Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1Cite this page: Ferreira J, Félix A. Carcinosarcoma (MMMT). PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/uterusMMMT.html. Accessed May 14th, 2024.
Definition / general
- Biphasic, malignant tumor with high grade epithelial and sarcomatous components
- Sarcomatous component is derived from the carcinomatous component as a result of metaplasia / transdifferentiation (epithelial to mesenchymal transition)
Essential features
- Rare, aggressive neoplasm
- Occurs in postmenopausal women, most frequently in the uterine corpus
- Biphasic tumor with malignant epithelial (more frequently high grade carcinoma) and sarcomatous (can be homologous and heterologous) components
- Staged like endometrial carcinomas according to the International Federation of Gynecology and Obstetrics and the American Joint Committee on Cancer staging classifications
Terminology
- Malignant mixed Müllerian tumor, malignant mesodermal mixed tumor, metaplastic carcinoma
ICD coding
- ICD-O: 8980/3 - Carcinosarcoma, NOS
Epidemiology
- Rare (< 5% of gynecological carcinomas)
- Almost always in postmenopausal women (mean age: 65 years)
- Higher incidence in black women
- References: J Gynecol Oncol 2018;29:e22, Gynecol Oncol 2004;93:204
Sites
- Most frequent in the uterine corpus
- Can also arise in the cervix, ovaries, fallopian tubes, vagina, peritoneum and extragenital sites
Pathophysiology
- 4 theories have been proposed (J Clin Pathol 2002;55:321):
- Collision theory: the sarcoma and carcinoma are two independent neoplasms
- Combination theory: both components are derived from a single stem cell that undergoes divergent differentiation early in the evolution of the tumor
- Conversion theory: the sarcomatous element derives from the carcinomatous element during the evolution of the tumor
- Composition theory: the spindle cell component is a pseudosarcomatous stromal reaction to the carcinoma
- The Cancer Genome Atlas (TCGA) data supports the conversion and combination theories (Nat Commun 2019;10:4965, Cancer Cell 2017;31:411)
- Carcinomatous cells convert themselves to sarcomatous cells via epithelial to mesenchymal transition; this is supported by high epithelial to mesenchymal transition gene signature scores and is likely due to epigenetic alterations at microRNA promoters and histone gene mutations and amplifications (Cancer Cell 2017;31:411, Proc Natl Acad Sci U S A 2016;113:12238)
Etiology
- Almost all are sporadic
- Tamoxifen use and pelvic radiation therapy have been associated with an increased incidence
