Table of Contents
Definition / general | Essential features | CPT coding | Sites | Etiology | Clinical features | Laboratory | Management | Case reports | Cytology description | Cytology images | Sample pathology report | Differential diagnosis | Practice question #1 | Practice answer #1 | Practice question #2 | Practice answer #2Cite this page: Chornenkyy Y, Choy B. HSIL (cytology). PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/cervixHSILcytology.html. Accessed August 14th, 2025.
Definition / general
- Changes in squamous cells associated with human papillomavirus (HPV), encompassing clinically actionable lesions previously referred to as moderate dysplasia, severe dysplasia, cervical intraepithelial neoplasia (CIN) 2, CIN 3 and carcinoma in situ (CIS) (Arch Pathol Lab Med 2012;136:1266, J Low Genit Tract Dis 2013;17:S1, Nayar: The Bethesda System for Reporting Cervical Cytology, 3rd Edition, 2015)
Essential features
- Virtually all women with high grade squamous intraepithelial lesion (HSIL) cytology are positive for high risk HPV (J Natl Cancer Inst 2001;93:293)
- Criteria for HSIL based on the 2014 Bethesda System for Reporting Cervical Cytology (Nayar: The Bethesda System for Reporting Cervical Cytology, 3rd Edition, 2015)
- Approximately the size of parabasal cells
- Nuclear atypia, including nuclear enlargement, irregular nuclear contours with frequent prominent indentations / grooves, generally hyperchromatic, lack of nucleoli
- Decreased cytoplasmic area leading to high N/C ratio
- Differential diagnosis ranges from benign to malignant entities and includes squamous / transitional cell metaplasia, atrophy, endocervical / endometrial cells, intrauterine device (IUD) effect, endocervical polyp atypia, adenocarcinoma in situ (AIS), squamous cell carcinoma, atypical squamous cells cannot exclude HSIL (ASC-H), atypical squamous cells of undetermined significance (ASC-US) associated with atrophy and inflammatory cells
CPT coding
- For screening Pap tests (routine and high risk): smear
