Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Flow cytometry images | Videos | Differential diagnosis | Additional references | Practice question #1 | Practice answer #1 | Practice question #2 | Practice answer #2Cite this page: Arshi J, Huber AR. Celiac sprue. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/smallbowelceliacsprue.html. Accessed September 13th, 2025.
Definition / general
- Celiac sprue / celiac disease (gluten sensitive enteropathy) is an immune mediated inflammatory disease of the small intestine seen in genetically predisposed individuals and is caused by sensitivity to prolamins, like wheat (gliadin), Barley (hordein), rye (secalin) and oats (avenin)
- Innate and adaptive immune response to prolamins leads to characteristic infiltration of the lamina propria and the epithelium with chronic inflammatory cells and villous atrophy, leading to malabsorption
Essential features
- Immune mediated disease characterized by intraepithelial lymphocytosis in the small bowel secondary to gluten ingestion
- Partial or total atrophy of intestinal villi and hyperplasia of intestinal crypts with chronic inflammation in the lamina propria
- Serologic testing in combination with biopsy confirmation required for diagnosis
Terminology
- Celiac disease
- Sprue
- Nontropical sprue
- Gluten sensitive enteropathy
- Gluten induced enteropathy
ICD coding
- ICD-10: K90.0 - celiac disease
Epidemiology
- Seroprevalence rate: 1.4% worldwide (1.3% in South America; 1.8% in Asia) (Am J Gastroenterol 2021;116:1148)
- Prevalence of biopsy diagnosed celiac disease: 0.7% worldwide (Gastroenterology 2021;160:63)
- M:F = 1:1.85 (Dig Dis Sci 2018;63:184)
- Mean age at diagnosis is 8.4 years
- Bimodal distribution: first at 8 - 12 months and second in third to fourth decades
- Associated with autoimmune diseases (dermatitis herpetiformis, type 1 diabetes mellitus, Hashimoto thyroiditis, Graves disease, etc.), idiopathic diseases (dilated cardiomyopathy, epilepsy, multiple sclerosis, etc.) and chromosomal diseases (Down syndrome, Turner syndrome and William syndrome) (BMC Med 2019;17:142)
