Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Prognostic factors | Treatment | Gross description | Gross images | Frozen section description | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Immunohistochemistry & special stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Practice question #1 | Practice answer #1 | Practice question #2 | Practice answer #2Cite this page: Sharma A, Lastra RR. Endometrial hyperplasia. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/uterusendometrialhyperplasiageneral.html. Accessed September 8th, 2025.
Definition / general
- Proliferation of endometrial glands with a resulting increase in gland to stroma ratio
- Atypical hyperplasia / endometrioid intraepithelial neoplasia (AH / EIN) is considered a premalignant condition
- Increased risk of both progression to and simultaneous endometrial endometrioid adenocarcinoma
Essential features
- Estrogen driven precursor lesion to endometrial endometrioid adenocarcinoma
- Increase in gland to stroma ratio (> 3:1 glandular to stromal elements)
- Divided into 2 groups: with or without atypia
- Definitive treatment for AH / EIN is hysterectomy; progestin therapy for fertility preservation
- Current system of classification
- Hyperplasia without atypia
- AH / EIN
- Prior terminologies (simple and complex) are no longer included
Terminology
- Obsolete terms
- Cystic hyperplasia
- Adenomatous hyperplasia
- Simple and complex hyperplasia
ICD coding
Epidemiology
- Similar to that of endometrial endometrioid adenocarcinoma
