Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Practice question #1 | Practice answer #1Cite this page: Allard FD. Adenocarcinoma-ampulla. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/ampullaadenocarcinoma.html. Accessed September 15th, 2025.
Definition / general
- Uncommon epithelial malignancy with glandular or mucinous differentiation that has an epicenter in the ampulla of Vater and displays an intestinal, pancreatobiliary or mixed phenotype
- While advanced duodenal, distal common bile duct or pancreatic carcinoma may extend to involve the ampulla, only those malignancies centered on or circumferentially surrounding the ampulla are regarded as ampullary carcinomas
Essential features
- Ampulla of Vater is a complex anatomical region that represents the junction of duodenal and pancreatobiliary type mucosa, resulting in a heterogenous group of malignancies that may arise from this site (Am J Surg Pathol 2012;36:1592)
- Distinguishing ampullary / periampullary primaries from duodenal, distal common bile duct and pancreatic ductal primaries is based on careful gross examination to assess the tumor epicenter
- Distinguishing intestinal from pancreatobiliary type tumors is an important prognostic factor; immunostains are helpful adjuncts to morphologic assessment (Int J Surg Pathol 2019;27:598)
ICD coding
- ICD-O
- ICD-11: 2B80.20 - adenocarcinoma of small intestine, site unspecified
Epidemiology
- Rare, with an incidence of 4 - 10 cases/1 million population (Eur J Gastroenterol Hepatol 2000;12:75, J Surg Oncol 2009;100:598, Cancer Causes Control 2007;18:415, Cancer 2019;125:1489)
- M:F = 1.48:1 in one large series (J Surg Oncol 2009;100:598, Am J Surg Pathol 2012;36:1592)
- Commonly presents between the ages of 60 and 80 (median: 65 years) (J Surg Oncol 2009;100:598, Am J Surg Pathol 2012;36:1592)
- Associated with familial adenomatous polyposis (FAP), Lynch syndrome, Gardner syndrome, Peutz-Jeghers syndrome and neurofibromatosis (Gastroenterology 2001;121:1127, Mod Pathol 2001;14:1169, Gastroenterology 1992;102:1980, Am J Med Genet 1980;6:205)
Sites
- Ampullary adenocarcinomas have 4 recognized subtypes (Am J Surg Pathol 2012;36:1592)