Sites
- Small bowel
Pathophysiology
- HLA class II genes HLA DQ2 and HLA DQ8 on chromosome 6 are implicated in the genetic susceptibility
- Up to 20% of the first degree relatives are affected by the disease indicating strong hereditary component
- HLA molecules on antigen presenting cells present the laminins to CD4+ T cells and activate them
- Environmental trigger is the ingestion of a diet containing gluten
- Fragments of gluten peptides resistant to degradation are transported across the epithelium by transcellular pathways
- Altered intestinal permeability and release of cellular contents, including the enzyme tissue transglutaminase (tTG)
- CD71 (transferrin) receptor is found to be upregulated in active celiac disease
- IFNγ and IL21 are released, which result in epithelial damage following activation of T cells by antigen presenting cells
- Innate immune response by intraepithelial lymphocytes (IELs), which express NK receptors MHC class I related chain A and B and HLE on epithelial cells and lead to destruction of epithelium
- IL15 upregulates NK receptors in cytotoxic intraepithelial lymphocytes and leads to T cell receptor independent killing
- Refractory sprue: where the disease persists despite avoidance of laminins, intraepithelial lymphocytes acquire a highly activated NK phenotype
- 2 types (Gastrointest Endosc Clin N Am 2012;22:639):
- Refractory celiac disease type I:
- Intraepithelial lymphocytes express CD3 and CD8, as well as TCRβ; similar to patients with celiac disease
- Good prognosis when combined with immunosuppressive therapy
- Refractory celiac disease type II:
- Lack CD8, CD4 and TCRαβ
- Have intracellular CD3ε and a clonal TCR gene rearrangement
- Carry dismal prognosis
- Refractory celiac disease type I:
- 2 types (Gastrointest Endosc Clin N Am 2012;22:639):
