13 September 2023 - Case of the Month #531
All cases are archived on our website. To view them sorted by case number, diagnosis or category, visit our main Case of the Month page. To subscribe or unsubscribe to Case of the Month or our other email lists, click here.
Thanks to Drs. Aaron Huber and Diana Agostini-Vulaj, University of Rochester Medical Center, Rochester, New York, USA for contributing this case and discussion and to Dr. Raul Gonzalez, Emory University School of Medicine, Atlanta, Georgia, USA for reviewing the discussion.

Case of the Month #531
Clinical history:
An 85 year old woman with a remote history of right sided colon cancer underwent a transverse colon resection for a newly diagnosed colon cancer.
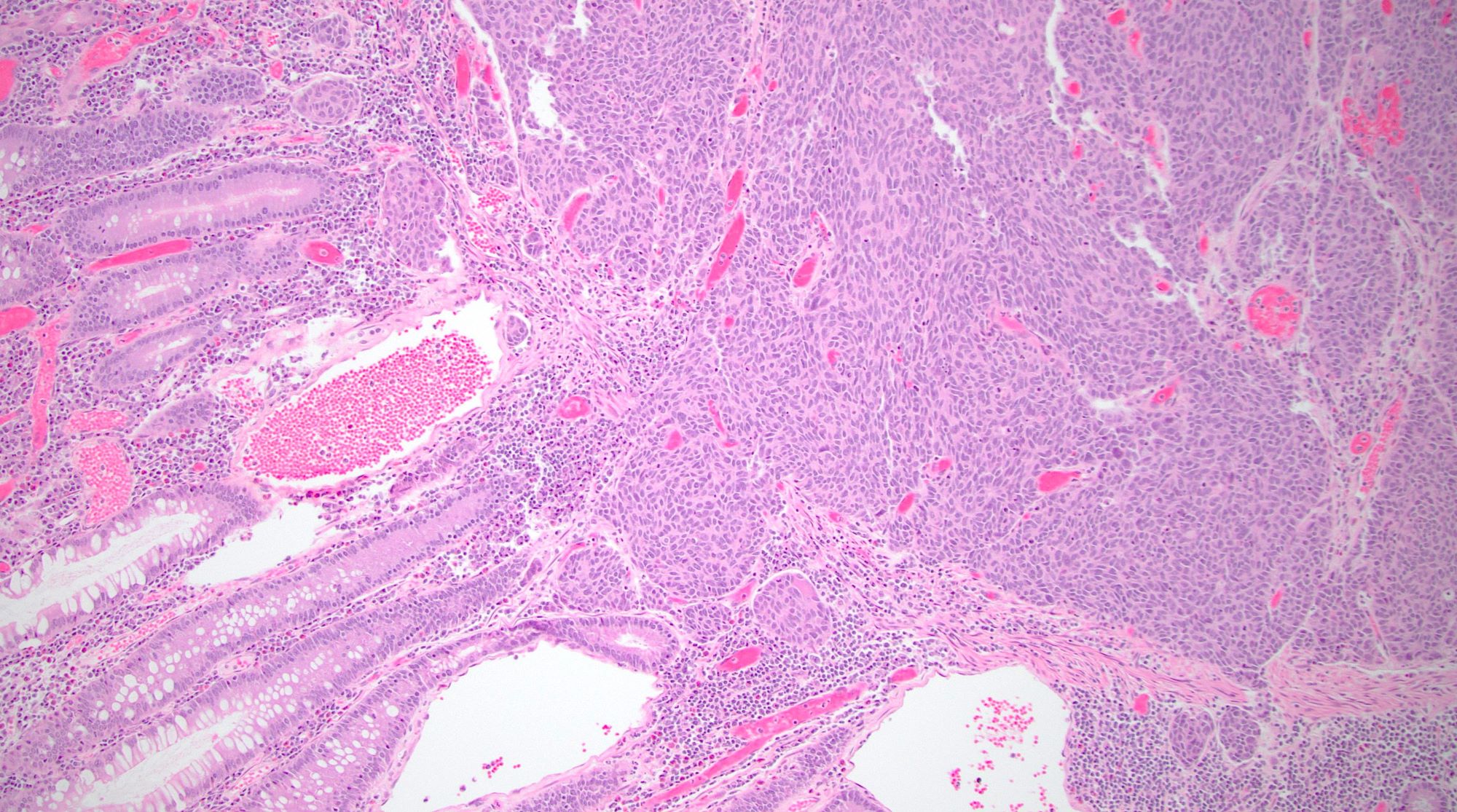
Histopathology images:
What is your diagnosis?