- Age: fourth to sixth decades (peak fifth)
- Increased circulating estrogen
- Body mass index (BMI): dose response relationship of BMI ≥ 25 and risk of hyperplasia (Am J Obstet Gynecol 2016;214:689.e1)
- Nulliparous women (Cancer 1985;56:403, Am J Epidemiol 2008;168:563)
- No race predilection
Sites
- Uterus: endometrium, endometrial polyps or adenomyosis
- Any tissue involved by endometriosis
- Ectopic endometrial glands / stroma are responsive to estrogen stimulation and can also develop an endometrial-like hyperplasia and subsequently carcinoma (Gynecol Oncol 2002;84:280, Gynecol Oncol 1996;60:238, Int J Gynecol Pathol 1996;15:1)
Pathophysiology
- Increased endogenous or exogenous estrogen, unopposed by progesterone (Semin Oncol Nurs 2019;35:157)
- Initially, estrogen has mitogenic effect on both endometrial glands and stroma
- Chronic estrogenic stimulation without progesterone affects glands to a greater extent → glandular overgrowth (hyperplasia)
Etiology
- Premenopausal
- Polycystic ovarian syndrome (PCOS): increased circulating androgens peripherally converted into estrogen
- Chronic anovulation / infertility: dysregulated estrogen without opposing progesterone secretion → simultaneous proliferation and breakdown
- Peri and postmenopausal
- Exogenous estrogen
- Estrogen supplementation: systemic therapy to alleviate symptoms of menopause → endometrial proliferation
- Tamoxifen: hormonal treatment for breast cancer acts as estrogen receptor antagonist in breast but agonist in endometrium