Etiology
- Genetic factors: HLA DQ2 and HLA DQ8
- Ingestion of gluten containing diet
- Innate and adaptive immune responses
- Several research studies suggested a reduction in the diversity of gut microbiota and increase in Firmicutes / Bacteroidetes (Adv Nutr 2020;11:160)
Diagrams / tables
Modified Marsh-Oberhuber classification of histologic findings in celiac disease:
| Marsh type | IEL/100 enterocytes: jejunum | IEL/100 enterocytes: duodenum | Crypt hyperplasia | Villi |
| 0 | < 40 | < 30 | Normal | Normal |
| 1 | > 40 | > 30 | Normal | Normal |
| 2 | > 40 | > 30 | Increased | Normal |
| 3a | > 40 | > 30 | Increased | Mild atrophy |
| 3b | > 40 | > 30 | Increased | Marked atrophy |
| 3c | > 40 | > 30 | Increased | Complete atrophy |
| 4 | > 40 | > 30 | Atrophic | Severe (flat) |
Images hosted on other servers:
Clinical features
- Celiac disease in pediatric population is characterized by diarrhea, loss of appetite, abdominal distension and failure to thrive
- Older children: diarrhea, bloating, constipation, abdominal pain or weight loss
- Adults: malabsorption syndrome with chronic diarrhea, weight loss and asthenia
- Extraintestinal manifestations:
- Iron deficiency anemia (microcytic)
- Macrocytic anemia due to vitamin B12 deficiency
- Osteopenia, osteoporosis due to altered absorption of calcium and vitamin D3
- Growth retardation; tooth enamel defects, aphthous stomatitis, hypertransaminasemia
- Dermatitis herpetiformis
- Nonspecific symptoms: headache, paresthesia, neuroinflammation, anxiety and depression
- Changes in reproductive system like late menarche, amenorrhea, recurrent miscarriages, premature birth, early menopause in females and changes in number and mobility of spermatozoa in males
- Refractory celiac disease (RCD) is a persistent malabsorption and villous atrophy despite strict adherence to a gluten free diet for at least 6 - 12 months in the absence of other causes and overt malignancy