- Periampullary carcinomas are exophytic masses that arise from the duodenal surface of the ampulla and engulf the ampullary orifice (~5%)
- Intra-ampullary carcinomas arise from intra-ampullary papillary tubular neoplasms (IAPN, ~25%)
- Ampullary ductal carcinomas arise from the portions of the distal common bile or pancreatic ducts located within the papilla of Vater and circumferentially involve the duct within the papilla (~15%)
- Ampullary carcinomas, NOS are ulceronodular tumors located at the papilla of Vater that do not show the specific features of the 3 categories listed above (~55%)
Pathophysiology
- Experts theorize that the tendency for small bowel adenocarcinomas as well as familial adenomatous polyposis related adenocarcinomas to occur in the ampullary region may be related to exposure to bile and pancreatic secretions
- Known precursor lesions include (Am J Surg Pathol 2012;36:1592)
- Intra-ampullary adenocarcinomas: intra-ampullary papillary tubular neoplasms
- Periampullary adenocarcinoma: intestinal type adenoma
- Ampullary ductal adenocarcinomas: some tumors show intraepithelial neoplasia; however, this is difficult to distinguish from colonization of the surface epithelium by invasive carcinoma cells
- Ampullary carcinoma, NOS: precursor lesion unclear
Etiology
- For the vast majority of tumors, a specific etiology cannot be determined (Am J Surg Pathol 2012;36:1592, Am J Surg Pathol 2010;34:1731, J Clin Oncol 2013;31:1348)
Clinical features
- Rare, accounting for 0.2% of gastrointestinal cancers and 6 - 9% of periampullary malignancies (Am Soc Clin Oncol Educ Book 2014:112)
- Patients often present with persistent jaundice, abdominal pain, pancreatitis or weight loss
- Overall 5 year survival is 39 - 45% for patients who undergo resection (Hepatobiliary Pancreat Dis Int 2018;17:443, J Surg Oncol 2009;100:598, Am J Surg Pathol 2012;36:1592)
Diagnosis
- Confirmation that a tumor is an ampullary carcinoma is best done on careful gross assessment of the resection specimen to determine the tumor epicenter (Am J Surg Pathol 2012;36:1592)