- Exogenous estrogen
- Any age
- Obesity: aromatase (enzyme converting circulating androgens to estrogen) is found in adipose tissue → peripheral hyperestrogenism (Mod Pathol 2000;13:295, Am J Obstet Gynecol 2016;214:689.e1)
- Ovarian pathology
- Stromal hyperplasia and hyperthecosis: stromal luteinization → hyperandrogenism → hyperestrogenism (BJOG 2003;110:690)
- Hormone secreting stromal tumors: granulosa cell tumor, thecoma
Clinical features
- Abnormal or dysfunctional uterine bleeding (Obstet Gynecol Surv 2004;59:368)
- Rare cases asymptomatic
Diagnosis
- Pelvic / transvaginal ultrasound
- Endometrial biopsy
- Hysteroscopy with endometrial curettage (Eur J Gynaecol Oncol 2007;28:400)
Laboratory
- No validated biomarker for endometrial hyperplasia
Radiology description
- Thickened endometrial stripe on pelvic / transvaginal ultrasound (Obstet Gynecol Sci 2016;59:192)
- Generally no discrete mass
Prognostic factors
- Endometrial hyperplasia
- Presence / absence of atypia is most important feature
- AH / EIN associated with
- Progression to endometrial endometrioid adenocarcinoma in up to 28% of cases without hysterectomy after 20 year followup (J Clin Oncol 2010;28:788)
- Concurrent endometrial carcinoma in up to 43% of cases (Cancer 2006;106:812)
- Majority are low grade (FIGO grade 1) and low stage (FIGO stage IA or IB) (J Obstet Gynaecol Can 2008;30:896)
- Hyperplasia without atypia: progression to endometrial endometrioid adenocarcinoma in up to 4.6% of cases after 20 year followup (J Clin Oncol 2010;28:788)
