Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Frozen section description | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Practice question #1 | Practice answer #1 | Practice question #2 | Practice answer #2Cite this page: Sharma A, Lastra RR. Endometrioid carcinoma. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/uterusendometrioid.html. Accessed September 29th, 2025.
Definition / general
- Endometrial endometrioid carcinoma arises in younger women and is considered to be estrogen dependent with a defined precursor lesion
Essential features
- Estrogen driven carcinoma of the endometrium that has a well defined precursor lesion - atypical hyperplasia / endometrioid intraepithelial neoplasia
- Back to back glands lacking intervening stroma, usually with mild to moderate but occasionally marked atypia
- Major prognostic factors are FIGO grade and stage
Terminology
- Endometrial endometrioid adenocarcinoma refers to a tumor arising from the endometrium, which resembles proliferative type endometrial glands
ICD coding
Epidemiology
- Mean age is sixth decade, with a range from the third to ninth decades (Am J Clin Pathol 2008;129:110)
- No definitive race predilection
- Increased circulating estrogen:
- Body Mass Index (BMI): dose response relationship of BMI ≥ 25 and increased risk of hyperplasia / carcinoma (Am J Obstet Gynecol 2016;214:689.e1)
- Nulliparous females (Cancer 1985;56:403, Am J Epidemiol 2008;168:563)
- Endometrioid histotype constitutes approximately 80% of all endometrial carcinomas, most of which are low grade (FIGO grade 1 - 2) (Int J Gynecol Pathol 2019;38:S25)
Sites
- Most common site is the uterine corpus - endometrium, endometrial polyps or adenomyosis
- Primary cervical endometrioid adenocarcinomas are extraordinarily rare and likely develop from cervical endometriosis (Histopathology 2020;76:112)
- Drop metastasis or contiguous extension from corpus should be ruled out in these cases
- Technically, any tissue involved by endometriosis
- Ectopic endometrial glands / stroma are responsive to estrogen stimulation and can also develop an endometrioid-like hyperplasia and subsequently carcinoma (Gynecol Oncol 2002;84:280, Gynecol Oncol 1996;60:238, Int J Gynecol Pathol 1996;15:1)
Pathophysiology
- Increased endogenous or exogenous estrogen, unopposed by progesterone:
- Initially, estrogen has mitogenic effect on both endometrial glands and stroma
- Chronic estrogenic stimulation without progesterone affects glands to a greater extent → glandular overgrowth (hyperplasia) → adenocarcinoma
Etiology
- Premenopausal:
- Polycystic ovarian syndrome (PCOS, Stein-Leventhal syndrome):
- Increased circulating androgens peripherally converted into estrogen
- Anovulatory cycles
- Chronic anovulation: dysregulated estrogen without opposing progesterone secretion → simultaneous proliferation and breakdown
- Polycystic ovarian syndrome (PCOS, Stein-Leventhal syndrome):
- Peri and postmenopausal:
- Exogenous estrogen:
- Estrogen supplementation: systemic therapy to alleviate symptoms of menopause → endometrial proliferation
- Tamoxifen: hormonal treatment for breast cancer acts as estrogen receptor antagonist in breast but agonist in endometrium