- It is also important to look for precursor lesions that can support the origin of a periampullary tumor: pancreatic intraepithelial neoplasms for pancreatic cancer, intestinal adenoma for duodenal cancer, biliary intraepithelial neoplasms or intraductal papillary neoplasms for biliary (distal) cancer (see Pathophysiology) (Am J Surg Pathol 2012;36:1592)
Radiology description
- Endoscopic retrograde cholangiopancreatography (ERCP) is the most useful endoscopic study for diagnosing ampullary carcinoma; it permits tumor identification, biopsy and biliary decompression with a single procedure (Am J Surg 1997;174:355)
- MRI plus magnetic resonance cholangiopancreatography (MRCP) has shown good performance in differentiating between malignant and benign ampullary lesions (BMC Med Imaging 2019;19:77)
- MRI diagnostic accuracy for ampullary lesions has been reported to be as high as 91.17% (BMC Med Imaging 2019;19:77)
Prognostic factors
- Ampullary adenocarcinomas with pancreaticobiliary histology have a much worse outcome than those with intestinal histology (J Clin Oncol 2013;31:1348, J Am Coll Surg 2008;207:210, BMC Cancer 2013;13:428, World J Surg Oncol 2022;20:406)
- Decreased overall survival is associated with advanced age, tumor grade, tumor size, lymph node ratio, higher stage disease (pT3 - 4), lymph node metastasis, lymphovascular invasion, higher histologic grade tumors, perineural invasion and elevated serum CA 19-9 and CEA levels (Int J Radiat Oncol Biol Phys 2006;66:514, Ann Surg Oncol 2019;26:1079, J Am Coll Surg 2008;207:210, Am J Surg 2000;180:13, Br J Surg 2004;91:1600, Ann Surg Oncol 2003;10:1176, Pancreas 2019;48:70, J Gastrointest Surg 2008;12:1422)
- Positive prognostic indicators include serum bilirubin of 75 micromol/L or less and age < 70 years (Br J Surg 2004;91:1600)
- Prognosis (survival) by subtype: periampullary carcinoma > intra-ampullary > ampullary, NOS > ampullary ductal carcinoma (Am J Surg Pathol 2012;36:1592)
Case reports
- 11 year old boy presented with obstructive jaundice due to ampullary adenocarcinoma (youngest patient reported) (J Pediatr Surg 1997;32:636)
- 45 year old man who presented with abdominal pain, nausea, vomiting and a fever was found to have ampullary adenocarcinoma (Cureus 2022;14:e29398)