Diagnosis: Poorly differentiated neuroendocrine carcinoma, large cell type
Test question (answer at the end):
Which of the following is true of poorly differentiated neuroendocrine carcinoma, large cell type in the colon?
A. INSM1 expression is a sensitive and specific immunohistochemical marker for neuroendocrine differentiation
B. Mixed neuroendocrine nonneuroendocrine neoplasm may be diagnosed if the poorly differentiated neuroendocrine carcinoma comprises only 5% of the total tumor volume
C. This tumor has an overall good prognosis
D. This tumor is a common morphologic subtype of colorectal cancer
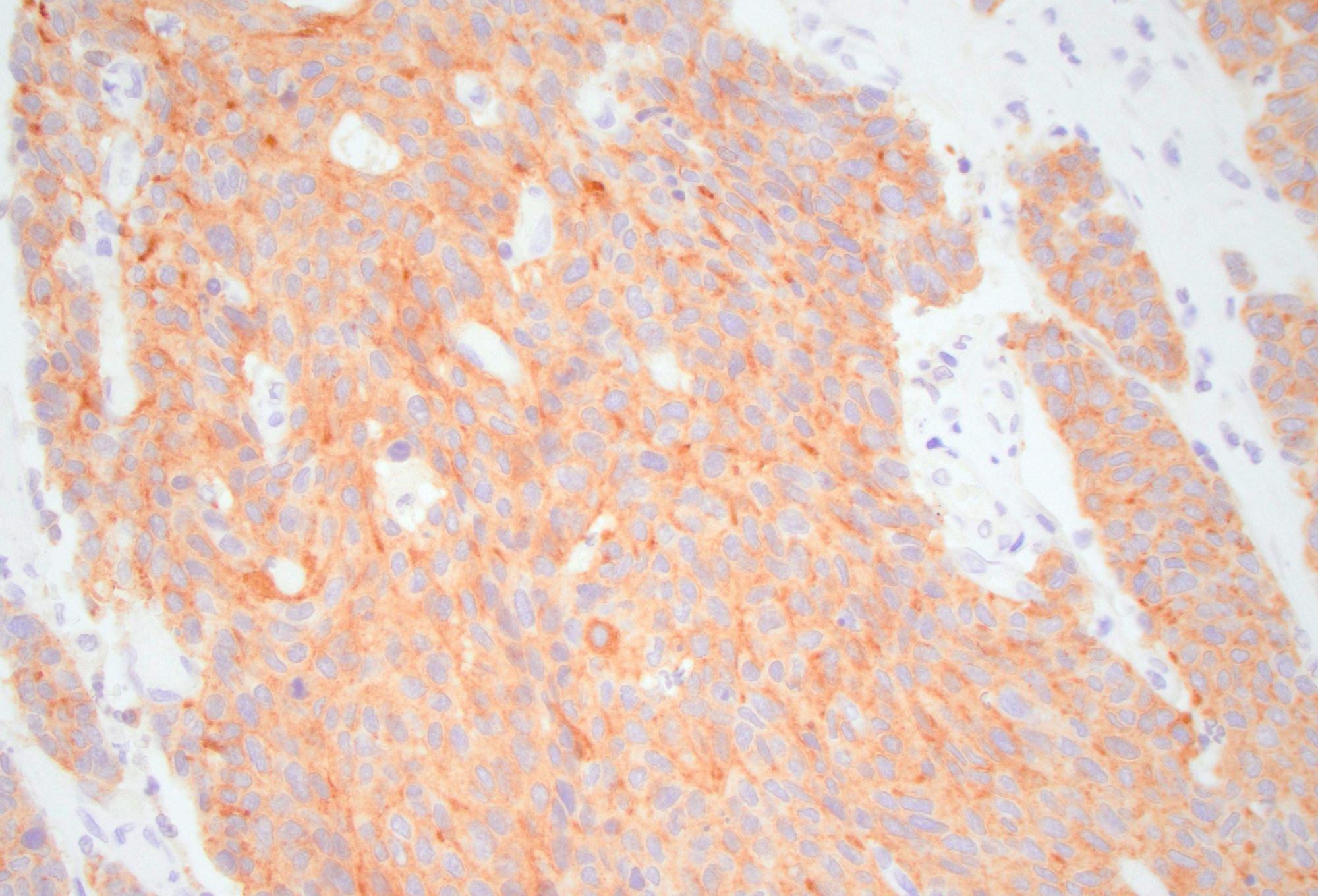
Stains:
Discussion:
In the current World Health Organization (WHO) classification of neuroendocrine neoplasms of the gastrointestinal tract, two types of poorly differentiated neuroendocrine carcinoma are recognized, small cell and large cell. Although only 5 - 7% of well differentiated neuroendocrine tumors of the gastroenteropancreatic tract arise in the colon, 25% of all gastroenteropancreatic neuroendocrine carcinomas arise at this site. Colonic neuroendocrine carcinoma is rare and comprises only ~0.6% of all colorectal carcinomas. Large cell neuroendocrine carcinoma (LCNEC) of the colon occurs at a mean age of 65 years, affects men slightly more than women and has a clinical presentation similar to that of conventional colorectal adenocarcinoma. Regardless of site, neuroendocrine carcinoma in the gastrointestinal tract behaves aggressively and the majority are at an advanced stage with metastatic disease at the time of diagnosis.
LCNEC is comprised of an infiltrative tumor that has an organoid architecture with trabecular, rosette-like and solid patterns. The nests of tumor cells commonly demonstrate central necrosis. There may be single cell necrosis or large areas of geographic necrosis. Apoptotic cells and mitotic figures are easily identifiable. The tumor cells in LCNEC are medium to large with amphophilic or basophilic cytoplasm and large, ovoid and hyperchromatic nuclei with nucleoli. Neuroendocrine differentiation can be confirmed with immunohistochemical stains. Some LCNEC are associated with overlying epithelial dysplasia (adenoma), which suggests that these may be a precursor lesion to LCNEC. Colonic LCNEC is positive for synaptophysin (usually