- Type I and type II: severe complications like ulcerative jejunitis and enteropathy associated T cell lymphoma is seen in some patients with refractory celiac disease
Diagnosis
- Gold standard for diagnosis is serology with confirmation of histology on duodenal biopsy
- Serologic testing: tTGA, EMA and IgA class antigliadin antibodies (AGA) are the serologic tests
- Endoscopy: hallmark endoscopic finding is patchy villous atrophy in the duodenum and is identified by magnification endoscopy or chromoendoscopy
- Scalloping or notching of mucosal folds can be seen
- Sensitivity is low
- For microscopic evaluation, 1 - 2 biopsy specimens should be taken from duodenal bulb and at least 4 specimens should be taken from postbulbar duodenum
- HLA DQ association test: identifies HLA DQ2 and HLA DQ8
- Negative test essentially rules out celiac disease but is also seen in populations without celiac disease
- See diagrams / tables
Laboratory
- tTGA has the highest sensitivity for celiac disease (98%); specificity is 90% (Arch Pathol Lab Med 2012;136:735)
- Positive even in individuals on gluten free diet
- EMA, while having a lower sensitivity, shows an almost absolute specificity (Arch Pathol Lab Med 2012;136:735)
- IgA class antigliadin antibodies (AGA) is now an obsolete test (Arch Pathol Lab Med 2012;136:735)
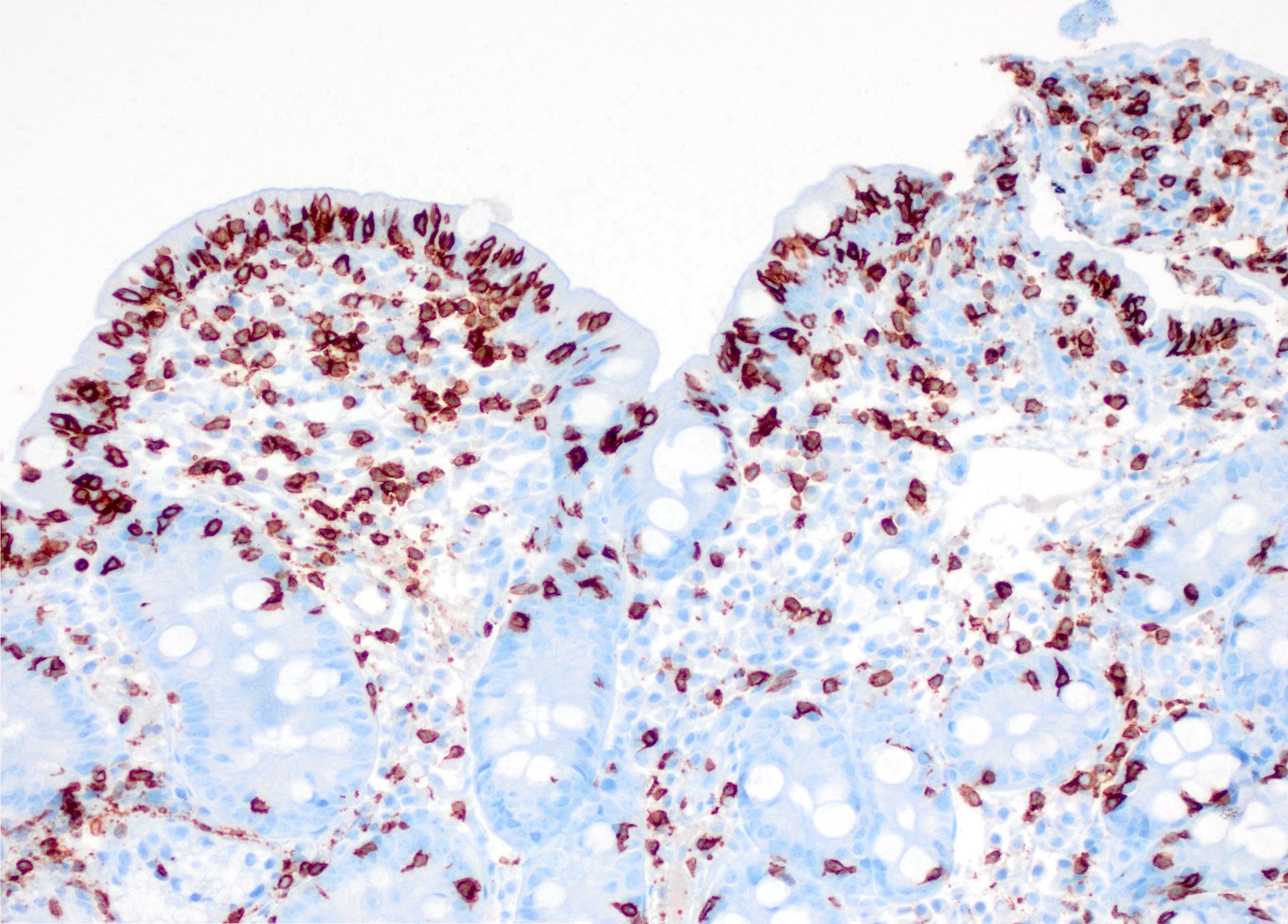
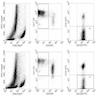
- Refractory celiac disease type 2 is characterized by loss of multiple surface T cell markers like CD3, CD7 and CD8 in more than 20% of the intraepithelial lymphocytes on flow cytometry
- Flow cytometry: quantification of intraepithelial lymphocytes by flow cytometry (called IEL lymphogram), demonstrates that the IELs are antigen experienced T lymphocytes and bear the αβ (> 90%) and γδ (< 10%) receptors
- In celiac disease, the total IELs are increased, along with a permanent increase in γδ IELs and a decrease in CD3 IELs (Auto Immun Highlights 2016;7:14)