Treatment
- Endometrial hyperplasia without atypia
- Hysterectomy too aggressive; risk of progression to or simultaneous endometrial endometrioid adenocarcinoma is low (refer to Prognostic factors)
- Treatments outlined below for AH / EIN acceptable within appropriate clinical context
- Endometrial hyperplasia without atypia arising in endometrial polyp: polypectomy curative if completely excised under hysteroscopic guidance
- Endometrial ablation can be used (not adequate alternate therapy for AH / EIN or refractory endometrial hyperplasia without atypia) (Am J Obstet Gynecol 1998;179:569)
- AH / EIN
- Hysterectomy with or without bilateral salpingo-oophorectomy is definitive treatment
- If patient desires fertility or is not a surgical candidate
- AH / EIN arising in endometrial polyp
- Polypectomy curative if completely excised under operative hysteroscopy
- Hysterectomy occasionally warranted in appropriate clinical context
- Progestin therapy: oral or intrauterine device (Am J Obstet Gynecol 2014;210:255.e1, Hum Reprod 2013;28:1231, Obstet Gynecol 2013;121:1165)
- Latter considered superior for efficacy, compliance and prevention of recurrence
- Can even be trialed for fertility preservation in cases up to nonmyoinvasive FIGO grade 1 endometrioid adenocarcinoma
- Metformin controversial (Cochrane Database Syst Rev 2017;10:CD012214)
- AH / EIN arising in endometrial polyp
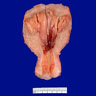
Gross description
- Usually not grossly appreciable