- 58 year old man with familial adenomatous polyposis presented with ampullary adenocarcinoma (Bratisl Lek Listy 2019;120:908)
- 74 year old man who presented with decompensated cirrhosis and choledocholithiasis associated with an ampullary adenocarcinoma (Cureus 2023;15:e37566)
- 77 year old man who presented with 2 rare synchornous primaries: ampullary adenocarcinoma and ileal gastrointestinal stromal tumor (World J Gastrointest Oncol 2022;14:2253)
Treatment
- Management of early stage disease is primarily surgical, typically pancreatoduodenectomy (Whipple procedure) followed by adjuvant chemoradiation (Am Soc Clin Oncol Educ Book 2014:112)
- Curative resection rates for early stage disease are as high as 80 - 90% with modern techniques at high volume centers (Am Surg 1999;65:1043, J Surg Oncol 2009;100:651)
- Local resection / ampullectomy is sometimes an option for patients who are poor surgical candidates / have significant comorbidities and is reported to offer lower surgical morbidity; however, recurrence rates are higher (Dig Surg 2003;20:511, Ann Surg Oncol 2005;12:971, Ann Surg 1996;224:621)
- Unresectable disease is treated systemically with gemcitabine combined with a platinum compound plus radiation therapy (Am Soc Clin Oncol Educ Book 2014:112, N Engl J Med 2010;362:1273)
- Small retrospective studies have suggested that patients with pancreatobiliary type carcinomas may benefit from gemcitabine therapy while those with intestinal type tumors may benefit from 5-fluorouracil (5-FU) based regimens (BMC Cancer 2008;8:170, Am J Surg Pathol 2014;38:1371)
- A large, multicenter European study group for pancreatic cancer (ESPAC)-3 periampullary randomized controlled trial demonstrated no significant overall survival benefit from adjuvant chemotherapy (fluorouracil or gemcitabine) (JAMA 2012;308:147)
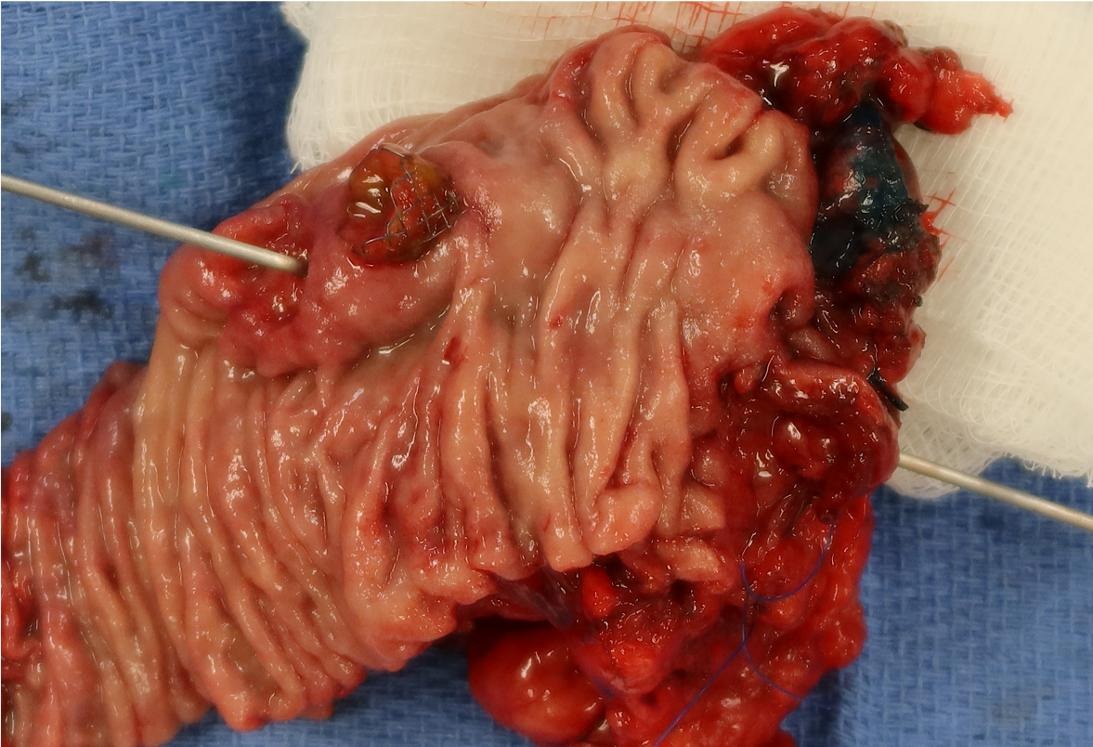
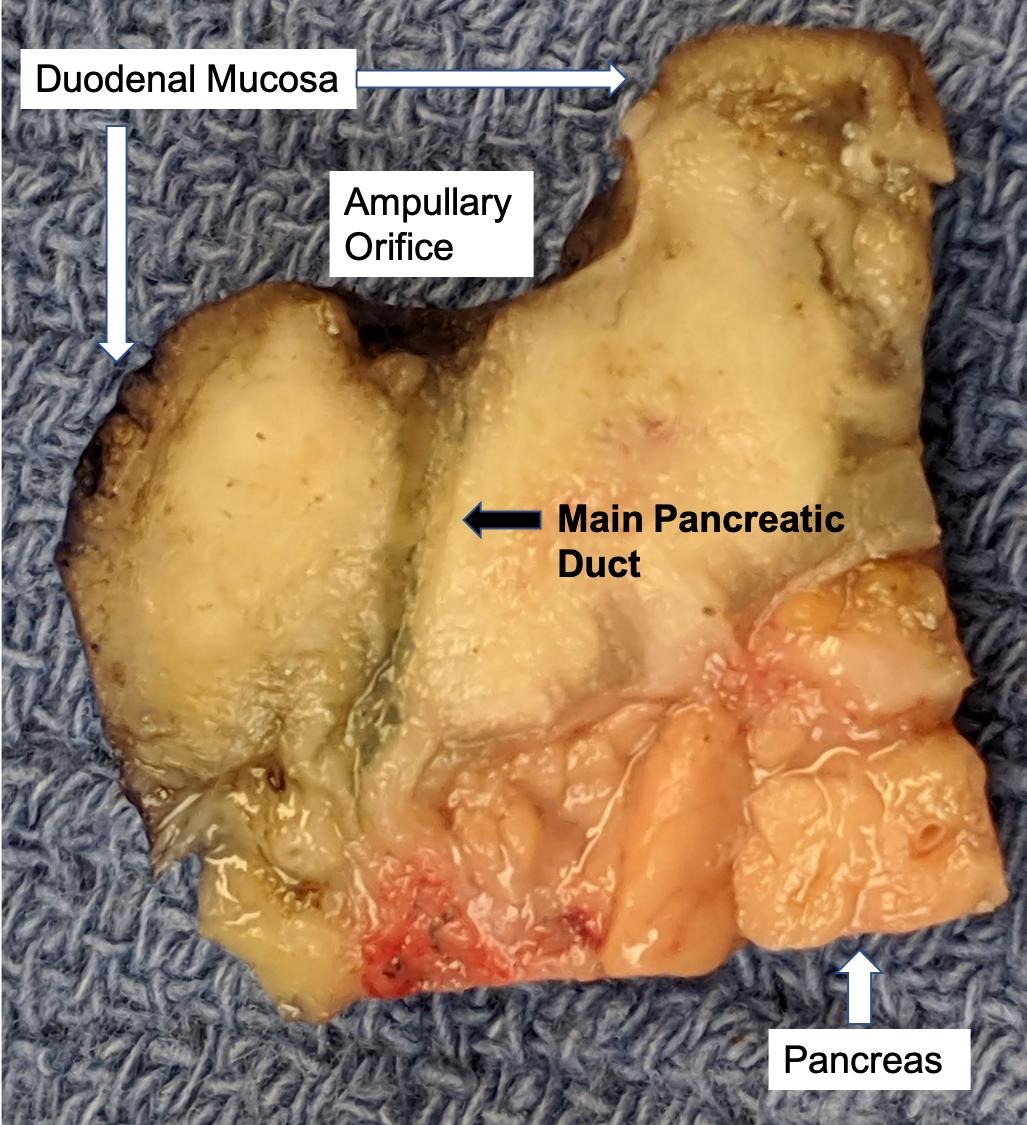
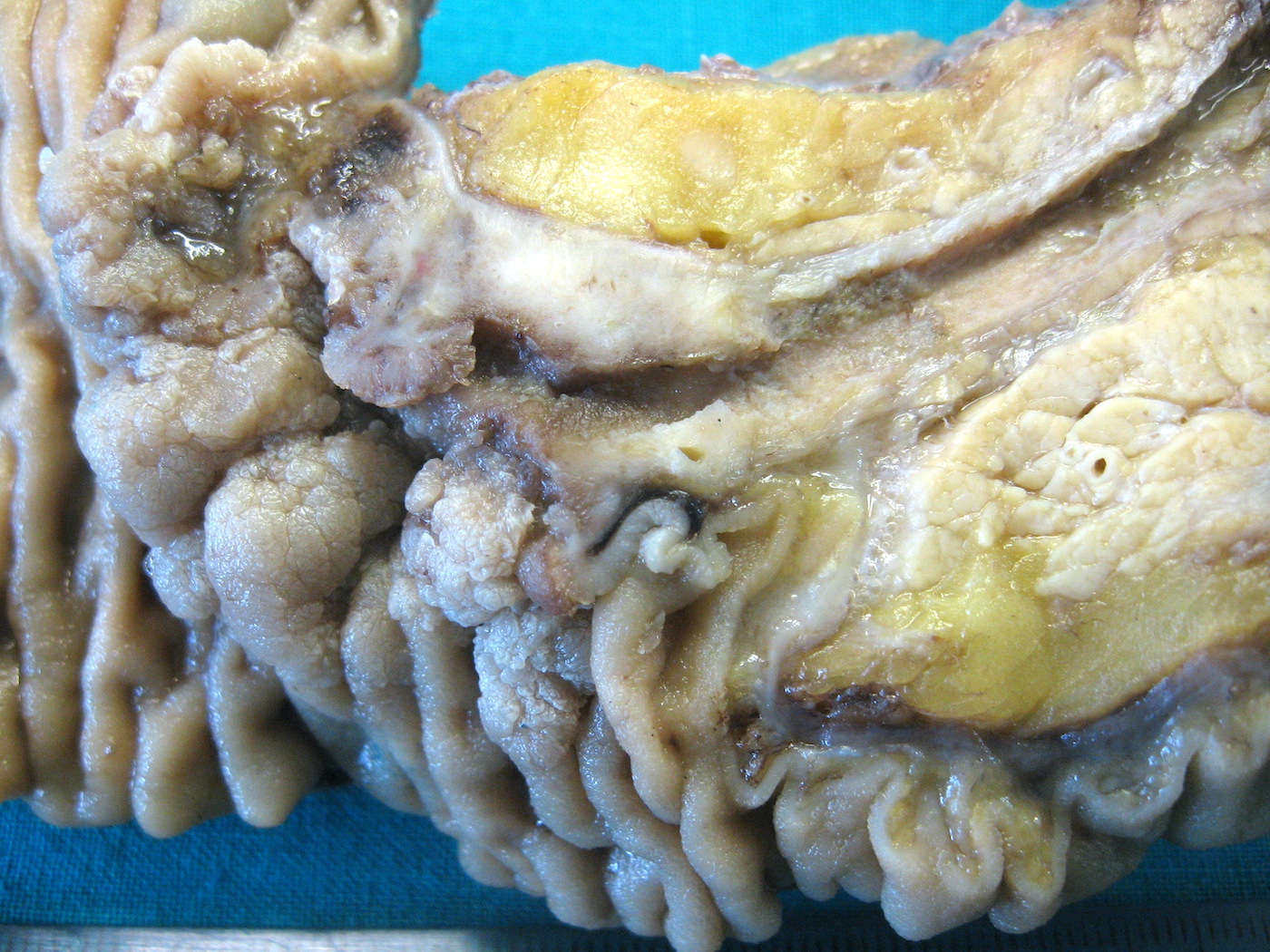
Gross description
- Gross appearance depends on the region of the ampulla involved (Am J Surg Pathol 2012;36:1592)
- Periampullary adenocarcinomas
- Exophytic mass arising from the duodenal aspect of the ampulla