Radiology description
- Fluoroscopy: small intestinal dilatation, dilution of contrast, multiple nonobstructing intussusceptions (coiled spring appearance), moulage sign (dilated jejunal loop with complete loss of jejunal folds) and flocculation (coarse clumps of disintegrated barium)
- CT / MRI: jejunoileal fold pattern reversal (highest specificity); ileal fold thickening, perienteric stranding, submucosal fat deposition in longstanding cases, lymphadenopathy and ascites (Eur J Radiol 2008;65:483)
Prognostic factors
- Excellent prognosis with gluten free diet
- Refractory celiac disease type 2 is refractory to gluten free diet, tends to have a poor prognosis and is associated with enteropathy associated T cell lymphoma (typically within 5 years of onset) (Gut 2010;59:547)
- Enteropathy associated T cell lymphoma may also be seen in patients with long lasting, well controlled celiac disease
- Higher risk of small bowel adenocarcinoma
Case reports
- 6 year old girl presenting with celiac disease and aplastic anemia (J Med Case Rep 2018;12:16)
- 9 year old boy with celiac disease and aplastic anemia (Pediatr Dev Pathol 2014;17:470)
- 11 year old girl with complicated primary intestinal lymphangiectasia (Waldmann disease) successfully treated with octreotide (Ann Med Surg (Lond) 2021;68:102588)
- 39 year old man with undifferentiated chronic pulmonary disease, chronic anemia, celiac disease, atrial fibrillation and PLA2R positive membranous nephropathy (Respir Med Case Rep 2021;33:101446)
- 40 year old woman with cerebral arterial and venous sinus thrombosis revealing celiac disease (BMC Gastroenterol 2020;20:327)
Treatment
- Repletion of nutritional deficiencies
- Prevention of bone loss
- Treatment with sulfones for dermatitis herpetiformis
- Gluten (laminin) free diet
- Transglutaminase 2 inhibitor
- Hepatitis B and pneumococcal vaccination
Gross description
- Flattening or blunting of villi in the small bowel