- Other predisposing factors include chronic estrogen exposure, nulliparity, diabetes and obesity
- References: Gynecol Oncol 2017;144:329, Cancer 1980;45:1625
Clinical features
- Vaginal bleeding, abdominal mass and pelvic pain are the usual presenting symptoms
- 10% of patients have distant metastasis at presentation
- Extrauterine spread in up to 45% of patients at presentation (Ann Oncol 2016;27:1257)
Diagnosis
- Frequently only rendered after surgical resection
Prognostic factors
- 5 year survival rate is approximately 30% (stage I - II: 59%; stage III: 25%; stage IV: 9%) (Gynecol Oncol 2010;116:419)
- This is a much more aggressive tumor than high grade endometrial carcinoma where the 5 year survival rate for stage I disease is over 80% (Am J Surg Pathol 2007;31:979)
- Stage is the most consistent independent predictor of outcome
- Presence of heterologous elements is a poor prognostic factor in early stage disease but its significance remains to be determined in advanced stage disease (Am J Surg Pathol 2007;31:1653)
- Metastatic component frequently of carcinomatous type but can be of sarcomatous component or of combinations of both; carcinoma components tended to spread lymphatically, while sarcoma components tended to spread locoregionally (Ann Oncol 2016;27:1257)
- Compared with other high grade endometrial carcinomas, lung metastasis are more frequent (Gynecol Oncol 2005;98:274)
Case reports
- 64 year old woman with carcinosarcoma of the uterine cervix (BMJ Case Rep 2019;12:e227050)
- 76 and 81 year old women with primary peritoneal carcinosarcomas (Taiwan J Obstet Gynecol 2019;58:288)
- 78 year old woman with a primary mesenteric carcinosarcoma with neuroendocrine differentiation (Mod Pathol 2001;14:515)
Treatment
- Total abdominal hysterectomy and bilateral salpingo-oophorectomy with pelvic lymphadenectomy
- Radiotherapy or chemotherapy
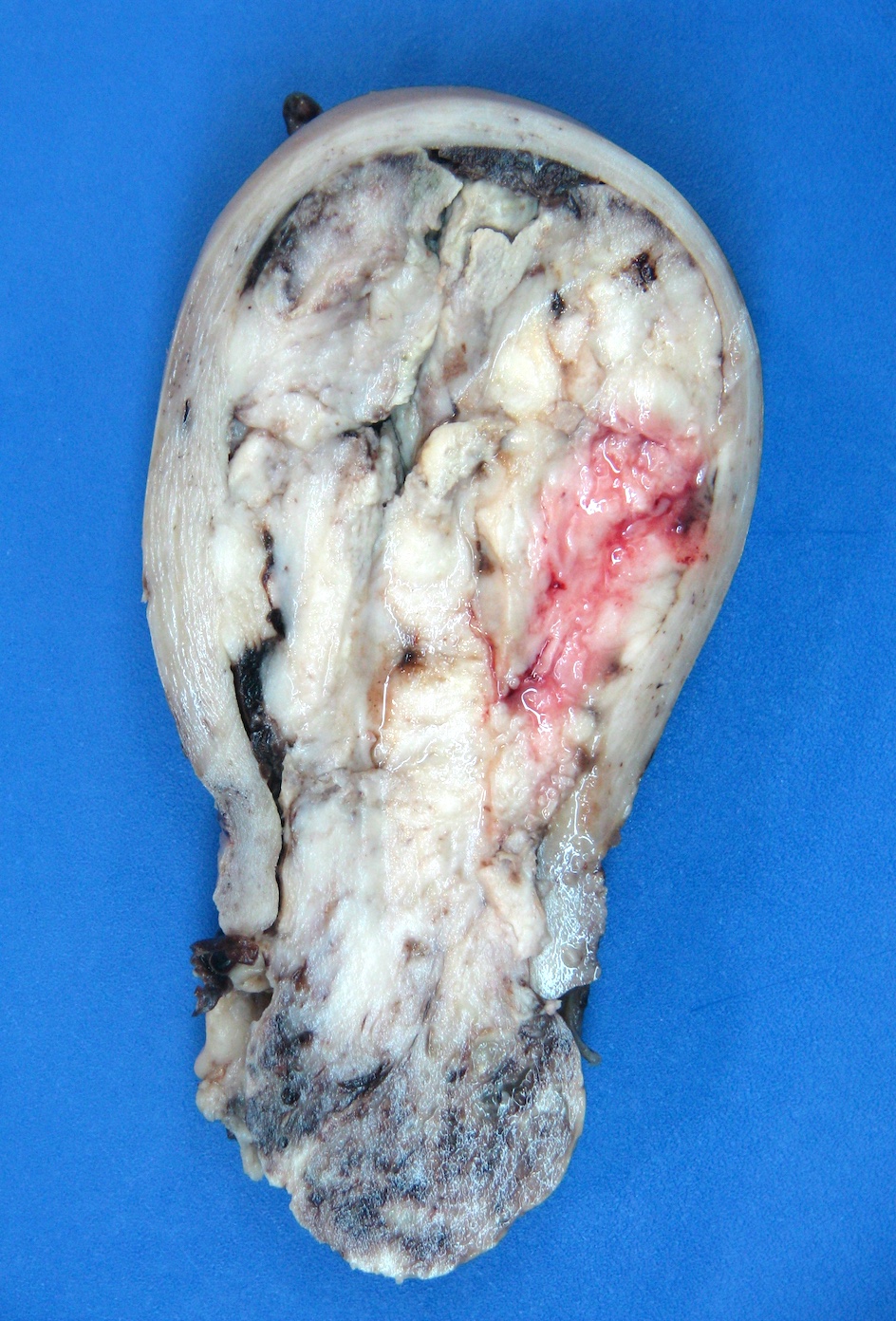
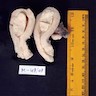
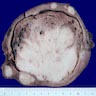
Gross description
- Polypoid lesions
- Fleshy, bulky and friable
- Hemorrhage and necrosis common
- May fill uterine cavity and protrude through cervical os
- Usually extensive myoinvasion and often extends beyond the uterus
- Reference: Semin Diagn Pathol 2010;27:274
Gross images
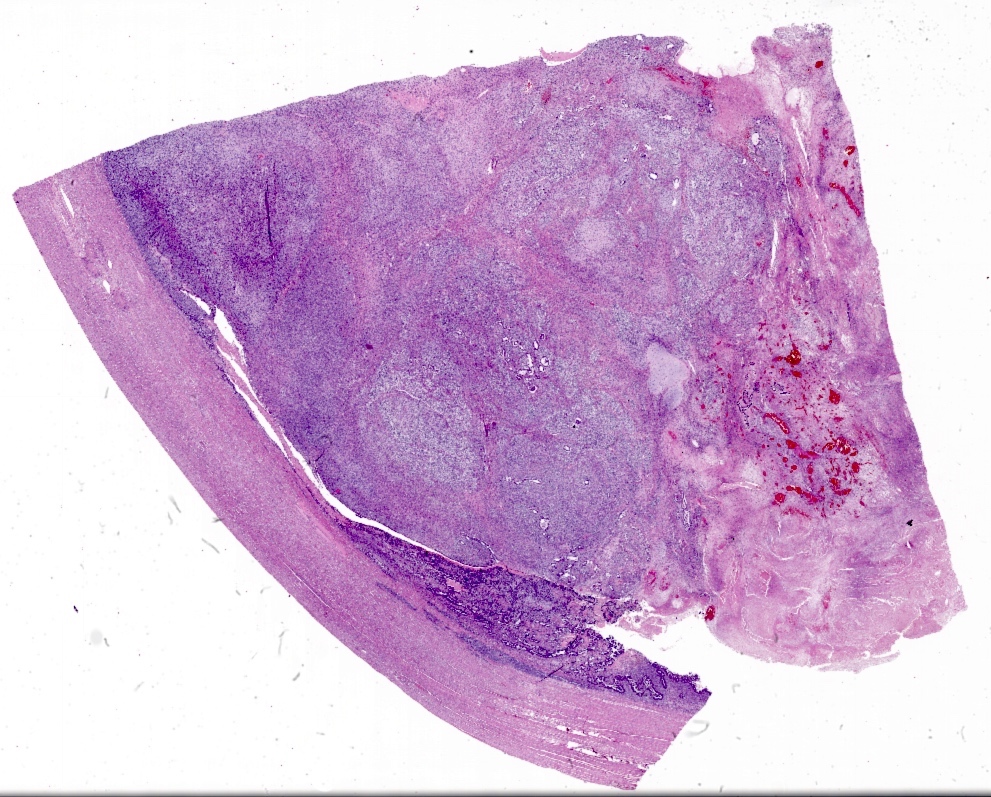
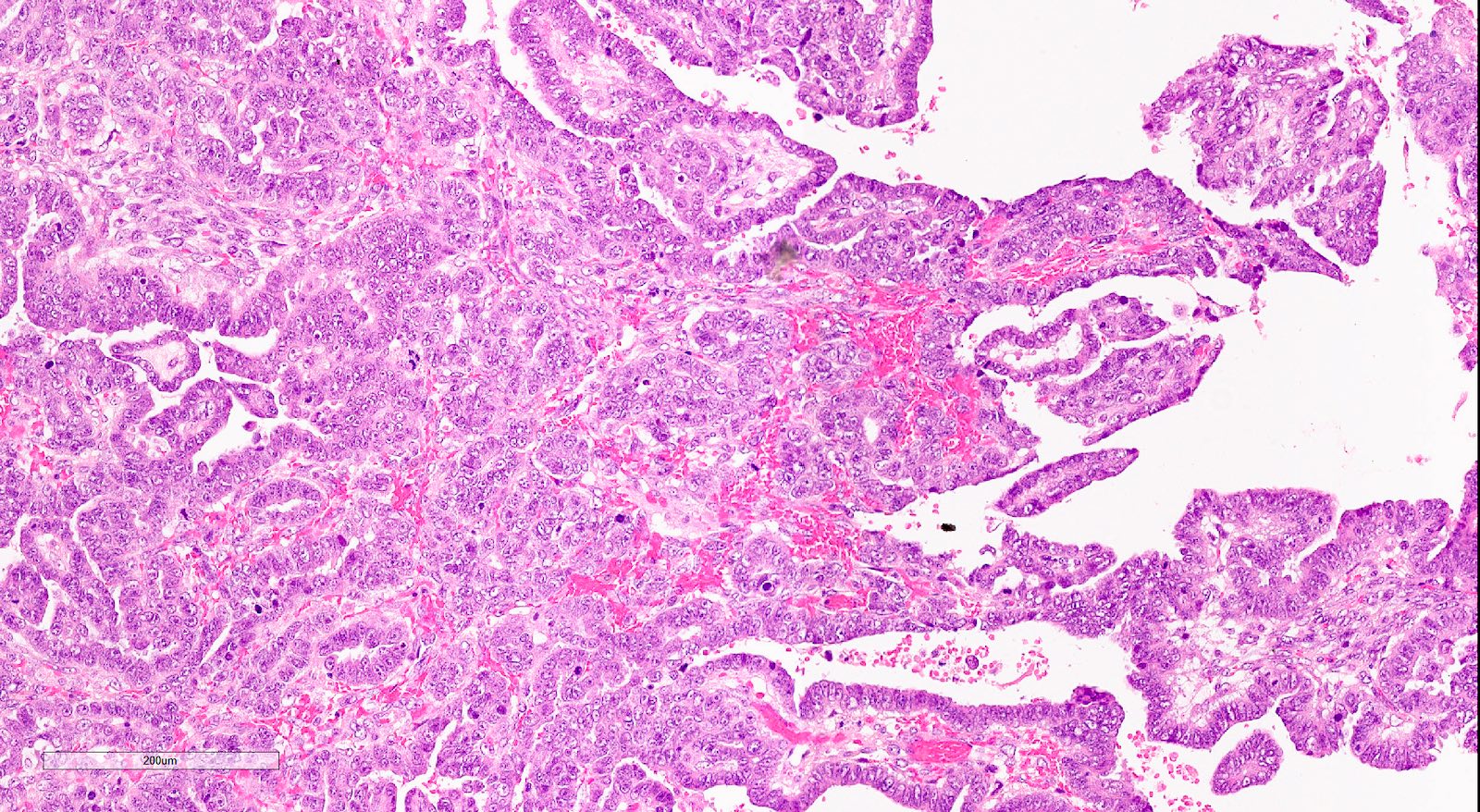
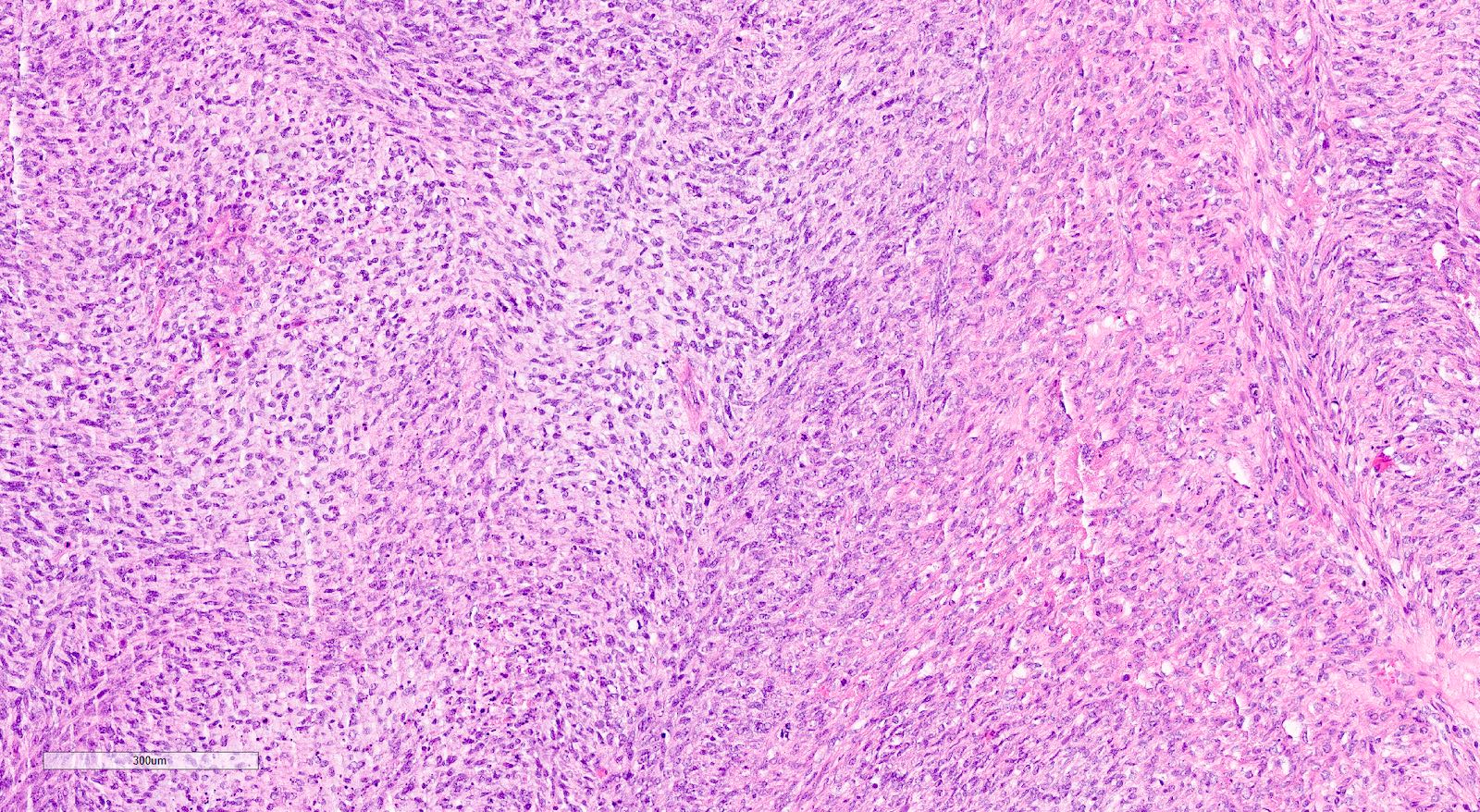
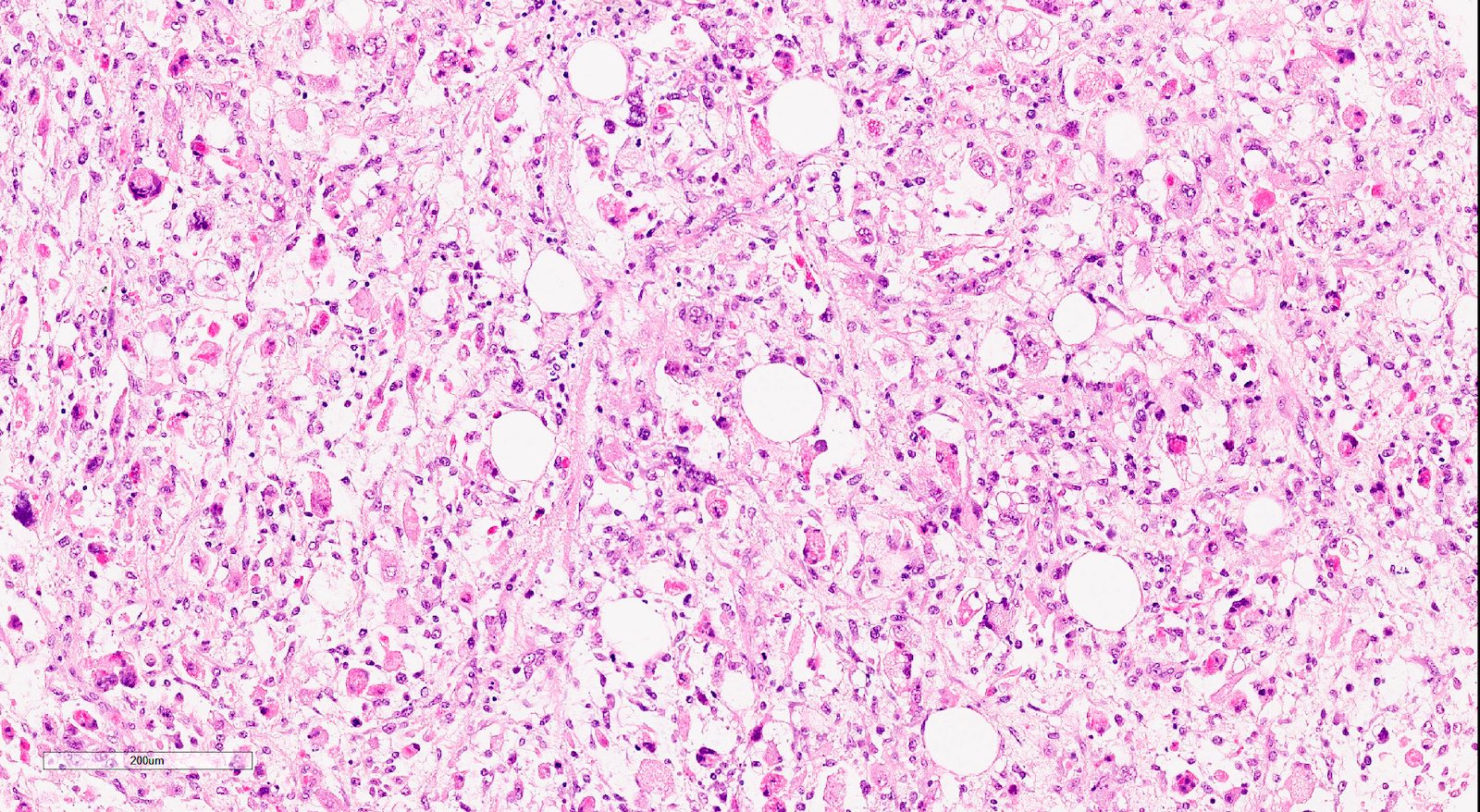
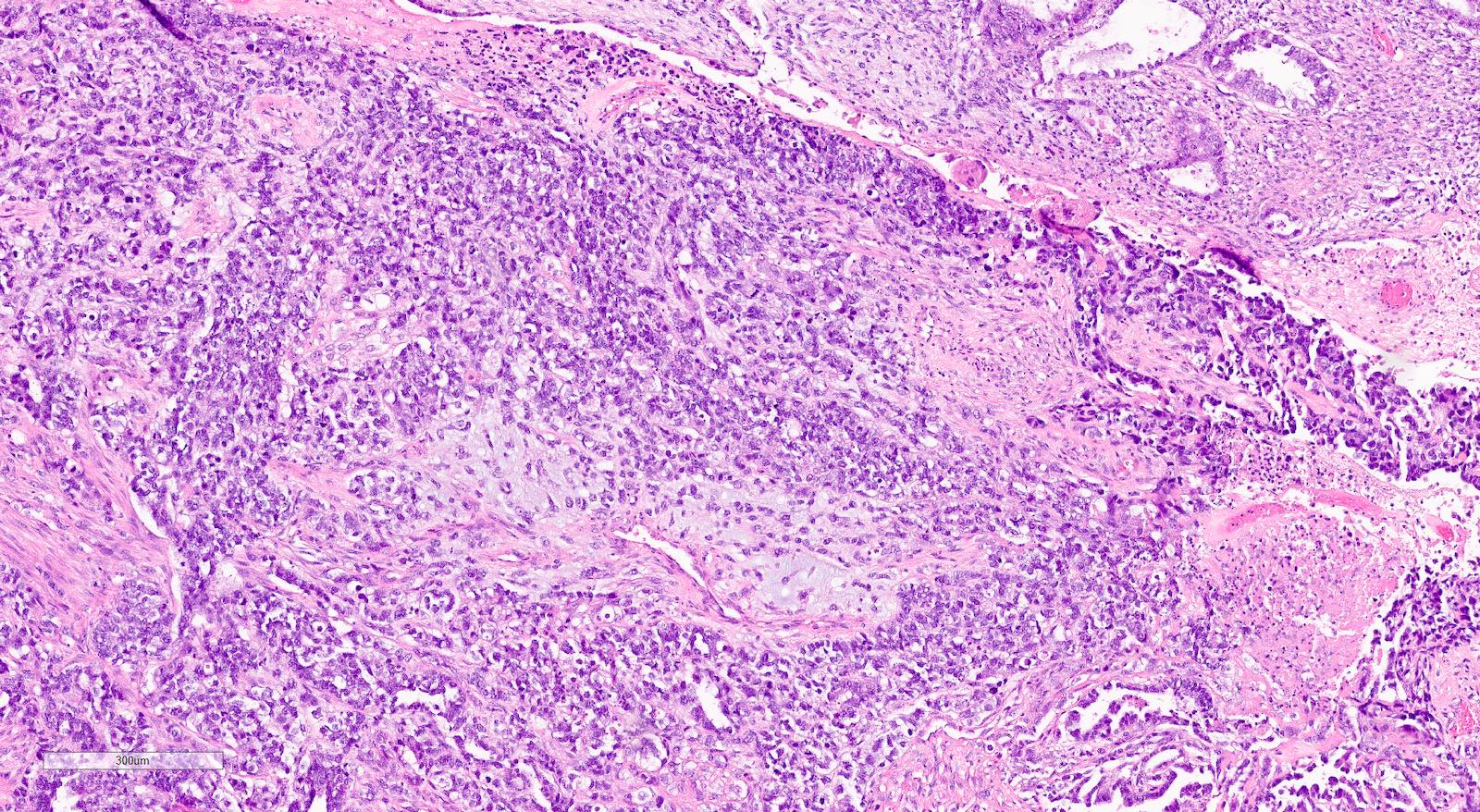
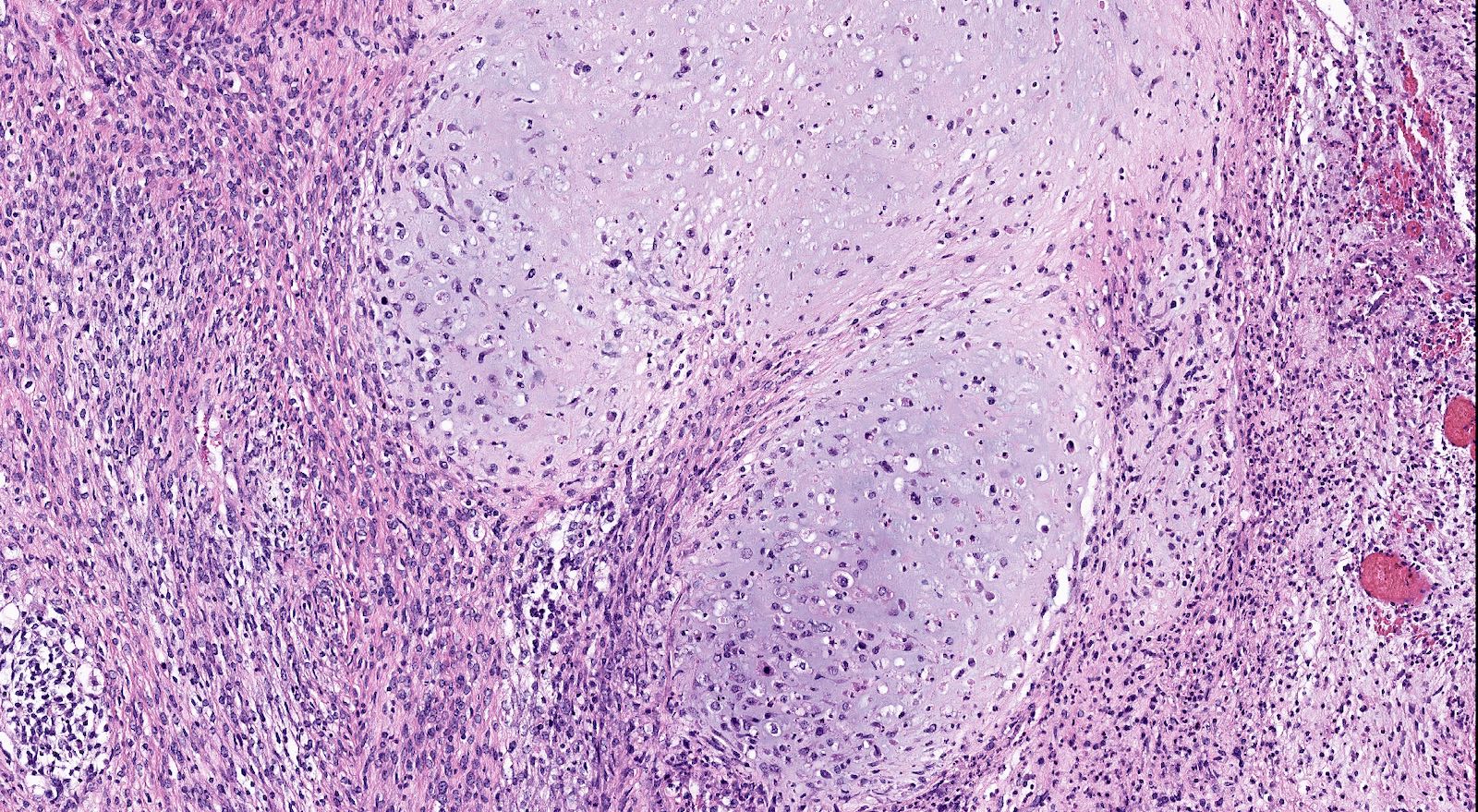
Microscopic (histologic) description
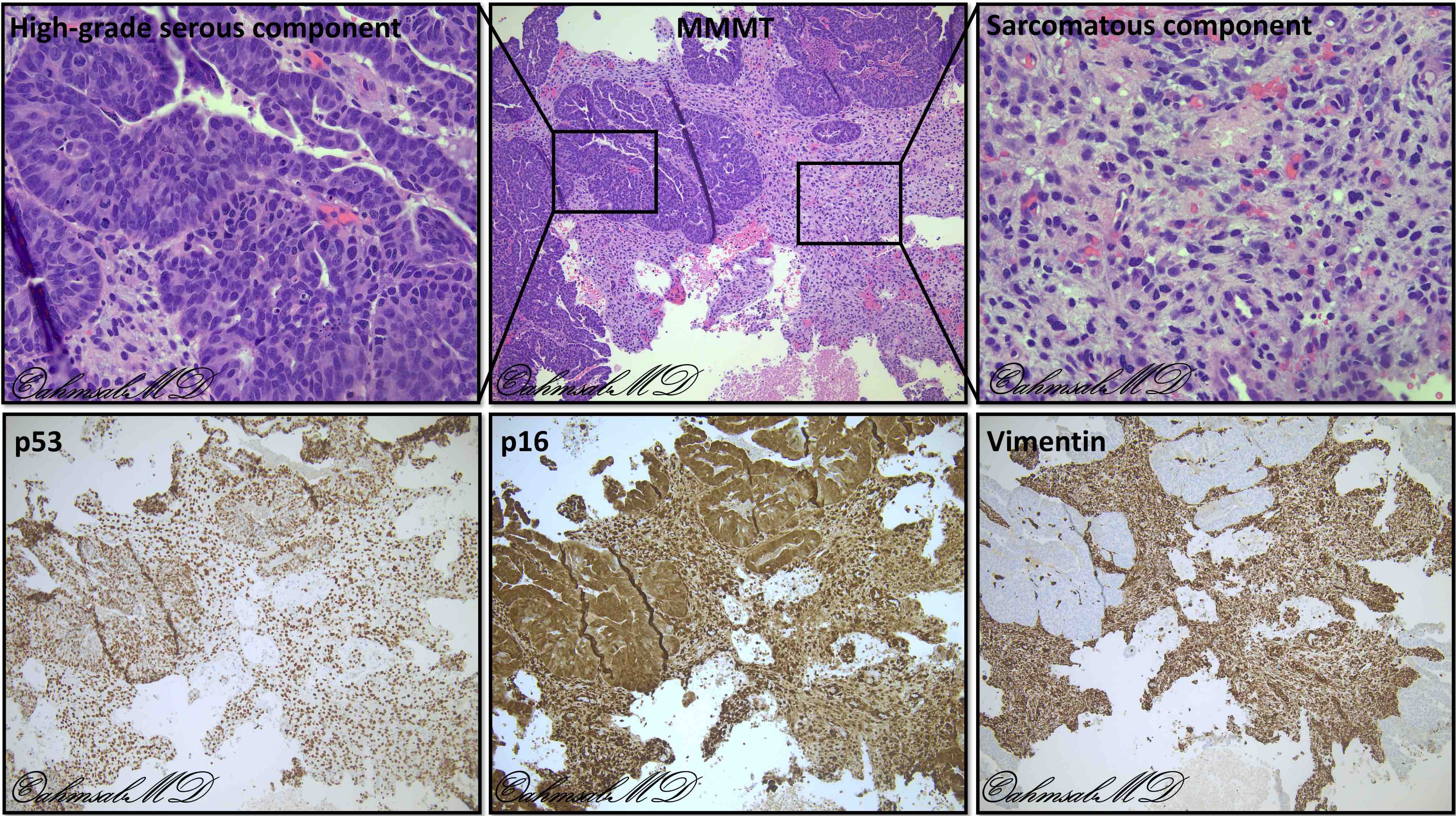