- Exogenous estrogen:
- Any age:
- Obesity: adipose tissue produces aromatase (enzyme converting circulating androgens to estrogen) → peripheral hyperestrinism (Mod Pathol 2000;13:295, Am J Obstet Gynecol 2016;214:689.e1)
- Ovarian pathology:
- Stromal hyperplasia and hyperthecosis: stromal luteinization → hyperandrogenism → hyperestrinism (BJOG 2003;110:690)
- Hormone secreting stromal tumors: granulosa cell tumor, thecoma
- Inherited cancer syndromes:
- Hereditary nonpolyposis colon cancer / Lynch syndrome: defect in mismatch repair proteins (see Molecular / cytogenetics description)
- Cowden syndrome: defect in PTEN tumor suppressor gene (Insights Imaging 2015;6:545)
Clinical features
- Abnormal, dysfunctional or postmenopausal uterine bleeding
- Pelvic pain or mass / compression effect on adjacent structures
- Abdominal bloating
- Dyspareunia, dysuria
- General stigmata of malignancy, i.e. weight and appetite loss, malaise, fatigue
- Rare cases asymptomatic
- Reference: Eur J Cancer 2001;37:64
Diagnosis
- Pelvic / transvaginal ultrasound
- Endometrial biopsy
- Hysteroscopy with endometrial curettage
- Hysterectomy:
- Incidental finding in specimens removed for benign pathology (up to 0.7% including other endometrial histotypes) (Int J Gynecol Cancer 2008;18:1065)
- Observed in 43% of specimens removed for atypical hyperplasia / endometrioid intraepithelial neoplasia (Cancer 2006;106:812)
- Incidental finding on cervical cytology screening or endocervical curettings
Laboratory
- In rare cases, CA-125 and CEA may be elevated (J Obstet Gynaecol Can 2011;33:844, Curr Oncol 2016;23:e439)
Radiology description
- Pelvic / transvaginal ultrasound
- Thickened endometrial stripe with heterogenous echotexture, increased vascularity and ill defined endomyometrial interface
- MRI
- Hypointense mass or heterogenous thickening of endometrium
- Best modality to detect integrity of endomyometrial junction
- CT abdomen / pelvis
- Enlarged uterus
- Hypoattenuating, hypoechoic mass in endometrial cavity
- Used mostly for staging of advanced disease (i.e. lymph node status and distant metastases)
- Reference: Indian J Radiol Imaging 2015;25:137
Prognostic factors
- Prognosis largely dependent on FIGO / TNM stage:
- Presence and extent of myometrial invasion (< 50% or > 50%)
- Cervical stromal involvement
- Serosal / vaginal / adnexal involvement
- Nodal metastases
- FIGO grade:
- Low grade (FIGO grades 1 and 2) have excellent survival compared with high grade (FIGO grade 3) tumors, the prognosis of which is similar to that of endometrial serous carcinoma
- However, other parameters such as age, tumor size, histologic features (lymphovascular invasion, microcystic elongated and fragmented glands / MELF pattern invasion) and most recently, molecular features (see Molecular / cytogenetics description) are implicated in prognosis (Int J Gynecol Pathol 2019;38:S93)
Case reports
- 31 year old woman with coexistent endometrioid and mesonephric-like endometrial carcinoma treated with progesterone (Diagn Pathol 2019;14:54)
- 49 year old woman with HER2-amplified tumor efficaciously treated with afatinib (Onco Targets Ther 2019;12:5305)