strongly positive) and chromogranin (this may be faintly or focally positive or entirely absent). Nonspecific neuroendocrine markers such as CD56 and neuron specific enolase (NSE) are commonly positive. The newer immunohistochemical marker INSM1 (insulinoma associated protein 1) is a very sensitive and specific marker of neuroendocrine differentiation in gastrointestinal neuroendocrine neoplasms. CDX2 and TTF1 may also be positive. The Ki67 labeling index is typically over 80% of neoplastic cells. Notably, a Ki67 is not necessary in these clearly high grade poorly differentiated neuroendocrine carcinomas. Mismatch repair (MMR) immunohistochemistry may demonstrate MMR deficiency in ~15% of cases. LCNEC tends to harbor a high mutational burden, with mutations in p53 and RB1 being most common. Other mutations that may be identified include SMAD4 (DPC4), KRAS, FHIT, DCC, MEN1 and BRAF.
The histologic differential diagnoses include small cell carcinoma, mixed neuroendocrine nonneuroendocrine neoplasm (MiNEN), and metastatic neuroendocrine carcinoma. Often, the most challenging morphologic differential diagnosis is with small cell carcinoma of the colon. Small cell carcinoma, as the name implies, is composed of small cells with scant cytoplasm and hyperchromatic nuclei, often with nuclear molding. The morphology between small cell and LCNEC may overlap considerably and it may be difficult to separate these two types of poorly differentiated neuroendocrine carcinoma; however, the clinical management and outcome for either type is similar. MiNEN is the new nomenclature for a mixed tumor typically composed of adenocarcinoma and poorly differentiated neuroendocrine carcinoma, that was previously known as mixed adenoneuroendocrine carcinoma (MANEC). However, since the nonneuroendocrine carcinoma is not always adenocarcinoma and the neuroendocrine component is not always a poorly differentiated neuroendocrine carcinoma, the term MiNEN was adopted. MiNEN is differentiated from a pure LCNEC by findings of at least 30% of a nonneuroendocrine carcinomatous component within the same tumor. Lastly, metastatic neuroendocrine carcinoma may be difficult to separate on morphologic and immunophenotypic findings alone. In this scenario, correlation with the clinical and radiographic picture is of utmost importance.