- Florid to pseudopolypoid endometrium (similar to that of secretory phase)
Frozen section description
- Not appropriate for preliminary diagnosis of hyperplasia or atypia
- Intraoperative consultation may be utilized for diagnosing adenocarcinoma in a patient with preoperative diagnosis of AH / EIN but this is not considered standard of care
- Concurrent carcinoma may be missed intraoperatively due to endometrial undersampling for lack of gross lesion (Am J Obstet Gynecol 2007;196:e40, Asian Pac J Cancer Prev 2012;13:1953)
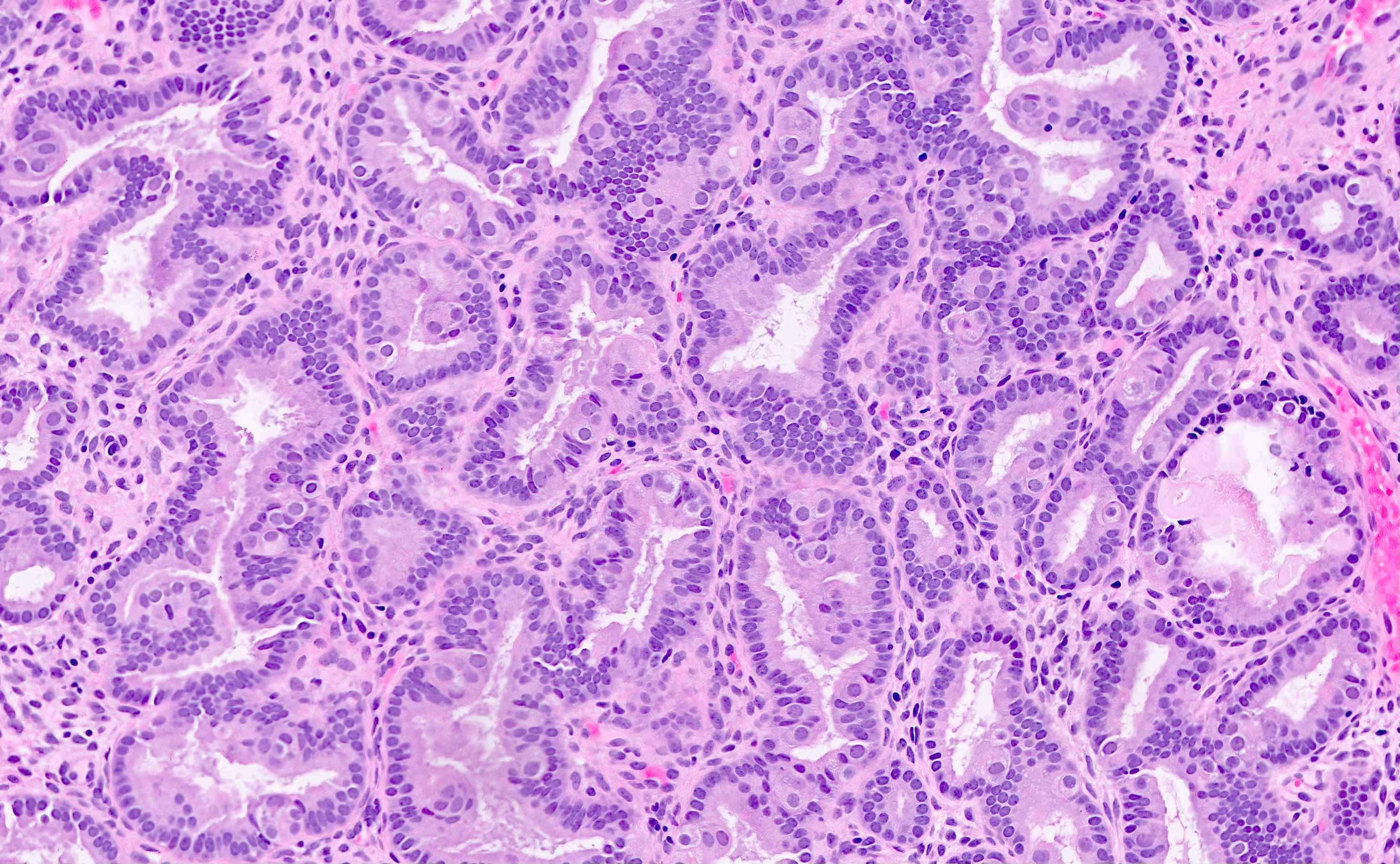
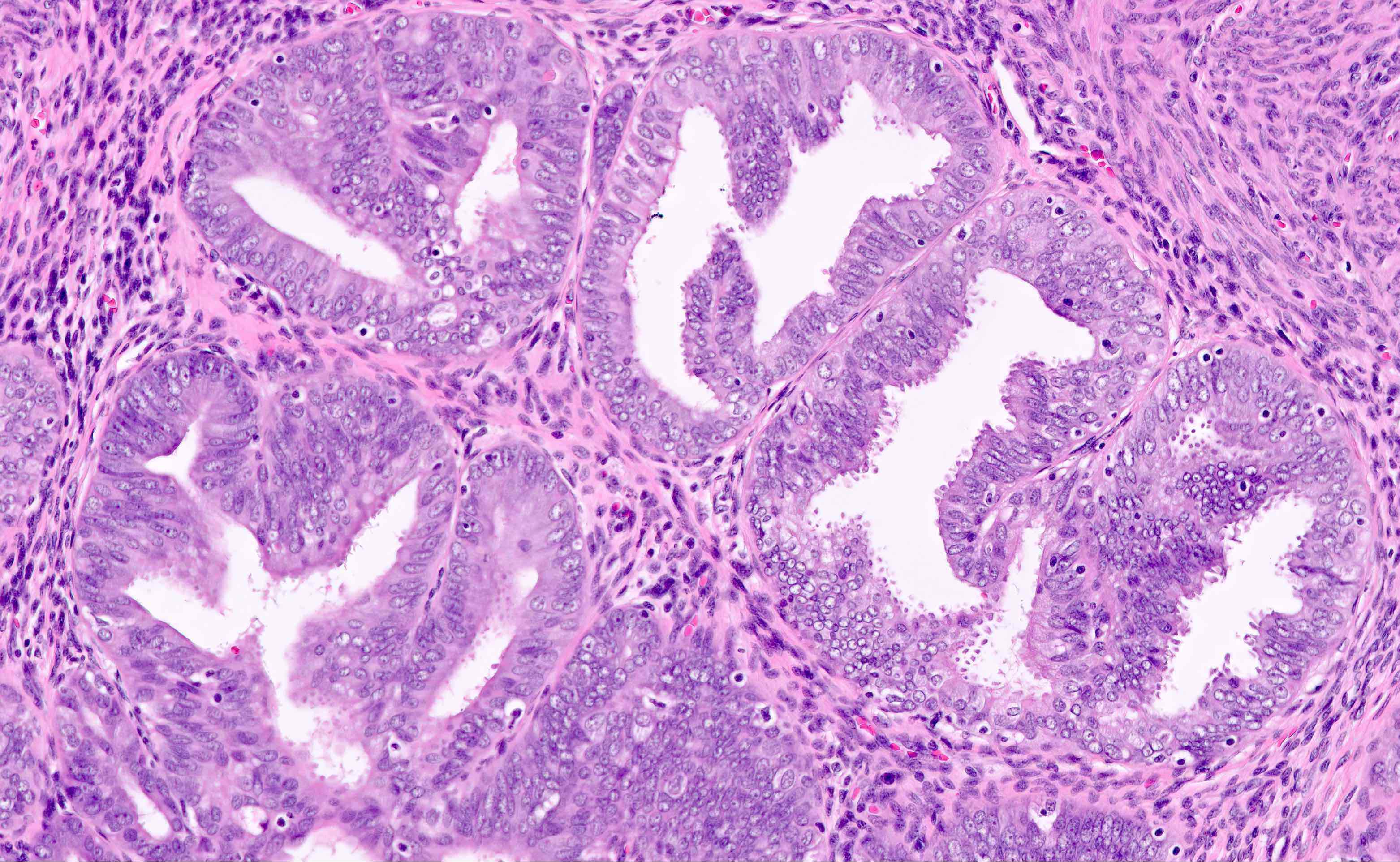
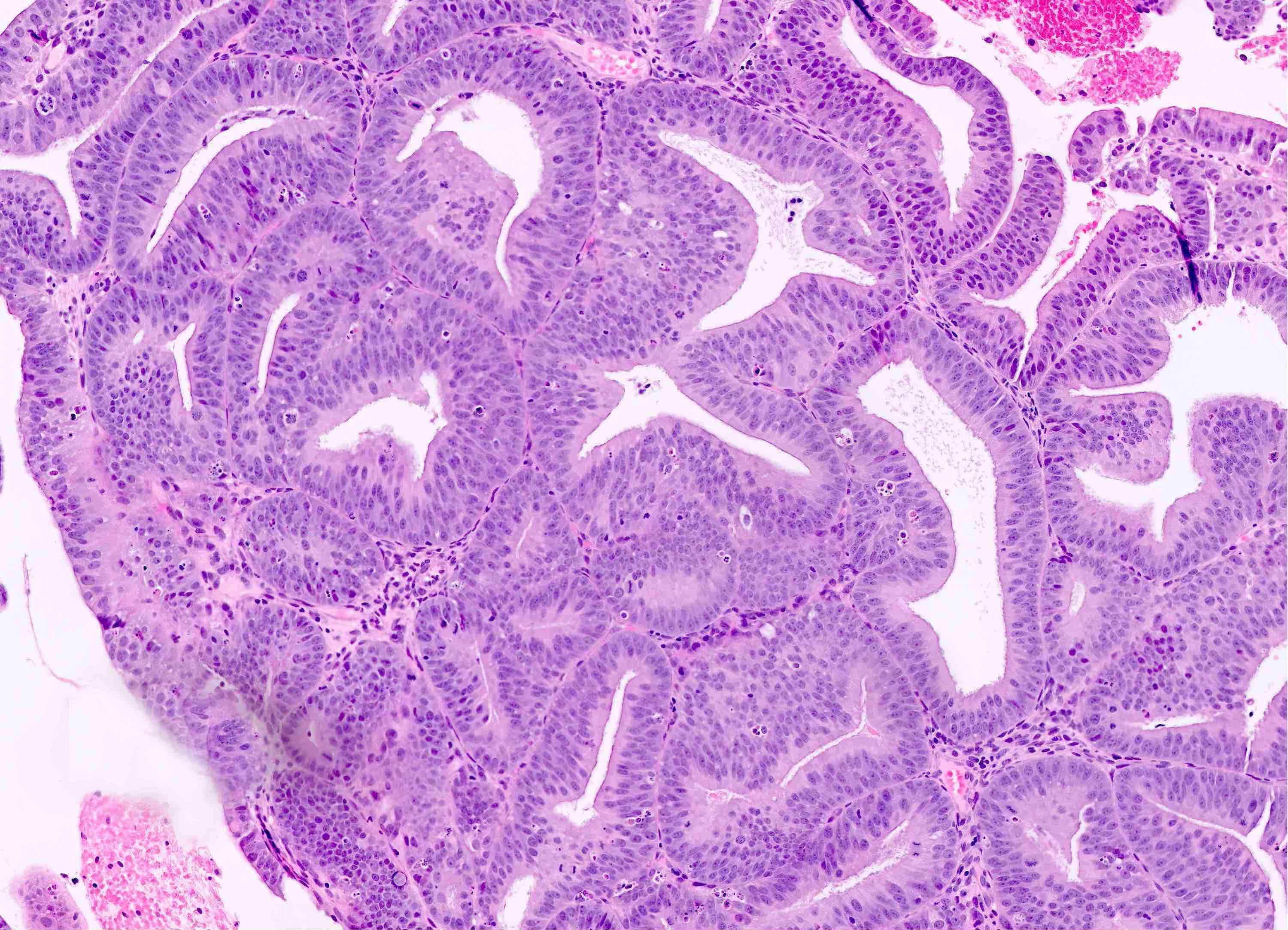
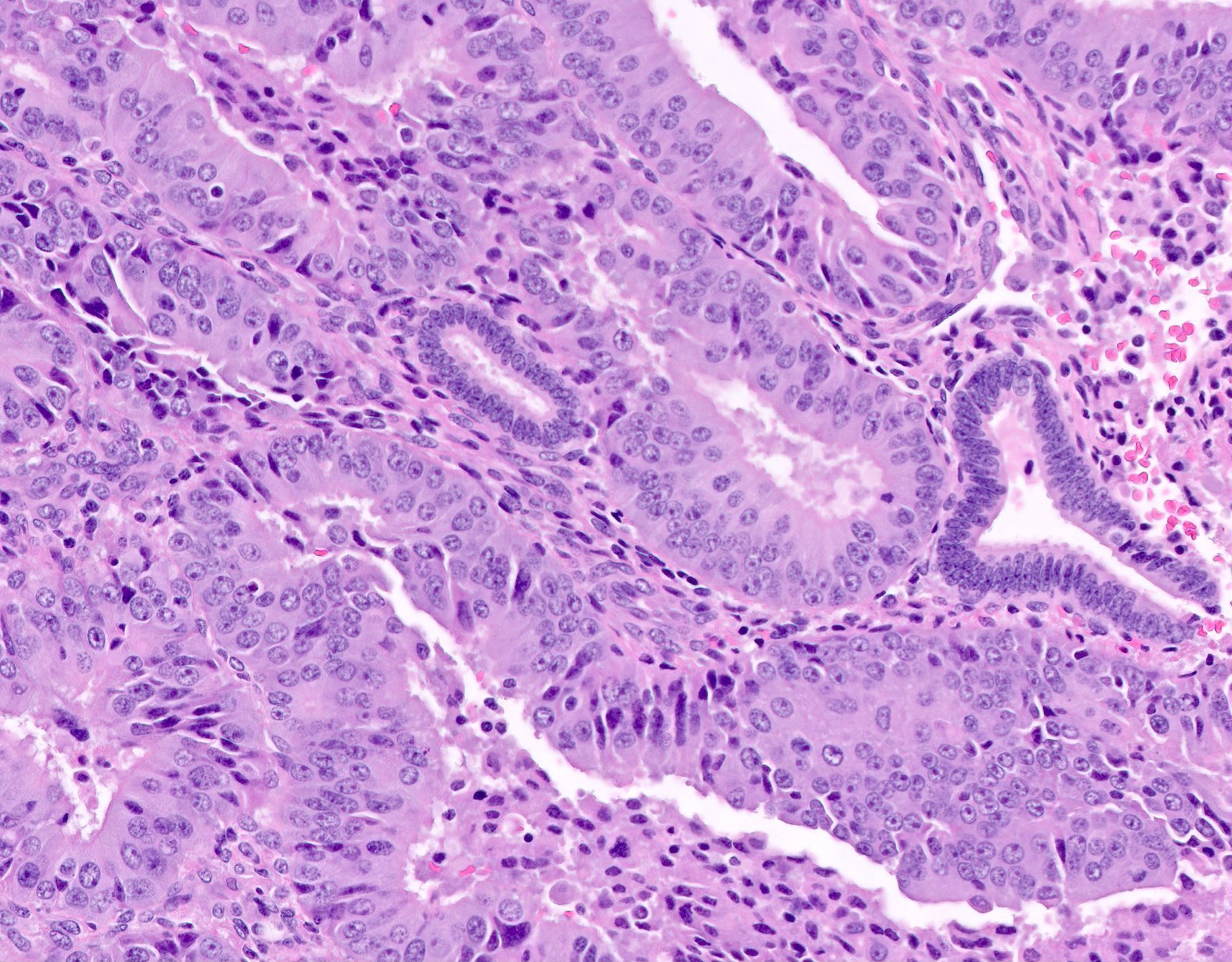
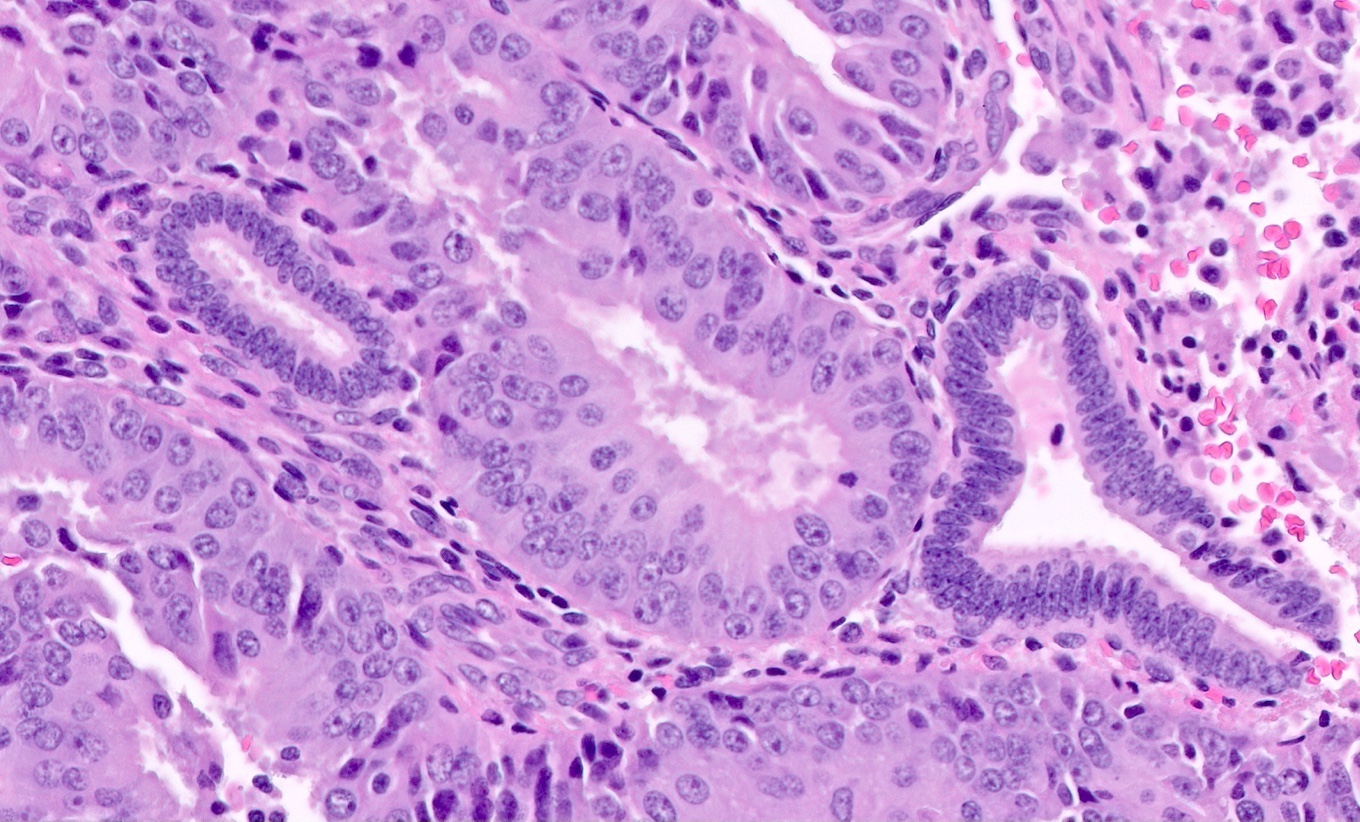
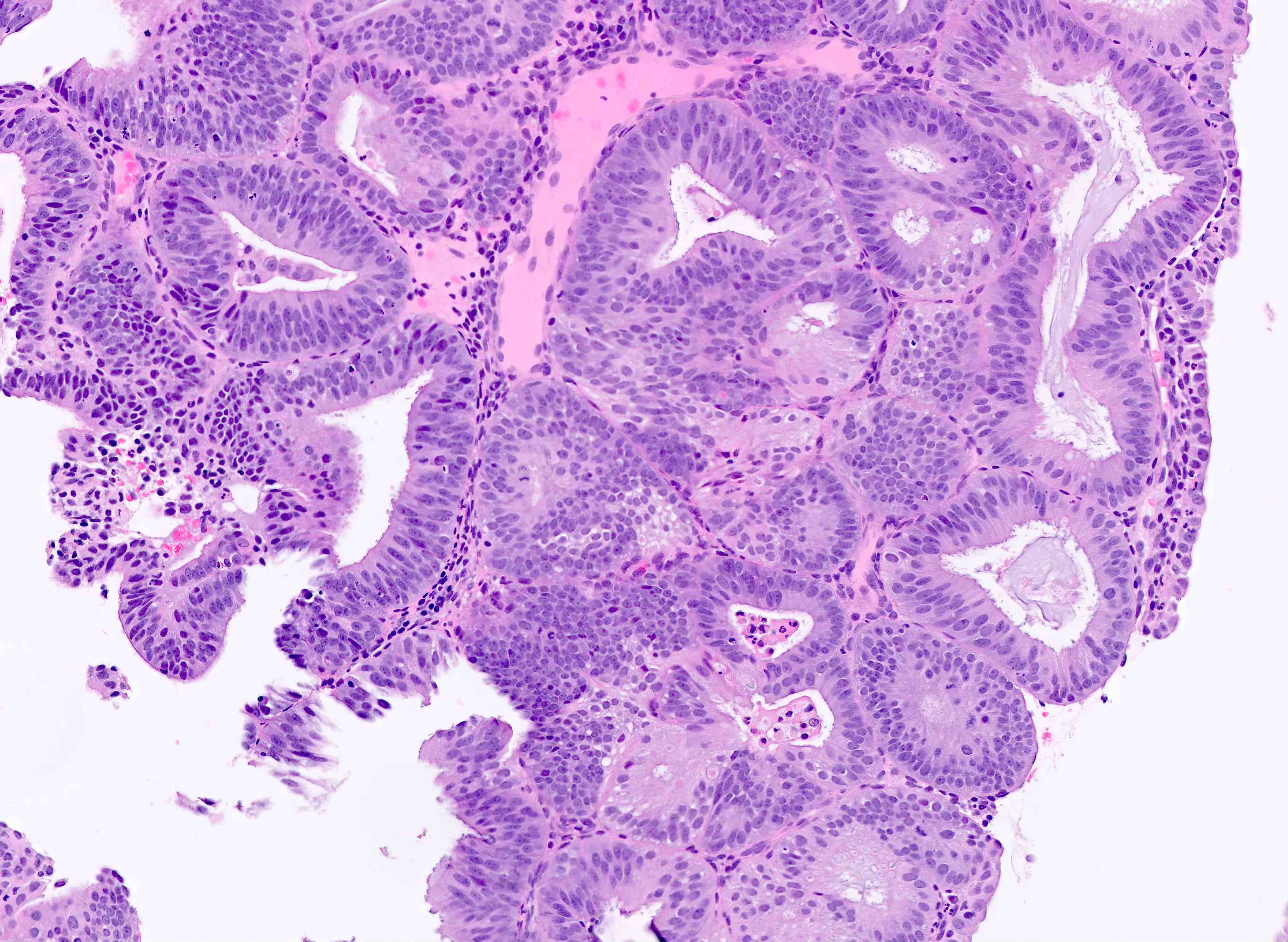
Microscopic (histologic) description
- Endometrial hyperplasia without atypia (Semin Diagn Pathol 2010;27:199)
- Architecture
- Closely packed glands such that gland to stroma ratio is > 3:1 but stroma is still present between glandular basement membranes (however minimal)
- Variation in gland size with cystic dilatation or irregular luminal contours (budding, angulation, invagination, outpouching, papillary projections)
- Associated with stromal breakdown (Diagn Cytopathol 2006;34:609)
- Increased volume of endometrial tissue on biopsy / curetting is typical (especially in postmenopausal patient) but not required for diagnosis
- Cytologic features
- Must be assessed in comparison to cytological features of background endometrium (which even in nonneoplastic states can show hyperchromasia, nuclear enlargement and nucleoli due to cyclic hormonal changes)