- 56 year old woman with paraneoplastic syndrome (PTHrP) and hypercalcemia (Gynecol Oncol Rep 2019;29:58)
- 61 year old woman with tarsal metastasis as the presenting lesion of well differentiated tumor (Eur J Gynaecol Oncol 1995;16:387)
- 71 year old woman with biphenotypic epithelial and sex cord differentiation (Int J Gynecol Pathol 2007;26:291)
Treatment
- Primary treatment is surgical (hysterectomy and bilateral salpingo-oophorectomy with staging), unless patient desires fertility
- Hormonal therapy (progesterone, leuprolide) alone can lead to complete remission in early stage, low grade tumor for women who want to preserve fertility but long term follow up studies not available (J Clin Oncol 2007;25:2798)
- Adjuvant chemo / radiotherapy largely dependent on postoperative surgical stage and histologic grade but incorporates other factors (lymphovascular invasion, age, tumor size and involvement of lower uterine segment / surface cervical glands)
- Reference: NCCN: Clinical Practice Guidelines in Oncology (NCCN Guidelines®) [Accessed 7 August 2020]
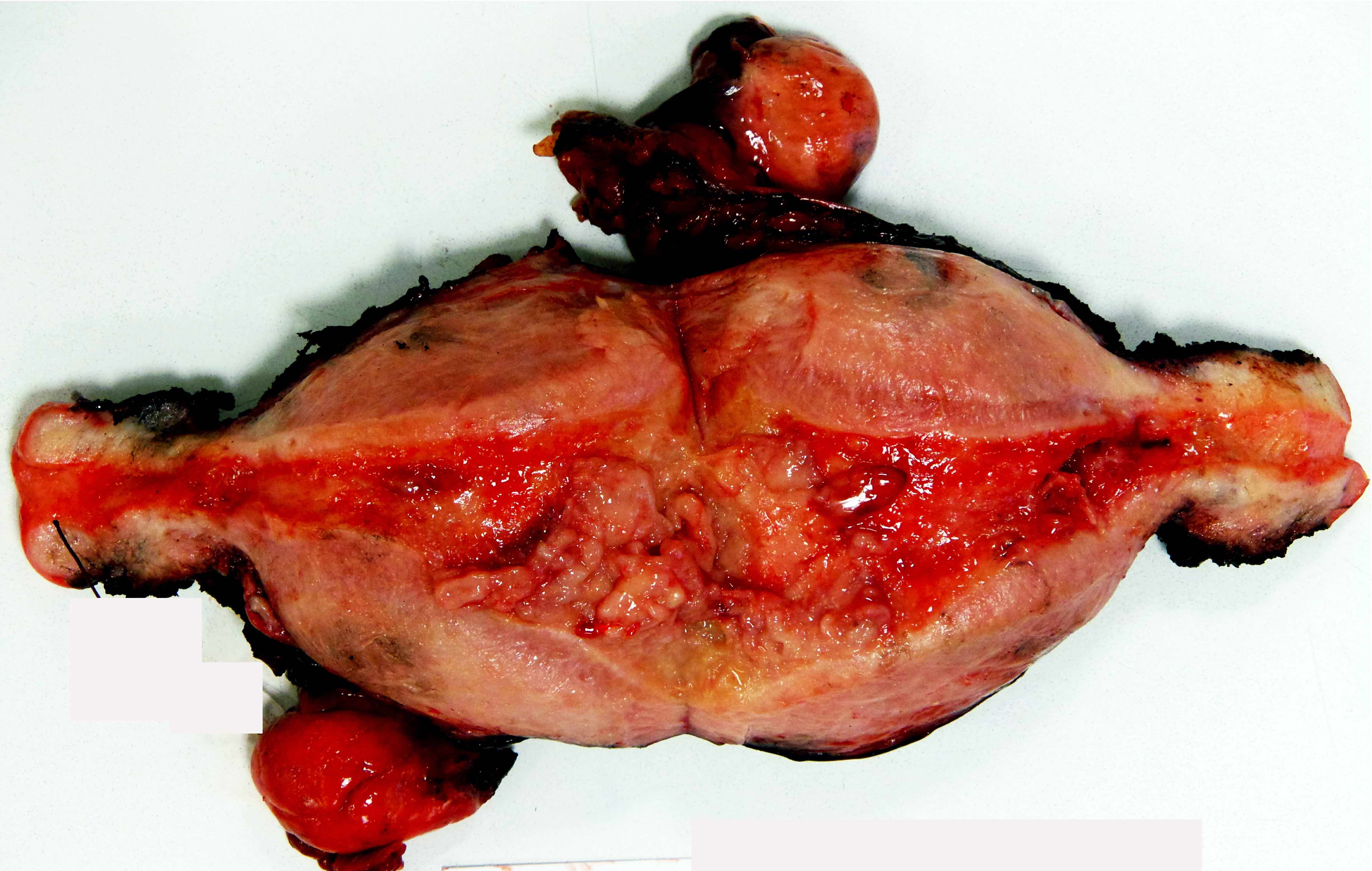
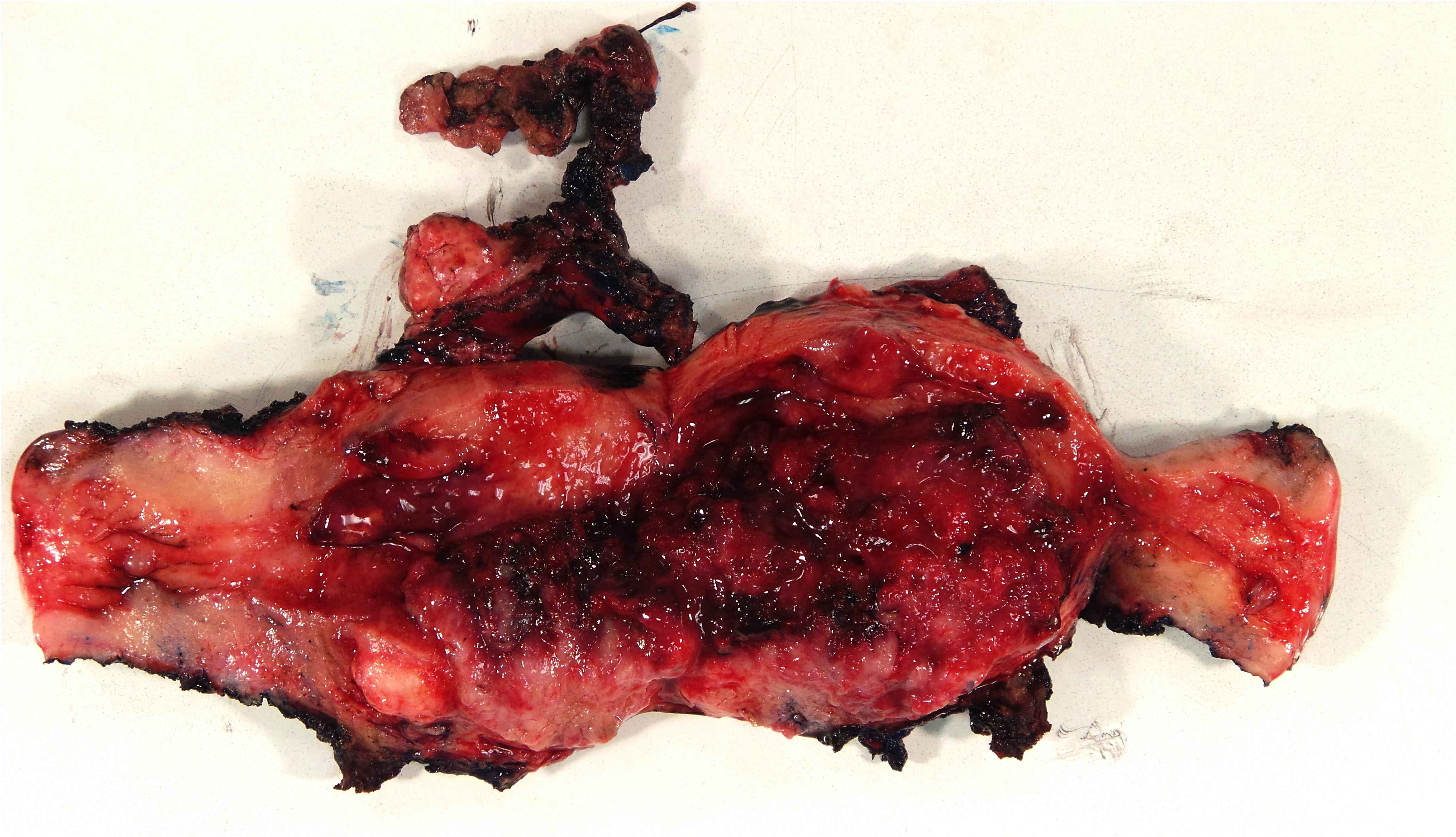
Gross description
- Mass arising from endometrial surface with varied appearances / sizes but usually exophytic and friable in texture
- Tumor / myometrial interface usually vaguely demarcated, which is useful to grossly assess depth of invasion during intraoperative evaluation
- Occasionally, no grossly appreciable mass, in which case the entire endometrium must be submitted for histologic evaluation (if prior biopsy showed carcinoma / atypical hyperplasia)
- Reference: Int J Gynecol Pathol 2019;38:S9
Gross images
Frozen section description
- Intraoperative consultation is not appropriate for:
- Diagnosing adenocarcinoma in a patient with a preoperative diagnosis of atypical hyperplasia / endometrioid intraepithelial neoplasia
- Diagnosing hyperplasia or atypia
- Section entire endometrium / mass to assess and freeze area of deepest apparent invasion
- Key information to report to surgeon (influences subsequent lymphadenectomy) (original Mayo Criteria: Am J Obstet Gynecol 2000;182:1506)
- FIGO grade of tumor (*1 or 2)
- Largest tumor dimension (*< 2 cm)
- Whether tumor is 1) *endometrium confined, 2) *< 50% myoinvasive or 3) > 50% myoinvasive
- *Cases meeting all criteria do not merit pelvic lymphadenectomy
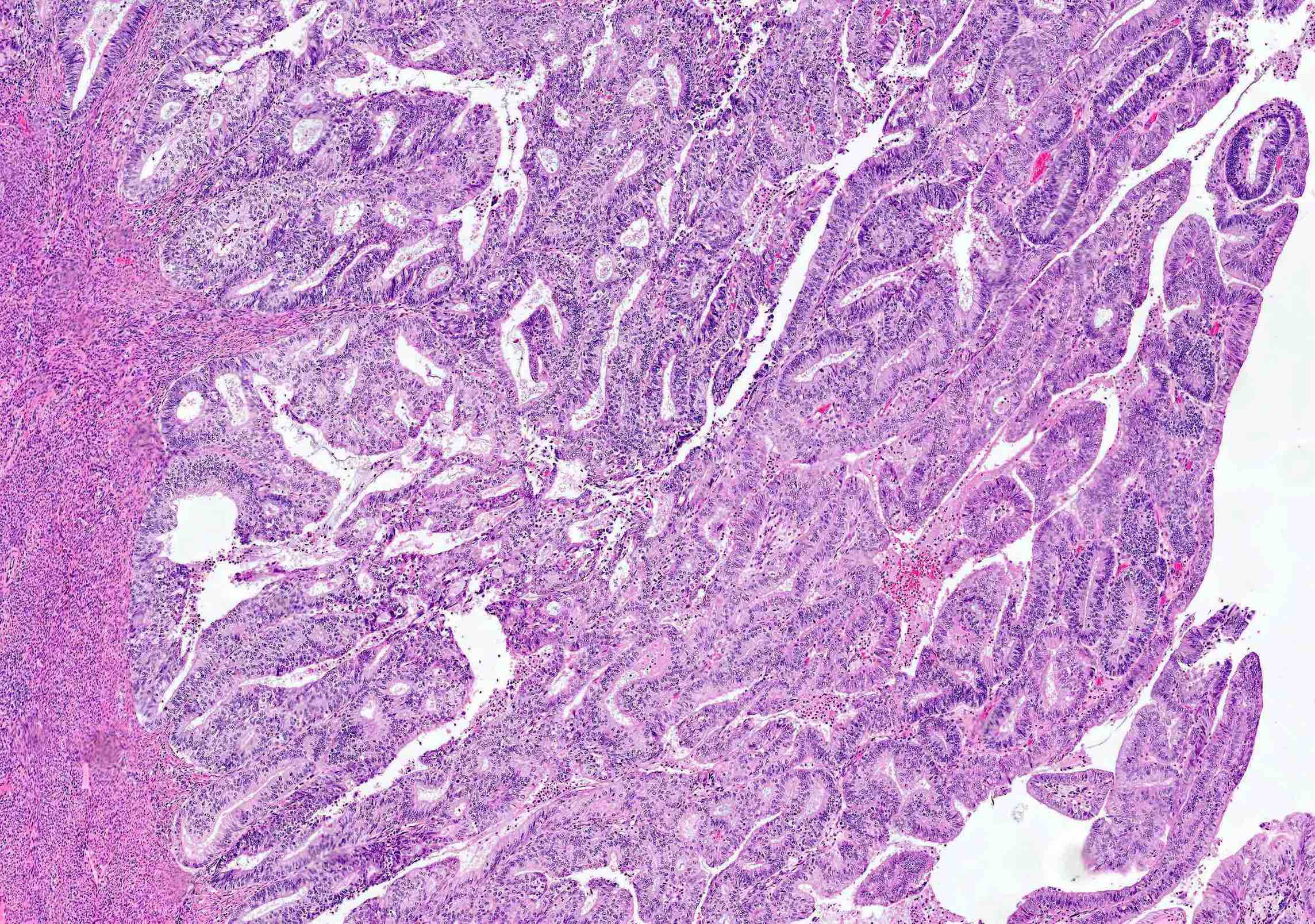
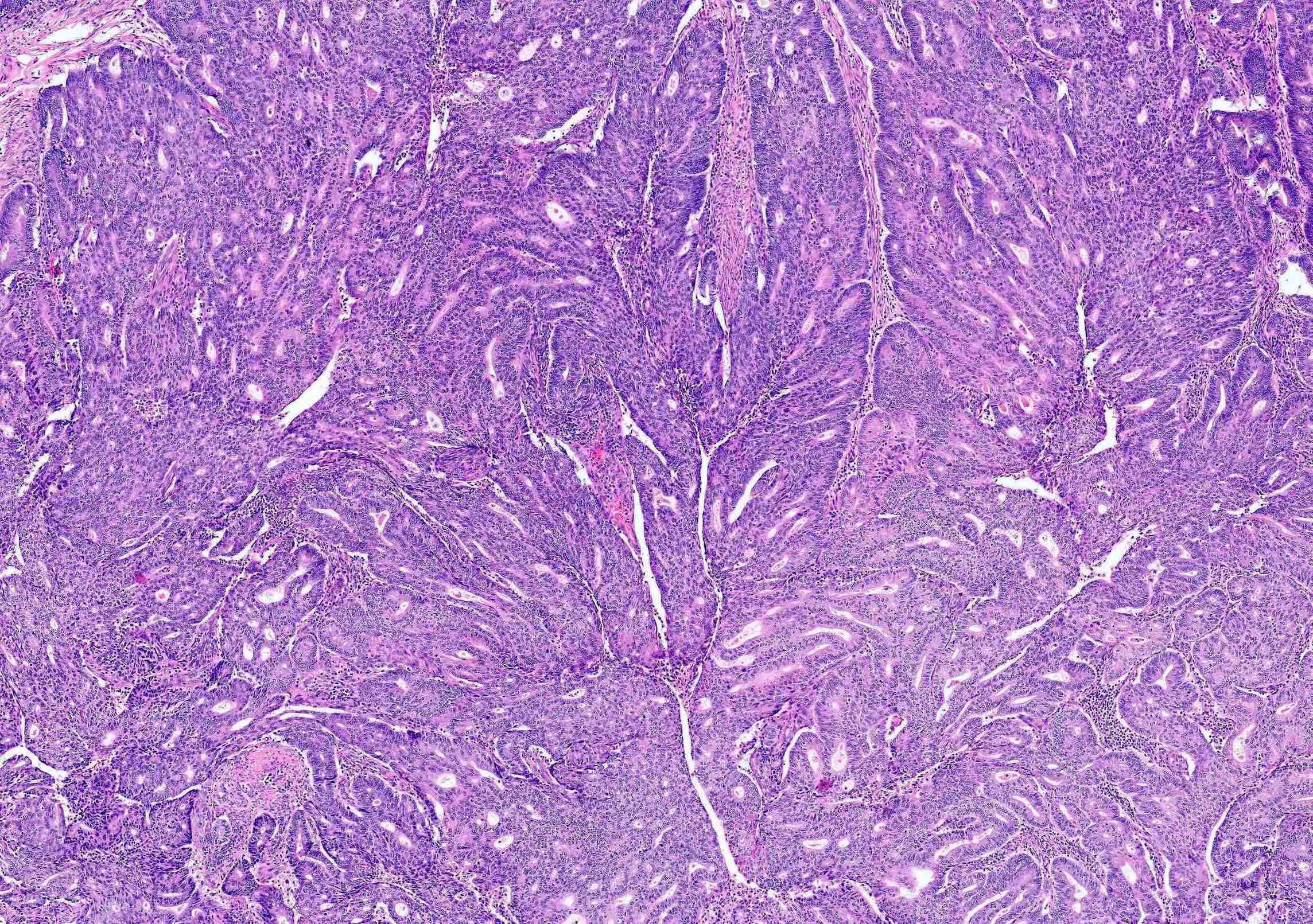
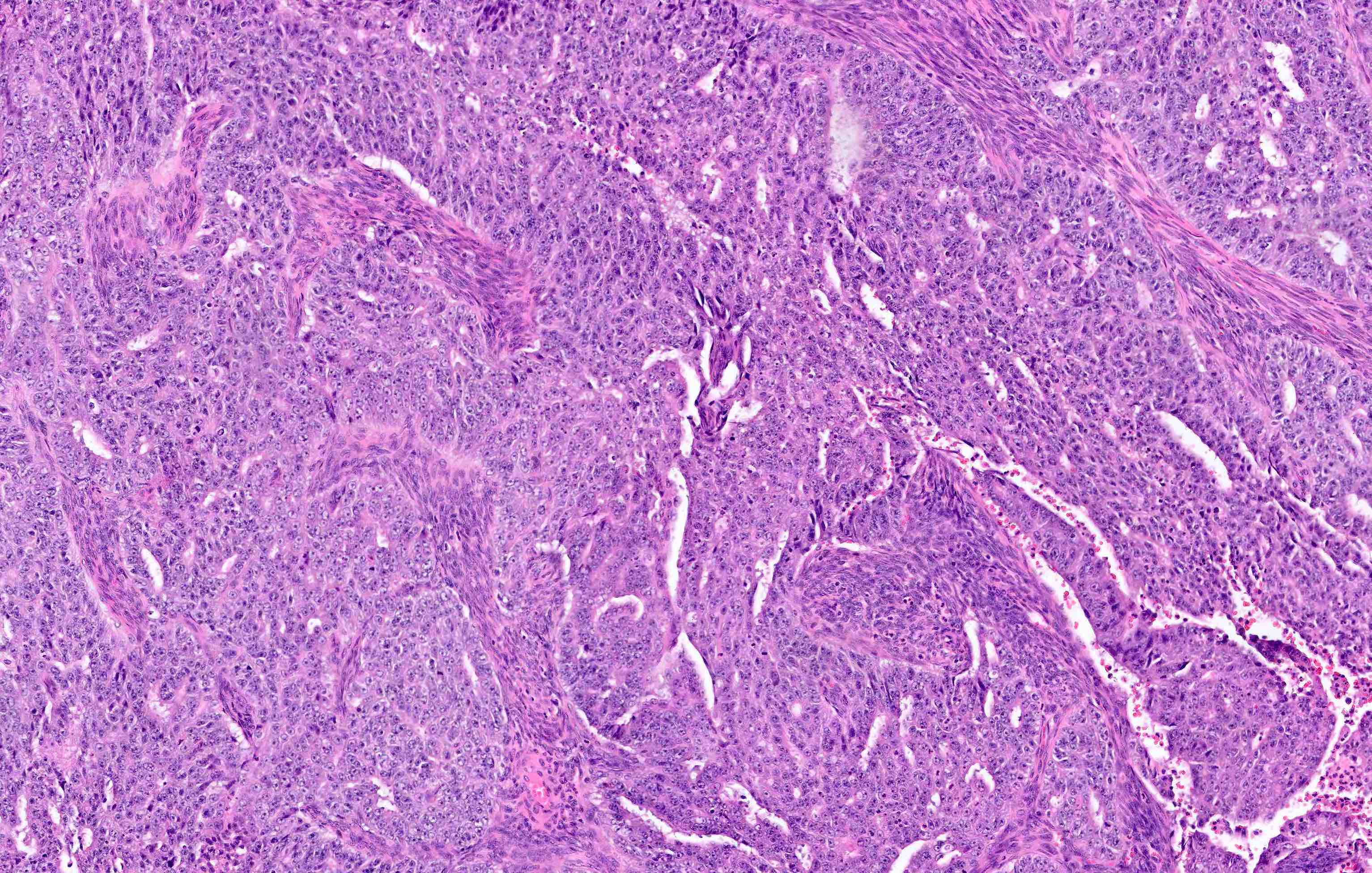
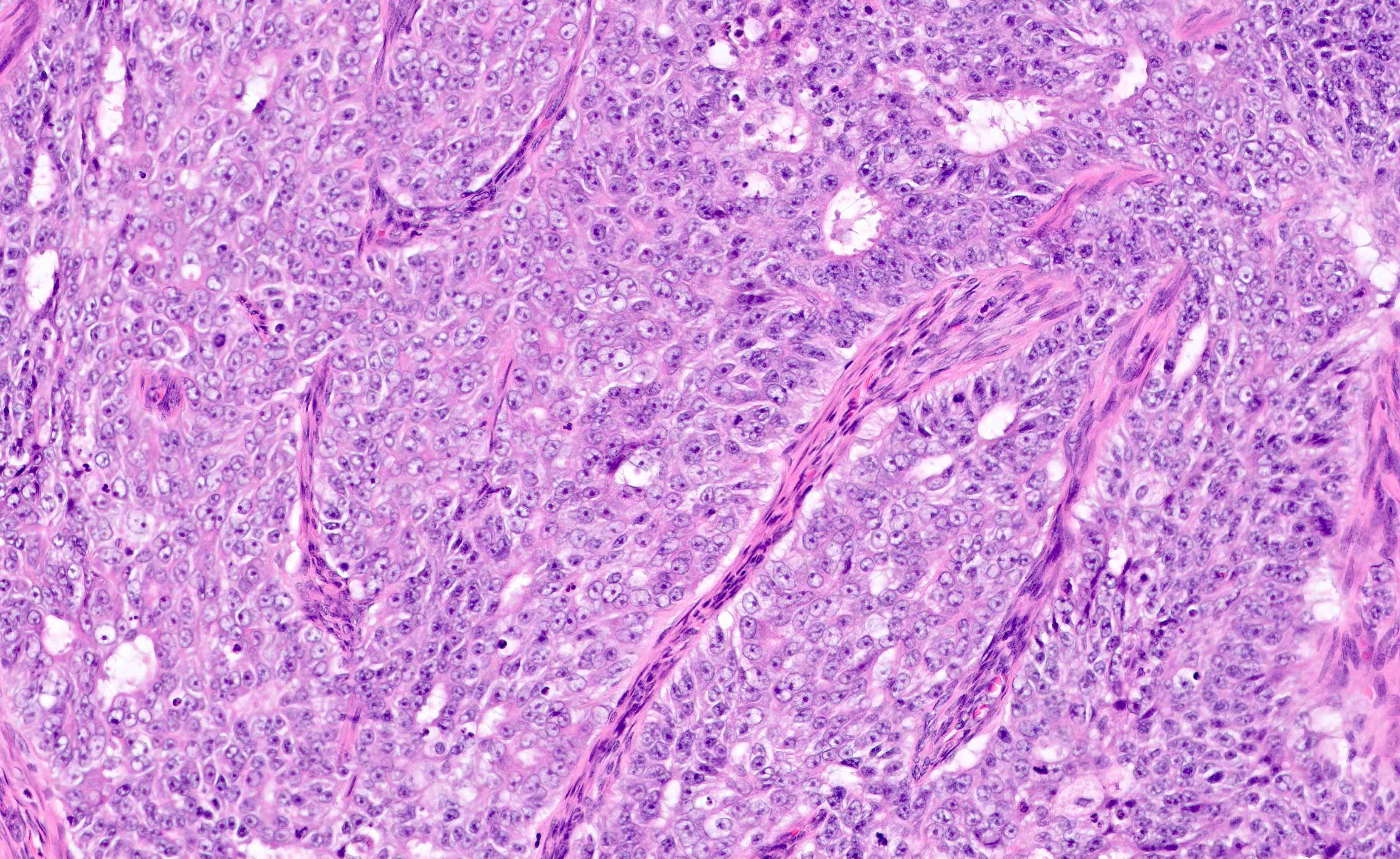
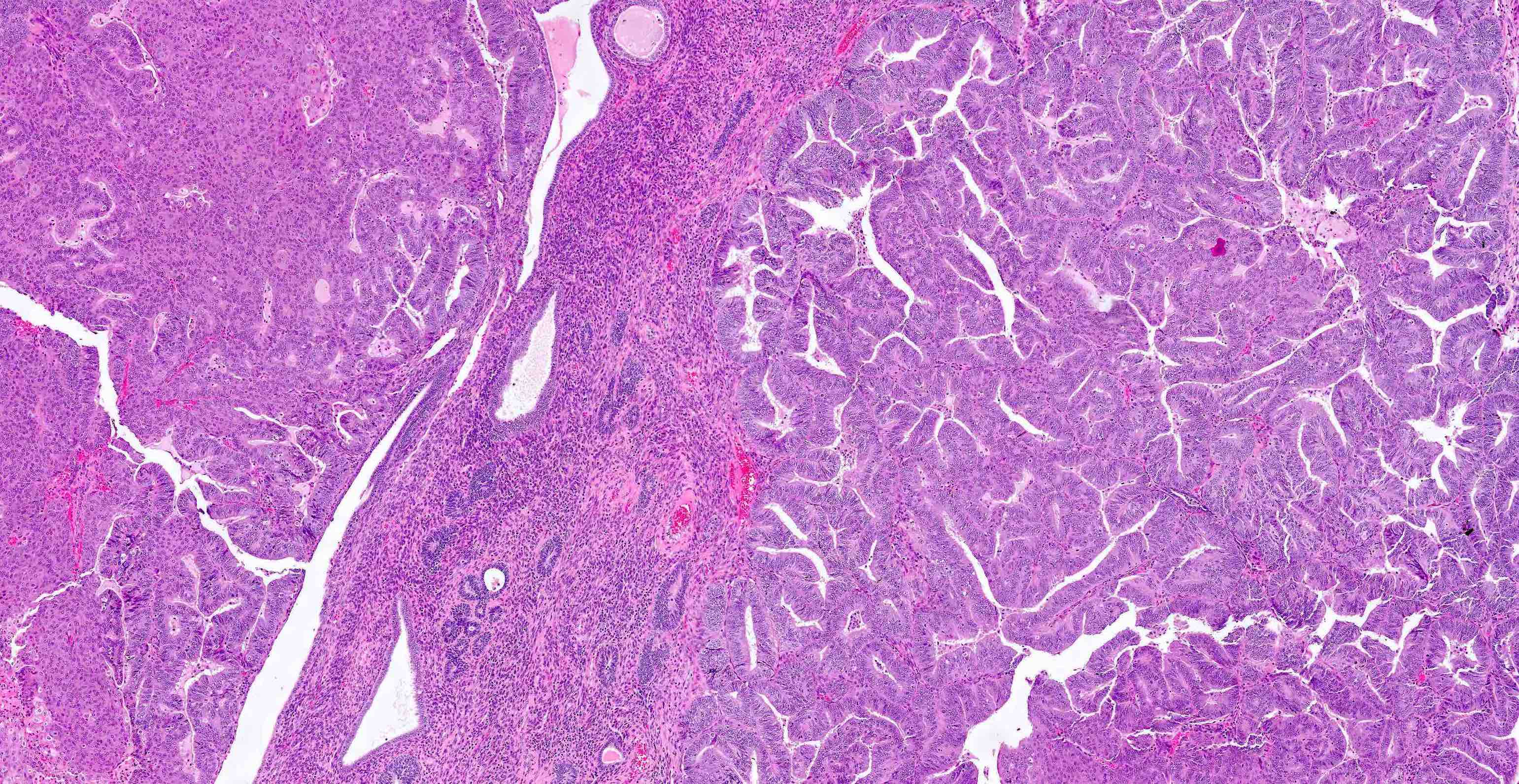
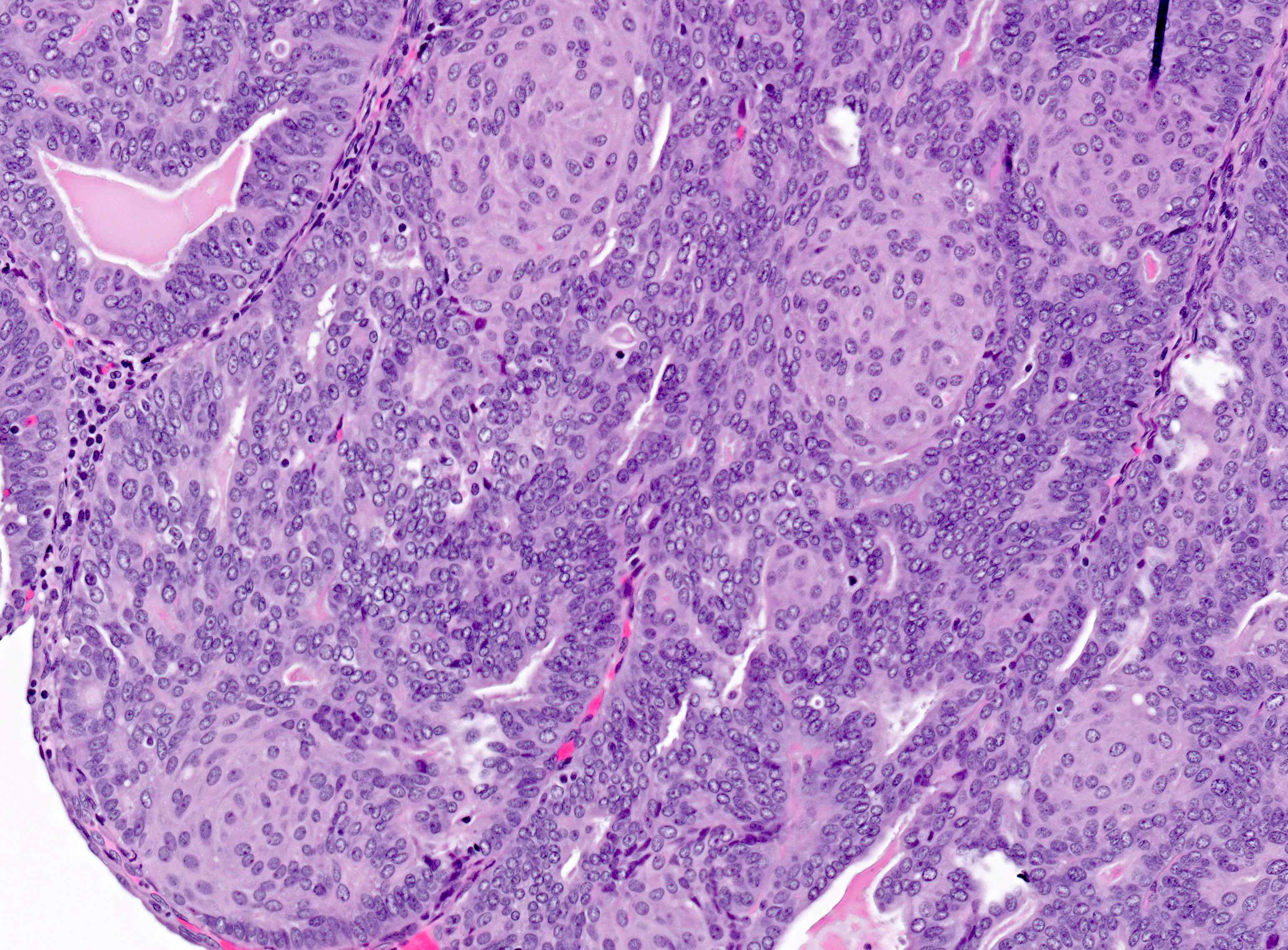
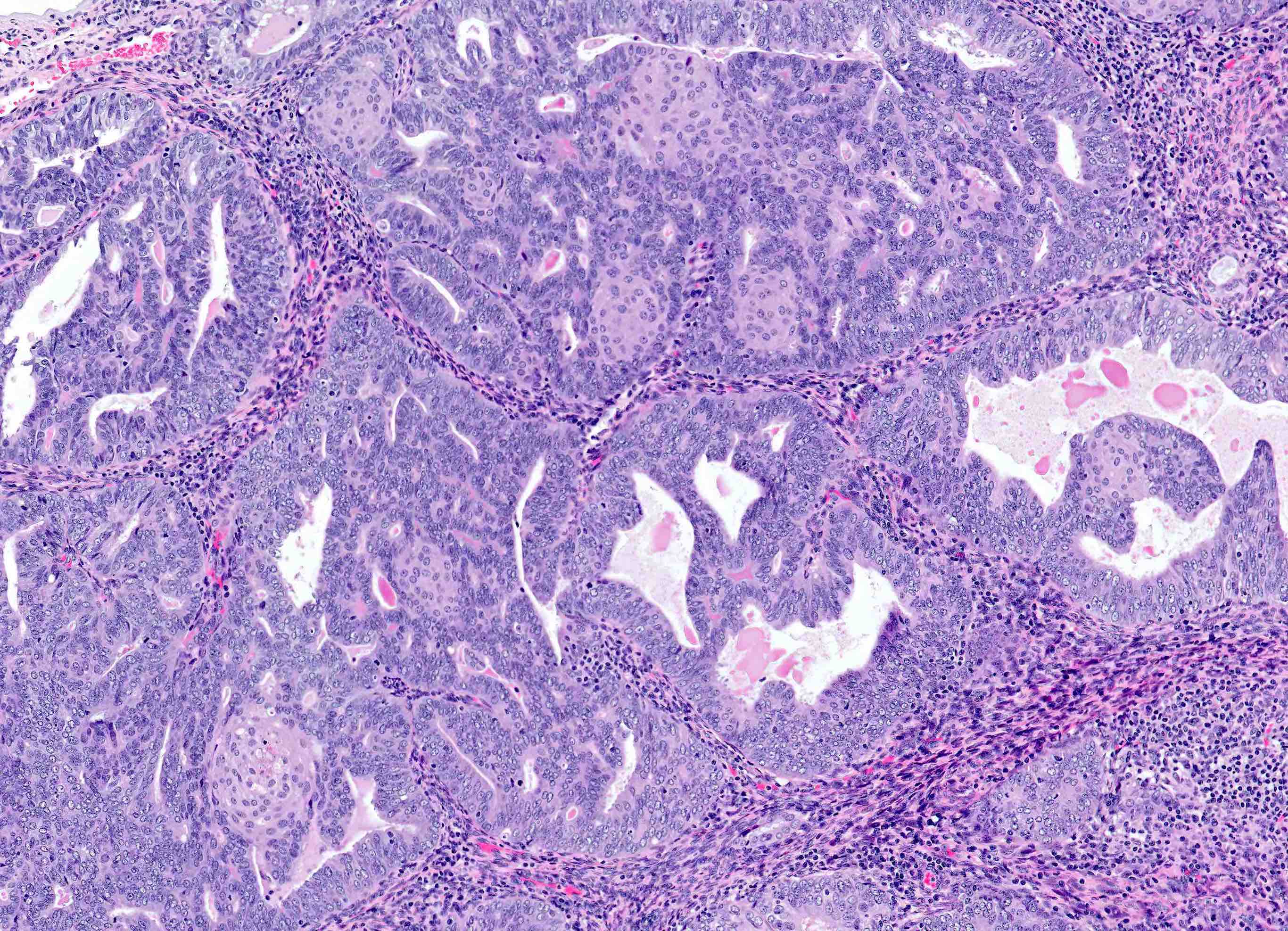
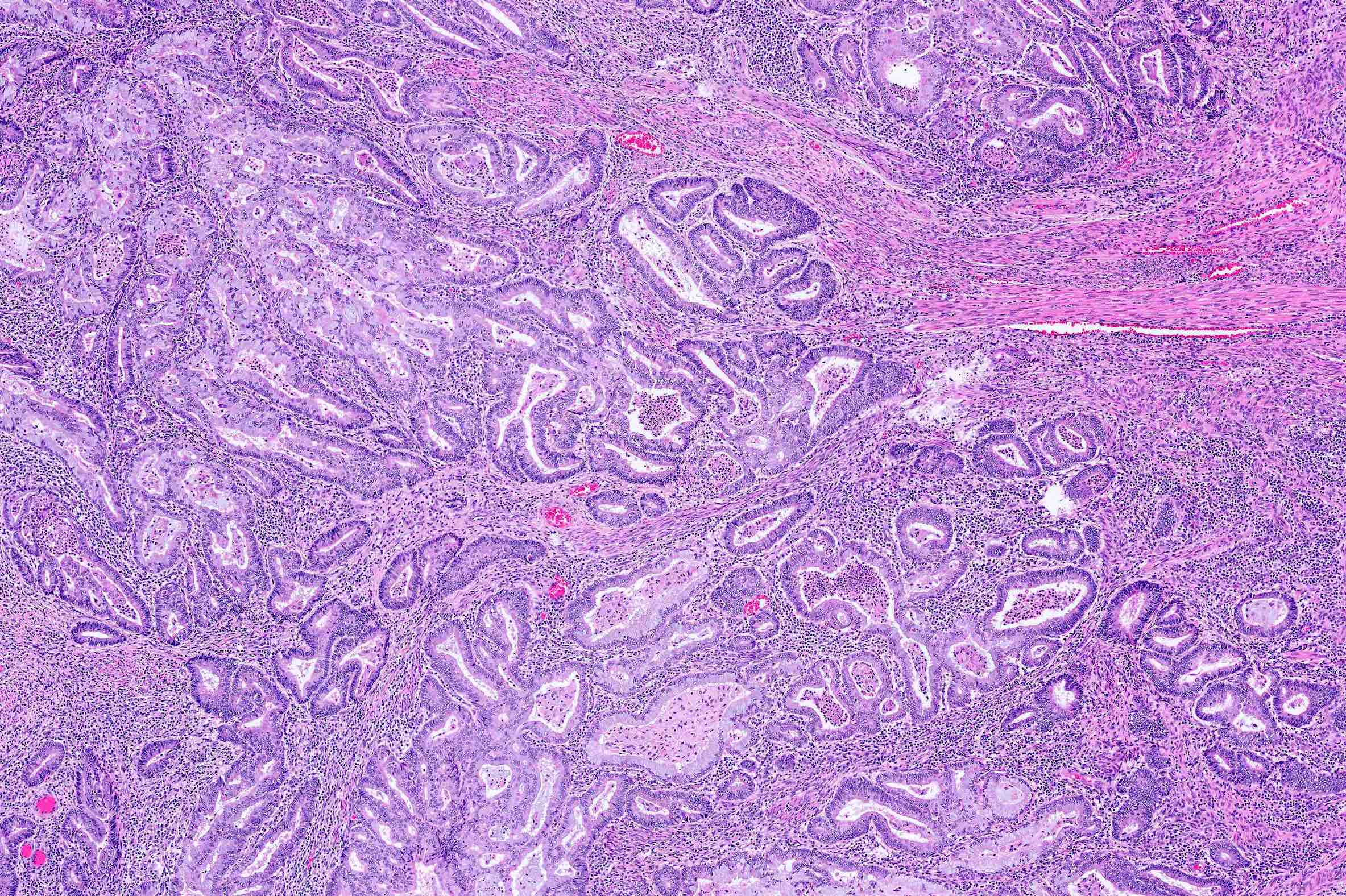
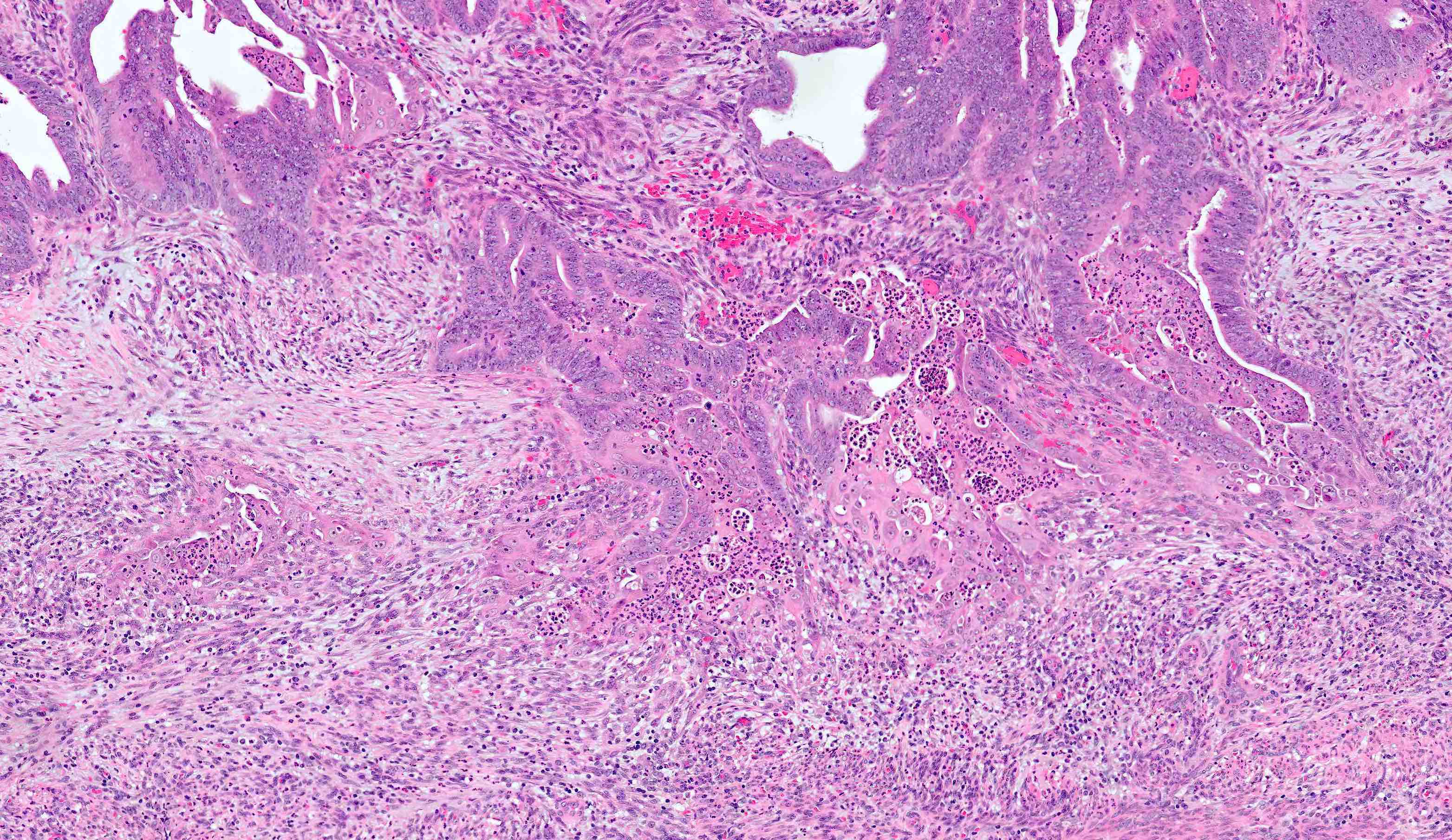
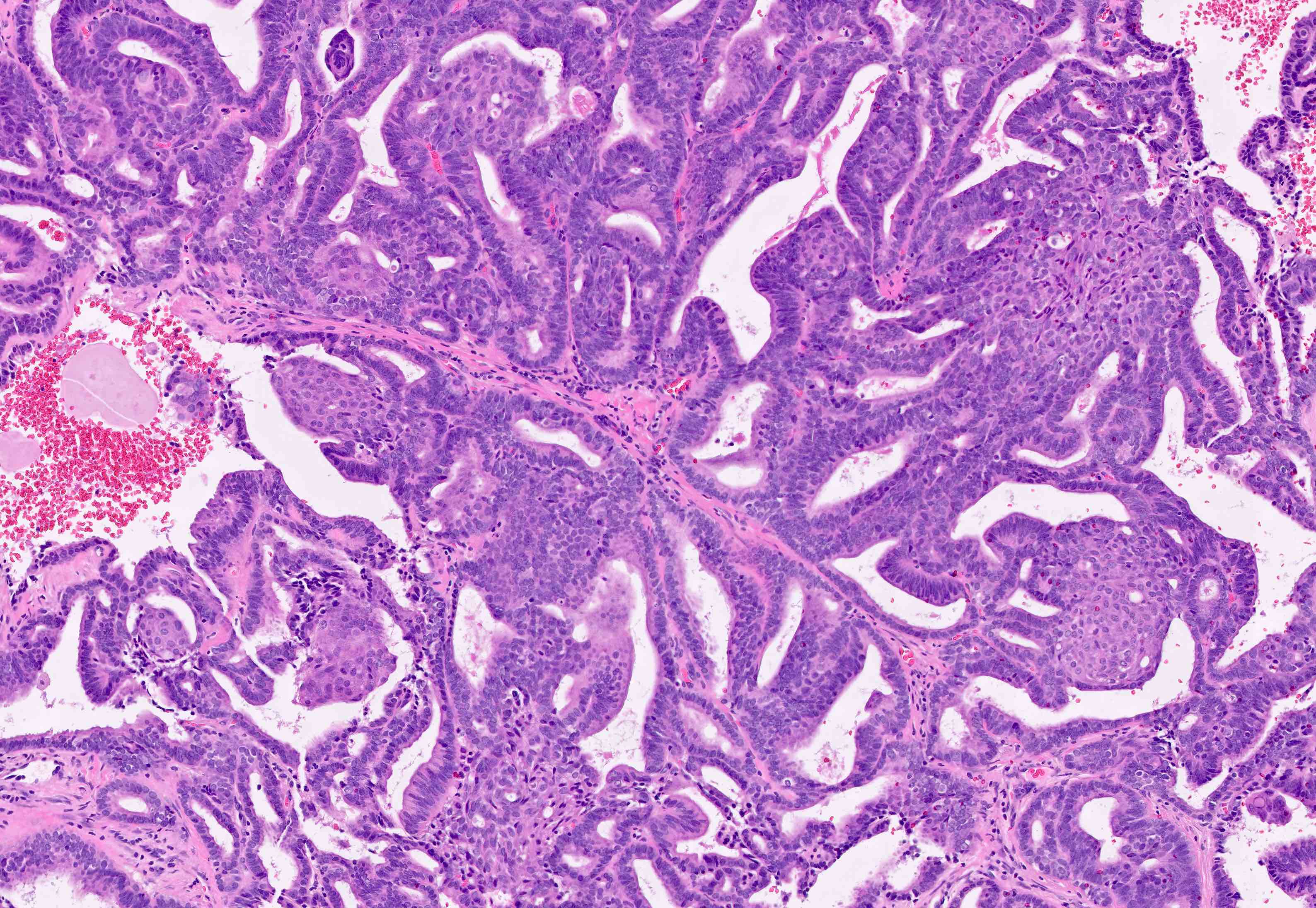
Microscopic (histologic) description
Overall
Myometrial invasion
FIGO grading system (based primarily on architecture)
Morphologic variants
- Architecture
- Key feature is confluent or back to back glands lacking intervening stroma
- Cribriform or microacinar configurations
- Complex papillary, micropapillary or villoglandular structures
- Cytologic features