- Vegetating mass around the ampulla that may obscure the ampullary orifice
- Invasive component of the lesion may extend beyond the ampulla to involve the adjacent duodenal wall
- Large tumors: average size of 4.7 cm with a 2.4 cm invasive component
- 50% of cases have lymph node involvement at time of resection
- Intra-ampullary adenocarcinomas
- Arising from an intra-ampullary papillary tubular neoplasm
- Appear as a mucosa covered bulge with a dilated ampullary orifice and bulky intraluminal growth within the ampulla
- Average tumor size of 2.9 cm with an invasive component of 1.5 cm
- 28% of cases have lymph node involvement at time of resection
- Ampullary ductal adenocarcinomas
- Appear as small concentric elevations and ulcerating retractions around the ampullary orifice
- Upon bivalving the specimen along the duct, concentric thickening with or without stricturing of the intra-ampullary duct will be seen
- Small tumor size: average of 1.9 cm
- Low incidence of lymph node spread
- Ampullary carcinoma, NOS
- Tumor is grossly centered in the ampulla
- Lacks the specific characteristics of the subtypes listed above
- Often presents as an ulceration of the papilla with dilation of both the common bile duct and main pancreatic duct
- Periampullary adenocarcinomas
- Careful gross assessment of tumor extension into the duodenal wall and the pancreas or peripancreatic soft tissue as well as any major vessels is important for correct staging
- Average gross size of tumors: 2.6 cm with invasive component measuring 1.8 cm (Am J Surg Pathol 2012;36:1592)
Gross images
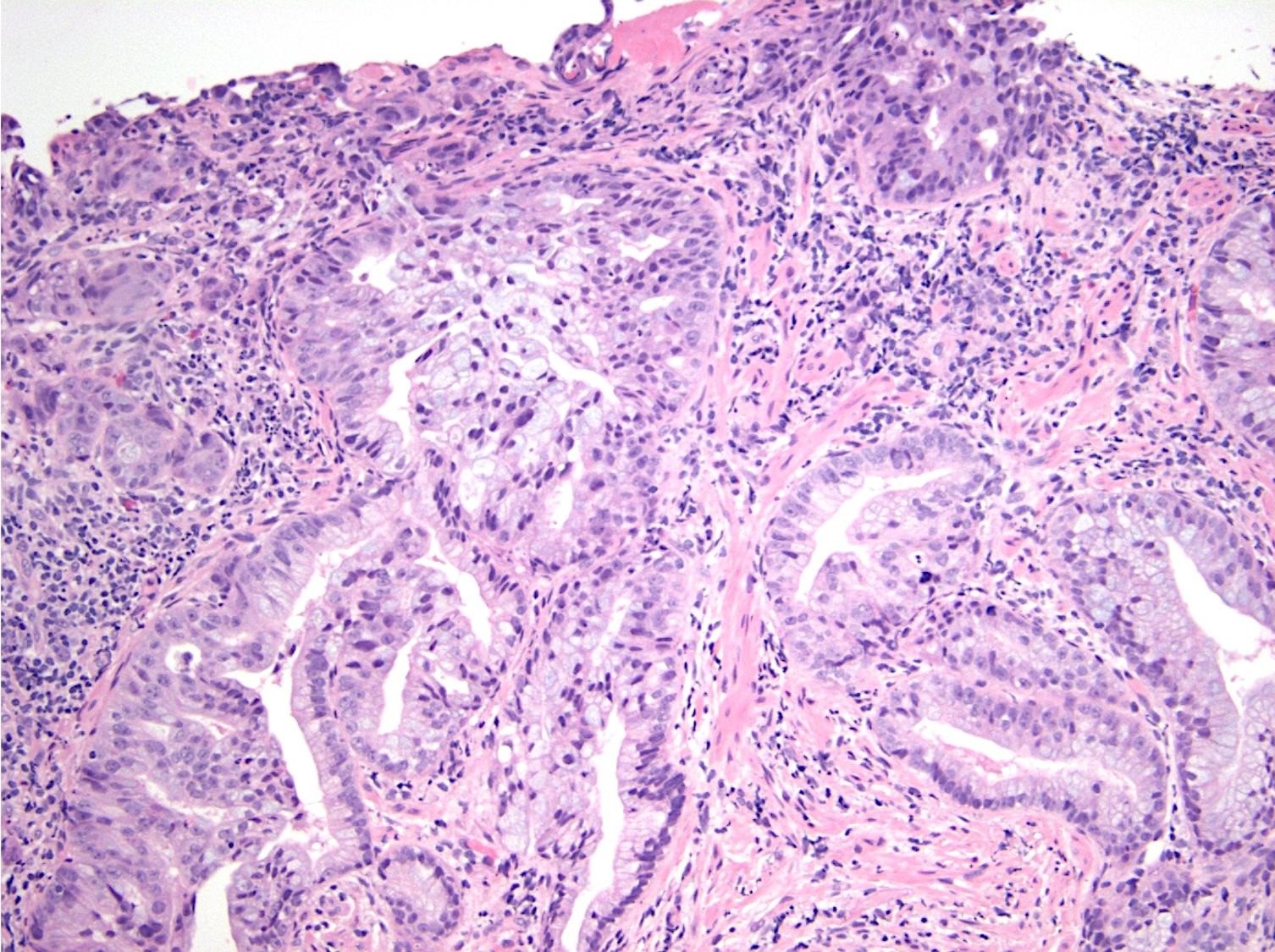
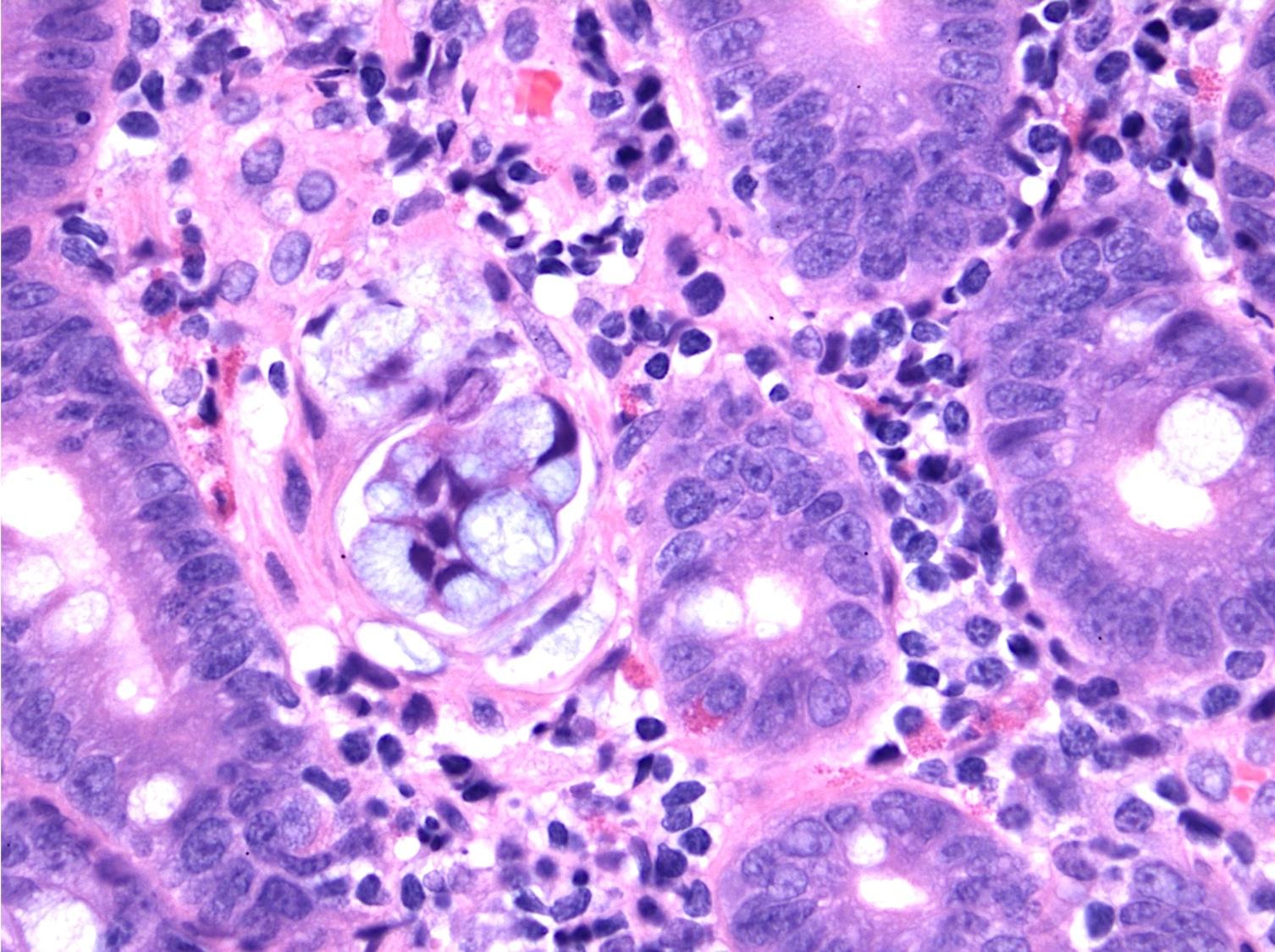
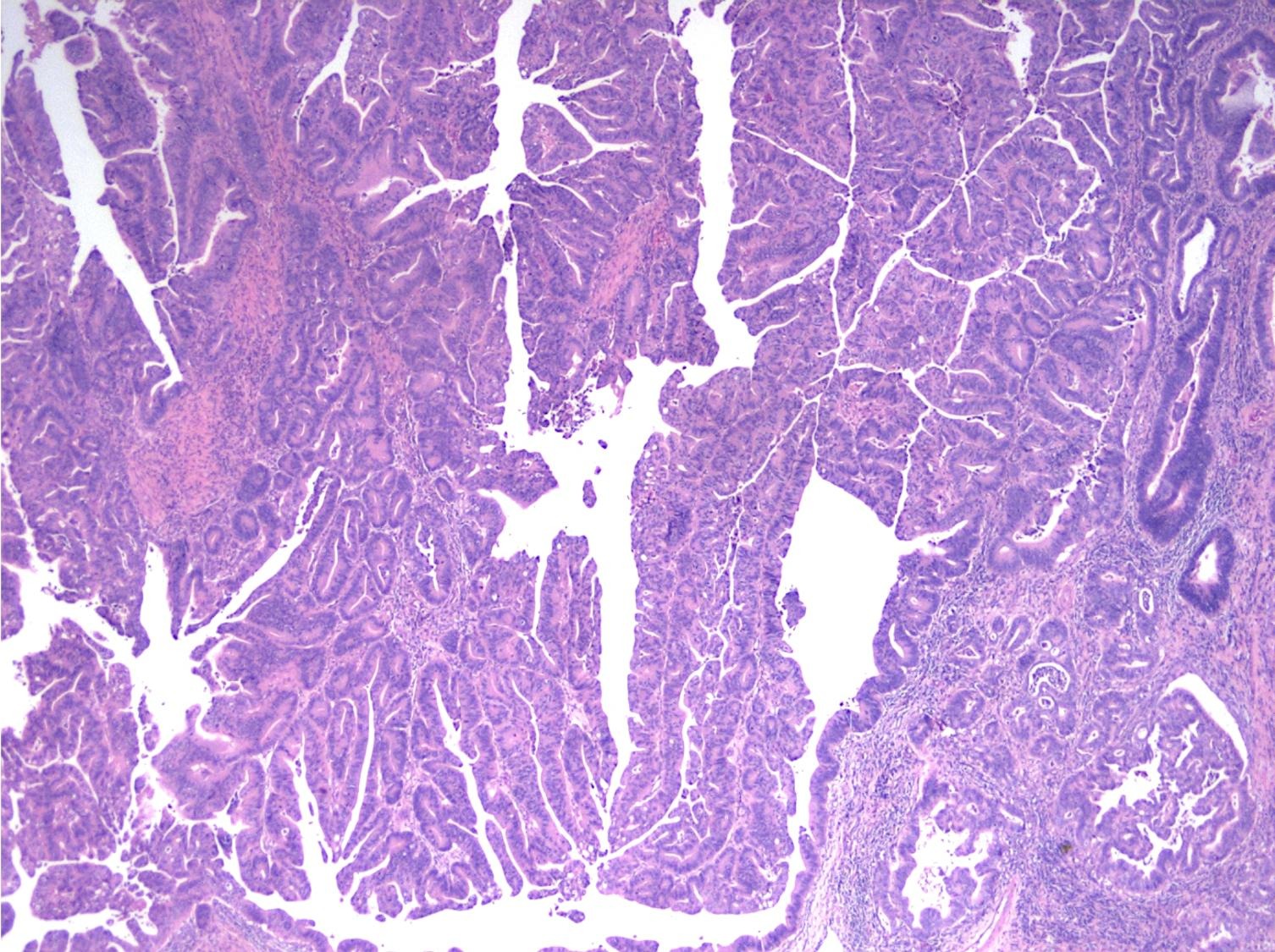
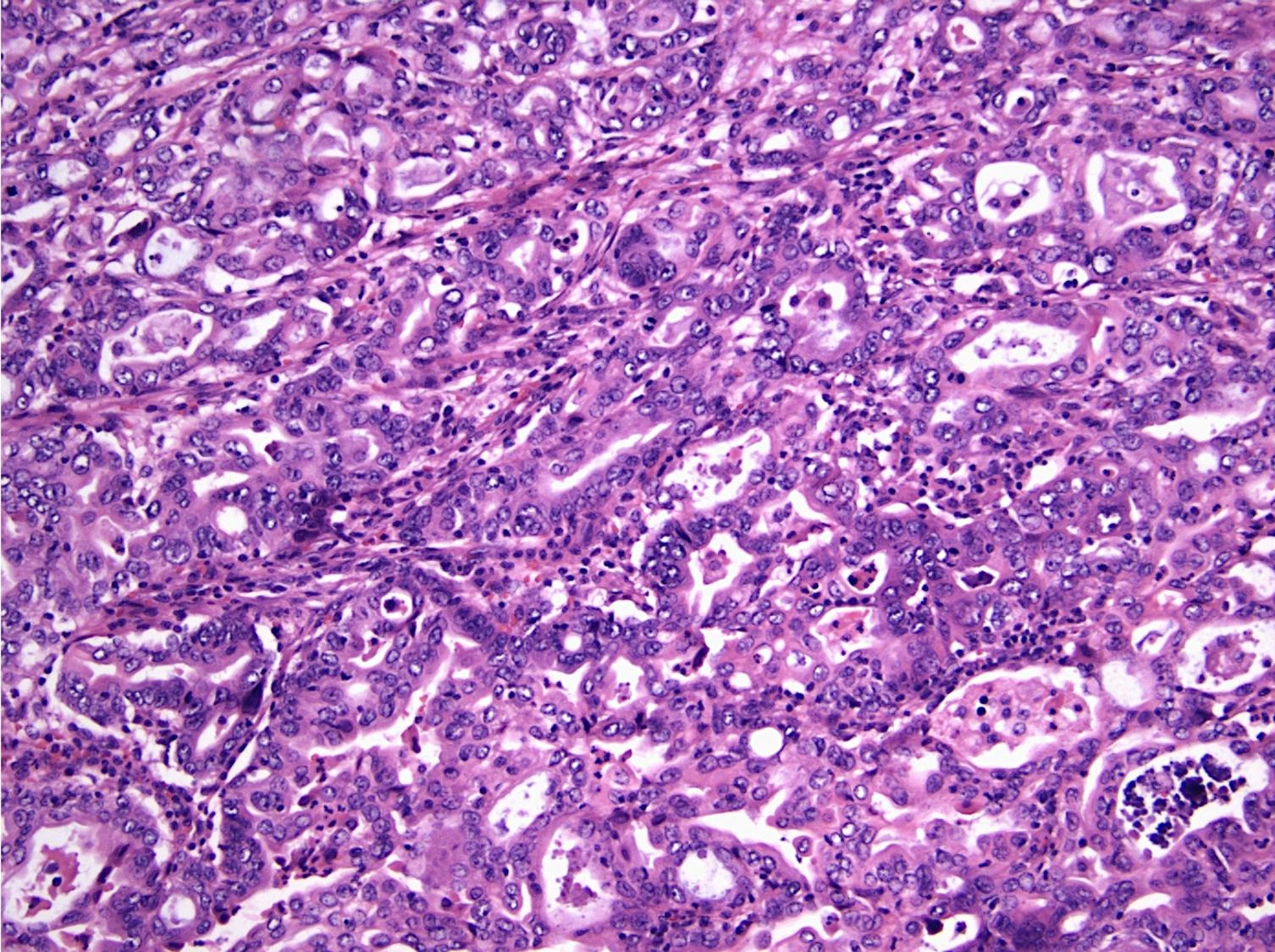
Microscopic (histologic) description
- Majority are gland forming (tubular adenocarcinoma)
- 60% show either intestinal or biliary phenotypes while 40% have a mixed phenotype (Mod Pathol 2016;29:1575)
- Intestinal type: columnar cells with elongated, pseudostratified nuclei with scattered goblet cells and Paneth cells
- Pancreatobiliary type: cuboidal cells with pleomorphism forming small glands in desmoplastic stroma