- Atypia is not defined with fixed cytologic descriptors but rather considered a cytologic distinction from the surrounding baseline endometrium; this may include metaplastic changes
- In endometrial hyperplasia without atypia, the cytologic features of the crowded glands must be identical to those of the background endometrium
- Architecture
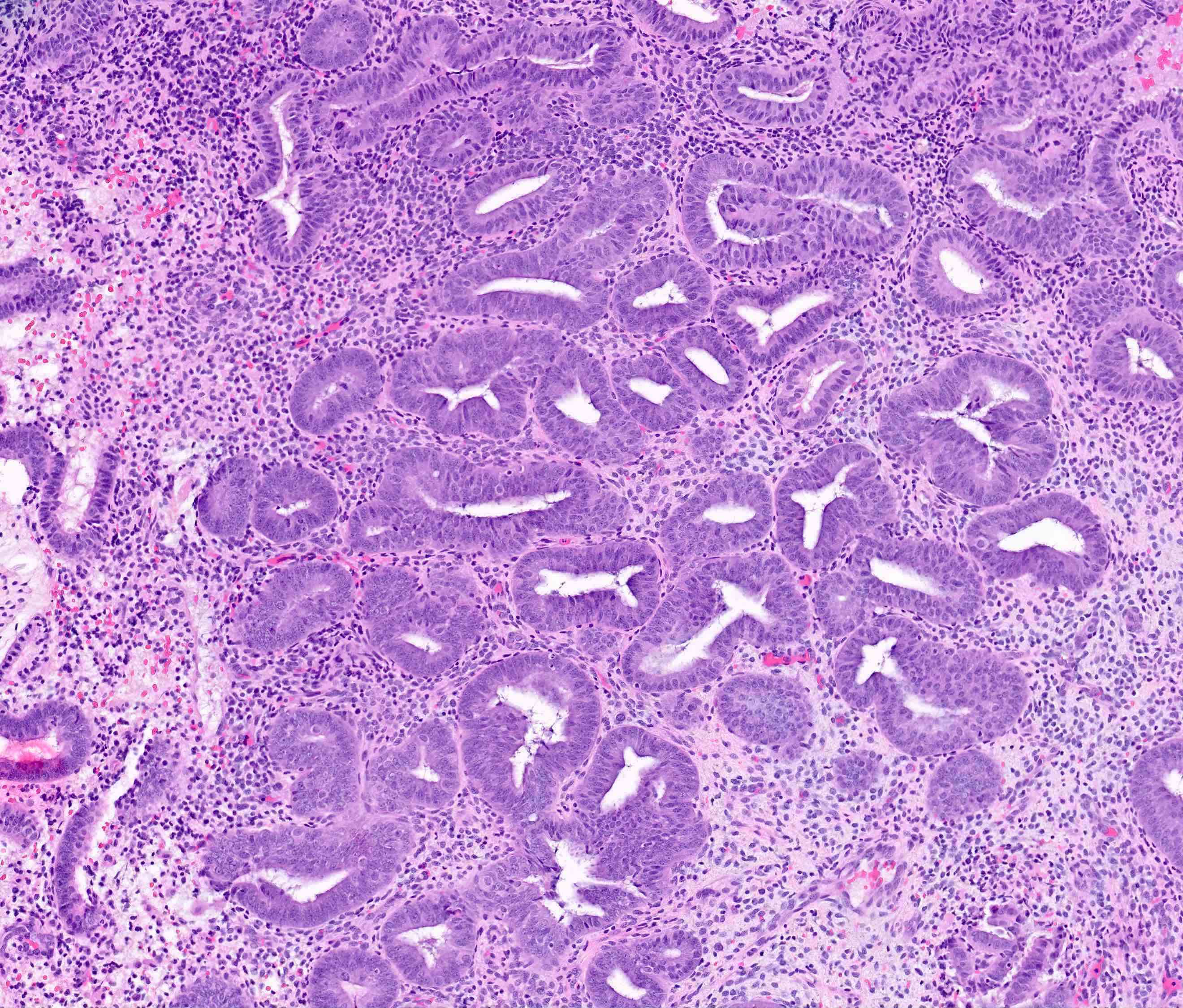
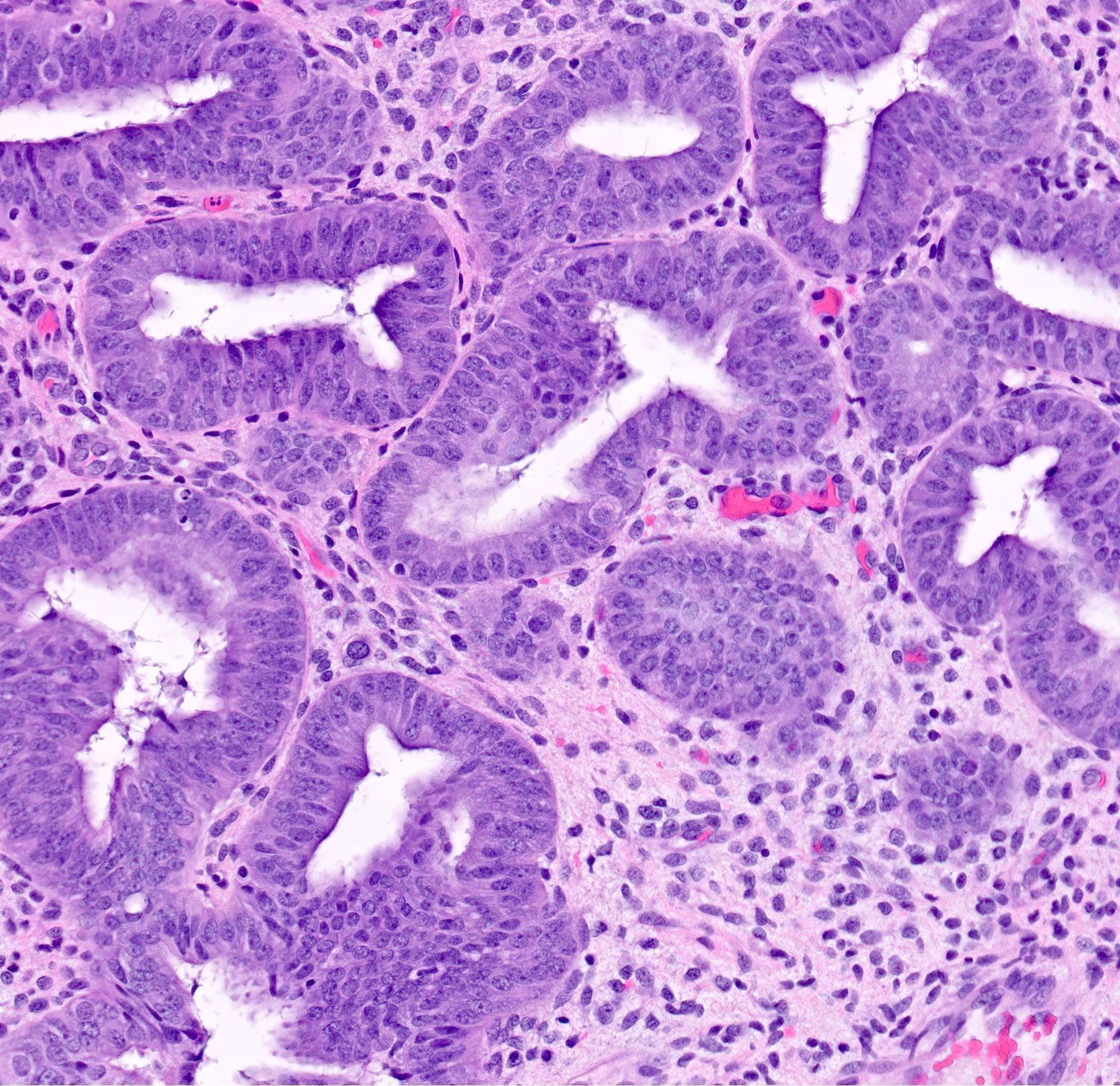
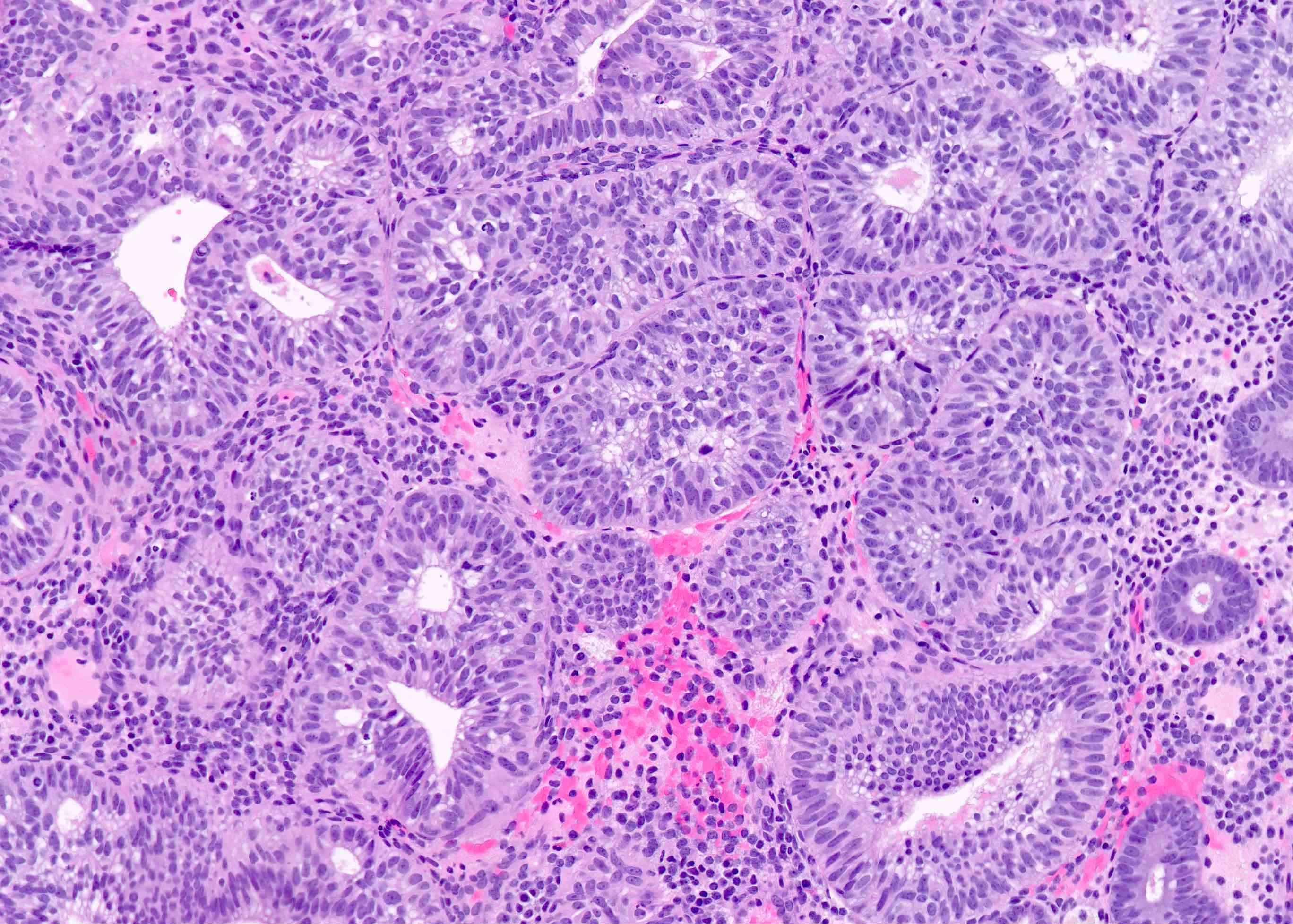
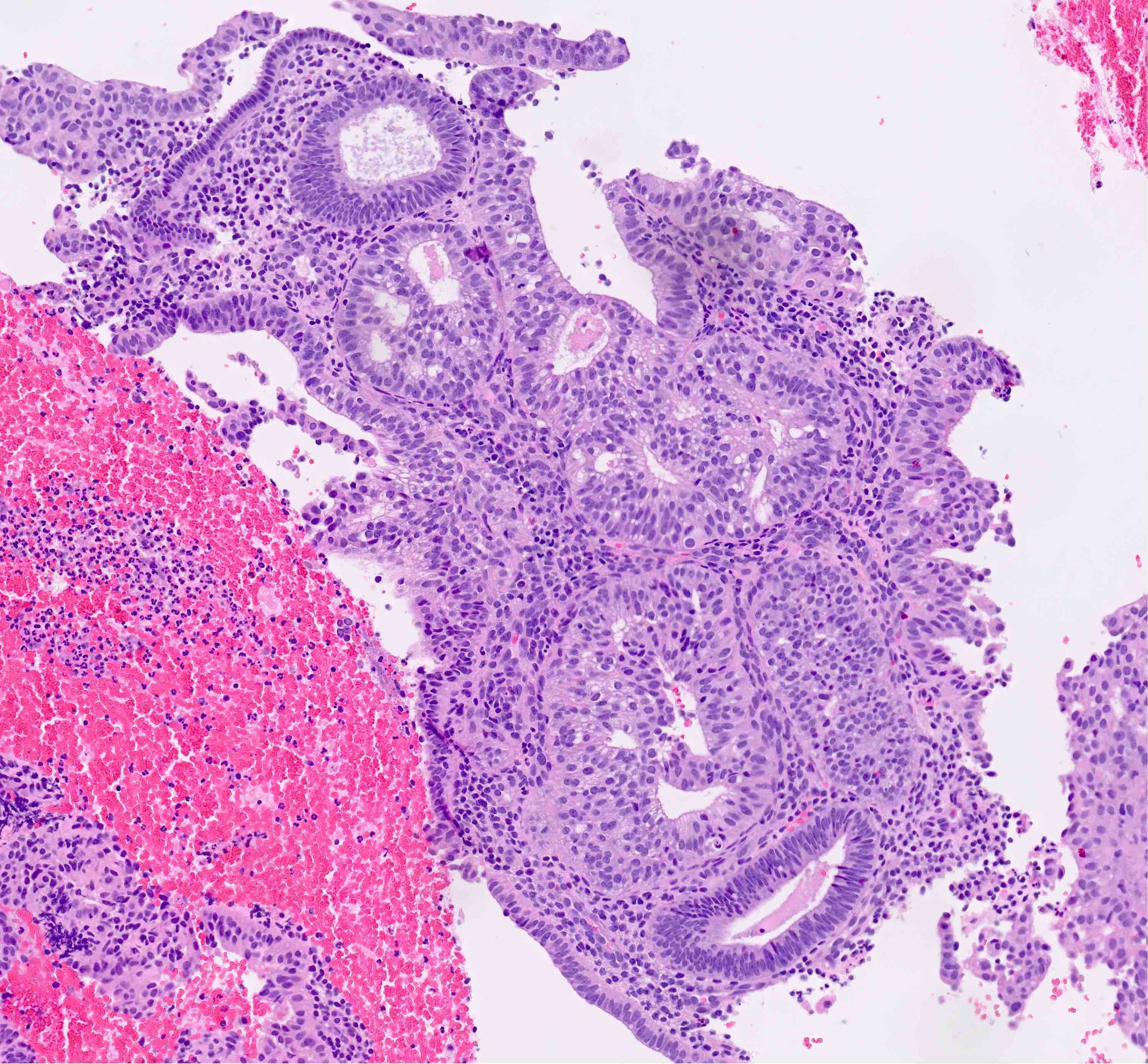
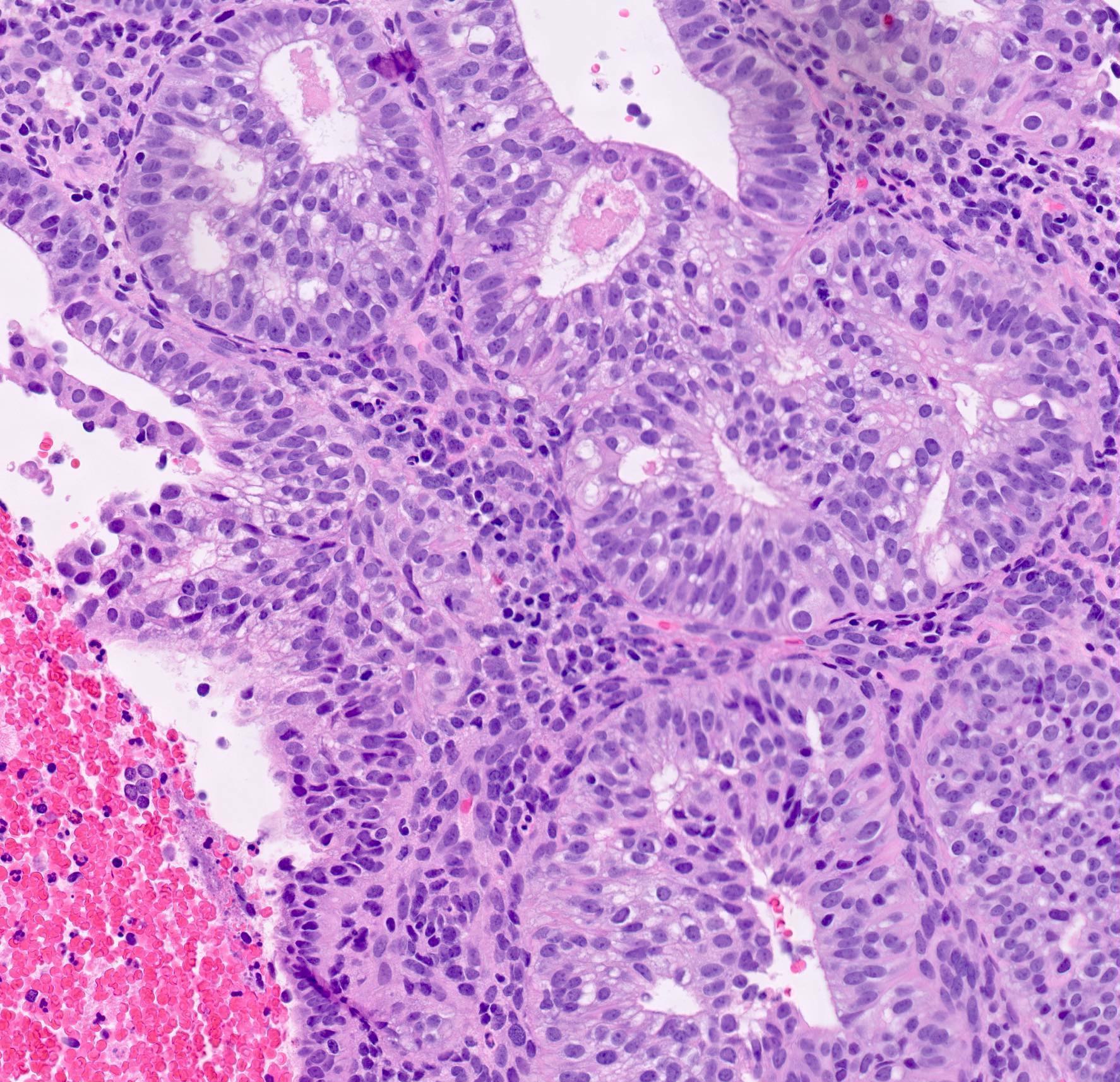
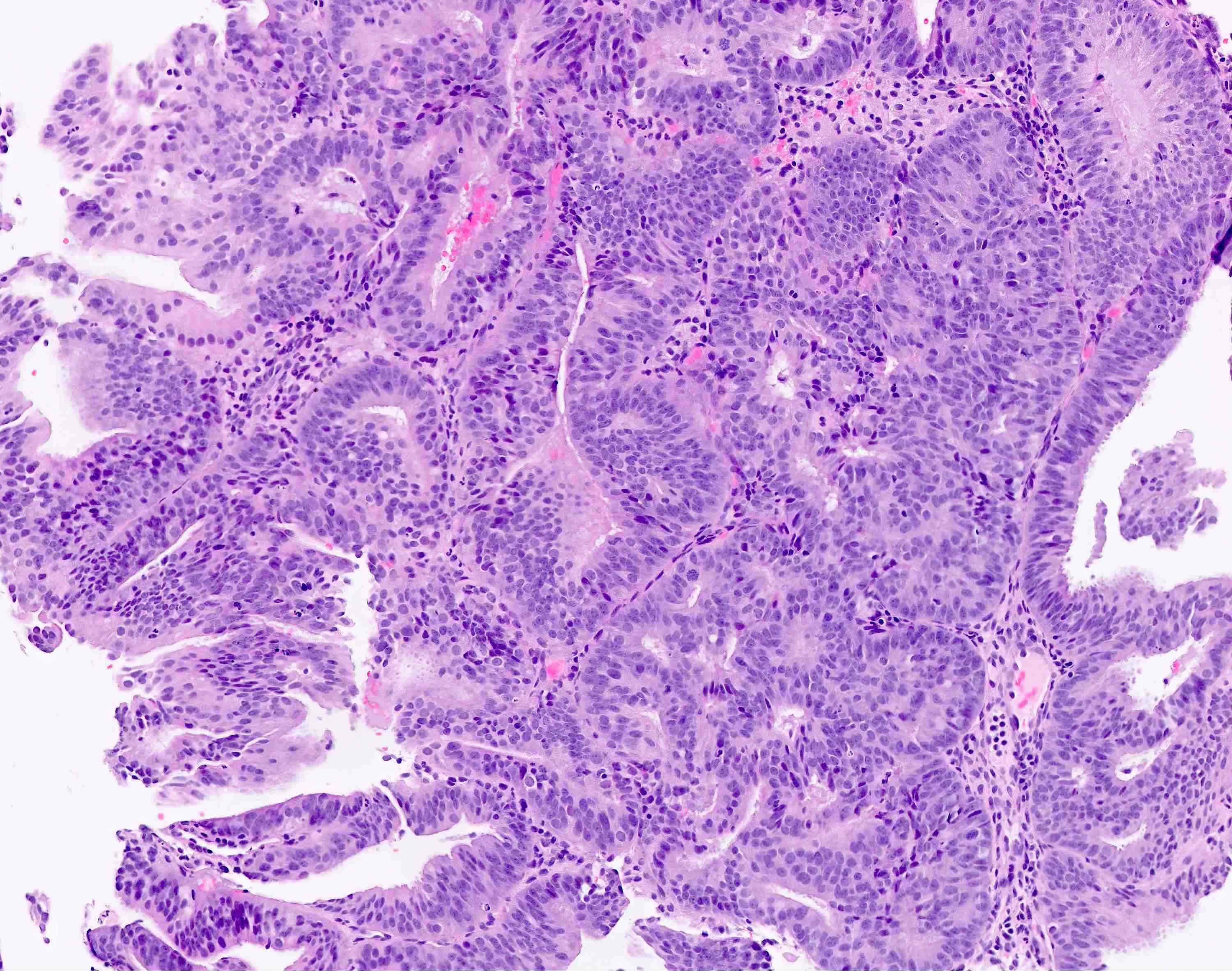
- AH / EIN
- Architecture
- Similar to the spectrum described above for hyperplasia without atypia
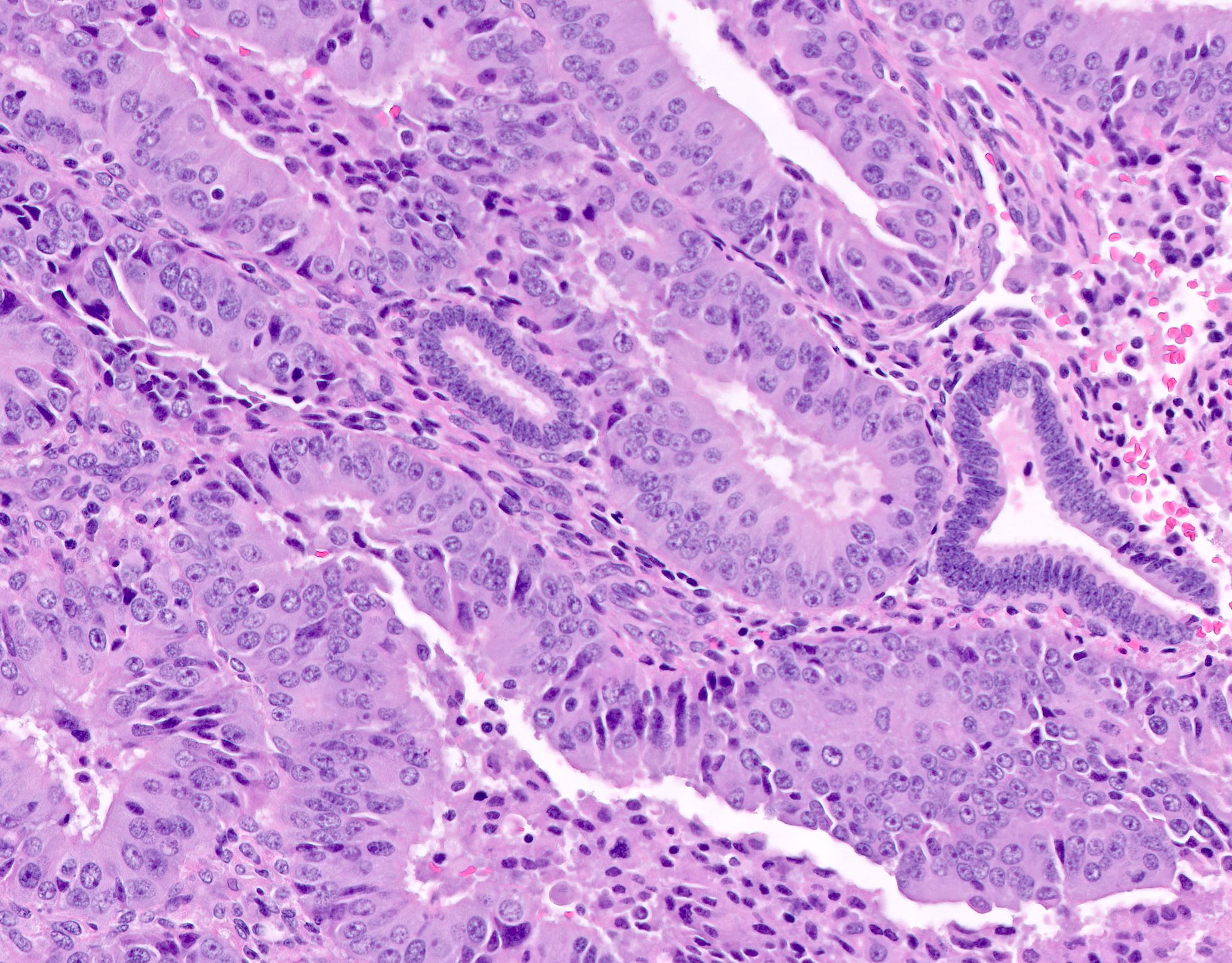
- Cytologic features
- Definition of cytologic atypia in the endometrium is not static; it is dependent on the appearance of the physiologic baseline endometrium
- AH / EIN is a clonal expansion of endometrial glands with different cytomorphology than that of surrounding normal endometrium
- Atypia may manifest as loss of cellular polarity, hyperchromasia, nuclear enlargement or even as benign appearing metaplastic changes (tubal, eosinophilic, mucinous) (Histopathology 2008;53:325)
- In a focus of crowded glands, the presence of ciliated metaplasia (usually considered reassuring) does not negate the possibility of AH / EIN
- Diagnosis of AH / EIN in a polyp should be made in cytologic comparison to polyp glands, not the nonpolyp endometrial tissue (Mod Pathol 2005;18:324)