- Resembles proliferative type endometrium with varying features / degrees of atypia but cytology must differ from that of surrounding nonneoplastic glands
- Cellular / nuclear enlargement
- Nuclear rounding (rather than elongation) with large nucleoli
- Loss of polarity
- Cytoplasmic eosinophilia
- Sharp glandular luminal borders
- Foamy histiocytes in residual stroma
- Resembles proliferative type endometrium with varying features / degrees of atypia but cytology must differ from that of surrounding nonneoplastic glands
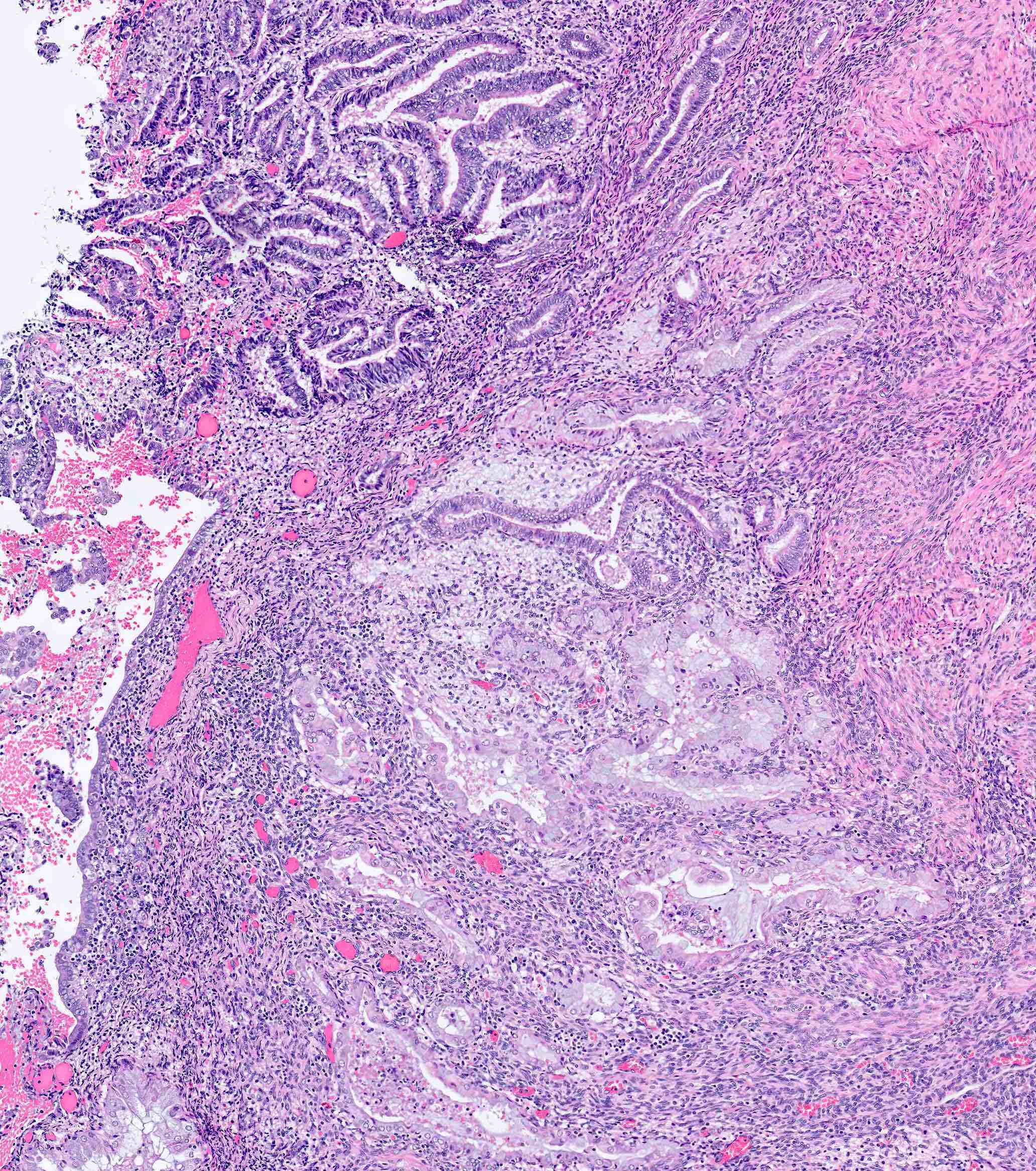
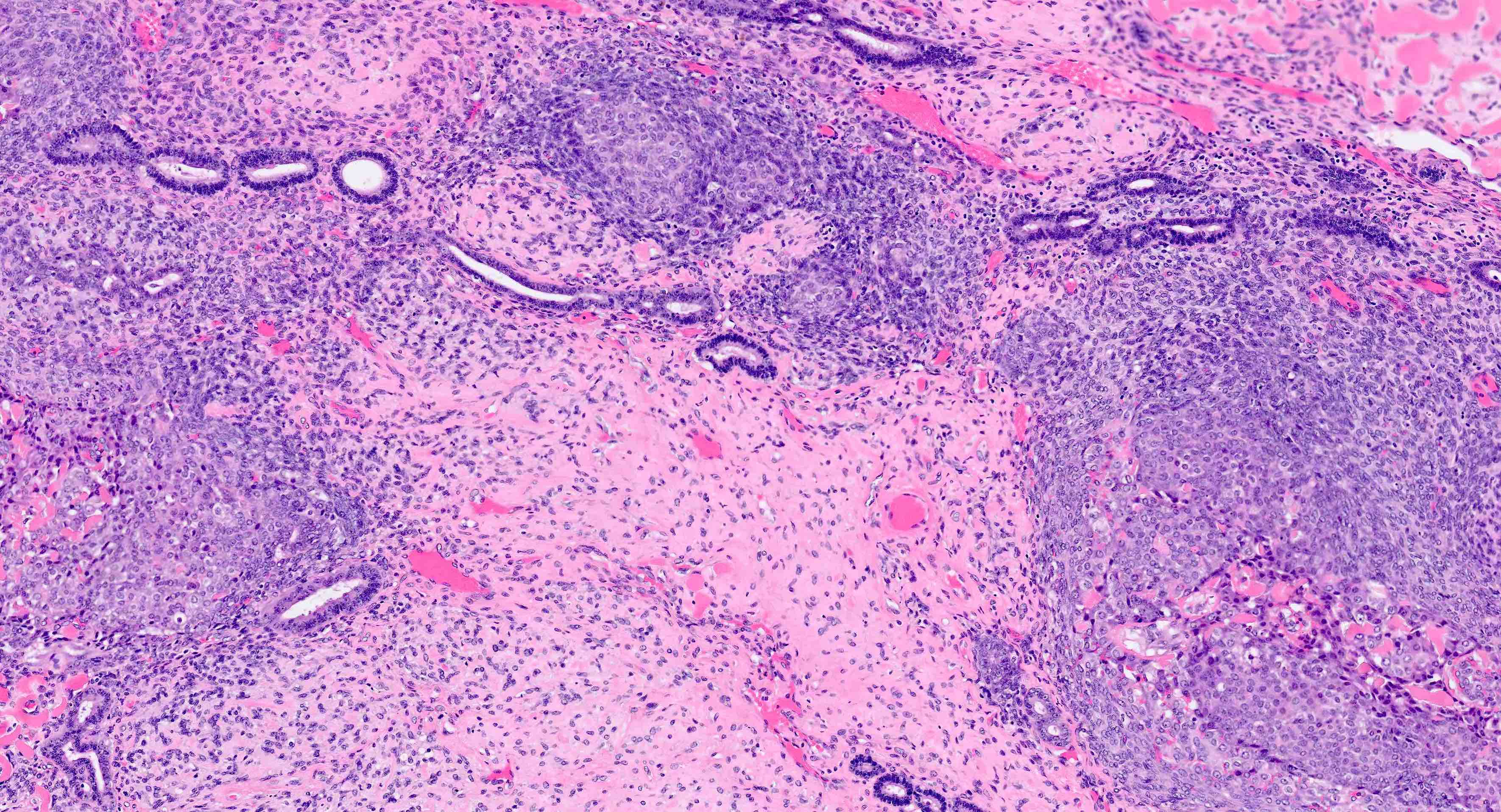
Myometrial invasion
- Conventional pattern:
- Traverses beyond confines of typically irregular endomyometrial junction without intervening rim of benign marker glands or endometrial stroma
- Rounded, smooth pushing invasive front, or
- Infiltrative extension of neoplastic glands
- Stromal response at invasive front variably consists of fibroblastic proliferation, edema and inflammatory cells
- This response may be absent
- Ratio of myoinvasion is crucial to staging:
- Numerator: depth of furthest invasion (endomyometrial junction to deepest focus of invasive glands)
- Denominator: myometrial thickness (distance from endomyometrial junction to uterine serosa)
- Traverses beyond confines of typically irregular endomyometrial junction without intervening rim of benign marker glands or endometrial stroma
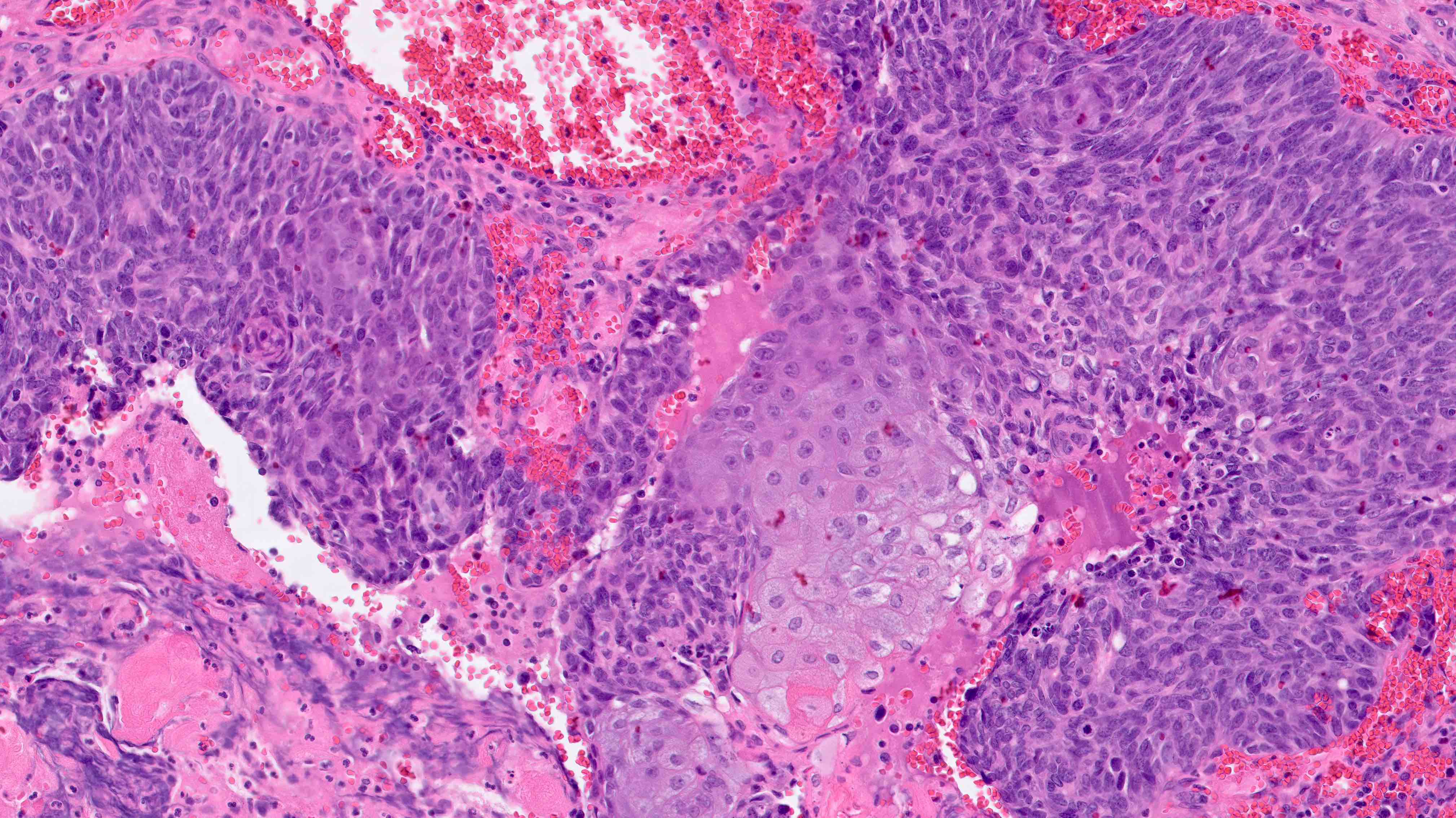
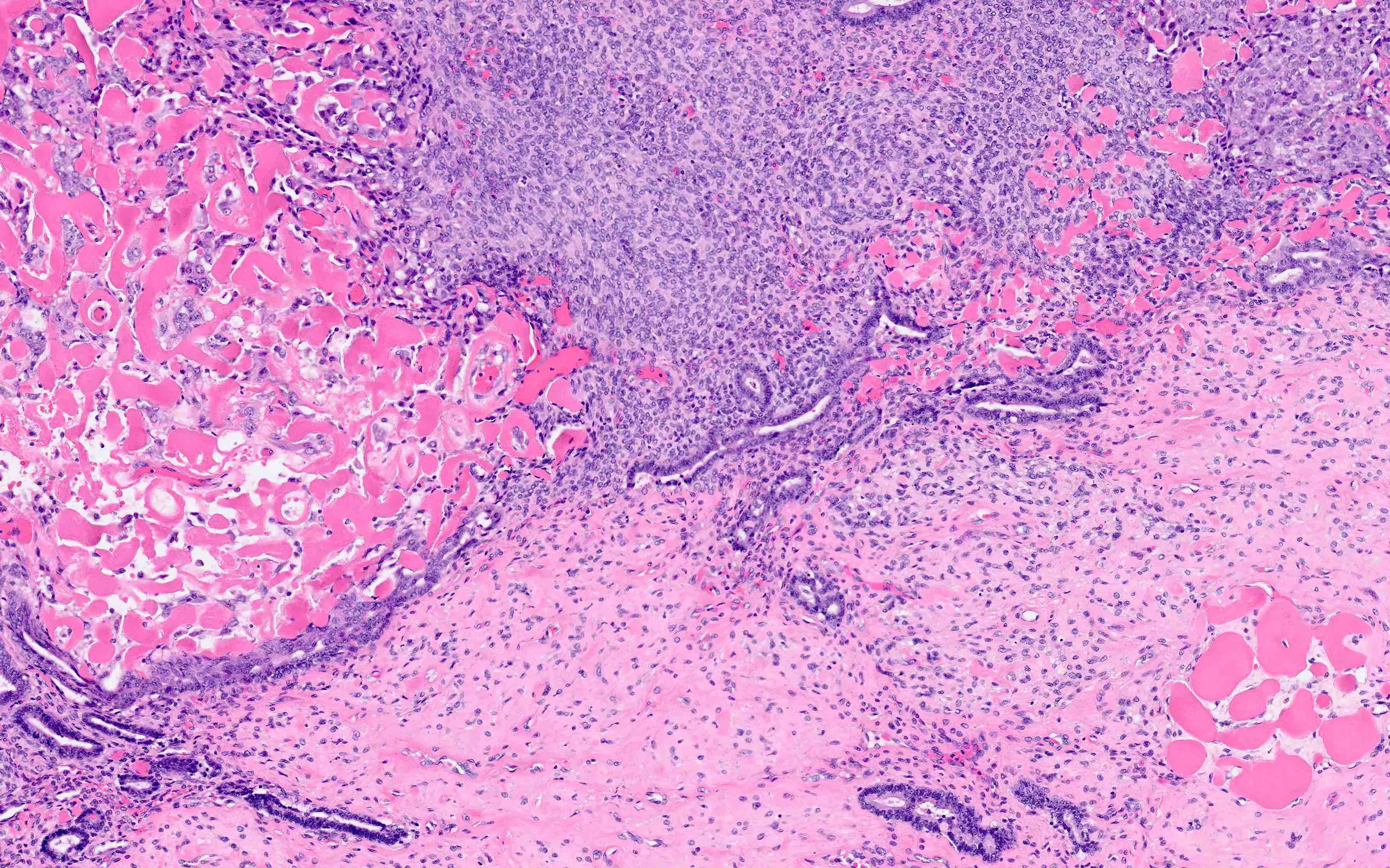
- Notable patterns of invasion
- Microcystic, ELongated and Fragmented (MELF):
- Generally associated with low grade (FIGO 1 - 2); associated with higher rate of lymphovascular invasion and lymph node metastases but not overall survival
- Fragmented microcystic, elongated glands lined by flattened or histiocytoid epithelium, which can lead to depth of invasion underestimation
- Distinctive fibromyxoid stromal reaction with acute inflammation
- Adenoma malignum:
- Diffusely infiltrative carcinomatous glands with irregular contours, invading myometrium in clusters without or with minimal associated stromal response
- Can lead to depth of invasion underestimation
- Distinguish from carcinoma involving adenomyosis, which should not be interpreted as invasion
- Will have nonneoplastic endometrial glands or stroma at periphery and conventional adenomyosis in other areas
- Microcystic, ELongated and Fragmented (MELF):
- Myoinvasion: notable caveats
- Adenomyosis (Int J Gynecol Pathol 2019;38:S93):
- Important distinction between 1) carcinoma involving adenomyosis and 2) carcinoma involving adenomyosis with invasion from that focus
- Regarding 1: depth of invasion = distance from endomyometrial junction to deepest point of invasion elsewhere (the nonmyoinvasive carcinoma within the adenomyotic focus is not considered invasion)
- Regarding 2: depth of invasion = distance from endomyometrial junction to the point of invasion arising in that specific focus of adenomyosis (irrespective of the deep or superficial location of that focus of adenomyosis within the myometrial wall)
- Polyp:
- Only invasion into underlying myometrium should be considered in depth of invasion from endomyometrial junction, not invasion into the polyp stroma itself