- Mixed type: shows mix of intestinal and pancreatobiliary types
- Nonglandular patterns include:
- Mucinous adenocarcinoma: > 50% stromal mucin pools containing floating tumor cells / glands with an intestinal phenotype
- Poorly cohesive cell carcinoma
- Medullary carcinoma
- Adenosquamous carcinoma: this extremely rare mixed tumor shows both morphologic and immunophenotypic evidence of both glandular and squamous differentiation (World J Surg Oncol 2015;13:287, World J Surg Oncol 2013;11:124)
- Undifferentiated carcinoma
- Undifferentiated carcinoma with osteoclast-like giant cells: tumor that is comprised of sarcomatoid appearing mononuclear cells and contains osteoclast-like giant cells
- Undifferentiated carcinoma with rhabdoid phenotype: discohesive tumor cells show abundant eosinophilic intracytoplasmic rhabdoid bodies and are present in a myxoid matrix (Am J Surg Pathol 2016;40:544)
- Histologic features by subtype (Am J Surg Pathol 2012;36:1592)
- Intra-ampullary adenocarcinomas
- Majority of the lesion often consists of the precursor intra-ampullary papillary tubular neoplasm
- Majority show an intestinal phenotype
- Growth patterns include papillary, tubular and tubulopapillary
- Noninvasive precursor component may display a different epithelial phenotype than the invasive component
- Periampullary adenocarcinomas
- Majority are intestinal type
- May show mucinous or signet ring cell patterns
- Ampullary ductal carcinomas
- Pancreatobiliary type
- May show focal micropapillary or sarcomatoid areas
- Ampullary carcinoma, NOS
- Lacks the specific characteristics of the above subtypes
- Heterogenous histologic types: 45% pancreatobiliary type, 27% intestinal type, 28% mixed or other type
- Intra-ampullary adenocarcinomas
Microscopic (histologic) images
Cytology description
- Cellular to moderately cellular preparations
- Malignant cells when grouped are typically crowded and present in 3 dimensional clusters
- Single pleomorphic cells are often present
- Nuclei are typically enlarged and irregular with an increased N:C ratio, coarse chromatin and prominent nucleoli are often present
- Necrosis can occasionally be seen (J Clin Pathol 2001;54:449, Cancer 2005;105:289)