- Architecture
Microscopic (histologic) images
Immunohistochemistry & special stains
- Not typically useful in differential diagnosis between normal endometrium and benign / malignant endometrial proliferations
- Loss of PTEN or PAX2 (Int J Gynecol Pathol 2015;34:40, Cancer Res 2010;70:6225)
- Most frequently mutated genes in endometrioid endometrial carcinoma and its precursors (tumor suppressor and transcription factor inactivation, respectively)
- Helpful but neither sensitive nor specific for AH / EIN
- Beta catenin also potentially useful in above panel (Am J Surg Pathol 2022;46:404)
Molecular / cytogenetics description
- No reproducible genomic alterations
- AH / EIN is variably associated with mutations in PTEN, PIK3CA, KRAS, CTNNB1 (Jpn J Cancer Res 1998;89:985, Cancer Res 1998;58:3254, Med Klin 1975;70:812)
- Similar to endometrial endometrioid adenocarcinoma
- Loss of mismatch repair proteins resulting in progressive accumulation of microsatellite unstable loci (Mod Pathol 2019;32:1508)
Sample pathology report
- Endometrium, curettage:
- Disordered proliferative endometrium with focus of hyperplasia without atypia
- Endometrium, biopsy:
- AH / EIN focally bordering on endometrial endometrioid adenocarcinoma (FIGO grade I) (see comment)
- Comment: There are rare minute foci suspicious for a FIGO grade 1 endometrioid endometrial adenocarcinoma. Recommend additional sampling with endometrial curettage for a more definitive diagnosis.