- Exophytic tumors:
- Thickness of exophytic component should not be considered, only invasion from endomyometrial junction
- Leiomyomas:
- Invasive carcinomas overlying / extending into a leiomyoma: wall thickness should incorporate (not subtract) the leiomyoma; unless there is a greater percentage of invasion elsewhere
- Adenomyosis (Int J Gynecol Pathol 2019;38:S93):
FIGO grading system (based primarily on architecture)
- Grade 1: 5% or less nonsquamous solid growth pattern
- Grade 2: 6 - 50% nonsquamous solid growth pattern
- Grade 3: > 50% nonsquamous solid growth pattern
- Nuclear atypia exceeding that expected for the architectural grade increases FIGO grade by 1
- Glandular variant of endometrial serous carcinoma or component thereof, must be excluded
Morphologic variants
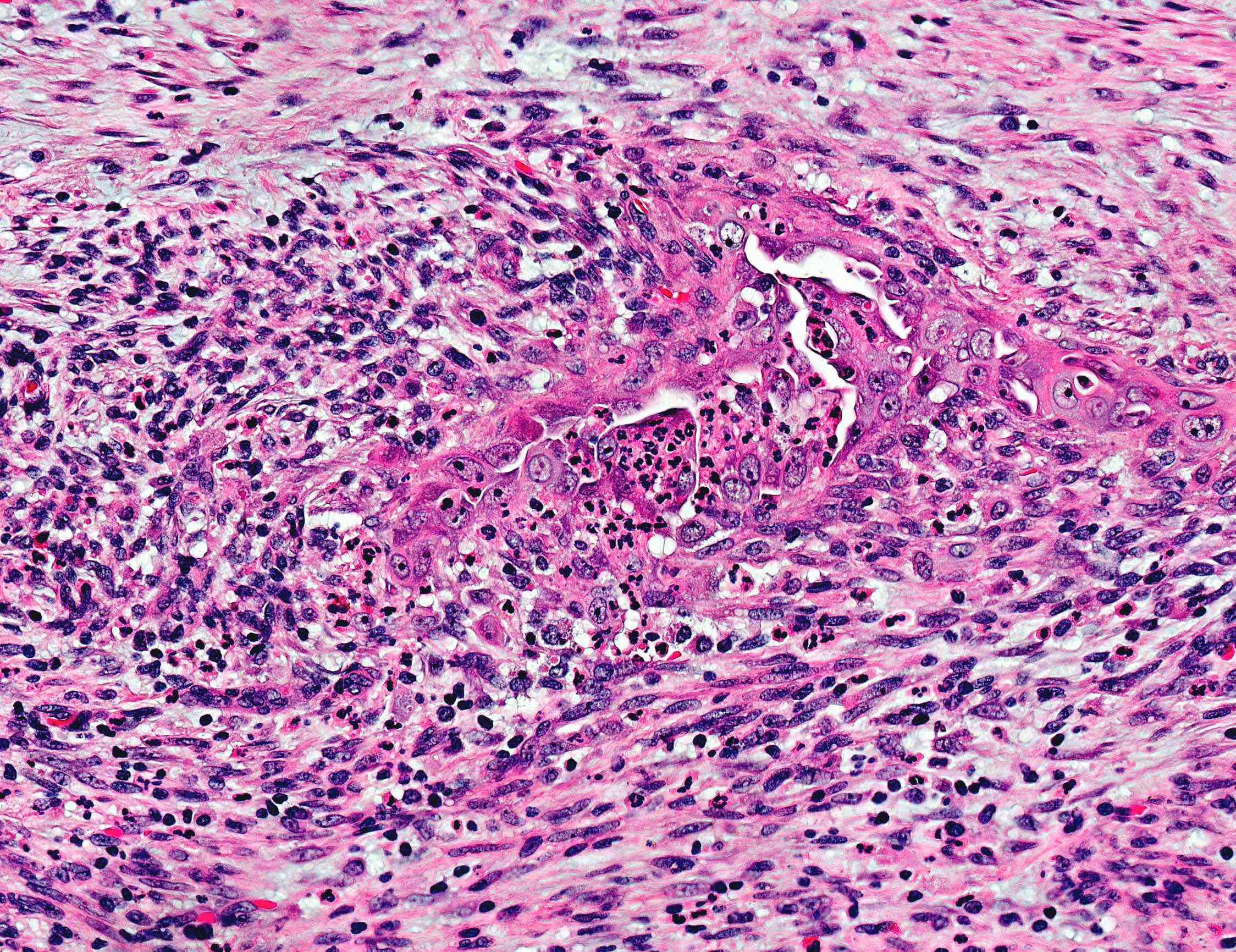
- Altered differentiation / metaplasia:
- Squamous, morular and mucinous differentiation are characteristically associated to endometrioid type adenocarcinomas; generally not observed in serous, clear cell or other histotypes
- Squamous or "squamous" morular: usually banal but occasionally cytologically malignant; former can be glycogenated which imparts appearance of clear cytoplasm
- Mucinous: intracytoplasmic mucin (intraluminal mucin pooling does not qualify)
- Secretory: sub / supranuclear vacuolization
- Ciliated / tubal: resembles fallopian tube lining; scattered cells with apical terminal bars and ciliation
- Papillary type variants:
- Villoglandular
- Small nonvillous papillae
- Micropapillae
- Microglandular hyperplasia-like: microcystic, microacinar glands with intraluminal neutrophils
- Spindled: bland spindling of carcinomatous cells merging with epithelioid carcinomatous component
- Corded and Hyalinized Endometrial Carcinoma (CHEC): linear cords of carcinoma cells molded by an abundant myxohyaline background
- Mixed endometrial carcinoma:
- Defined as combination of at least 2 endometrial histologic subtypes (most commonly endometrioid and serous), the minor component of which must constitute at least 5% of tumor volume on resection specimen (WHO 2014)
- Distinction important as prognosis is similar to that of the higher grade component (i.e. serous, clear cell, neuroendocrine)
- Dedifferentiated endometrial carcinoma:
- Abrupt transition from well differentiated (FIGO 1 - 2) to undifferentiated carcinoma
Microscopic (histologic) images
Cytology description
- Not typically or ideally a diagnosis made on cytologic specimens but can be identified incidentally on standard Papanicolaou smears
- No histologic criteria to differentiate between well to moderately differentiated tumor in which atypia is usually not prominent and (Obstet Gynecol 1988;71:242, Obstet Gynecol 1990;76:1000):
- Normal proliferative endometrium
- Cells exfoliated from endometrial polyp
- Endometrial hyperplasia
- Cytologically unremarkable endometrial cells in cervical Pap of a woman ≥ 45 years is considered abnormal and should be reported (Cancer Cytopathol 2015;123:271)
- Positive pelvic washings or Pap smears have no effect on staging but are considered adverse prognosticators
Negative stains