- For screening Pap tests (routine and high risk): liquid based
- Manual screening only
- ThinPrep imager assisted screening
- FocalPoint (instrument only)
- FocalPoint (with manual screening)
- For diagnostic Pap tests: smear
- For diagnostic Pap tests: liquid based
- Manual screening only
- ThinPrep imager assisted screening
- FocalPoint (instrument only)
- FocalPoint (with manual screening)
Sites
- Cervix, vagina, anus
Etiology
- HSIL is attributable to high risk HPV infection
- High risk HPV DNA subtypes include: 16, 18, 31, 33, 34, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68 and 70 (J Clin Pathol 2002;55:244, N Engl J Med 2003;348:518)
Clinical features
- Accounts for 0.5% of all Pap test results (Arch Pathol Lab Med 2010;134:331)
Laboratory
- HPV testing may be used as part of screening, triage and surveillance (J Am Soc Cytopathol 2020;9:291)
- Initially endorsed as triage test for ASCUS cytologic result in 2001
- Approved for:
- Cotesting in 2003
- Postcolposcopic / posttreatment follow up and risk stratification using partial genotype (HPV 16 / 18) in 2006
- Primary screening option in 2014
- 5 FDA approved HPV testing platforms:
- Qiagen Hybrid Capture
- Hologic Cervista
- Hologic Aptima
- Roche Cobas - FDA approved for primary screening
- Becton Dickinson Onclarity - FDA approved for primary screening
- Note: HPV result plays no role in the cytologic examination or grading of SIL
Management
- 2019 American Society of Colposcopy and Cervical Pathology (ASCCP) risk based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors (J Low Genit Tract Dis 2020;24:102)
- Personalized risk based recommendations based on a patient's risk of CIN 3+, as determined by a combination of current results and past history (including unknown history)
- Those with immediate risk of CIN 3+ 60% or greater based on history and current results, expedited treatment is preferred
- Clinical situation exceeding this risk threshold: HSIL cytology and concurrent positive testing for HPV 16