In summary, LCNEC of the colon is a rare type of colorectal carcinoma that has an aggressive course and overall poor prognosis. These tumors are composed of intermediated to large sized cells with enlarged and hyperchromatic nuclei with nucleoli. Neuroendocrine markers such as synaptophysin, chromogranin, INSM1 and to a lesser extent CD56 and NSE may be used to confirm neuroendocrine differentiation. Common mutations identified in LCNEC are p53 and RB1; however, numerous other mutations have been identified. The main differential diagnoses are colonic small cell carcinoma, metastatic neuroendocrine carcinoma and mixed neuroendocrine nonneuroendocrine neoplasm (MiNEN).
References:
Surg Pathol Clin 2020;13:377, Pathologica 2021;113:19, Dis Colon Rectum 2004;47:163, Adv Clin Exp Med 2016;25:719, Am J Clin Pathol 2015;144:579, WHO Classification of Tumours Editorial Board: Digestive System Tumours, 5th Edition, 2019
Test question answer:
A. INSM1 (insulinoma associated protein 1) is a sensitive and specific marker of neuroendocrine differentiation in neuroendocrine neoplasms. LCNEC of the colon is a rare highly aggressive subtype of colorectal cancer with a poor prognosis. The WHO criteria for mixed neuroendocrine nonneuroendocrine neoplasm requires at least 30% of each component.
All cases are archived on our website. To view them sorted by case number, diagnosis or category, visit our main Case of the Month page. To subscribe or unsubscribe to Case of the Month or our other email lists, click here.
Thanks to Drs. Aaron Huber and Diana Agostini-Vulaj, University of Rochester Medical Center, Rochester, New York, USA for contributing this case and discussion and to Dr. Raul Gonzalez, Emory University School of Medicine, Atlanta, Georgia, USA for reviewing the discussion.

Case of the Month #531
Clinical history:
An 85 year old woman with a remote history of right sided colon cancer underwent a transverse colon resection for a newly diagnosed colon cancer.
Histopathology images:
What is your diagnosis?
Click here for diagnosis, test question and discussion:
Diagnosis: Poorly differentiated neuroendocrine carcinoma, large cell type
Test question (answer at the end):
Which of the following is true of poorly differentiated neuroendocrine carcinoma, large cell type in the colon?
A. INSM1 expression is a sensitive and specific immunohistochemical marker for neuroendocrine differentiation
B. Mixed neuroendocrine nonneuroendocrine neoplasm may be diagnosed if the poorly differentiated neuroendocrine carcinoma comprises only 5% of the total tumor volume
C. This tumor has an overall good prognosis
D. This tumor is a common morphologic subtype of colorectal cancer
Stains:
Discussion:
In the current World Health Organization (WHO) classification of neuroendocrine neoplasms of the gastrointestinal tract, two types of poorly differentiated neuroendocrine carcinoma are recognized, small cell and large cell. Although only 5 - 7% of well differentiated neuroendocrine tumors of the gastroenteropancreatic tract arise in the colon, 25% of all gastroenteropancreatic neuroendocrine carcinomas arise at this site. Colonic neuroendocrine carcinoma is rare and comprises only ~0.6% of all colorectal carcinomas. Large cell neuroendocrine carcinoma (LCNEC) of the colon occurs at a mean age of 65 years, affects men slightly more than women and has a clinical presentation similar to that of conventional colorectal adenocarcinoma. Regardless of site, neuroendocrine carcinoma in the gastrointestinal tract behaves aggressively and the majority are at an advanced stage with metastatic disease at the time of diagnosis.