- p53 wild type (patchy and weak expression in scattered nuclei)
- To be considered aberrant (also known as mutation type), p53 must be either 1) diffusely positive in > 80% of nuclei, 2) completely negative (null type mutant pattern) or 3) any amount of unequivocal cytoplasmic staining (blush does not qualify)
- Caveat: high grade (FIGO 3) tumors can show aberrant expression (Gynecol Oncol 2004;94:449, Am J Surg Pathol 2009;33:1504)
- p16 mosaic (patchy and weak nuclear / cytoplasmic expression)
- To be considered positive, p16 must have strong, diffuse, block-like nuclear and cytoplasmic positivity throughout the area of interest
- Caveat: high grade (FIGO 3) tumors can show aberrant expression (Gynecol Oncol 2004;94:449, Am J Surg Pathol 2009;33:1504)
- CK20, CEA, HNF-1B, Napsin A (rarely positive in endometrioid adenocarcinoma with clear cell change) (Am J Surg Pathol 2015;39:1061)
Molecular / cytogenetics description
- The Cancer Genome Atlas (TCGA, 2013) defined 4 molecular and prognostic subgroups of endometrial carcinoma (endometrioid (EEC) and serous histotypes) (Nature 2013;497:67):
- POLE mutated (ultramutated): very high mutational burden, microsatellite stability (~10% of EEC)
- Comprised by a majority of grade 3 EEC
- Best prognosis of all subgroups (despite high grade histology)
- Microsatellite instability (MSI) (hypermutated): high mutational burden with aberrant mismatch repair protein (MMR) IHC
- More common in grade 3 EEC (but can be seen in all grades) (Arch Gynecol Obstet 2020;301:1117)
- Intermediate prognosis similar to copy number low group
- Copy number low: low mutational burden, low copy number alterations, no alterations in TP53 gene and wild type p53 IHC
- Comprised by a majority of grade 1 - 2 EEC
- Intermediate prognosis similar to hypermutated group, correlated with copy number alteration level
- Copy number high (serous-like): low mutational burden with elevated copy number alteration rate and high frequency of TP53 gene mutations with aberrant p53 IHC
- Comprised by a majority of serous and a minor proportion of grade 3 endometrioid carcinomas
- Worst prognosis of all subgroups, correlated with high copy number alteration level
- POLE mutated (ultramutated): very high mutational burden, microsatellite stability (~10% of EEC)
- Hereditary nonpolyposis colon cancer / Lynch syndrome
- Universal screening of all endometrial carcinomas for Lynch syndrome (endometrioid and clear cell histotypes, but testing endometrial serous carcinomas is controversial):
- Endometrial carcinoma (not colonic) is more frequently the presenting neoplasm for female patients with Lynch syndrome
- MMR IHC (MLH1, PMS2, MSH2, MSH6) validated on both EEC biopsies / curettings and resections (Gynecol Oncol 2018;149:570)
- Loss of any component of MSH2 / MSH6 complex → likely Lynch syndrome, refer for genetic testing
- Loss of MLH1 / PMS2 complex → likely sporadically derived → reflex hypermethylation testing of MLH1
- Hypermethylation of MLH1 negative → likely Lynch syndrome, refer for genetic testing
- Histologic features of Lynch syndrome associated endometrial carcinoma:
- Most are of endometrioid histotype and frequently arise in lower uterine segment