- Exceptions to this recommendation include: patients who are pregnant, younger than 25 years or have concerns about potential effects of treatment on future pregnancy outcomes that outweigh concerns about cancer
- Expedited treatment = excisional procedure without preceding colposcopic biopsy demonstrating CIN 2+ ("see and treat" approach)
- Those with risks 25% or greater and less than 60% based on history and current results, expedited treatment or colposcopy is acceptable
Case reports
- 33 year old woman with a double cervix and a single uterine corpus, diagnosed with bilateral HSIL (Acta Cytol 2004;48:273)
- 39 year old woman with persistent HSIL cytology and HPV 16 positivity, diagnosed with vaginal intraepithelial neoplasia (VAIN) 3 without uterine cervix involvement on total hysterectomy (J Med Cases 2020;11:246)
Cytology description
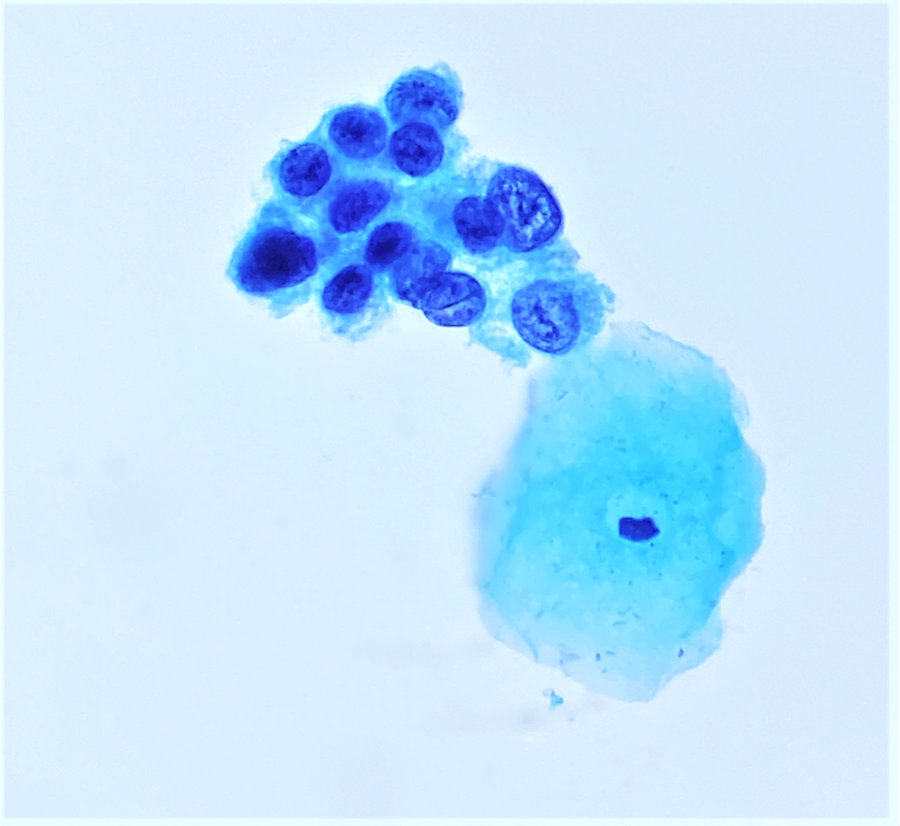
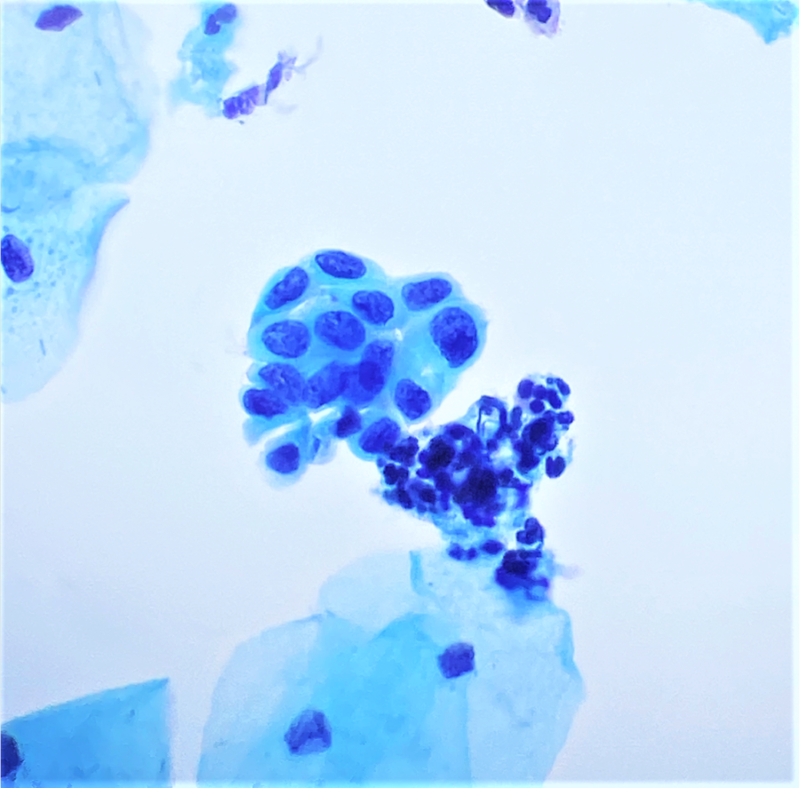
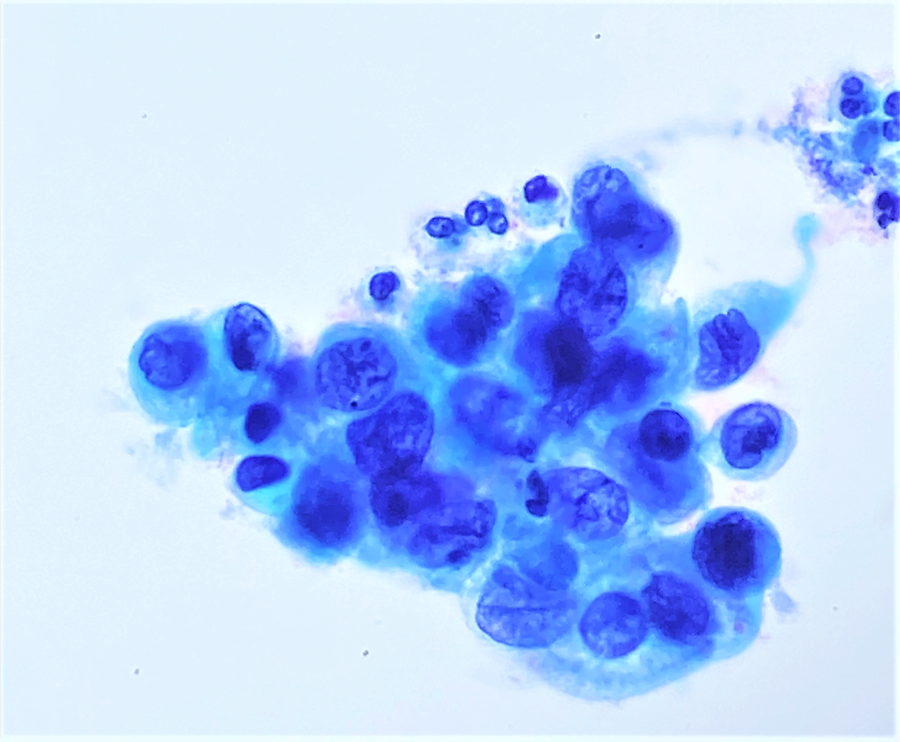
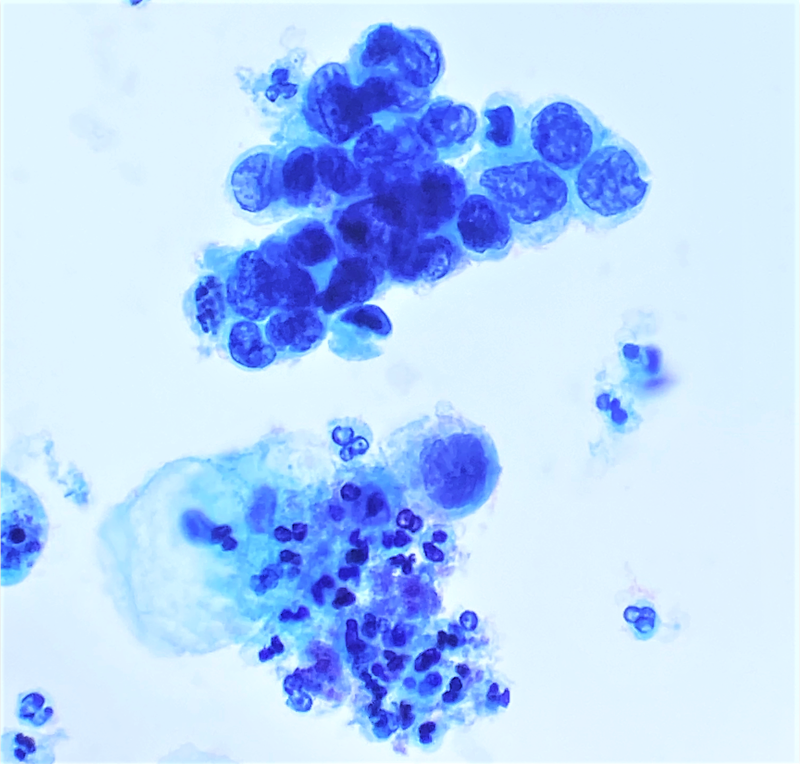
- Diagnostic criteria (Nayar: The Bethesda System for Reporting Cervical Cytology, 3rd Edition, 2015)
- Approximately the size of parabasal cells
- Smaller than low grade squamous intraepithelial lesion (LSIL) cells
- Cells seen singly, in sheets or in syncytium-like clusters (appearing as hyperchromatic crowded groups)
- Nuclear atypia
- Nuclear enlargement: more variable than in LSIL; some in same range, while others may be smaller
- Irregular nuclear contour and frequently demonstrates prominent indentations / grooves
- Generally hyperchromatic but can be normochromatic or hypochromatic
- Chromatin may be fine or coarsely granular and evenly distributed
- Lack of nucleoli
- Nucleoli can be seen when HSIL extends into endocervical gland spaces or in the background of reactive or reparative change
- Cytoplasm
- Decreased cytoplasmic area leading to high N/C ratio
- May be immature / lacy / delicate or densely metaplastic or mature / densely keratinized