- Ulceration may be seen in severe cases
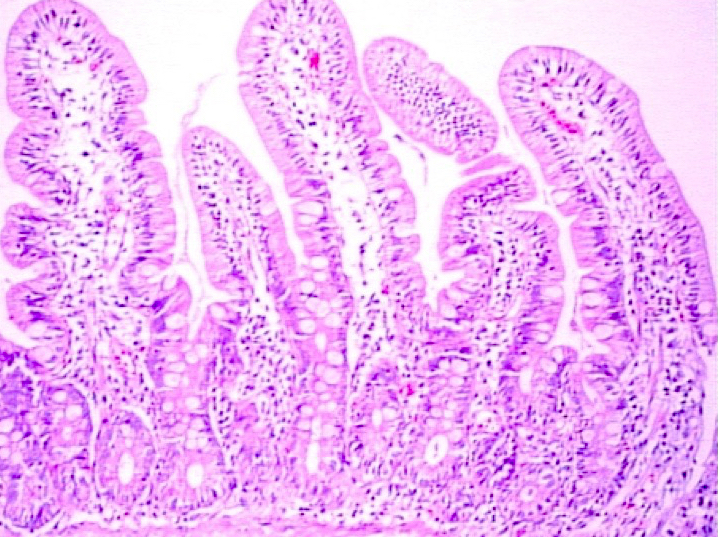
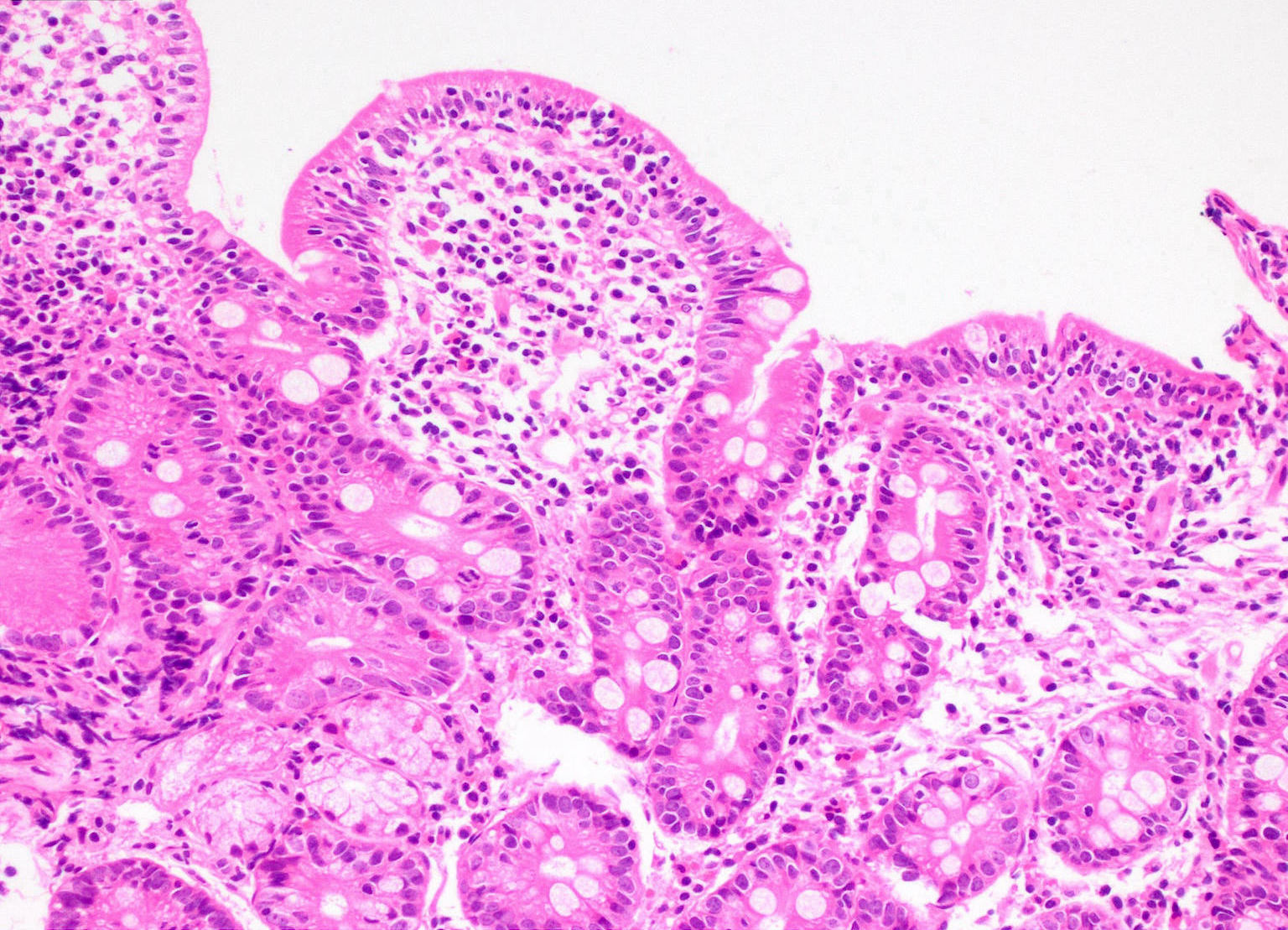
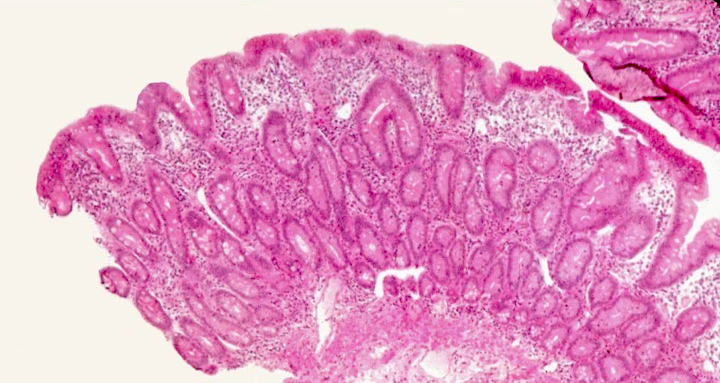
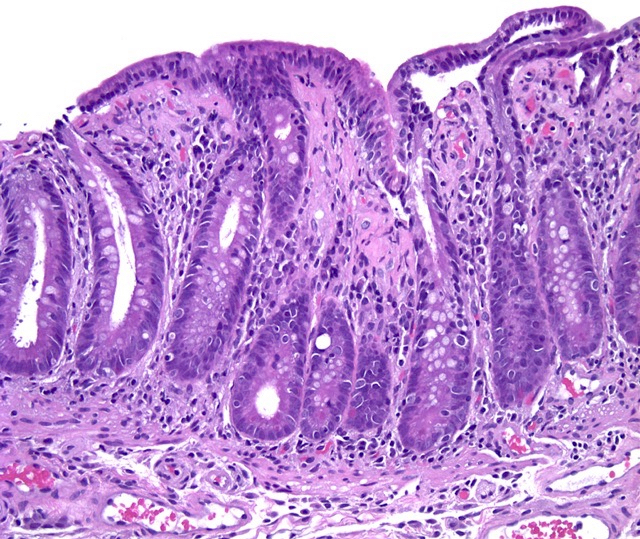
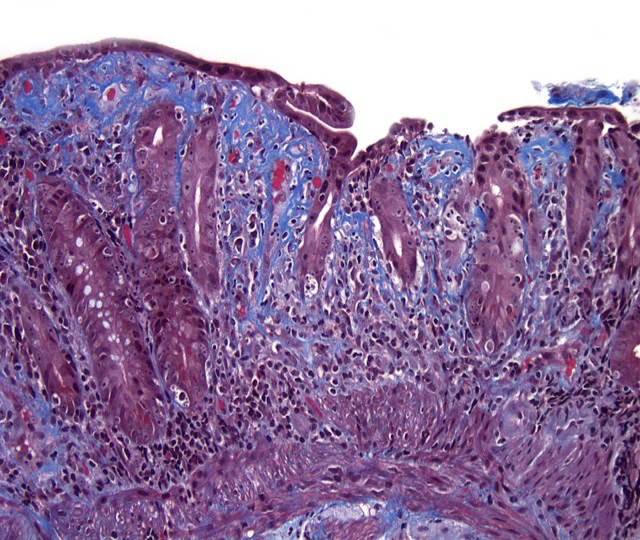
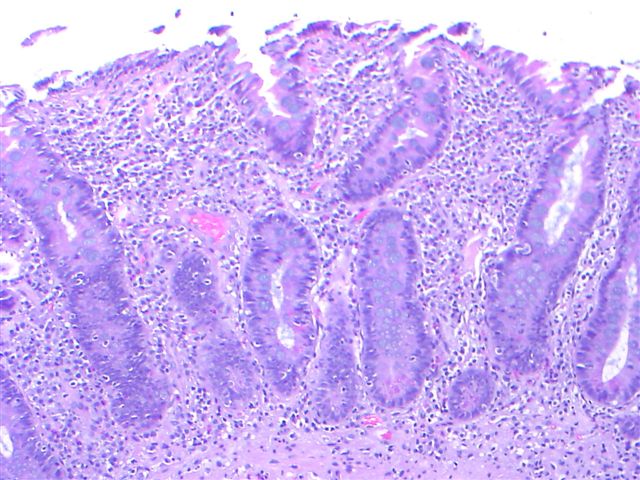
Microscopic (histologic) description
- Histological elementary lesions (Dig Liver Dis 2011;43:S385, Semin Diagn Pathol 2014;31:124):
- Increased intraepithelial T lymphocytes (IEL):
- 25 - 29 IEL/100 enterocytes is considered borderline
- > 30 IEL/100 enterocytes represents a pathological lymphocytosis
- Decreased enterocyte height, flattening of enterocytes, intracytoplasmic vacuolation and reduction or absence of brush border are suggestive but not specific
- Crypt hyperplasia:
- Extension of the regenerative epithelial crypts associated with changes in the presence of more than 1 mitosis per crypt
- Villous atrophy:
- Decrease in villous height, normal villous:crypt ratio (3:1) until total disappearance of villi
- This assessment requires proper orientation of the biopsies
- Increased intraepithelial T lymphocytes (IEL):
- Diagnostic categories are based on these elementary lesions:
- Modified Marsh-Oberhuber classification of histologic findings in celiac disease
- Simplified systems (Corazza & Villanaci or Ensari), which may be more reproducible (Arch Pathol Lab Med 2010;134:826, Pathol Res Pract 2016;212:1174)
- Different grades of duodenal mucosal lesions:
- Grade A / type 1: increased intraepithelial lymphocytes but no villous atrophy
- Grade B1 / type 2: villi still present but shortened
- Grade B2 / type 3: complete villous atrophy
Microscopic (histologic) images
Videos
Celiac Disease: A Fairly Advanced Lecture for Primary Healthcare Providers
Differential diagnosis
- Tropical sprue:
- Variable villous blunting and contains more lamina propria eosinophils than celiac disease
- Typically involves proximal duodenum and spares terminal ileum