- Associated with tumor infiltrating lymphocytes and peritumoral lymphocytes
- Association with dedifferentiated / undifferentiated histotypes
- Universal screening of all endometrial carcinomas for Lynch syndrome (endometrioid and clear cell histotypes, but testing endometrial serous carcinomas is controversial):
Sample pathology report
- Endometrium, curettings:
- Endometrial endometrioid adenocarcinoma, FIGO grade 2, with squamous differentiation
- Background endometrium with extensive atypical hyperplasia / endometrioid intraepithelial neoplasia
- Uterus and cervix, hysterectomy:
- Endometrial endometrioid adenocarcinoma, FIGO grade 1, with deep myometrial invasion (> 50%), focal lymphovascular invasion and extension to lower uterine segment (see synoptic report and comment)
- Comment: Immunohistochemical stains for mismatch repair proteins (with appropriate controls) demonstrate loss of MLH1 and PMS2, with retention of MSH2 and MSH6. Molecular testing for MLH1 promotor hypermethylation has been requested and results will be issued as an addendum.
Differential diagnosis
Benign
Malignant / premalignant
- Endometrial glandular stromal breakdown:
- Artifactual crowding due to glandular collapse, predecidual necrosis and regenerative atypia
- Microglandular hyperplasia (MGH) of cervix:
- Has basal squamous metaplasia, prominent subnuclear vacuolization, relatively low proliferation index, lacks stromal foam cells and does not have a conventional EEC pattern present (Int J Gynecol Pathol 2003;22:261, Adv Anat Pathol 2009;16:1)
- Benign endometrial metaplasias:
- Squamous metaplasia:
- "Squamous" morular metaplasia - not truly squamous (Histopathology 2008;53:156):
- Mimics solid growth of EEC but should not factor into FIGO scoring
- Frequently beta catenin, CD10, SATB2 and CDX2+ but ER and p63-
- Caveat: some EECs may demonstrate nuclear expression of beta catenin (i.e. positive) due to CTNNB1 mutation
- Papillary syncytial metaplasia / eosinophilic syncytial change:
- Can have variable cellular atypia
- Abortive pseudopapillary structures with abundant eosinophilic cytoplasm in background of stromal breakdown
- Tubal / ciliated metaplasia:
- Can be hyperchromatic and atypical but presence of cilia is (usually) a clue to benign nature
- Secretory endometrium:
- Florid tortuosity of glands may appear disorganized and crowded, hence mimicking hyperplasia / carcinoma
- In comparison with endometrial intraepithelial neoplasia (EIN), lacks (Int J Gynecol Pathol 2014;33:114):
- Glandular crowding distinct from background secretory endometrium
- Architectural disorder - long axis of glands in different directions; budding / branching / staghorn contours
- Increased Ki67 proliferation index (Int J Gynecol Pathol 2014;33:114)
Malignant / premalignant
- Atypical hyperplasia / endometrioid intraepithelial neoplasia (AH / EIN):
- Distinction challenging on small biopsies
- Controversial but 1) cribriforming, 2) solid growth, 3) complex labyrinthine architecture should occupy a diameter ≤ 2.1 mm (half of one 4x low power field) (Cancer 1982;49:2547)
- Atypical polypoid adenomyoma (APA):