- Approximately the size of parabasal cells
- Problematic HSIL patterns (Nayar: The Bethesda System for Reporting Cervical Cytology, 3rd Edition, 2015, Am J Clin Pathol 2021;156:300)
- Syncytium-like aggregates / hyperchromatic crowded groups
- Tight clusters should be examined with care for cytomorphologic features of HSIL
- Mitoses may be seen within these clusters
- Flattening at the edge of the groups, whorling in the center and lack of glandular architectural features favor HSIL over glandular abnormality
- HSIL with endocervical gland involvement
- Nucleoli may be seen in HSIL within glands
- May have peripheral palisading of cells and nuclear pseudostratification (features usually seen in glandular lesions)
- Central cells with spindling or whorling and flattening of the nuclei at the edge of the clusters favor HSIL over glandular origin
- HSIL resembling endometrial cells and repair
- Small cells with degenerated nuclei showing pyknosis and scant cytoplasm, resembling shedding endometrial cells
- Cells with more abundant cytoplasm and may have elongated taffy pull cytoplasmic appendages, enlarged nuclei and prominent nucleoli, resembling repair
- Careful examination for cells with more classic features of HSIL if suspicion is high
- Single and rare small HSIL cells
- Few small single cells with HSIL cytomorphologic features may be missed
- Close inspection, especially in the empty spaces between cells, is important
- Abnormal stripped nuclei
- Large, cytologically abnormal stripped nuclei should prompt a closer examination for more classic HSIL cells
- Should be differentiated from cytolysis and small blue cells seen in atrophy / tamoxifen therapy
- Keratinizing high grade lesions
- HSIL cells with more cytoplasm that is abnormally keratinized
- Often with anisokaryosis and marked variation of cellular shape, including elongate, spindle, caudate and tadpole cells
- Seen singly or in clusters
- HSIL in atrophy
- HSIL cells are generally small in background of atrophy, making it difficult to differentiate from benign atrophic cells