- Biphasic tumor with carcinomatous and sarcomatous elements, both high grade (Int J Gynecol Pathol 1990;9:1)
- Carcinomatous and sarcomatous components are juxtaposed
- Carcinomatous elements:
- Often high grade endometrioid or serous carcinoma, frequently admixed
- More uncommonly clear cell carcinoma
- 50 - 75% of cases have serous or mixed serous and high grade endometrioid carcinoma (Am J Surg Pathol 2007;31:1653)
- Hybrid morphology between endometrioid and serous carcinoma is frequent, as is undifferentiated carcinoma (Mod Pathol 2010;23:781)
- Other components that can be rarely found are squamous, mucinous and neuroendocrine
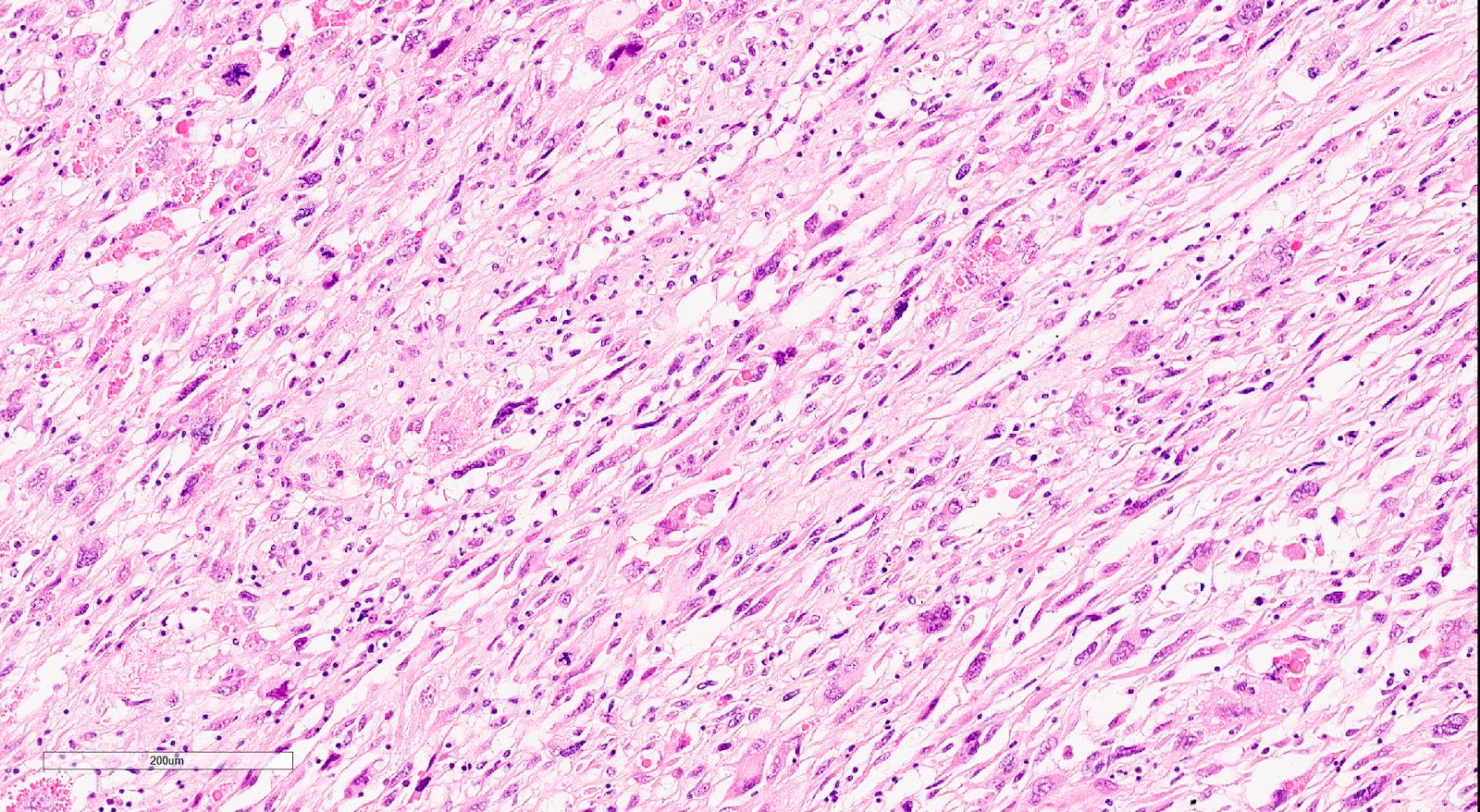
- Sarcomatous elements:
- Often spindle and pleomorphic
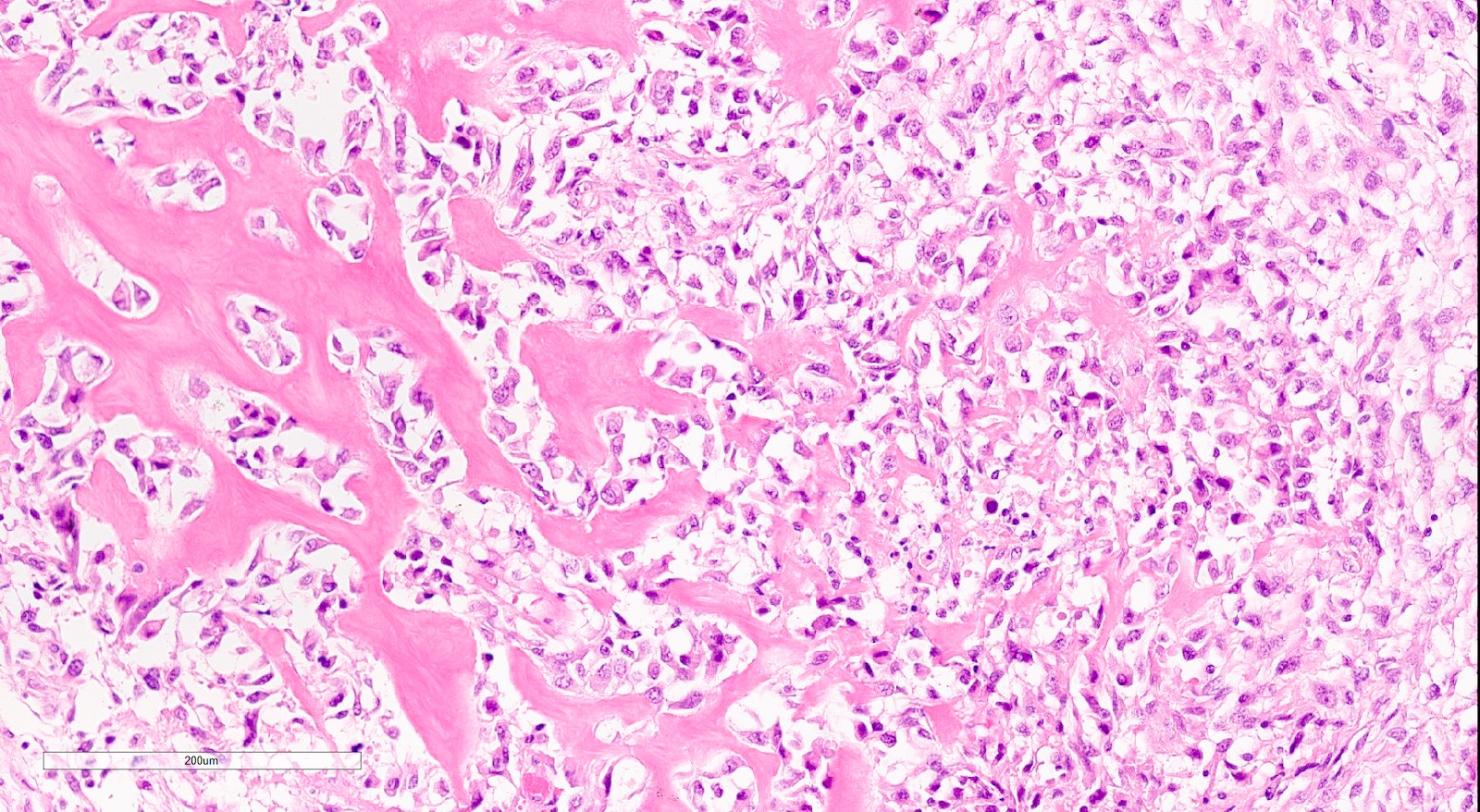
- 50% contain heterologous elements (most commonly rhabdomyosarcoma and chondrosarcoma) (Am J Surg Pathol 2007;31:1653)
- Osteosarcomatous, liposarcomatous and angiosarcomatous differentiation are less common (Semin Diagn Pathol 1988;5:199, Arch Pathol Lab Med 1991;115:583, Int J Gynecol Pathol 2017;36:140)
- Carcinomatous elements:
- Angiolymphatic invasion common, more commonly of the carcinomatous component
Microscopic (histologic) images
Cytology description
- Cytologic findings have been described on cervicovaginal smears, endometrial and peritoneal aspirates (Diagn Cytopathol 2019;47:547)