Differential diagnosis
- Benign:
- Compression artifact:
- Telescoping and pseudocompression of glands due to procedure / processing artifact may create appearance of packed and back to back glands
- Absence of peripheral stromal elements to lesion in question is a clue to artificial density
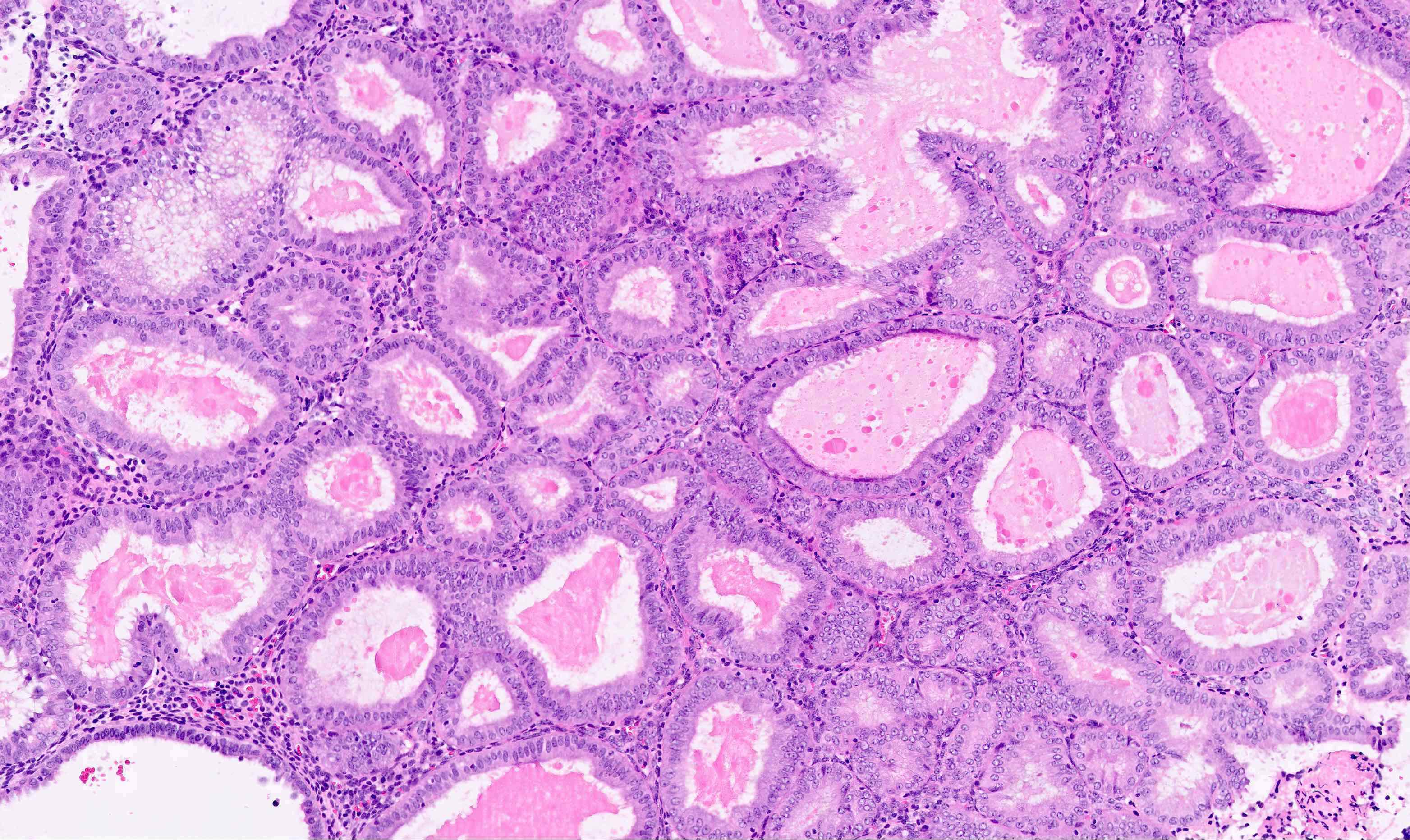
- Cystic atrophy:
- Can have similar low power appearance to hyperplastic endometrium with closely apposed and cystically dilated glands but these do not have the irregular contours of hyperplasia
- Glandular lining is low cuboidal to flattened without mitotic activity, in contrast to proliferative endometrium
- Stroma is dense and resembles that of endometrium basalis
- Endometrial polyp:
- Similar low power appearance in biopsies (by definition, altered, disorganized or irregular glands)
- Glands can be slightly crowded at baseline in endometrial polyp and should not be diagnosed as hyperplasia
- Distinct densely fibrotic stroma with thick walled blood vessels
- Endometrial polyps can contain foci of AH / EIN (see above in Microscopic (histologic) description)
- Disordered proliferative / anovulatory endometrium:
- No well delineated criteria
- Histologically considered as degree below hyperplasia without atypia on a shared morphologic spectrum and distinction is often not reproducible
- Cystic glandular dilation with focal / diffuse loss of spatial organization
- Changes are secondary to excess estrogenic influence on the endometrium and should prompt pathologist to search carefully for AH / EIN
- Both have similar treatment (exogenous progestin)
- Metaplastic changes:
- Metaplastic changes must always be interpreted within the context of glandular architecture and background endometrium (as above); their presence is not definitionally benign despite bland cytology
- Squamous and squamous morular metaplasia
- When involving nonhyperplastic glands, can create false appearance of solid crowding
- As in endometrial endometrioid adenocarcinoma, squamous component should be subtracted in assessment of glandular architecture
- Surface syncytial and eosinophilic metaplasia
- Similar low power appearance due to cytoplasmic eosinophilia and epithelial proliferation
- Metaplasia is usually cytologically bland
- Endometrial stromal / glandular breakdown:
- Menstrual endometrium may demonstrate altered cytology, such as loss of polarity due to nuclear piling and coarsening of chromatin
- Collapse of glands creates artificial crowding without stromal scaffolding
- Presence of glandular aggregation amidst necrotic predecidua can mimic carcinoma
- Compression artifact:
- Malignant:
- Endometrioid adenocarcinoma, FIGO grade 1:
- Degree of atypia between the two is usually similar
- AH / EIN should not have
- Cribriforming, confluent glands
- Labyrinthine intraluminal connections
- Areas of purely solid epithelium
- Stromal alteration suggesting invasion - desmoplasia (myofibroblasts, edema, inflammation) or necrosis (intervening endometrial stroma replaced by pools of neutrophilic debris)
- Endometrioid adenocarcinoma, FIGO grade 1:
Additional references
Practice question #1
Practice answer #1
A. Anovulatory menstrual cycles. The photomicrograph shows an image of endometrioid intraepithelial neoplasia (EIN). Answer B is incorrect because EIN is not grossly appreciable. Answer C is incorrect because progestin eluting IUD is one avenue of treatment for EIN; however, it is not considered definitive. Answer D is incorrect because EIN is associated with an increased risk (up to 40%) of concurrent or subsequent carcinoma. Answer E is incorrect because immunohistochemical stains for WT1 and GATA3 have no role in the diagnosis of EIN.
Comment Here
Reference: Endometrial hyperplasia
Comment Here
Reference: Endometrial hyperplasia
Practice question #2
Which of the following features is one of the requirements of a diagnosis of endometrial hyperplasia?
- Crowded glands with minimal residual intervening stroma
- Diffuse nuclear staining for p53
- Documentation of a PTEN mutation or loss of PTEN by IHC
- Glands with cribriforming architecture and cytologic alterations distinct from surrounding glands
- Loss of mismatch repair proteins
Practice answer #2
A. Crowded glands with minimal residual intervening stroma. Answer A is correct because the diagnostic criteria for endometrial hyperplasia, as outlined by WHO, includes the presence of a crowded proliferative endometrium and increased gland to stroma ratio. Answer B is incorrect because p53 has no role in the diagnosis of endometrioid intraepithelial neoplasia (EIN). Answer C is incorrect because PTEN abnormality by mutational analysis or IHC is nonspecific and not considered a requirement for the diagnosis of EIN. Answer D is incorrect because glands with cribriform architecture are features of endometrioid carcinoma rather than EIN. EIN, however, does require distinct cytology from the background endometrium. Answer E is incorrect because while loss of mismatch repair proteins may be observed in EIN, it is neither a requirement nor should be employed towards the diagnosis in the absence of appropriate morphologic features.
Comment Here
Reference: Endometrial hyperplasia
Comment Here
Reference: Endometrial hyperplasia