- Syncytium-like aggregates / hyperchromatic crowded groups
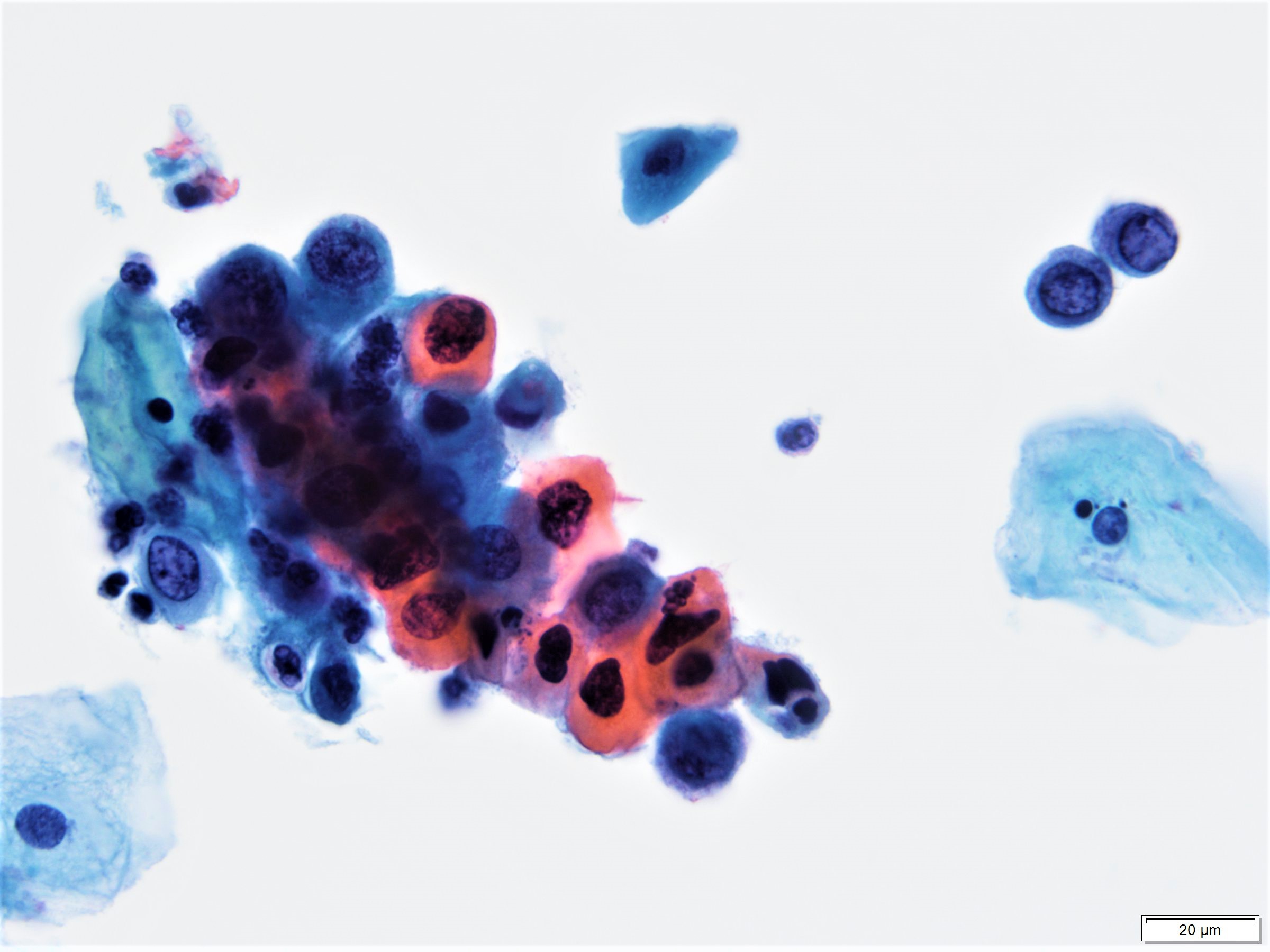
Cytology images
Sample pathology report
- Statement of adequacy:
- Satisfactory for evaluation
- Transformation zone component present
- Final interpretation:
- Epithelial cell abnormality, squamous cell
- High grade squamous intraepithelial lesion (HSIL)
Differential diagnosis
- Squamous metaplasia:
- Less nuclear enlargement and lower N/C ratio
- Minimal to mild nuclear membrane abnormalities
- If reactive, may have nucleoli
- Transitional cell metaplasia:
- Characteristic nuclear morphology showing longitudinal grooves (frequent coffee bean shaped nuclei suggest metaplastic changes)
- Minimal hyperchromasia
- Tubal metaplasia:
- Apical terminal bar and cilia
- Same sized nuclei as squamous metaplastic nuclei
- Basally placed nucleus with smooth nuclear contours
- May have higher N/C ratio than normal endocervical cells
- Endometrial cells:
- Exfoliated:
- Degenerated, small nuclei with high N/C ratio
- Small nucleoli may been seen
- Apoptotic bodies may been present within shedding endometrial groups
- Directly sampled:
- Nuclei slightly larger than those of intermediate cells
- Lower N/C ratio
- Smooth nuclear membranes
- Maintain nuclear polarity when seen in clusters with associated endometrial stromal cells
- Exfoliated:
- Endocervical cells:
- Presence of small nucleoli
- Finely granular and evenly distributed chromatin
- Smooth nuclear contours
- Granular or finely vacuolated cytoplasm, occasionally with some elongation
- Exfoliated cells may have rounded up appearance and high N/C ratio
- Retain columnar cytoplasmic configuration with eccentrically placed nuclei
- Endocervical polyp atypia:
- Single cells with highly atypical and hyperchromatic nuclei
- Correlation with clinical history and histology is important
- IUD effect:
- Variable amount of cytoplasm and N/C ratio
- Degenerative nuclei with wrinkled or smudgy dark chromatin