- Fine needle aspiration can be especially useful documenting recurrent or metastatic disease; demonstration of both sarcomatous and carcinomatous components can be difficult
Positive stains
- Immunohistochemistry is not necessary to establish the diagnosis
- Can be useful to demonstrate heterologous differentiation (skeletal muscle)
- Immunohistochemical loss of mismatch repair (MMR) proteins can occur but is infrequent (Am J Surg Pathol 2020;44:782)
- Some are HER2 positive (Mod Pathol 2020;33:118)
- Aberrant neuroendocrine markers expression is frequent (Pathology 2019;51:369)
- Carcinomatous component:
- PAX8, EMA, cytokeratin positive
- Both serous and high grade endometrioid carcinomas may show aberrant p53 expression (Int J Gynecol Pathol 2017;36:412)
- Endometrioid components can show ER, PR positivity
- Sarcomatous component:
- Often shows aberrant p53 expression (Int J Gynecol Pathol 2017;36:412)
- Rhabdomyosarcoma: desmin and myogenin
- Liposarcoma and chondrosarcoma: S100
Molecular / cytogenetics description
- TCGA endometrial carcinoma molecular subgroups can be applied to carcinosarcomas (Nat Commun 2019;10:4965):
- Proportions of POLE and MSI subtypes are similar to other types of endometrial carcinoma
- Copy number high (CNH) are much more common in carcinosarcomas