- Autoimmune enteropathy:
- Marked villous atrophy with apoptosis at bases of crypts and lymphocytes infiltrating the crypts but not surface epithelium
- Goblet cells, paneth cells and endocrine cells may be lost
- Common variable immunodeficiency:
- Apoptotic enterocytes with reduced or absent plasma cells
- Villous blunting and increased intraepithelial lymphocytes are present
- Food allergy:
- Hypersensitivity to food antigens other than gluten, including cows milk, soy protein, fish, rice and chicken
- May also be associated with increased numbers of intraepithelial lymphocytes, as well as architectural changes in the form of patchy or diffuse disease
- Crohn’s disease:
- Neutrophils in lamina propria and epithelium of duodenum with patchy involvement
- Collagenous sprue:
- Patchy, excessive, subepithelial collagen deposits
- Eosinophilic gastroenteritis:
- Dense eosinophilic and mast cell infiltrate
- Inflammatory bowel disease
- HIV enteropathy:
- Increased mitosis in glandular epithelial cells and increased number of apoptotic enterocytes at the surface
- Giardiasis:
- Variable villous blunting with tear drop shaped organisms with paired nuclei (size similar to enterocyte nuclei)
- Drugs (sartans, mycophenolate, NSAIDs):
- Serum tTg is not elevated
- Intestinal lymphoma:
- Monomorphic lymphocytes with irregular nuclear membranes
Additional references
Practice question #1
Practice answer #1
C. Intraepithelial lymphocytosis. All celiac disease patients, except for those with Marsh type 0, show intraepithelial lymphocytosis.
Comment Here
Reference: Celiac sprue
Comment Here
Reference: Celiac sprue
Practice question #2
What is the immunophenotype of intraepithelial lymphocytes increased in celiac disease?
- CD2+, CD3-, CD45- aberrant T cell population
- CD3+, CD7+, CD103+, granzyme B and TCRαβ
- CD3+, T cells with αβ and γδ receptors
- CD4+, CD8+, CD3- T cells
- CD4+, T cells with γε and αδ receptors
Practice answer #2