- Vacuolated cytoplasm, may have signet ring appearance
- Atrophy:
- Variable N/C ratio
- Chromatin is uniformly distributed and finely textured
- Nuclear contour / membrane is smooth
- Smudgy or degenerated nuclear chromatin
- No mitoses
- Decidualized stromal cells:
- More granular, less dense cytoplasm
- Prominent basophilic nucleolus
- Lack of any evidence of HPV cytopathic effect
- Larger nucleus and higher N/C ratio can mimic HSIL
- Clinical history of pregnancy
- Atypical squamous cells, cannot exclude HSIL (ASC-H):
- Recommended in cases with cells showing features of squamous metaplasia and nuclear atypia for which it is difficult / impossible to exclude HSIL
- Atypical squamous cells of undetermined significance (ASC-US) associated with atrophy:
- Recommended in cases with marked squamous atypia associated with atrophy that is difficult / impossible to distinguish from HSIL
- Adenocarcinoma in situ (AIS):
- Hyperchromatic nuclei with fine to coarse chromatin
- Nuclear membrane irregularities and notching
- High N/C ratio
- Feathering or rosette formation
- Squamous cell carcinoma:
- Prominent nucleoli
- Irregular chromatin distribution
- Necrotic debris
- Inflammatory cells:
- Histiocytes:
- Small to medium sized, oval kidney bean nuclei
- Sometimes prominent longitudinal groove
- Finely textured, normochromatic
- Abundant foamy vacuolated cytoplasm
- Lymphocytes:
- Small round nuclei with coarse chromatin to larger nuclei with open chromatin
- Minimal cytoplasm
- May be seen with tingible body macrophages, plasma cells and dendritic cells
- Histiocytes:
Practice question #1
A 37 year old woman with a previous history of recent pregnancy, recurrent sexually transmitted infections, intrauterine device use, persistent high risk HPV and LSIL, undergoes cervical cytology testing as part of her routine preventative health care visit. Cytomorphology is shown in the image above. Taking into account the clinical and cytomorphological findings, what is the most correct diagnosis?