- Most share common features with high grade serous ovarian and serous endometrial carcinomas, although a minority has features consistent with an endometrioid subtype
- TP53 is frequently mutated
- Mutations in the phosphatidylinositol3-kinase pathway genes (PIK3CA, PTEN, PIK3R1) are also common
- Other significantly mutated genes include FBXW7, PPP2R1, CDH4, KRAS, ARID1A, ARHGAP35, SPOP, RB1, U2AF1, ZBTB7B (Cancer Cell 2017;31:411)
Sample pathology report
- Uterus, total hysterectomy and bilateral salpingo-oophorectomy:
- Uterine carcinosarcoma with a heterologous component (see synoptic report and comment)
- Comment: There is a malignant biphasic cell proliferation composed of carcinomatous elements: (serous carcinoma) intimately admixed with sarcomatous elements (chondrosarcoma and osteosarcoma). The constellation of morphological features supports the diagnosis of carcinosarcoma (malignant mixed Müllerian tumor). These are considered malignant epithelial tumors and behaves like a high grade carcinoma.
Differential diagnosis
- Endometrial carcinoma with spindle cell differentiation and corded and hyalinized endometrial carcinoma (Am J Surg Pathol 2005;29:157):
- No high grade malignant nuclear spindle cell differentiation
- Smooth transition between the malignant epithelial to benign spindle cell component