- In one study, 13/35 ampullary adenocarcinomas were identified via EUS FNA sampling (Cancer 2005;105:289)
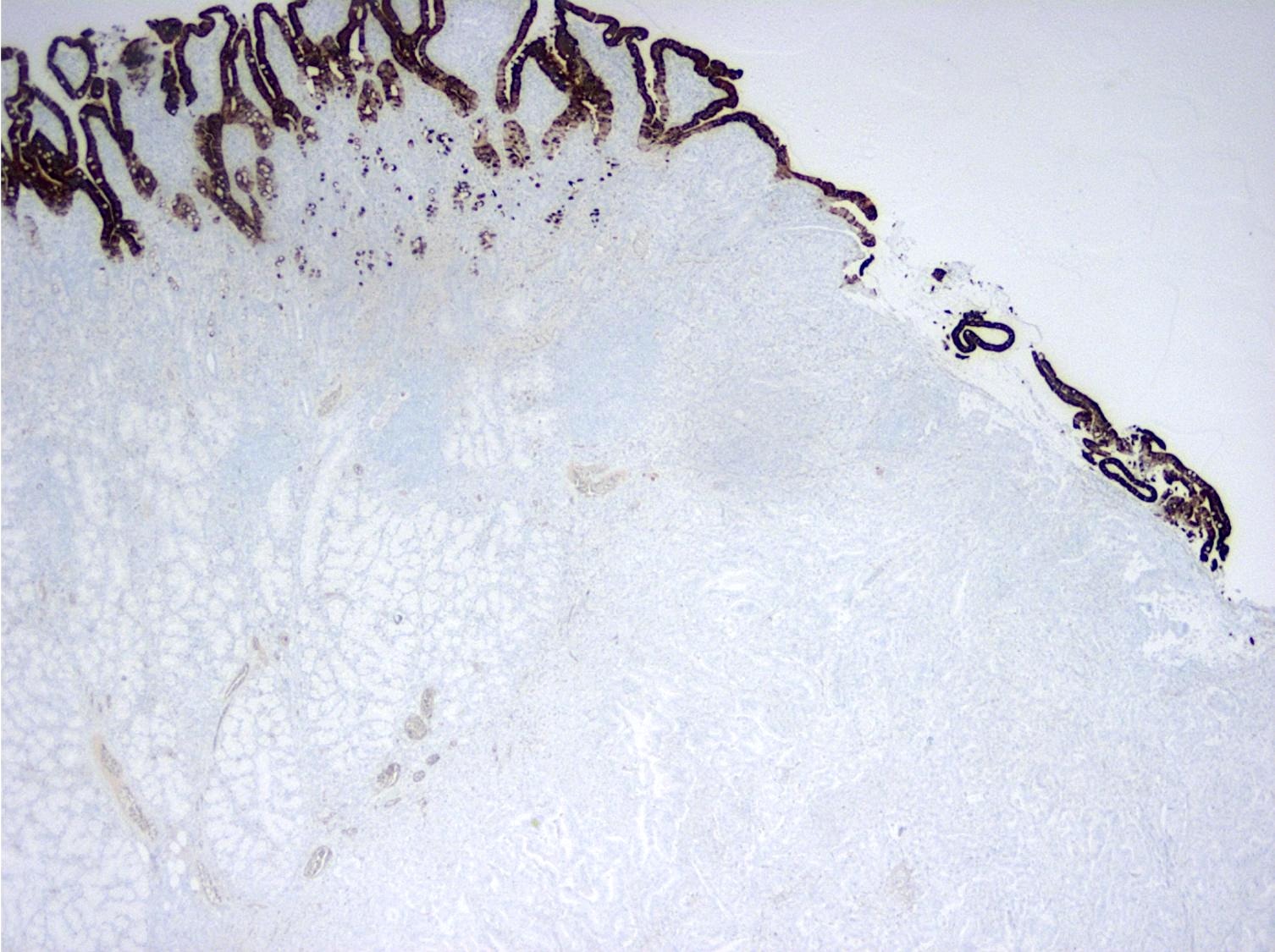
Positive stains
- Intestinal type tumors are typically positive for CK20 (50%), CDX2 (> 25% of tumor staining, 89.5%) and S100P (63%) with variable CK7 (70 - 80%) (Am J Surg Pathol 2014;38:1371, Hum Pathol 2013;44:2213, J Clin Pathol 2019;72:762)
- Pancreatobiliary type tumors are positive for MUC1 (100%), CK7 (91%) (Am J Surg Pathol 2014;38:1371)
- 18 - 20% can be ambiguous (mixed type or a nonglandular tumor subtype) by IHC (Am J Surg Pathol 2014;38:1371, Br J Cancer 2019;120:697)
- Undifferentiated carcinoma with osteoclast-like giant cells: often show mutational p53 staining pattern with giant cells showing immunoreactivity for CD68 (Am J Surg Pathol 1998;22:1247)
- Undifferentiated carcinoma with rhabdoid phenotype: loss of nuclear SMARCB1 / INI1 expression (Am J Surg Pathol 2016;40:544)
Negative stains
- Intestinal type tumors are typically positive for MUC2 (39.5%), MUC5AC (41%) (Am J Surg Pathol 2014;38:1371, Hum Pathol 2013;44:2213, J Clin Pathol 2019;72:762)
- Intestinal type tumors are typically negative for MUC1 (EMA) (Am J Surg Pathol 2014;38:1371)
- Pancreatobiliary type tumors are typically negative for CDX2 (< 25% of tumor staining), MUC2 and CK20 (Am J Surg Pathol 2014;38:1371, Hum Pathol 2013;44:2213)
Molecular / cytogenetics description
- KRAS mutations are present in 30 - 40%, may be associated with worse disease free survival and are more frequently found in pancreatobiliary type tumors (Oncotarget 2016;7:58001, Br J Cancer 2019;120:697)
- TP53 is also a negative predictor of survival, regardless of phenotype (Ann Surg 2018;267:149)
- Microsatellite instability is present in 10 - 15%, usually in intestinal type tumors (Am J Surg Pathol 2009;33:691)
- Other mutations detected include: APC, PIK3CA, SMAD4, BRAF, CDKN2A, FBXW7, TP53, APC, ELF3 and RAS mutations (Br J Cancer 2019;120:697, J Pathol Transl Med 2021;55:192, Cancer Cell 2016;29:229)
- Targetable mutations have been identified in both intestinal and pancreatobiliary phenotype tumors (J Pathol Transl Med 2021;55:192, Ann Surg 2018;267:149)