- Atrophic changes
- High grade squamous intraepithelial lesion (HSIL)
- Intrauterine device related changes
- Tubal metaplasia
Practice answer #1
B. High grade squamous intraepithelial lesion (HSIL). High grade squamous intraepithelial lesion (HSIL) changes consist of immature, delicate or metaplastic cytoplasm. The nuclear features generally contain high N/C ratios, nuclear envelope irregularities and generally hyperchromatic nuclei, although hypochromatic forms can be present. The chromatin is usually evenly dispersed. Nucleoli are generally absent but can be seen when HSIL extends into endocervical gland spaces or in the background of reactive or reparative change. Atrophic changes (A) generally have a clinical history of advanced age or menopause. The morphologic features include variable N/C ratio, degenerated nuclear chromatin, smooth nuclear membranes and no mitotic activity. Intrauterine device related changes (C): while this patient has history of IUD use, she also has history significant for LSIL and positive hrHPV increasing pretest probability for HSIL. Generally IUD changes consist of degenerative nuclei, smudgy dark chromatin and vacuolated cytoplasm. Tubal metaplasia (D) is an HSIL mimic and generally contains apical terminal bar and cilia, nuclear size roughly similar to squamous metaplastic cells, basally located nucleus, smooth nuclear contours and can have higher N/C ratios than normal endocervical cells.
Comment Here
Reference: HSIL cytology
Comment Here
Reference: HSIL cytology
Practice question #2
Practice answer #2
B. High grade squamous intraepithelial lesion (HSIL). An immunocompromised state increases a patient's risk of persistent infection and development of a squamous intraepithelial lesion (SIL). Specifically, smoking, immunosuppression (e.g. transplant or human immunodeficiency virus [HIV / AIDS]) or radiation therapy are risk factors for low grade squamous intraepithelial lesions (LSIL) progression to HSIL. In this example, HSIL cells demonstrate delicate cytoplasm, variation of nuclear size and shape, nuclear grooves, nuclear envelope irregularities and abnormal chromatin. Low grade squamous intraepithelial lesion (LSIL) (C) would be expected to have lower nuclear to chromatin ratio (for nuclear enlargement more than ≥ 3, the area of normal intermediate nuclei results in a slightly increase nuclear to cytoplasmic ratio), mature cytoplasm, koilocytic change (perinuclear cavitation consisting of a broad, sharply delineated clear perinuclear zone and a peripheral rim of densely stained cytoplasm). Atrophy (A) distinguishing features are absent. These include clinical history of menopause or advanced age, uniformly distributed chromatin, smooth nuclear contours, nuclear to chromatin ratio can be increased, degenerated nuclear chromatin, no mitoses. Tubal metaplasia (D) is an HSIL mimic and generally contains apical terminal bar and cilia, nuclear size roughly similar to squamous metaplastic cells, basally located nucleus, smooth nuclear contours and can have higher N/C ratios than normal endocervical cells.
Comment Here
Reference: HSIL cytology
Comment Here
Reference: HSIL cytology