- Undifferentiated and dedifferentiated carcinoma (Surg Pathol Clin 2019;12:343):
- No sarcomatous areas
- No recognizable glandular, trabecular, nested (if undifferentiated carcinoma) areas
- Well or moderately differentiated endometroid carcinoma are usually the only epithelial component (if dedifferentiated carcinoma)
- Desmoplastic stromal reaction in high grade endometrial or undifferentiated carcinoma:
- Atypical fibromyxoid areas associated with inflammatory cells
- No merging between malignant areas and stromal reaction
- Endometrioid adenocarcinoma with metaplastic osteoid elements (Am J Surg Pathol 2005;29:157):
- No cytological atypia in cells surrounded by osteoid type matrix
- Müllerian adenosarcoma (Hum Pathol 1990;21:363):
- Epithelial component is benign
- Malignant stroma predominantly in periglandular cuffs
- Sarcoma typically low grade
- Combined adenocarcinoma and neuroendocrine carcinoma (Am J Surg Pathol 2016;40:577):
- Neuroendocrine markers positive
- No sarcomatous areas, especially heterologous elements
- Mesonephric carcinomas (Am J Surg Pathol 2020;44:429, Am J Surg Pathol 2004;28:601):
- Undifferentiated uterine sarcoma (Surg Pathol Clin 2019;12:363):
- No recognizable epithelial areas
Board review style question #1
Board review style answer #1