answer E is incorrect because patients < 18 years old at transplant have an improved 5 year graft survival compared to adults and age at transplant is not associated with chronic rejection. Answer D is incorrect because shorter ischemic times have been associated with improved graft outcomes and ischemic time is not directly related to the development of chronic rejection.
Comment Here
Reference: Rejection
Comment Here
Reference: Rejection