- Distinction especially challenging with and should not be made on biopsy material, where lobular / polypoid architecture of APA can be lost
- Atypical, angulated endometrial glands with centrally necrotic squamous morules embedded in fibromyomatous stroma are usual features of APA
- Appearance of characteristic APA glands in muscular stroma can mimic myometrial invasion by well differentiated EEC
- Endometrial serous carcinoma (ESC):
- Both can have papillary architecture
- Prominent pleomorphism and mitotic activity
- Arises in background of atrophy and often within endometrial polyps
- Apical borders are hobnailed and exfoliative, i.e. they appear to shed cells
- Positive for both p16 (diffuse, block-like) and p53 (aberrant patterns, see Negative stains)
- Mimics:
- EEC with papillary architecture (villoglandular, small nonvillous papillae, micropapillae):
- EEC with ciliated / tubal metaplasia:
- Hyperchromatic metaplastic cells lack prominent mitotic activity
- Appear atypical as they are cytologically distinct from adjacent epithelium and hence mimic ESC (or its precursor - serous endometrial intraepithelial carcinoma)
- ESC usually not ciliated
- Endometrial clear cell carcinoma (CCC):
- Clear to oxyphilic cytoplasm, uniform but moderate atypia and distinct hobnail appearance often with prominent nucleoli and hyaline globules
- Papillary cores are often hyalinized
- HNF-1B+ and Napsin A+ and ER / PR-
- Mimics:
- EEC with glycogenated squamous metaplasia:
- EEC with secretory features:
- Resembles progestational endometrium with well differentiated, nonstratified columnar cells and prominent cytoplasmic vacuolization (Cancer 1982;49:1511)
- Caution - this variant is not necessarily restricted to premenopausal females
- ER / PR+; HNF-1B-, Napsin A-
- Endocervical usual type adenocarcinoma (ECA):
- Uterine carcinosarcoma (CS):
- Mesenchymal component is unequivocally malignant, with cellular atypia and mitotic activity
- Sarcomatous component usually negative for cytokeratins, EMA and beta catenin (latter may help distinction from CHEC pattern EEC)
- Often heterologous sarcomatous differentiation (rhabdo, osteo, chondrosarcoma)
- Mimics:
- Corded and Hyalinized Endometrial Carcinoma (CHEC):
- Lacks prominent sarcomatous atypia and mitotic activity
- Vague sertoliform or trabeculated growth (not patternless like CS) in abundant hyaline matrix
- Cells may show nuclear expression of beta catenin (i.e. positive)
- Spindled variant of EEC:
- Corded and Hyalinized Endometrial Carcinoma (CHEC):
Practice question #1
Practice answer #1
Practice question #2
Which of the following immunophenotypes is consistent with a well differentiated endometrial endometrioid adenocarcinoma?
- PAX8+, CK7+, CK20+, ER / PR+, wild type p53, patchy / focal p16
- PAX8+, CK7+, CK20-, ER / PR+, wild type p53, strong / diffuse p16
- PAX8+, CK7+, CK20-, ER / PR-, wild type p53, strong / diffuse p16
- PAX8+, CK7+, CK20-, ER / PR+, wild type p53, patchy / focal p16
- PAX8+, CK7-, CK20+, ER / PR+, wild type p53, patchy / focal p16
Practice answer #2
D. PAX8+, CK7+, CK20-, ER / PR+, wild type p53, patchy / focal p16
Comment Here
Reference: Endometrioid carcinoma
Comment Here
Reference: Endometrioid carcinoma