LCNEC is comprised of an infiltrative tumor that has an organoid architecture with trabecular, rosette-like and solid patterns. The nests of tumor cells commonly demonstrate central necrosis. There may be single cell necrosis or large areas of geographic necrosis. Apoptotic cells and mitotic figures are easily identifiable. The tumor cells in LCNEC are medium to large with amphophilic or basophilic cytoplasm and large, ovoid and hyperchromatic nuclei with nucleoli. Neuroendocrine differentiation can be confirmed with immunohistochemical stains. Some LCNEC are associated with overlying epithelial dysplasia (adenoma), which suggests that these may be a precursor lesion to LCNEC. Colonic LCNEC is positive for synaptophysin (usually strongly positive) and chromogranin (this may be faintly or focally positive or entirely absent). Nonspecific neuroendocrine markers such as CD56 and neuron specific enolase (NSE) are commonly positive. The newer immunohistochemical marker INSM1 (insulinoma associated protein 1) is a very sensitive and specific marker of neuroendocrine differentiation in gastrointestinal neuroendocrine neoplasms. CDX2 and TTF1 may also be positive. The Ki67 labeling index is typically over 80% of neoplastic cells. Notably, a Ki67 is not necessary in these clearly high grade poorly differentiated neuroendocrine carcinomas. Mismatch repair (MMR) immunohistochemistry may demonstrate MMR deficiency in ~15% of cases. LCNEC tends to harbor a high mutational burden, with mutations in p53 and RB1 being most common. Other mutations that may be identified include SMAD4 (DPC4), KRAS, FHIT, DCC, MEN1 and BRAF.
The histologic differential diagnoses include small cell carcinoma, mixed neuroendocrine nonneuroendocrine neoplasm (MiNEN), and metastatic neuroendocrine carcinoma. Often, the most challenging morphologic differential diagnosis is with small cell carcinoma of the colon. Small cell carcinoma, as the name implies, is composed of small cells with scant cytoplasm and hyperchromatic nuclei, often with nuclear molding. The morphology between small cell and LCNEC may overlap considerably and it may be difficult to separate these two types of poorly differentiated neuroendocrine carcinoma; however, the clinical management and outcome for either type is similar. MiNEN is the new nomenclature for a mixed tumor typically composed of adenocarcinoma and poorly differentiated neuroendocrine carcinoma, that was previously known as mixed adenoneuroendocrine carcinoma (MANEC). However, since the nonneuroendocrine carcinoma is not always adenocarcinoma and the neuroendocrine component is not always a poorly differentiated neuroendocrine carcinoma, the term MiNEN was adopted. MiNEN is differentiated from a pure LCNEC by findings of at least 30% of a nonneuroendocrine carcinomatous component within the same tumor. Lastly, metastatic neuroendocrine carcinoma may be difficult to separate on morphologic and immunophenotypic findings alone. In this scenario, correlation with the clinical and radiographic picture is of utmost importance.
In summary, LCNEC of the colon is a rare type of colorectal carcinoma that has an aggressive course and overall poor prognosis. These tumors are composed of intermediated to large sized cells with enlarged and hyperchromatic nuclei with nucleoli. Neuroendocrine markers such as synaptophysin, chromogranin, INSM1 and to a lesser extent CD56 and NSE may be used to confirm neuroendocrine differentiation. Common mutations identified in LCNEC are p53 and RB1; however, numerous other mutations have been identified. The main differential diagnoses are colonic small cell carcinoma, metastatic neuroendocrine carcinoma and mixed neuroendocrine nonneuroendocrine neoplasm (MiNEN).
References:
Surg Pathol Clin 2020;13:377, Pathologica 2021;113:19, Dis Colon Rectum 2004;47:163, Adv Clin Exp Med 2016;25:719, Am J Clin Pathol 2015;144:579, WHO Classification of Tumours Editorial Board: Digestive System Tumours, 5th Edition, 2019
Test question answer:
A. INSM1 (insulinoma associated protein 1) is a sensitive and specific marker of neuroendocrine differentiation in neuroendocrine neoplasms. LCNEC of the colon is a rare highly aggressive subtype of colorectal cancer with a poor prognosis. The WHO criteria for mixed neuroendocrine nonneuroendocrine neoplasm requires at least 30% of each component.