- General: ERBB, WNT and PI3K
- Intestinal phenotype: PI3 / AKT
- Pancreatobiliary phenotype: RAS / RAF and PI3 / AKT
- BRAF and KRAS are mutually exclusive (Br J Cancer 2019;120:697)
Sample pathology report
- Pancreas, small bowel and distal common bile duct, pancreatoduodenectomy:
- Ampullary adenocarcinoma, pancreatobiliary type, poorly differentiated, invasive into the pancreatic head (pT3a) (see synoptic report)
- 3 of 15 lymph nodes positive for carcinoma and 1 tumor deposit (3/15, pN1)
- Perineural as well as extensive lymphovascular and large vessel invasion is present
- Resection margins are free of high grade dysplasia and carcinoma (closest approximation: 0.3 cm to retroperitoneal margin)
- Use ampulla pTMN for staging
Differential diagnosis
- Intra-ampullary papillary tubular neoplasm:
- No invasion present
- Adenomatous changes in submucosal glands / ductules simulating invasion:
- No desmoplastic stroma or single cell invasion present to indicate a truly invasive process
- Dysplastic ducts will have round, regular contours and be present in a lobular configuration in most cases
- Extrahepatic cholangiocarcinoma:
- Tumor may show concentric thickening of the distal common bile duct but tumor will not be centered in the ampulla
- Duodenal adenocarcinoma:
- Tumor may also arise in intestinal type adenomatous mucosa near the ampullary orifice and extend to involve the ampulla but the tumor will not be centered around the orifice
Additional references
Practice question #1
Practice answer #1
C. Pancreatobiliary. The image shows a mass arising at the convergence of the main pancreatic duct and the duodenal mucosal surface, circumferentially surrounding the main pancreatic duct with depression of the duodenal surface. No mass forming lesion is present within the lumen of the duct. Answers A, B and D are incorrect because the gross image is most consistent with an ampullary ductal carcinoma, and the majority of ampullary ductal carcinomas show a pancreatobiliary phenotype, histologically.
Comment Here
Reference: Adenocarcinoma - ampulla
Comment Here
Reference: Adenocarcinoma - ampulla